Abstract
Alzheimer’s disease (AD) is the fourth major cause of mortality in the elderly in the US and the leading cause of dementia worldwide. While pharmacological targets have been discovered, there are no true disease-modifying therapies. We have recently discovered that multiple low-dose infusions of human umbilical cord blood cells (HUCBCs) ameliorate cognitive impairments and reduce Aβ-associated neuropathology in PSAPP transgenic mice. However, the mechanism for these effects of HUCBCs remains unclear. In the present study, we examined whether monocytes, as important components of HUCBCs, would have beneficial outcomes on the reduction of AD-like pathology and associated cognitive impairments in PSAPP transgenic AD model mice. PSAPP mice and their wild-type littermates were treated monthly with an infusion of peripheral human umbilical cord blood cell (HUCBC)-derived monocytes over a period of 2 and 4 months, followed by behavioral evaluations, biochemical, and histological analyses. The principal findings of the present study confirmed that monocytes derived from HUCBCs (CB-M) play a central role in HUCBC-mediated cognition-enhancing and Aβ pathology-ameliorating activities. Most importantly, we found that compared with CB-M, aged monocytes showed an ineffective phagocytosis of Aβ, while exogenous soluble amyloid precursor protein α (sAPPα) could reverse this deficiency. Pretreating monocytes with sAPPα upregulates Aβ internalization. Our further studies suggested that sAPPα could form a heterodimer with Aβs, with the APP672–688 (Aβ1–16) region being responsible for this effect. This in turn promoted binding of these heterodimers to monocyte scavenger receptors and thus promoted enhanced Aβ clearance. In summary, our findings suggest an interesting hypothesis that peripheral monocytes contribute to Aβ clearance through heterodimerization of sAPPα with Aβ. Further, declined or impaired sAPPα production, or reduced heterodimerization with Aβ, would cause a deficiency in Aβ clearance and thus accelerate the pathogenesis of AD.
Keywords: Cord blood, Monocyte, Amyloid β, Soluble amyloid precursor protein α (sAPPα), Heterodimerization
INTRODUCTION
Currently, 26 million people worldwide suffer from Alzheimer’s disease (AD). Indeed, it is predicted that the number of AD cases will quadruple and reach worldwide epidemic proportions by 2050. Amyloid-β (Aβ) plaques, resulting from defective removal or upregulated buildup, are a central manifestation of AD (39). It has been proposed that mechanisms involved in Aβ clearance might be the key to providing insights into therapeutic interventions. The binding of Aβ to receptors that can mediate its clearance has therefore been a major therapeutic focus (10,24,35,41,43). Additionally, there is a possibility that the amyloid precursor protein (APP) metabolite, soluble amyloid precursor protein α (sAPPα), may bind to Aβ, and this may confer beneficial effects for the treatment of AD via increasing its clearance from the brain parenchyma (2,11).
Although recent advances in cellular and molecular targets have yielded strategies for enhancing Aβ clearance and reversing AD-associated Aβ deposition, a cure remains elusive. We previously reported human umbilical cord blood cells (HUCBCs) modulated inflammation, diminished Aβ pathology, and reduced behavioral deficits in PSAPP (APPswe/PS1dE9) transgenic mice (9). In the present study, we attempt to determine which mononuclear cell (MNC) population was conferring these effects. Accumulated AD research has suggested that monocytes among MNCs are contributory to promoting Aβ clearance (9,20,31,32). Therefore, we examined whether monocytes purified from HUCBCs would have beneficial outcomes on the reduction of AD-like pathology and rescue of cognitive impairments in PSAPP transgenic AD mice. PSAPP mice and their wild-type littermates were treated monthly with a peripheral HUCBC-derived monocyte infusion over a period of 2 and 4 months, followed by behavioral evaluations as well as biochemical and histological analyses. The present study extends on the HUCBC AD therapy to (1) examine whether monocytes are responsible for the beneficial effects against AD observed in HUCBC therapeutics and (2) to determine the mechanism by which these salutary effects occur.
MATERIALS AND METHODS
Ethics Statement
All experiments were performed in accordance with the guidelines of the National Institutes of Health, and all animal studies were approved by the University of South Florida (USF) Institutional Animal Care and Use Committee. Animals were humanely cared for during all experiments, and all efforts were made to minimize animal suffering. Animals were anesthetized with isoflourane (50 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) and euthanized by transcardial perfusion with ice-cold physiological saline containing heparin (10 units/ml) (Sigma-Aldrich). In this study, use of human cord blood cells was involved. Ninety-five to 98% of mononuclear cells from HUCBCs were provided by Saneron CCEL Therapeutics Inc. (Tampa, FL, USA). Saneron used de-identified HUCBC donations from certified commercial sources.
Animals
PSAPP (APPswe/PS1dE9) mice and their wild-type (WT) littermates were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). PSAPP mice overproduce human Aβ1–40 and Aβ1–42 peptides (APP Swedish mutation with a PS1 deletion in exon 9) and from about 4 months develop progressive cerebral Aβ deposits with learning and memory impairment, albeit without neuronal loss (1,5,9,17,23). All mice were characterized by PCR genotyping for mutant human APP and presenilin 1 (PS1) transgenes. Thus, all mice used in this study are genetically comparable. Both PSAPP mice and their WT littermates were maintained on a 12-h light/12-h dark cycle at ambient temperature and humidity. All cohorts were housed in the animal facility at the USF, Morsani College of Medicine (Johnnie B. Byrd Sr. Alzheimer’s Center and Research Institute, Tampa, FL, USA). Animals were fed standard rodent chow and water ad libitum. For the cord blood-derived monocyte experiments, 5.5-month-old PSAPP mice and WT litter-mates were used. All cohorts were female, and this served to eliminate the possibility of gender discrepancies among groups. All animals in these experiments were observed in a blinded, randomized approach.
HUCBC Preparation
HUCBCs, comprised of 95–98% mononuclear cells, were obtained using a proprietary density gradient solution (DSS-001) developed by Saneron and GE Healthcare. Our purified HUCBCs were cryopreserved and stored in liquid nitrogen at −210°C. HUCBCs were thawed prior to transplantation, at 37°C for 4 min, washed in 0.05 M phosphate-buffered saline (PBS), quantified and assessed for viability (Cell-Dyn; Abbott Laboratories, Abbott Park, IL, USA and Vi-CELL; Beckman Coulter, Indianapolis, IN, USA), and suspended in PBS to achieve a cell concentration of 2 × 105 cells per 100 μl for each infusion.
HUCBC-Derived Monocyte Acquisition
The HUCBC suspension was centrifuged and resuspended in monocyte buffer, containing 0.5% bovine serum albumin (BSA) (Sigma-Aldrich) and 2 mM EDTA (Cell Signaling, Beverly, MA, USA) in PBS. Monocytes were separated via positive selection using the MACS Miltenyi Biotec CD14+MicroBeads human kit (Miltenyi Biotec Inc., San Diego, CA, USA) (44). CD14+MicroBeads were added to the cell suspension and incubated for 15 min at 4°C. Cells were washed, resuspended in PBS, and run through a column in a magnetic separator. Cells were allowed to flow into a collection flask, and this comprised the monocyte-deficient cord blood (MD-CB) infused in the cohorts. The column was removed and flushed with buffer to obtain an enriched population of cord blood-derived monocytes (CB-M). Acquired monocyte populations were characterized using flow cytometry. Cell counts and viability were determined as described. Cells were centrifuged, resuspended in PBS twice, and aliquots of 2 × 105 cells per 100-μl concentration per mouse were made.
