Abstract
Objectives
Menthol is often added to cigarettes and e-cigarette solutions for its cooling and anti-irritant effects, and may contribute to development of nicotine dependence, particularly in vulnerable populations such as adolescents, and among African Americans. Menthol is rapidly metabolized to menthol glucuronide (MG) with little or no unconjugated menthol measurable in venous blood. Human challenge studies of the effects of inhaled menthol, and of its interactions with nicotine, would benefit from a quantitative measure of acute menthol exposure. Our objective was to determine whether plasma MG concentrations might be a suitable quantitative biomarker of acute menthol exposure following its inhalation.
Methods
We performed a secondary analysis of plasma MG concentrations obtained during a study of the effects of inhaled menthol on behavioral responses to intravenous nicotine. MG concentrations were followed over time in venous plasma from 48 participants following inhalation of aerosols from e-cigarettes employing solutions containing either of 2 menthol concentrations or placebo.
Results
Whereas plasma MG concentrations were variable, they showed a dose-dependent increase following menthol inhalation.
Conclusions
Measurement of plasma MG may be useful to assess inter-individual differences in acute menthol exposure in human challenge studies involving menthol inhalation.
Keywords: menthol, menthol glucuronide, e-cigarettes, ENDS
Menthol is a common additive to electronic nicotine delivery systems (ENDS) as well as conventional cigarettes, that is preferred by youth and African Americans.1-3 Data suggest that menthol vapors also may cause inhalation cytotoxicity when produced by high voltage output ENDS.4 Although menthol’s cooling and anti-irritant effects5 are thought to be mediated primarily by peripheral TRP channels,6-8 menthol also has been reported to bind to nicotinic receptors.9 Human challenge studies of menthol’s behavioral effects and interactions with nicotine, including the latter’s reinforcing properties and risk for developing addiction10-12 would benefit from a quantitative, real-time biomarker of menthol exposure to control for inter-individual differences in intake and disposition.
Menthol is rapidly metabolized to menthol glucuronide (MG) (approximately half of the menthol dose) and various oxidative metabolites, and unchanged menthol has not been measurable in human venous plasma following its administration by oral routes.13 Urinary MG has been shown to be a biomarker for menthol exposure following cigarette smoking,14,15 and MG concentrations in plasma have been reported following various forms of oral menthol administration.13 Relative changes in plasma MG were reported to correlate with those of various metabolome constituents in smokers, but actual MG concentrations were not reported.16,17
In this report, we present a secondary analysis of plasma MG concentrations measured in conjunction with a study of the effects of inhaled menthol on behavioral responses to intravenous nicotine. Plasma MG levels were quantified at multiple time-points following inhalation (in 3 separate test sessions) of aerosols from e-cigarettes containing either of 2 different menthol concentrations or placebo.
METHODS
Participants
A total of 48 non-treatment-seeking cigarette smokers, 39 men and 9 women, ages 18-30 (mean = 24.3, SD = 3.63) who were medically healthy participated. The breakdown by race was 27 Caucasians and 21 African Americans. Preference for ‘menthol’ (N = 25) or ‘non-menthol’ cigarettes (N = 23) was by self-report. Smoking status was defined as at least one cigarette per day for the past year, and verified by urinary cotinine measurements. Criteria for exclusion included history of drug or alcohol abuse, unstable psychiatric conditions, regular use of psychotropic medications, and pregnancy.
Study Design
Participants were asked to avoid mentholated products such as mouthwashes, toothpaste, and mints for 24 hours prior to test sessions, and provided with non-mentholated tooth paste. A tanktype e-cigarette, the Joyetech eGo-C™, with a single coil atomizer (2.2-ohm), 2-ml tank, and a 650-mAH battery operating at 3.7 volts (6.2 W) was employed. Prior to participation in the test sessions, participants familiarized themselves with the e-cigarette during a practice session. At the start of each test session, a 20-gauge indwelling venous catheter was placed for blood sampling and nicotine delivery.
