Abstract
Migraine is one of the most common neurological disorders, leading to more than 1% of total disability reported and over 68 million visits to emergency rooms or physician’s offices each year in the United States. Three times as many women as men have migraine, and while the mechanism behind this is not well understood, 17β-estradiol (estradiol) has been implicated to play a role. Studies have demonstrated that exposure to estrogen can lead to activation of inflammatory pathways, changes in sodium gated channel activity, as well as enhanced vasodilation and allodynia. Estradiol receptors are found in trigeminal nociceptors, which are involved in signaling during a migraine attack. The purpose of this study was to investigate the role of estradiol in migraine pathogenesis utilizing a multibehavioral model of migraine in rat. Animals were surgically implanted with a cannula system to induce migraine and behavior was assessed following exposure to a proestrous level of estradiol for total locomotor activity, light and noise sensitivity, evoked grooming patterns, and enhanced acoustic startle response. Results demonstrated decreased locomotor activity, increased light and noise sensitivity, altered facial grooming indicative of allodynia and enhanced acoustic startle. Further examination of tissue samples revealed increased expression of genes associated with inflammation and vasodilation. Overall, this study demonstrates exacerbation of migraine-like behaviors following exposure to estradiol and helps further explain the underlying mechanisms behind sex differences found in this common neurological disorder.
Keywords: Estrogen, inflammation, vasodilation, migraine mechanisms
Introduction
Migraine is a common neurological disorder which causes significant personal and societal burdens due to the high prevalence, loss of productivity, and the cost of treatments, which are not always effective for patients (Akerman, et al., 2013, Law, et al., 2013, Shapiro and Goadsby, 2007). Furthermore, there is relatively little basic research conducted on migraine (Schwedt and Shapiro, 2009), and as a result, available treatment options and understanding of this condition are limited.
Migraine is diagnosed in women three times more frequently than men (Buse, et al., 2013), and while the mechanisms behind this sex difference are not well understood, 17β-estradiol (estradiol) has been implicated to play a role (Gupta, et al., 2011, Somerville, 1975, Somerville, 1975). It is established that estradiol receptors are present in trigeminal nociceptors and studies have demonstrated that binding of estradiol leads to downstream activation of extracellular signal regulated kinase (ERK), a known inflammatory response pathway (Liverman, et al., 2009). Furthermore, it has been shown that estradiol exposure can lead to other nociceptive-related downstream effects; including, increased primary neuron activity (Cairns, et al., 2001, Cairns, et al., 2002), increased allodynia (Liverman, et al., 2009), altered sodium gated channel activity (Wang, et al., 2013), and enhanced vasodilation (Levy and Burstein, 2011, Sarajari and Oblinger, 2010).
Previous work in our laboratory has shown that exposure to the environmental estrogen bisphenol A (BPA) leads to exacerbated migraine-like behaviors and elevation in mRNA levels of genes related to estradiol and inflammation signaling (Vermeer, 2014). In order to better understand these outcomes and to further investigate the mechanistic role of estradiol in migraine pathogenesis the following study was carried out. Ovariectomized rats were exposed to a proestrus level of estradiol and a multibehavioral model of migraine was utilized as previously described. Animals were tested for total locomotor activity, photo- and phonophobia, evoked facial grooming, and acoustic startle reflex following a dural stimulation of inflammatory soup (IS) to induce migraine-like behaviors (Burstein and Jakubowski, 2004). Tissues were tested for changes in mRNA levels of genes related to estradiol, inflammation, and nociception signaling following behavioral experiments; including, estrogen receptors alpha (ERalpha) and GPR30, extracellular signal regulated kinase (ERK1 and ERK2), fatty acid amide hydrolase (FAAH), calcitonin gene-related peptide (CGRP), and sodium gated channel 1.8 (Nav1.8).
Several previous studies have examined how estradiol exposure alters behavioral outcomes and gene expression related to pain disorders; including, increased hind paw allodynia following partial sciatic nerve ligation (Sarajari and Oblinger, 2010), increased TRP channel activation after injection of complete Freund’s adjuvant bilaterally into the temporomandibular joint (Wu, et al., 2010), and changes in craniofacial pain (Cairns, 2007). This study was designed to further clarify the underlying mechanisms behind estradiol signaling in migraine and discuss how this compares to BPA exposure. Furthermore, this work helps develop a better understanding of migraine pathogenesis in humans, and more specifically, the mechanism behind female predominance found in this neurological condition.