Flow Cytometry Analysis of HUCBC-Derived Monocytes
HUCBC-derived monocytes were stained for identification of the subset monocyte population expressing CD14+ using an anti-CD14+ monoclonal antibody (1 μg/million cells) (Sigma-Aldrich) and FACS analysis by Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA, USA). Only monocyte populations composed of ≥~93% CD14+ cells were used in the study. In addition, further flow cytometry analysis of HUCBC-derived monocytes for membrane surface-associated APP and full-length APP was conducted using an anti-Aβ N-terminal monoclonal antibody (6E10, 1:2,000; Covance Research Products, Emeryville, CA, USA) and an anti-APP C-terminal polyclonal antibody (pAb751/770, 1:1,000; Calbiochem, Billerica, MA, USA) according to manufacturer specifications.
Western Blot Analysis for APP Levels
Cell lysates from cultured HUCBCs and HUCBC monocytes were assayed by Western blot (WB) analysis. In brief, cells were cultured then lysed in ice-cold lysis buffer (Cell Signaling) (1 mM Na3VO4, 150 mM NaCl, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 20 mM Tris pH 7.5, 1 mM EGTA, 1% v/v Triton X-100, 1 mM β-glycerolphosphate, 1 μg/ml leupeptin), plus 1 mM PMSF (Sigma-Aldrich). Inherent proteins were separated using 10% gel, transferred to 0.2-μm nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and visualized using standard immunoblotting protocol. All antibodies were diluted in 0.05 M Tris-buffered saline (TBS) (Sigma-Aldrich), containing 5% (w/v) nonfat dry milk (Bio-Rad). Membranes were immunoblotted with appropriate primary antibody and then probed using an anti-mouse IgG (1:2,000; Cell Signaling) or an anti-rabbit IgG (1:10,000; Thermo Fisher Scientific, Waltham, MA, USA) secondary antibody conjugated with horseradish peroxidase. Proteins were detected with Super Signal West Femto Maximum Sensitivity Substrate and BIOMAX-MR Film (Thermo Fisher Scientific). Primary antibodies include an anti-APP N-terminal monoclonal antibody (22C11, 1:2,000, EMD Millipore, Temecula, CA, USA), an anti-APP C-terminal polyclonal antibody (pAb751/770, 1:1,000) to evaluate APP expression, an anti-Aβ1–16 monoclonal antibody (6E10, 1:2,000), and an anti-β-actin monoclonal antibody (1:4,000; Sigma-Aldrich).
HUCBC-Derived Monocyte Infusion
Briefly, 23 PSAPP mice and WT littermates were randomly assigned into the following four treatment groups: whole HUCBCs (WCB, n = 6), HUCBC-derived monocytes (CB-M, n = 6), monocyte-deficient HUCBC (MD-CB, n = 6), or PBS (n = 5). With respect to the number of PSAPP and WT mice used in the study, each group, with the exception of the PBS-treated cohorts, comprised n = 3 animals per strain. The PBS-treated group consisted of WT (n = 2) and PSAPP (n = 3). The right tail vein of PSAPP mice or their WT littermates was identified and vasodilated using warm water, and then 2 × 105 cells/100 μl WCB, CB-M, MD-CB or 100 μL PBS per mouse was delivered via right tail vein injection. The injection was performed four times over 2 months and six times over 4 months. HUCBCs were infused at the end of weeks 1, 3, 5, and 7 for the 2-month treatment and at the end of weeks 1, 3, 5, 7, 9, 11, 13, and 15 for the 6-month treatment. We chose to administer these agents intravenously since it is a readily available approach and shown previously in our laboratory to be effective in reducing AD pathology in mice (9).
Behavioral Tests
Motor and cognitive evaluations were conducted at the end of the 2-month treatment (7.5 months of age) or at the end of the 4-month treatment (9.5 months of age) using the rotarod test for motor activity, as well as the radial arm water maze (RAWM) test and the visible platform in an open pool test for cognitive ability.
Rotarod Test
For 2 consecutive days, mice underwent rotarod test. Mice were positioned on the rod (diameter 3.6 cm) of the equipment (Rotarod 47650 accelerating model; Ugo Basile, Varese, Italy). The rod was set at 4 rpm, and mice were placed five at a time on the rod. Trial time was 5 min, and the rod steadily accelerated from 4 rpm up to 40 rpm. Mice were evaluated by the amount of time they were able to retain their balance on the rod.
Radial Arm Water Maze Test
All mice received 2 days of 15 swims or trials per day. Each swim culminated either when a visible or a submerged underwater goal was located or after 1 min had elapsed. Briefly, the mouse was dropped into a random start arm (predetermined on a score sheet) and allowed to swim until it located and climbed onto the platform (goal) over a period of 1 min. Errors were recorded as any entry into an incorrect arm or failure to enter any arm for the initial 15 s of the trial. Results were analyzed as number of errors made.
Visible Platform in an Open Pool Swim Test
For verification of whether the animals possessed the skills sufficient to complete the water maze task, we used the visible platform in an open pool swim test. In brief, it was performed in the same pool as the RAWM; however, the triangular wedges were removed, and the pool was left open with a visible platform in an imagined quadrant. Latency to find and ascend the platform was measured (60 s maximum).
Tissue Preparation
Subsequent to neurocognitive evaluations, cohorts were anesthetized with isoflourane and sacrificed at either 7.5 or 9.5 months of age. Hindlimbs (for bone marrow) and 500 μl peripheral blood were initially collected, and the mice were then perfused transcardially with an ice-cold physiological saline. Brains were rapidly isolated, and the left hemispheres were frozen immediately in liquid nitrogen and stored at −80°C. For molecular analysis, the left hemispheres were sonicated in RIPA buffer (Cell Signaling Technology) and centrifuged at 14,000 rpm for 1 h at 4°C. Supernatant was transferred to a new tube for soluble Aβ analysis, and the pellet was used for insoluble Aβ extraction as described previously (42). The right hemispheres were placed in 4% paraformaldehyde (Sigma-Aldrich) in PBS at 4°C overnight and then transferred to a graded series of sucrose solutions (10%, 20%, and 30%, each at 4°C overnight) for cryostat sectioning. Sequential 25-μm coronal sections were cut, and free-floating sections were then stored at 4°C in 24-well plates containing PBS with 100 mM sodium azide (Sigma-Aldrich).
Immunohistochemical Analysis
Sections were immunohistochemically stained using an anti-Aβ17–26 monoclonal antibody (4G8, 1:200; Covance Research Products) in conjunction with the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA, USA) coupled with 3,3′-diaminobenzidine (DAB) (Sigma-Aldrich) substrate. For all the staining, a set of sections without adding primary antibody was used as negative staining control. Aβ burden (Aβ immunoreactive area) was determined by quantitative image analysis of Aβ plaques burden in the retrosplenial cortex (RSC), entorhinal cortex (EC), and hippocampus (H) brain regions of PSAPP mice and their WT littermates for each of the groups treated. Images of five 25-μm sections (150 μm apart) through hippocampus and neocortex (RSC, EC brain regions) were captured, and a threshold optical density was obtained that discriminated staining from background. Quantification of 4G8-positive Aβ burden is reported as a percentage of immunolabeled area captured (positive pixels divided by total pixels captured). Quantitative image analysis was performed by a single examiner blinded to sample identities. Data are represented as mean ± SD (n = 4 females).
Enzyme-Linked Immunosorbent Assay
The enzyme-linked immunosorbent assay (ELISA) was performed according to our previous methods (37). Soluble Aβ1–40/42 levels in brain homogenates were analyzed by ELISA using Aβ1–40/42 ELISA kits (Invitrogen, Grand Island, NY, USA) in accordance with the manufacturer’s instructions. Data are represented as mean ± SD of Aβ1–40/42 (ng/mg of total protein).