In each of 3 test sessions, on different days, participants were assigned, in random sequence, to one of 3 different e-cigarette conditions (0.5% menthol, 3.2% menthol or placebo), each containing control tobacco flavor. The 3 e-liquid solutions containing the designated weighed-in concentrations of menthol were prepared by Pace Engineering Concepts (Delafield, WI, 53018) in a matrix comprised of 70/30 propylene glycol/vegetable glycerin and checked by in-house analysis. The doses of menthol selected were derived from a published dose-finding study employing solutions from the same source.18 The high menthol dose produced a cooling effect comparable to that of a commercial solution (AmericaneLiquidStore™) of comparable concentration; the lower dose produced a minimally discernable effect when inhaled from a nicotine free solution. Commercial suppliers of e-cigarette solutions rarely indicate menthol concentrations. The limited published data report concentrations in the range of 0.5% - 2.2%.19,20
Each session was composed of 3 bouts of inhalation, each consisting of 6 puffs, one every 15 seconds. Although not a focus of this secondary analysis, participants also received, in random order, intravenous delivery of saline, nicotine 0.25 mg, or nicotine 0.5 mg/70kg, immediately following each of the 3 bouts of menthol or control inhalation.
Blood Sampling
Samples were collected into heparinized Vacutainer® tubes without separator gel, and stored at −70°C. Eleven samples were drawn during each 4-hour session. Samples were drawn at baseline, and at 10, 30, and 60 minutes following each puffing bout. An additional sample was drawn 2 hours following the last puffing bout.
Plasma MG Assay
MG was measured by LC/MS/MS employing MG-d4 (Cambridge Isotopes) as an internal standard. The assay was adapted to plasma from that reported for urine14 with the following change: a protein precipitation/extraction step with methanol rather than simple dilution was used for sample preparation, and all standards and controls were prepared in MG-free plasma.
The following ion transitions were monitored:
MG: 331 → 85 (quantifier ion); 331 → 75 (qualifier ion)
MG d4: 335 → 85 (quantifier ion)
The lower limit of quantitation (LLOQ) was 4 ng/mL and between-day reproducibility (CV’s) at 4ng/ml (LLOQ), 10 ng/mL and 40 ng/ml were 17, 10 and 8% respectively.
Data Analysis
The area under the MG concentration versus time curve (AUC) was determined by the linear trapezoidal rule. We restricted AUC calculations to the 5 time-points (2 hours) which followed the last bout of smoking in each session to minimize possible contributions of baseline MG levels. For the same reason, we restricted AUC analyses to sessions with baseline MG concentrations of less than 11 ng/mL. Thus, 23 sessions out of 134 were excluded. Assuming exponential decay and a 50-minute MG half-life,13 extrapolation indicated that a baseline of 11 ng/mL or less would contribute negligibly to the AUC estimated from the time-points following the last puffing bout.
Apart from the high-menthol dose, the distributions of the AUC and peak change measures were highly skewed with floor effects, and hence, the non-parametric method for repeated measures analysis21 was used. Menthol was a within-subject factor with 3 levels (high, low and placebo) and history of type of cigarettes smoked was a between-subject factor with 2 levels (mentholated vs non-mentholated). Interactions between menthol and cigarette preference were also tested. We considered session order effects and nicotine sequence effects within session as factors, but because they were non-significant, these were dropped from the models. Mixed models were fit to the ranked data with method of moments variance estimators and unstructured variance-covariance matrix within subject. ANOVA-type statistics (ATS) were used to assess statistical significance of the effects in the models. Separate models were fit for AUC and Cmax. Post hoc comparisons were used to interpret statistically significant effects.
RESULTS
Forty-one of 48 participants completed all 3 test sessions, 4 completed 2 sessions, and 3 completed one test session. Peak plasma MG concentrations and AUC values following each of the menthol conditions are shown in Table 1 and AUCs are compared in Figure 1. Plasma concentrations (AUC values and Cmax) at each of the 2 doses were significantly different (p < .001) from one another and from placebo respectively (all pairwise p-values < .001). There was no statistically significant effect on AUC or peak concentration of prior smoking preference for mentholated versus non-mentholated cigarettes, p > .20. The change in the mean values of the AUC and peak MG concentrations (5.7-fold and 5.6-fold respectively) following the high/low menthol doses was dose proportional, closely approximating the 6.4 ratio of the high/low menthol e-liquid concentrations.