Monocyte Phagocytosis Assay
Monocytes were acquired as described earlier from both HUCBCs and aged human blood cells (from senior adults over 70 years old). These cells were further characterized for cell surface CD14+ and APP biomarkers using flow cytometry analysis. Phagocytosis of Aβ was conducted as previously described (52). Briefly, primary HUCBC-derived monocytes (CB-M) and aged human blood cell-derived monocytes (aged BC-M) were incubated with 1 μM FITC-Aβ1–42 for 1 h in the absence or presence of sAPPα (100 ng/ml), scavenger receptor class A ligand (SR-A), or unlabeled Aβ (naked Aβ42). Both extracellular and cell-associated FITC-Aβ1–42 from cellular supernatants and lysates were quantified using fluorometric analysis to determine mean fluorescence value for each sample as previously described (53). For each condition, relative fold change values were calculated as mean fluorescence value for each sample at 37°C/mean fluorescence value for each sample at 4°C. In all of the conditions, an additional control without cells was carried out to account for nonspecific adherence of Aβ to the plastic surface of culture plates, and the mean values were normalized to these controls. Monocytes were then fixed and imaged in independent channels using a confocal microscope equipped with Normarski optics.
Cell Culture and Immunoprecipitation
Chinese hamster ovary (CHO) cells overexpressing either WT human APP (CHO/APPwt) or Swedish mutant APP (APPswe) were donated by Dr. Stefanie Hahn and Dr. Sascha Weggen (University of Heinrich Heine, Düsseldorf, Germany). These cells were cultured for 3 h in 10% FBS in DMEM medium, and then the conditioned media were collected, immunoprecipitated using an anti-Aβ17–26 monoclonal antibody (4G8) (1:1,000 dilution; Covance Research Products) and then analyzed by WB using 22C11 (1:1,000 dilution; EMD Millipore) or 6E10 (1:1,000 dilution; Covance Research Products). Alternatively, the conditioned media were immunoprecipitated with 22C11 and then analyzed by WB using 6E10. For our parallel heterodimerization study, truncated sAPPα peptide was incubated with synthesized Aβ1–42 (Sigma-Aldrich), immunoprecipitated with 4G8, and analyzed by WB using 82E1 (1:1,000 dilution; IBL, Minneapolis MN, USA) or 22C11. In addition, CHO/APPswe-derived conditioned media were collected, immunoprecipitated with 4G8, and then analyzed by WB using 82E1 or 22C11.
Statistical Analysis
All data were presented as mean ± SEM or mean ± SD and normally distributed. For the RAWM test, the one-way analysis of variance (ANOVA) followed by post hoc LSD test was performed to compare differences between groups. For the rotarod test, data were analyzed by ANOVA followed by post hoc Bonferroni test. For Aβ burden as well as both soluble and insoluble Aβ1–40/42, a t-test for independent samples followed by a post hoc Bonferroni was used to determine the significant difference between each MD-CB-, WCB-, CB-M-, and PBS-treated group. A value of p < 0.05 was considered significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS), release 18.0 (IBM, Armonk, NY, USA).
RESULTS
Characterization of Purified Human Umbilical Cord Blood-Derived Monocytes
CD14+ monocytes were isolated from HUCBCs using a MACS Miltenyi Biotec CD14+MicroBeads human kit via positive selection and then characterized by FACS analysis. Prior to enrichment, there was a 13% CD14+ monocyte population (Fig. 1A, upper panel), whereas after positive selection the population increased to 95% (Fig. 1A, lower panel). HUCBC-derived monocytes were further investigated for surface and full-length APP using 6E10 and pAb751/770 antibodies, respectively. Antibody 6E10 recognizes total APP, Aβ, and β-CTF, while pAb751/770 antibody specifically recognizes full-length APP. Unfractionated HUCBCs and purified CD14+ monocytes were 48.0% and 93.8% positive for surface APP (Fig. 1B). Conversely, pAb751/770 antibody detected only 0.98% and 3% full-length APP in unfractionated HUCBCs and purified CD14+ monocytes, respectively, indicating full-length APP may have been truncated (Fig. 1C). To further confirm these data, HUCBCs and HUCBC-derived monocyte lysates were analyzed by WB analysis, indicating that HUCBC-derived monocytes express dramatically more total APP than the whole HUCBCs. The pAb751/770 antibody did not detect any band in both whole HUCBCs and HUCBC-derived monocyte fractions (Fig. 1D).
Figure 1.
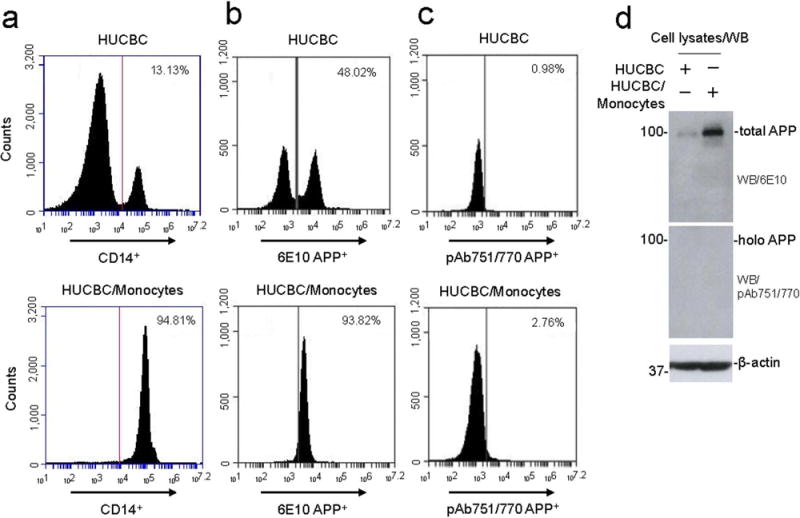
FACS analysis of expression markers in human umbilical cord blood monocytes. Surface expression marker for monocytes such as CD14+, total surface APP, and full-length APP were analyzed using anti-CD14+ (a), anti-Aβ N-terminal (6E10) (b), and anti-APP C-terminal (pAb751/770) (c) antibodies by flow cytometry from HUCBC and HUCBC-derived monocytes. (d) Cell lysates obtained from HUCBC and HUCBC-derived monocytes were subjected to Western blot (WB) analysis. The percentage of CD14+, 6E10+, and pAb751/770 APP+ cells in each quadrant is indicated. Overall, the purity of HUCBC-derived monocytes was found to be almost 95%.
Short-Term (2-Month) Administration of HUCBC-Derived Monocytes Improves Learning, Memory, and Motor Function in PSAPP and WT Mice
To investigate whether HUCBC-derived monocytes could improve locomotive and cognitive function, we administered PBS, whole HUCBCs (WCB), HUCBC-derived monocytes (CB-M), or monocyte-deficient HUCBC (MD-CB) intravenously in PSAPP and control (aged-matched WT) mice over a 2-month time period and then subjected them to rotarod and RAWM testing (Fig. 2A). Beginning with the block 2 trial, CB-M- and WCB-treated PSAPP mice showed significantly fewer errors in RAWM than PBS and MD-CB-treated PSAPP mice (p < 0.01 vs. PBS-PSAPP mice) (Fig. 2B). Likewise, WCB- and CB-M-treated WT mice located the target with significantly fewer errors than PBS-treated WT mice during blocks 3 to 5 and 8 (p < 0.05, p < 0.01 vs. PBS-WT and MD-CB-WT littermates). Interestingly, MD-CB-treated PSAPP mice were cognitively inflexible, and MD-CB-treated WT mice learned less at blocks 6 and 7 when compared to their PBS-treated WT littermates (Fig. 2B). Notably, increased latency to fall over time appeared standard for all mice, while CB-M-treated PSAPP and WT mice displayed overall greater latency to fall. This signified that CB-M-treated PSAPP and WT mice developed enhanced coordination and balance abilities over PBS-treated PSAPP and WT mice.
Figure 2.
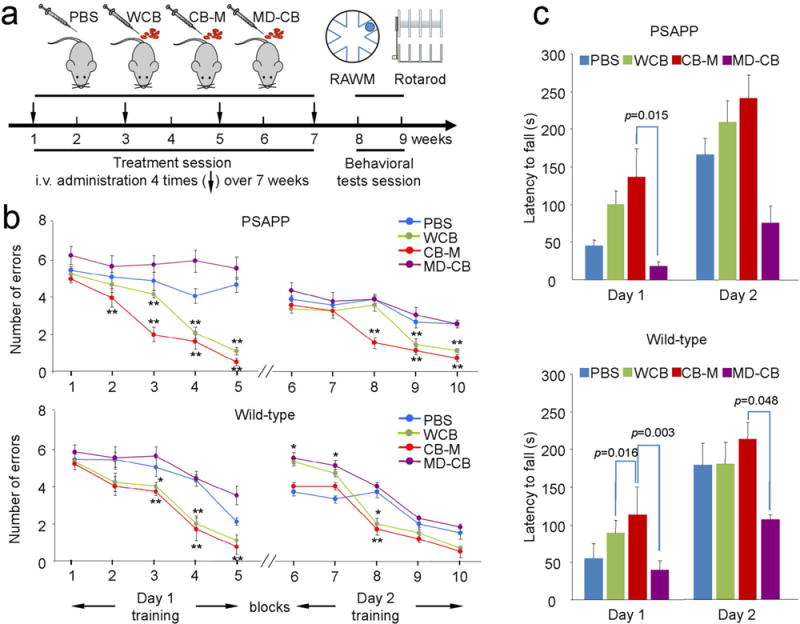
Short-term (2-month) administration of HUCBC-derived monocytes improves learning, memory, and motor function. Both PSAPP and age-matched WT control mice were tested after short-term systemic administration of monocytes (a). Schematic illustration of HUCBC treatment schedule and testing for cognitive and motor function in PSAPP and WT mice. (b) The cognitive function was presented as numbers of entry arm errors before finding the platform. WCB- and CB-M-treated PSAPP mice showed better behavioral performance than PBS-treated group. In addition, CB-M-treated PSAPP mice showed improve behavioral performance than WCB-treated group. In contrast, MD-CB-treated group showed no cognitive improvement. WT littermates also showed similar tendency at each treatment condition. (c) Motor function was also assessed using rotarod test after treatment. WCB-treated PSAPP mice showed enhance motor activity, reflected as enhanced latency to fall, but not statistically significant compared to PBS-treated group. CB-M-treated PSAPP mice showed significantly improved motor activity compared to MD-CB-treated mice on day 1. Similarly, CB-M-treated WT control mice showed significant improvement in motor activity compared to MD-CB-treated group on both days 1 and 2. All data are presented as mean ± SEM (*p < 0.05; **p < 0.01) and analyzed by one-way analysis of variance (ANOVA) with LSD post hoc test (b), or one-way ANOVA with Bonferroni post hoc test (c).
Long-Term (4-Month) Administration of HUCBC-Derived Monocytes Improves Learning, Memory, and Motor Function in PSAPP and WT Mice
To investigate whether long-term (4-month) monocyte administration improves learning, memory, and motor functions, we administered PBS, whole HUCBCs (WCB), HUCBC-derived monocytes (CB-M), or monocyte-deficient HUCBC (MD-CB) intravenously in PSAPP and control (aged-matched WT) mice over a 4-month time period. The behavioral data at 4 months of each treatment were reminiscent of the 2-month treatment results (Fig. 3A–C). In the RAWM, CB-M- and WCB-treated PSAPP mice showed significantly fewer errors (blocks 2 to 5 on day 1; blocks 6 to 10 on day 2; p < 0.05 and p < 0.01) than all other groups. CB-M- and WCB-treated WT mice likewise showed significantly fewer errors in blocks 4, 5, and 9 compared to other groups. Surprisingly, PBS-treated WT mice showed better performance than the MD-CB-treated group in blocks 3, 7, 9, and 10 (Fig. 3B). These findings were further reinforced using the open pool platform swim test to assess whether animals possessed the motor skills sufficient to complete the water maze tasks. Data indicate both 2- and 4-month CB-M treatment outperformed PBS treatment of PSAPP and WT mice at short- and long-term treatment periods (data not shown).
Figure 3.
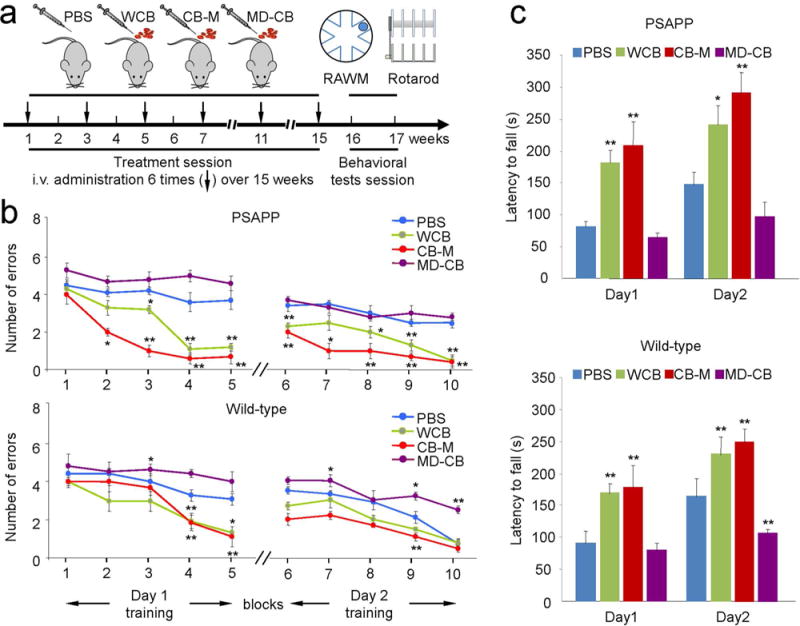
Long-term (4-month) administration of HUCBC-derived monocyte improves learning, memory, and motor function. Both PSAPP and aged-match WT control mice were cognitively tested after long-term monocyte administration. (a) Schematic diagram of HUCBC treatment schedule and behavioral testing in PSAPP and WT mice. (b) Hippocampal-dependent learning and memory function was assessed by RAWM test after treatment completion. The cognitive activity was presented as numbers of entry arm errors before finding the platform. Consistent with the 2-month treatment data, WCB- and CB-M-treated PSAPP mice showed improved cognitive function compared with PBS-treated PSAPP mice. WT littermates also showed similar effects with each treatment paradigm. MD-CB treatment did not show behavioral improvement and even exacerbated the cognitive function of WT mice. (c) Motor activity was assessed using rotarod test after monocyte administration. WCB- or CB-M-treated PSAPP mice significantly improve the motor activity, assessed as latency to fall, compared to PBS-treated group, while MD-CB treatment progressively impaired the motor function of WT control group. All data are shown as the mean ± SEM (*p < 0.05; **p < 0.01) and analyzed by one-way analysis of variance (ANOVA) with LSD post hoc test (b), or one-way ANOVA with Bonferroni post hoc test (c).