Table 1.
Plasma MG Concentrations Following Inhalation of Menthol
| Variable | Menthol “Dose”: E-Liquid Concentration | Test Statistic | p-value | ||
|---|---|---|---|---|---|
| Menthol Dose (%) | 3.2 % (N = 37) |
0.5% (N = 39) |
0% (N = 35) |
||
|
| |||||
| AUC (ng.min/mL) | |||||
| Median | 2091.9 | 292.6 | 0.0 | ||
| (IQR) | (1229.7–3108.0) | (0.0–698.0) | (0.0–0.0) | ATS (1.95) = 246.8 | < .001 |
| Mean (SD) | 2179.6 (1128.0) | 381.7 (359.3) | 74.6 (174.5) | ||
|
| |||||
| Cmax (ng/mL) | |||||
| Median | 31.7 | 7.0 | 0.0 | ||
| (IQR) | (23.4–45.5) | (0.0–9.6) | (0.0–4.1) | ATS (1.92) = 238.3 | < .001 |
| Mean (SD) | 33.9 (16.7) | 6.1 (4.9) | 1.6 (3.0) | ||
Note:
IQR = Inter-quartile range;
SD = standard deviation;
ATS = ANOVA Type Statistic AUC and Cmax are calculated for the period 120-240 minutes into the infusion.
Figure 1. Plasma Menthol Glucuronide Concentrations (AUC) for each Menthol Condition.
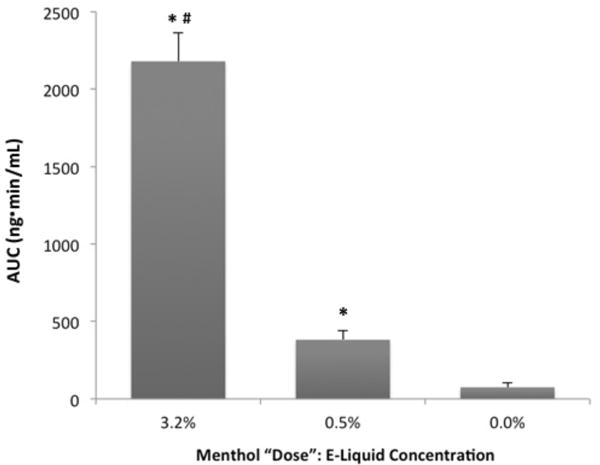
Note:
*Indicates a statistically significant difference from placebo.
#Indicates a statistically significant difference from the 0.5% menthol condition (all pairwise p values < .001).
Error bars = SEM
Controlling for within-session order of nicotine dose and between-session sequence of menthol dose did not alter these outcomes. Peak MG concentrations most often occurred at 130 minutes (first sample after the third puffing bout) following the high dose. There were no statistically significant sex effects on MG concentrations. There was a 9.6-fold and 8.5-fold difference between the highest and lowest values for plasma AUC and Cmax following the highest dose. In several participants, MG concentrations did not exceed the LLOQ following the low dose.
DISCUSSION
Our intent was to explore the usefulness of plasma MG measurements as a quantitative biomarker of acute menthol exposure during menthol inhalation challenges as, for example, in studies of its interactions with nicotine. Our data demonstrate that plasma MG may serve as a measure of acute menthol exposure following its inhalation via e-cigarettes, and by inference, mentholated conventional cigarettes. With its short half-life, however, plasma measurements are not likely to be useful for quantifying chronic or intermittent menthol exposure. For identification of prior menthol intake or for estimation of chronic menthol exposure, quantifying of MG’s urinary excretion should be more useful.14
Following inhalation of 2 doses of menthol, mean peak MG concentration and AUC showed dose dependency. Although the proportional change in mean AUC values following the 2 menthol doses approximated that of the menthol solution concentrations, there was considerable inter-subject variability. Thus, measurement of plasma MG should allow consideration of inter-subject differences in menthol exposure during data analysis following human challenge studies.