Rotarod data show that WCB- and CB-M-treated PSAPP and WT mice displayed superior coordination and advanced to the point of sustaining their position on the rod for the entire trial time of 5 min (Fig. 3C; day 2) compared to both PBS-treated PSAPP and WT groups (p < 0.05, p < 0.01 vs. PBS-PSAPP or WT mice).
HUCBC-Derived Monocytes Markedly Reduce Aβ Deposits
To investigate whether HUCBC-derived monocytes could reduce amyloid pathology, the brains of each mouse were investigated after four different treatments (i.e., PBS, whole HUCBCs (WCB), HUCBC-derived monocytes (CB-M), or monocyte-deficient HUCBC (MD-CB) treatments). WCB- and CB-M-treated PSAPP mice showed reduced amyloid plaques in hippocampal (H), retrosplenial cortex (RSC), and entorhinal (EC) regions compared with PBS- and MD-CB-treated mice using 4G8 immunohistochemical staining (Fig. 4A). Quantitative image analysis shows that percentage of plaques in the H region in CB-M- and WCB-treated groups was significantly less than PBS- and MD-CB groups (Fig. 4B, upper panel, mean ± SD, n = 4).
Figure 4.
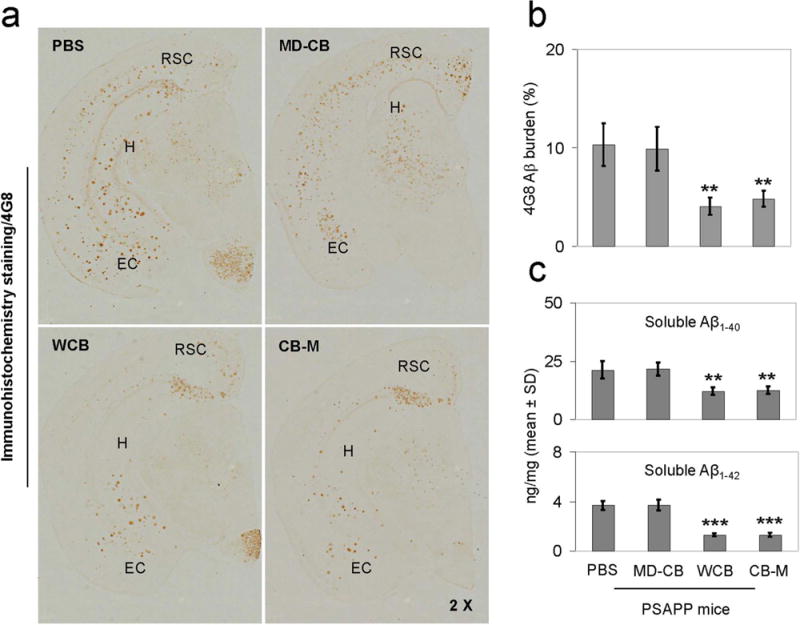
HUCBC-derived monocytes reduce Aβ pathology. (a) HUCBC (WCB) and HUCBC-derived monocytes (CB-M) significantly reduce Aβ plaques in retrosplenial cortex (RSC), entorhinal cortex (EC), and hippocampus (H) of PSAPP mice, as determined by immuno histochemical staining with anti-Aβ17–24 antibody (4G8). (b) The percentage quantification of immunoreactive areas in area of interest (H region) by image analysis for each treatment group using 4G8 antibody (n = 4). (c) Detergent-soluble Aβ1–40 and Aβ1–42 levels in brain homogenates of PSAPP mice were analyzed by Aβ ELISA. Data are represented as mean ± SD of Aβ1–40,42 (ng/mg of total protein). A t-test for independent samples reveals significant differences between HUCBC or HUCBC-derived monocyte (CB-M) treatment and PBS control groups (**p < 0.01), but no significant difference between whole HUCBC (WCB) and CB-M treatment groups (p > 0.05).
In addition, brain homogenates from the 4-month treatment groups were measured for both soluble Aβ1–40 and Aβ1–42 levels by ELISA. WCB- and CB-M-treated PSAPP mice showed significantly decreased levels of both soluble Aβ1–40 and Aβ1–42 compared to PBS and MD-CB-treated group (Fig. 4C, middle and lower panels, mean ± SD, n = 4). The 2-month treatment resulted in similar outcomes (data not shown).
Aβ Phagocytosis by Aged and Cord Blood Cell-Derived Monocytes
Since phagocytosis by monocytes may be a possible pathway for Aβ clearance from the brain (14,31), we hypothesized that HUCBC-derived monocytes might phagocytose Aβ. HUCBC-derived monocytes (CB-M) and aged human blood cell-derived monocytes (aged BC-M) were cultured with FITC-Aβ1–42 for 1 h in the presence or absence of sAPPα (100 ng/ml), scavenger receptor class A ligand (SR-A ligand) or unlabeled Aβ1–42 (naked Aβ1–42). Extracellular (Fig 5A, upper panel) and cell-associated (lower panel) FITC-Aβ1–42 was then measured from supernatants and lysates, respectively, using a fluorimeter. Data are represented as relative mean fluorescence (mean ± SD) for each sample at 37°C divided by mean fluorescence at 4°C (n = 4 for each condition; p < 0.05, p < 0.01, p < 0.001 vs. aged BC-M). Aβ internalization was reflected by the level of cell-associated FITC-Aβ1–42. Interestingly, CB-M internalized Aβ much more than aged BC-M, while sAPPα pretreatment (100 ng/ml) enhanced Aβ internalization by aged BC-M. Primary monocytes were then fixed and imaged using confocal microscopy equipped with Normarski optics (Fig. 5B). The merged image showed significant Aβ42 internalization inside the cytoplasm of CB-M as shown by most of the green fluorescence localized inside the cytoplasmic compartment (left upper panel), while aged CB-M showed much less FITC-Aβ1–42 internalization (left lower panel). Treatment of aged CB-M with sAPPα showed a significant increase in Aβ1–42 internalization in the cytoplasm by these cells (right upper panel), while SR-A ligand reduced this effect (right middle panel). As expected, competition by naked Aβ1–42 significantly decreased FITC-Aβ1–42 internalization by CB-M (left middle panel).
Figure 5.
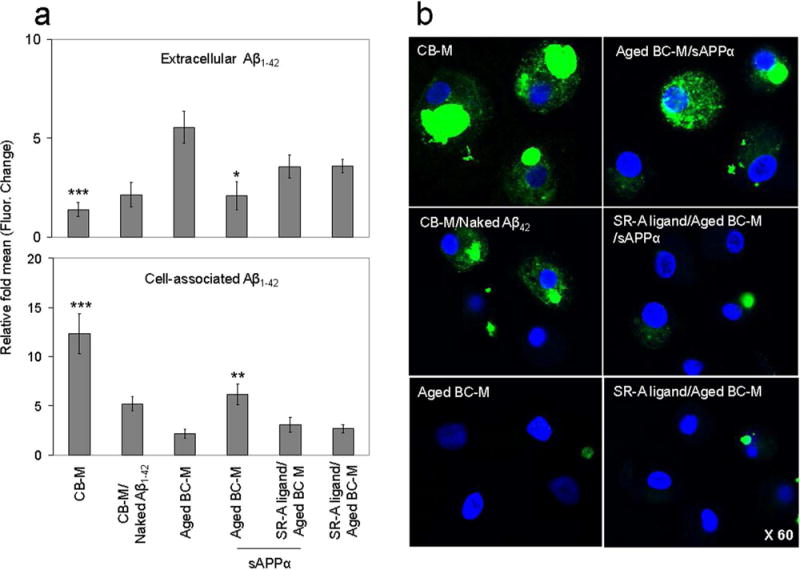
In vitro confocal images of Aβ phagocytosis by cord and aged blood-derived monocytes. Monocytes were obtained, characterized by flow cytometry, incubated with purified FITC-Aβ1–42 peptide (1 μM) for 60 min, and then assessed by confocal microscopy. (a) Quantitative measurement of Aβ1–42 internalization by cord and aged blood monocytes. Extracellular (upper panel) and cell-associated (lower panel) FITC-Aβ1–42 was measured from supernatants and lysates, respectively, using fluorimeter. HUCBC-derived monocytes (CB-M) shows lower level of extracellular Aβ1–42 (top left panel) and as expected higher level of cell-associated Aβ1–42 than aged BC-M (bottom left panel). Internalization of Aβ1–42 by aged human blood cell-derived monocytes (BC-M) was enhanced by sAPPα treatment (100 ng/ml). Monocyte scavenger receptor class A (SR-A) appears to be the cell surface receptor that account for the internalization Aβ1–42. SR-A ligand decreased Aβ1–42 internalization during sAPPα treatment. Data are represented as relative mean fluorescence (mean ± SD) for each sample at 37°C divided by mean fluorescence at 4°C (n = 4 for each condition) (*p < 0.05, **p < 0.01, ***p < 0.001). (b) Primary monocytes were treated, fixed, and imaged using confocal microscopy equipped with Normarski optics. Blue fluorescence represents monocytes, and green fluorescence represents FITC-labeled Aβ1–42. Merged image shows significant internalization of Aβ1–42 within the cytoplasm of CB-M as shown by most of the green fluorescence localized in the cytoplasmic compartment (left upper panel), while aged BC-M shows significant reduction of Aβ1–42 internalization (left lower panel). Aged BC-M treated with sAPPα shows significantly increased Aβ1–42 internalization in the cytoplasm (right upper panel), while SR-A ligand decreased Aβ1–42 internalization inside the cytoplasm during sAPPα treatment (right middle panel). Unlabeled (naked) Aβ1–42 competitively reduced internalization of FITC-Aβ42 by CB-M (left middle panel).
In Vitro Heterodimerization sAPPα With Aβ
In this study, we have shown that sAPPα treatment improves Aβ phagocytosis by aged blood monocytes. It is known that APP undergoes homodimerization at the cell surface, while sAPPα disrupts this homodimerization by binding with APP, thereby preventing starvation-induced cell death (18). We hypothesized that sAPPα might also form a heterodimer with Aβ, which is then internalized inside the monocytes. To prove sAPPα/Aβ heterodimerization, 3-h cultured conditioned media of CHO/APPwt or CHO/APPswe cells were immunoprecipitated with anti-Aβ17–27 antibody (4G8), followed by WB detection using either anti-Aβ1–16 antibody (6E10) (Fig. 6A, left panel) or anti-APP N-terminal antibody (22C11) (Fig. 6A, right panel). The 22C11 blot showed only the sAPPα band (~100 kDa), while the 6E10 blot showed both Aβ (~4 kDa) and sAPPα (~100 kDa). Moreover, immunoprecipitation with 22C11 followed by WB detection using 6E10 showed both Aβ (~4 kDa) and sAPPα (~100 kDa) bands (Fig. 6B). Taken together, these data suggest a possible sAPPα/Aβ heterodimerization in the cell culture system.
Figure 6.
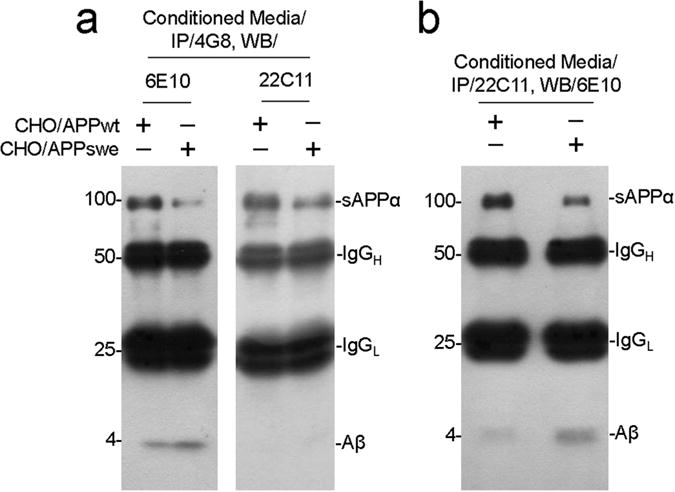
sAPPα and Aβ heterodimerization at monocyte’s cell surface. Immunoprecipitation of sAPPα and Aβs in cell culture. (a) Conditioned media of CHO/APPwt and CHO/APPswe cells expressing APP, Aβs, and sAPPα were immunoprecipitated with anti-Aβ17–27 antibody (4G8) followed by WB detection with anti-Aβ1–16 monoclonal antibody (6E10) (left panel) or an anti-APP N-terminal antibody (22C11) (right panel). WB with 6E10 shows both sAPPα and Aβ bands (left panel), but WB with 22C11 only shows sAPPα band (right panel). (b) Further confirmed by immunoprecipitating with anti-APP N-terminal antibody (22C11) and WB detection with 6E10 reveals both sAPPα and Aβ bands.
sAPPα/Aβ Heterodimerization at APP672–687 (Aβ1–16) Region
To investigate whether APP672–687 region is responsible for sAPPα/Aβ heterodimerization, truncated sAPPα was incubated with synthetic Aβ1–42 (Fig. 7A), immunoprecipitated with 4G8, and then analyzed by WB detection using either anti-Aβ1–16 antibody (82E1) (Fig. 7B) or anti-APP N-terminal antibody (22C11) (Fig. 7C). In parallel, CHO/APPswe conditioned media received the same treatments as a negative control. Truncated sAPPα incubated with synthetic Aβ1–42 showed two attached bands with the presence of not only Aβ1–42 (~4 kDa, upper band) but also truncated sAPPα (~4 kDa, lower band) (Fig. 7B), while CHO/APPswe conditioned media showed only a single band for Aβ1–42 (~4 kDa). In addition, CHO/APPswe conditioned media clearly show one band for sAPPα (~100 kDa), while truncated sAPPα incubated with synthetic Aβ1–42 clearly shows the presence of truncated sAPPα (~4 kDa) in the same blot. The presence of sAPPα was substantiated by 6E10 WB (data not shown).
Figure 7.