Inter-subject differences in menthol’s disposition consequent to polymorphisms of the UGT isoform(s) responsible for menthol conjugation may, in part, explain the range of MG concentrations observed.22,23 Whereas menthol has been shown to affect nicotine disposition,24 we know of no reports of the effect of nicotine on menthol’s disposition. Nevertheless, we verified that the sequence of nicotine doses had no effect on observed MG concentrations.
Inter-individual differences in puffing/inhalation technique and variable performance of the e-cigarette model employed undoubtedly contributed to the range of MG concentrations seen at each dose despite regulated puffing frequency. Moreover, different e-cigarette models may differ in menthol delivery. Thus, quantitation of e-liquid consumption by each participant and evaluation of the menthol content of the vapor produced would have allowed a more accurate estimate of menthol dosage than e-liquid menthol concentrations alone and should be addressed in future studies.
Whereas plasma MG concentrations may reflect menthol exposure, they are not route specific. Intake from mouthwash, toothpaste, tea, and candies is common. Measures were taken to avoid this confounder, and to minimize its impact during data analysis.
Additional caveats need to be considered in the interpretation of plasma MG concentrations. As is the case with most, although not all, glucuronide metabolites, MG is presumably inactive. Thus, caution is indicated regarding attempts to correlate plasma concentrations directly with behavioral effects or possible toxicity. Menthol’s cooling and anti-irritant effects are mediated through local TRP channel subtypes,6-8 that occurs prior to menthol’s absorption, although some effects may be mediated by brain receptors. The bioavailability of unconjugated menthol to central nervous system receptor sites is uncertain, but analogous to such other inhaled drugs as nicotine and cocaine25,26 rapid access to the brain is likely. These concerns should not preclude the use of plasma MG measurements as a quantitative biomarker of the parent compound, menthol, for evaluating differences in exposure between individuals, experimental groups and different delivery systems in challenge studies involving menthol inhalation.
IMPLICATIONS FOR TOBACCO REGULATION
Menthol is a common additive to tobacco products, including ENDS, and is the only characterizing flavor permitted in cigarettes other than tobacco flavor.27 Adolescents particularly favor mentholated products, and scientific evidence indicates that menthol likely promotes development of nicotine addiction in young smokers.1,3 Smokers of mentholated products have been reported to have more difficulty quitting.28 Objective evidence regarding the effects of menthol and the need to regulate menthol in tobacco products can be derived from human behavioral studies of menthol/nicotine interactions. Interpretation of such drug challenge studies might be enhanced by obtaining objective information regarding differences between participants in acute menthol exposure through measurement of plasma MG.
Human Subjects Statement
Participants provided written consent prior to participation. The Human Subjects Committees of the VA Connecticut Healthcare System and Yale University approved this study. Participants were paid for participation.
Acknowledgments
Research reported in this publication was supported by a TCORS (P50DA036151) grant from NIDA and the FDA Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Conflict of Interest Statement
All authors have no conflict of interests to declare.
Contributor Information
Peter Jatlow, Professor Emeritus in Laboratory Medicine and in Psychiatry, Yale School of Medicine, New Haven, CT.
Gerald Valentine, Clinician in Psychiatry, Department of Psychiatry, Yale School of Medicine, New Haven, CT.
Ralitza Gueorguieva, Senior Research Scientist in Biostatistics, Director of Biostatistics in Psychiatry, Yale School of Public Health, New Haven, CT.
Haleh Nadim, Research Associate, Department of Laboratory Medicine, Yale School of Medicine, New Haven, CT.
Ran Wu, Statistician, Department of Psychiatry, Yale School of Medicine, New Haven, CT.
Stephanie S. O’Malley, Professor, Department of Psychiatry, Yale School of Medicine, New Haven, CT.
Mehmet Sofuoglu, Professor, Department of Psychiatry, VA CT Healthcare System and Yale School of Medicine, New Haven, CT.