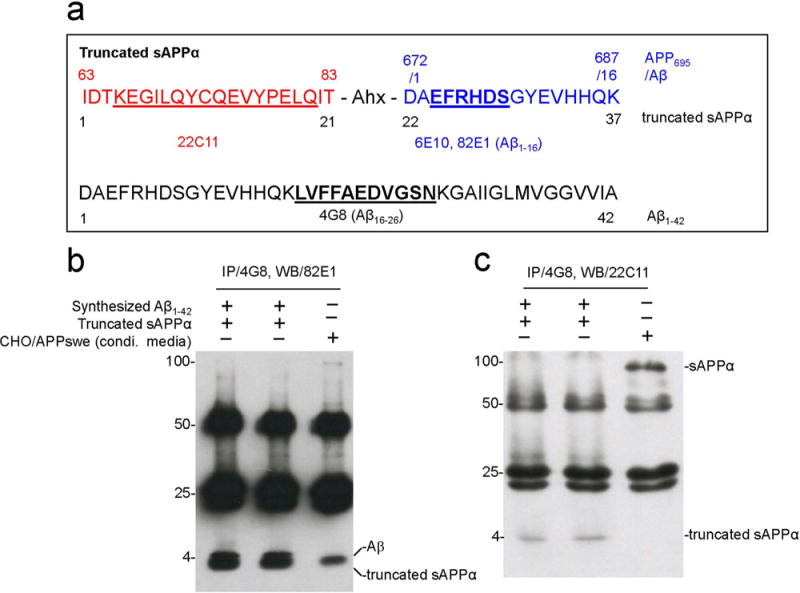
APP672–687 (Aβ1–16) region is responsible for sAPPα/Aβ heterodimerization. (a) Diagram showing the sequence of truncated sAPPα (top) and Aβ42 (bottom). (b) Truncated sAPPα was incubated with synthesized Aβ1–42, immunoprecipitated with anti-Aβ17–27 antibody (4G8), and analyzed by WB with anti-Aβ1–16 antibody (82E1) or (c) an anti-APP N-terminal monoclonal antibody (22C11). For negative control, conditioned media from CHO/APPswe cells underwent the same treatment in this experiment. 82E1 picked up both Aβ1–42 (~4 kDa, upper band) and truncated sAPPα (~4 kDa, lower band) after incubating truncated sAPPα with synthesized Aβ1-42 (b, lane 1 and 2) but only Aβ1–42 band showed up in control (b, lane 3). (c) 22C11 only picks up truncated sAPPα (~4 kDa) (c, lanes 1 and 2) after truncated sAPPα was incubated with synthesized Aβ1-42 and as expected only sAPPα (~100 kDa) (c, lane 3) in CHO/APPswe conditioned media. Note: Ahx (aminohexanoic acid), a common hydrophobic spacer.
DISCUSSION
The role of Aβ in AD progression has already been established (19,34). The toxic function of this peptide in the brain is reported in many studies. However, the brainspecific receptor for this peptide has not yet been characterized. In many studies, it has been shown that scavenger receptors and LRP internalize Aβ. Yazawa and colleagues, for example, reported that formyl peptide receptor like-1, a G protein-coupled receptor usually found in monocytes and microglia, interacts with Aβ species causing both internalization and inflammation (51). Generally, Aβ accumulation in the brain stimulates an influx of microglia, monocytes, and various proinflammatory cytokines adjacent to that area, which causes localized inflammation and chemoattracts many more immune cells to that area. Aβ clearance via enzyme-mediated degradation, anti-Aβ autoantibodies, receptor-mediated Aβ transport across the BBB, or even therapeutic Aβ clearance has been encouraging, but not curative (4,27). We have shown that HUCBC mononuclear cells (MNCs) could modulate inflammation and reduce Aβ pathology in combination with markedly rescuing cognitive deficits (9,36). However, we could not identify the immune cell type or cells that attributed the above beneficial effects.
HUCBC administration has gained positive acceptance over the years in research and clinical settings as a source of hematopoietic stem cells to treat acquired and genetic diseases. HUCBCs have been used successfully to improve outcomes in many diseases, especially in hematological malignancies (6), spinal cord injury (38), traumatic brain injury (29), stroke (7), cardiovascular disease (21,28), and preclinical models of AD (9,36). Brunstein et al. (6) summarized a significant report on the use of allogenic umbilical cord blood cells in hematological malignancies especially in acute leukemia patients. The report suggested the use of cord blood cells under certain conditions, especially when a suitable sibling donor or a conventional donor is unavailable. However, the report data were taken from a single institution. Cord blood stem cells were used successfully in one study involving a spinal cord injured rat animal model, showing that infusion of human cord blood cells can reverse behavioral deficits even after 5 days of administration. Exogenous delivered cord blood cells were found around the injured area (38). Intravenous delivery of human cord blood cells after 1 day in a stroke animal model improved behavioral effects, and stem cells were found around the injured area. In this study the animal was immunocompromised with cyclosporine before administration of cells (46). Human umbilical cord blood progenitor cells were also used to treat acute myocardial infarction in a rat model. It was shown that exogenous cells could reduce infarction size significantly (21). Intravenous delivery of HUCBC-derived CD133+ cells improves functional recovery by preventing scar thinning and systolic dilation in a rat myocardial infarction model (28). In our lab we have also used HUCBCs in AD mouse models, showing that multiple low-dose infusions of HUCBCs could improve cognitive function and reduce amyloid pathologies (9).
HUCBCs comprise a population of hematopoietic stem and progenitor cells. HUCBCs consist of 95% to 98% MNCs, which may contribute to the beneficial effects observed in AD. As part of their immune surveillance, monocytes phagocytose dead cells and other cellular debris (40). Therefore, monocytes may be fundamental to developing effective therapies for AD. It has been postulated that monocytes may be instrumental for enhancing Aβ clearance (31). We know that resident microglia and perivascular macrophages are responsible for phagocytic clearance of Aβ from the brain parenchyma. A very early study in a rat model by Miyake and colleagues in 1984 showed that peripheral monocytes can enter into the brain after birth and can be detected for up to 3 weeks. They also found that those cells become morphologically changed in brain microenvironment and form ameboidlike microglia (33). Moreover, peripheral monocytes have been reported to infiltrate the brain parenchyma in various disease conditions such as experimental allergic encephalomyelitis (15), trauma (3,26), neurological disease (47), and infection (13,25,30). Recent studies also show trafficking and infiltration of peripheral monocytes into the brain in psychological stress (47–49). It has been shown that peripheral inflammation and tumor necrosis-α (TNF-α) signaling also recruit monocytes into the brain from blood (12). Based on the literature review and our previous study (9,36), our present study sought to modify our previous HUCBC study in such a way that only the CD14+ monocyte fraction (HUCBC-derived monocytes), isolated from total HUCBCs, would be infused peripherally over short-and long-term periods of time in PSAPP and WT mice.
The findings of this study suggest that peripheral administration of HUCBC-derived monocytes can improve hippocampal-dependent learning, memory, and motor function in transgenic PSAPP mice. Histological and biochemical analyses of brain tissue reveal that both short (2-month)- and long-term (4-month) administration of HUCBC monocytes reduces amyloid pathology as well as soluble and insoluble Aβ in the brain. In future studies, we are planning to explore the fate of HUCBC-derived monocytes in animal models.
Confocal microscopy of culture data clearly shows that the phagocytic capacity of aged monocytes decreased significantly and was restored by sAPPα treatment (Fig. 5), indicating that this protein might have an important therapeutic role in reducing Aβ from the brain parenchyma. Furthermore, the lower phagocytic capacity of aged blood monocytes could be explained by significantly less APP surface expression in aged blood monocytes and therefore correspondingly much lower sAPPα secretion than HUCBC-derived monocytes (shown in Fig. 5). It has been shown that the SR-A receptor binds with Aβ and promotes internalization and clearance (16,22,50). In addition, there is an increase in microglial expression of SR-A surrounding areas of human brain plaques (8).
The most intriguing part of this study was to identify one of the plausible mechanisms involved in Aβ phagocytosis by monocytes. Confocal microscopy and immunoprecipitation technique show that sAPPα binds with Aβ42 species at the cell surface, which promote internalization of Aβ peptide inside the monocyte’s cytoplasm. We hypothesized that heterodimerization of sAPPα/Aβ could be a plausible mechanism by which monocytes or peripheral macrophages reduce amyloid burden from brain parenchyma. Our in vitro experiment showed that the APP672–687 (Aβ1–16) region is responsible for sAPPα/Aβ heterodimerization. Many researchers have emphasized that the size of Aβ aggregates is crucial for internalization. Weltzien et al. found that smaller aggregates internalized efficiently, but larger aggregates usually spend more time on the monocyte’s cell surface (45). The physical limit of Aβ aggregated mass size needs to be considered for phagocytosis and inflammation aggravation. Future studies will be required to confirm this sAPPα/Aβ heterodimerization in vivo as well. In sum, HUCBC monocytes could open a new therapeutic option in AD.