References
- 1.Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. Rockville, MD: US Food and Drug Administration; 2011. [Google Scholar]
- 2.Ahijevych K, Garrett BE. Menthol pharmacology and its potential impact on cigarette smoking behavior. Nicotine Tob Res. 2004;6(Suppl 1):S17–S28. doi: 10.1080/14622200310001649469. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL, Samet JM. The threat of menthol cigarettes to U.S. public health. N Engl J Med. 2011;364(23):2179–2181. doi: 10.1056/NEJMp1103610. [DOI] [PubMed] [Google Scholar]
- 4.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS) Tob Control. 2016;25(Suppl 2):ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46(8):618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Fan L, Balakrishna S, et al. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154(10):2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 8.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 9.Ashoor A, Nordman JC, Veltri D, et al. Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PLoS One. 2013;8(7):e67674. doi: 10.1371/journal.pone.0067674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henningfield JE, Benowitz NL, Ahijevych K, et al. Does menthol enhance the addictiveness of cigarettes? An agenda for research. Nicotine Tob Res. 2003;5(1):9–11. doi: 10.1080/1462220031000070543. [DOI] [PubMed] [Google Scholar]
- 11.Henningfield JE, Djordjevic MV. Menthol cigarettes: research needs and challenges. Nicotine Tob Res. 2004;6(Suppl 1):S11–S16. doi: 10.1080/14622200310001649450. [DOI] [PubMed] [Google Scholar]
- 12.Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine Tob Res. 2010;12(Suppl 2):S110–S116. doi: 10.1093/ntr/ntq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelal A, Jacob P, 3rd, Yu L, Benowitz NL. Disposition kinetics and effects of menthol. Clin Pharmacol Ther. 1999;66(2):128–135. doi: 10.1053/cp.1999.v66.100455001. [DOI] [PubMed] [Google Scholar]
- 14.Benowitz NL, Dains KM, Dempsey D, et al. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W, Blount BC, Watson CH, et al. Quantitative analysis of menthol in human urine using solid phase microextraction and stable isotope dilution gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1044-1045:200–205. doi: 10.1016/j.jchromb.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu PC, Lan RS, Brasky TM, et al. Metabolomic profiles of current cigarette smokers. Mol Carcinog. 2017;56(2):594–606. doi: 10.1002/mc.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu P-C, Lan RS, Brasky TM, et al. Menthol smokers: metabolomic profiling and smoking behavior. Cancer Epidemiol Biomarkers Prev. 2017;26(1):51–60. doi: 10.1158/1055-9965.EPI-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosbrook K, Green BG. Sensory effects of menthol and nicotine in an e-cigarette. Nicotine Tob Res. 2016;18(7):1588–1595. doi: 10.1093/ntr/ntw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisko JG, Tran H, Stanfill SB, et al. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine Tob Res. 2015;17(10):1270–1278. doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tierney PA, Karpinski CD, Brown JE, et al. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(e1):e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Designs. New York, NY: Wiley; 2002. [Google Scholar]
- 22.Bhasker CR, McKinnon W, Stone A, et al. Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics. 2000;10(8):679–685. doi: 10.1097/00008571-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Green MD, Belanger G, Hum DW, et al. Glucuronidation of opioids, carboxylic acid-containing drugs, and hydroxylated xenobiotics catalyzed by expressed monkey UDP-glucuronosyltransferase 2B9 protein. Drug Metab Dispos. 1997;25(12):1389–1394. [PubMed] [Google Scholar]
- 24.Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310(3):1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 25.Henningfield JE, London ED, Benowitz NL. Arterial-venous differences in plasma concentrations of nicotine after cigarette smoking. JAMA. 1990;263(15):2049–2050. [PubMed] [Google Scholar]
- 26.Evans SM, Cone EJ, Henningfield JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279(3):1345–1356. [PubMed] [Google Scholar]
- 27.Family Smoking Prevention and Tobacco Control Act. 21 USC. 2009;30 [Google Scholar]
- 28.Foulds J, Hooper MW, Pletcher MJ, Okuyemi KS. Do smokers of menthol cigarettes find it harder to quit smoking? Nicotine Tob Res. 2010;12(Suppl 2):S102–S109. doi: 10.1093/ntr/ntq166. [DOI] [PMC free article] [PubMed] [Google Scholar]