Acknowledgments
This work was supported by the NIH/ NIA [R01AG032432 (J.T) and the USF Health Byrd Institute Small Program BRD210 (J.T.)]. We would like to thank Dr. Doug Shytle for his helpful discussion.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, Age-related behavioral impairments in transgenic mice carrying both mutant myloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891(1–2):42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for ttreatment of Alzheimer’s disease. Curr Med Chem. 2007;14(27):2848–2864. doi: 10.2174/092986707782360060. [DOI] [PubMed] [Google Scholar]
- 3.Bao Y, Kim E, Bhosle S, Mehta H, Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation. 2010;7(92) doi: 10.1186/1742-2094-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barten DM, Albright CF. Therapeutic strategies for Alzheimer’s disease. Mol Neurobiol. 2008;37(2–3):171–186. doi: 10.1007/s12035-008-8031-2. [DOI] [PubMed] [Google Scholar]
- 5.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG. Umbilical cord blood transplantation for the treatment of hematologic malignancies. Cancer Control. 2011;18(4):222–236. doi: 10.1177/107327481101800403. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 8.Christie RH, Freeman M, Hyman BT. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am J Pathol. 1996;148(2):399–403. [PMC free article] [PubMed] [Google Scholar]
- 9.Darlington D, Deng J, Giunta B, Hou H, Sanberg CD, Kuzmin-Nichols N, Zhou HD, Mori T, Ehrhart J, Sanberg PR, Tan J. Multiple low-dose infusions of human umbilical cord blood cells improve cognitive impairments and reduce amyloid-beta-associated neuropathology in Alzheimer mice. Stem Cells Dev. 2013;22(3):412–421. doi: 10.1089/scd.2012.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of abeta isoforms. Neuron. 2004;43(3):333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Dewachter I, Van Leuven F. Secretases as targets for the treatment of Alzheimer’s disease: The prospects. Lancet Neurol. 2002;1(7):409–416. doi: 10.1016/s1474-4422(02)00188-6. [DOI] [PubMed] [Google Scholar]
- 12.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29(7):2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7(3):221–232. doi: 10.3233/jad-2005-7304. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- 15.Floris S, Blezer EL, Schreibelt G, Dopp E, van der Pol SM, Schadee-Eestermans IL, Nicolay K, Dijkstra CD, de Vries HE. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: A quantitative MRI study. Brain. 2004;127(Pt 3):616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 16.Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND, Weiner HL, El Khoury J. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat Commun. 2013;4(2030) doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1d E9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24(3):516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem. 2009;284(22):15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved? Curr Alzheimer Res. 2006;3(1):71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 20.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages For clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA. 2009;106(4):1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henning RJ, Burgos JD, Ondrovic L, Sanberg P, Balis J, Morgan MB. Human umbilical cord blood progenitor cells are attracted to infarcted myocardium and significantly reduce myocardial infarction size. Cell Transplant. 2006;15(7):647–658. doi: 10.3727/000000006783981611. [DOI] [PubMed] [Google Scholar]
- 22.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: Their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 23.Jankowsky JL, Slunt HH, Gonzales V, Jenkins NA, Copeland NG, Borchelt DR. APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol Aging. 2004;25(7):885–892. doi: 10.1016/j.neurobiolaging.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106(9):1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014;34(8):1411–1419. doi: 10.1038/jcbfm.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6(2):108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leor J, Guetta E, Chouraqui P, Guetta V, Nagler A. Human umbilical cord blood cells: A new alternative for myocardial repair? Cytotherapy. 2005;7(3):251–257. doi: 10.1080/14653240510027163. [DOI] [PubMed] [Google Scholar]
- 29.Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11(3):275–281. [PubMed] [Google Scholar]
- 30.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11(5):318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaud JP, Bellavance MA, Prefontaine P, Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013;5(3):646–653. doi: 10.1016/j.celrep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, Priller J, Prinz M. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J Neurosci. 2011;31(31):11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake T, Tsuchihashi Y, Kitamura T, Fujita S. Immunohistochemical studies of blood monocytes infiltrating into the neonatal rat brain. Acta Neuropathol. 1984;62(4):291–297. doi: 10.1007/BF00687611. [DOI] [PubMed] [Google Scholar]
- 34.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27(3):301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008;30(1):94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17(3):423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saporta S, Kim JJ, Willing AE, Fu ES, Davis CD, Sanberg PR. Human umbilical cord blood stem cells infusion in spinal cord injury: Engraftment and beneficial influence on behavior. J Hematother Stem Cell Res. 2003;12(3):271–278. doi: 10.1089/152581603322023007. [DOI] [PubMed] [Google Scholar]
- 39.Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8(11):447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 40.Seta N, Kuwana M. Human circulating monocytes as multipotential progenitors. Keio J Med. 2007;56(2):41–47. doi: 10.2302/kjm.56.41. [DOI] [PubMed] [Google Scholar]
- 41.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Inves. 2000;106(12):1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat Neurosci. 2002;5(12):1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 43.Van Uden E, Mallory M, Veinbergs I, Alford M, Rockenstein E, Masliah E. Increased extracellular amy-loid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein. J Neurosci. 2002;22(21):9298–9304. doi: 10.1523/JNEUROSCI.22-21-09298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale S, Schmid-Alliana A, Breuil V, Pomeranz M, Millet MA, Rossi B, Schmid-Antomarchi H. Soluble fractalkine prevents monocyte chemoattractant protein-1-induced monocyte migration via inhibition of stress-activated protein kinase 2/p38 and matrix metalloproteinase activities. J Immunol. 2004;172:58–592. doi: 10.4049/jimmunol.172.1.585. [DOI] [PubMed] [Google Scholar]
- 45.Weltzien RB, Pachter JS. Visualization of beta-amyloid peptide (abeta) phagocytosis by human mononuclear phagocytes: Dependency on abeta aggregate size. J Neurosci Res. 2000;59(4):522–527. doi: 10.1002/(SICI)1097-4547(20000215)59:4<522::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73(3):296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 47.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. Beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75(12):970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang CN, Shiao YJ, Shie FS, Guo BS, Chen PH, Cho CY, Chen YJ, Huang FL, Tsay HJ. Mechanism mediating oligomeric abeta clearance by naive primary microglia. Neurobiol Dis. 2011;42(3):221–230. doi: 10.1016/j.nbd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Yazawa H, Yu ZX, Takeda, Le Y, Gong W, Ferrans VJ, Oppenheim JJ, Li CC, Wang JM. Beta amyloid peptide (abeta42) is internalized via the G-protein-coupled receptor FPRL1 and forms fibrillar aggregates in macrophages. FASEB J. 2001;15(13):2454–2462. doi: 10.1096/fj.01-0251com. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y, Bickford PC, Sanberg P, Giunta B, Tan J. Blueberry opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activation protein kinase. Rejuvenation Res. 2008;11(5):891–901. doi: 10.1089/rej.2008.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, Glabe CG, Ehrhart J, Bickford P, Mori T, Obregon D, Town T, Tan J. CD45 Deficiency Drives Amyloid-Beta Peptide Oligomers and neuronal loss in Alzheimer’s disease mice. J Neurosci. 2011;31(4):1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