Materials and Methods
Materials
Prostaglandin E2, bradykinin acetate, histamine dihydrochloride, serotonin hydrochloride, 17β-estradiol, sterile water, and all other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise noted.
Animals
Animal care and use procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee and conducted according to Institute of Laboratory Animal Research guidelines. Ten-week-old, female, sexually mature, ovariectomized Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, Massachusetts, USA). The animals were housed on a 12-hour light-dark cycle and given water and food ad libitum. Animals were supplied a diet of soy and alfalfa free pellets (Teklad 2020x, Harlan Laboratories, Indianapolis, IN, USA) to minimize the presence of dietary phytoestrogens. Rats were divided into groups based on application of inflammatory soup (IS, 1.0 mM bradykinin acetate, 1.0 mM histamine dihydrochloride, 1.0 mM serotonin hydrochloride, and 0.1 mM prostaglandin E2 in sterile PBS, pH 5.5, (Burstein, et al., 1998)) or phosphate buffered saline (PBS, adjusted to a pH of 7.4) and exposure to estradiol or vehicle corn oil. Animal numbers per group: IS control: 10 rats, IS+Estradiol: 7 rats, PBS control: 7 rats, PBS+Estradiol: 4 rats (data not shown). All behavioral experiments were conducted between 8:30 and 14:00 h in a dedicated, temperature-controlled room.
Cannula Implantation
A Plastics One (Roanoke, VA, USA) cannula made of biocompatible material was used for dural delivery of IS or PBS. The cannula was implanted through the skull under isoflurane anesthesia as previously described in detail. (Stucky, et al., 2011). Briefly, the cannula was placed on the right side of the skull over the occipital lobe 5 mm lateral to midline and halfway between bregma and lambda. One stainless steel screw was placed in rostral and caudal positions 3 mm from the center of the cannula as anchors for the dental cement (Ortho-Jet, Lang Dental Mfg. Co., Inc., Wheeling IL, USA) used to stabilize the cannula.
Treatment
Estradiol was dissolved in 100% ethanol. An aliquot was then diluted 1000-fold (ethanol final concentration 0.1%) in corn oil to a final concentration of 10 µg/ml. Rats were dosed subcutaneously at 2 µg/kg/day 30 min prior to migraine induction in order to achieve proestrus levels of estradiol in their system during behavioral analysis. This dose was based on both on previous literature values demonstrating levels of circulating estradiol during this stage to be 80–140 pg/mL during proestrus (Butcher, et al., 1974, He, et al., 2002, Nequin, et al., 1979, Smith, et al., 1975) and ELISA measurements performed for this study showing circulating levels of estradiol of 112.1 ± 14.1 pg/mL. Rats were divided into groups which received either estradiol or a vehicle (corn) oil injection. Animals received IS or PBS through the cannula implant. During IS or PBS application, the obturator cap was removed and an internal delivery cannula was placed as previously described (Stucky, et al., 2011, Vermeer, 2014). All animals were weighed before behavioral testing throughout the study. No significant differences were found in weight gain between rats treated with estradiol and vehicle treated rats.
Timeline
The same timeline was followed as previously described (Vermeer, 2014). Briefly, rats were given one week to acclimate following arrival and then were subjected to two days of presurgical baseline testing. Animals then underwent the cannula implantation surgery and were given a further week to recover. Finally, rats were given two days of postsurgical baseline testing prior to dural IS application. As previously described, behavioral experiments using IS applications and estradiol exposure were performed every third day for seven total applications. Rats were injected with estradiol 30 min prior to IS application and behavioral testing was completed 30 min following dural stimulation by the IS (Oshinsky and Gomonchareonsiri, 2007). Following recommendations of the Panel on Euthanasia, American Veterinary Medical Association, rats were dosed with 60 mg/kg pentobarbital sodium (Beuthanasia D, Schering-Plough, Summit, NJ, USA) and decapitated approximately 1 hr after the final behavioral experiment. The skull, brain, and cervical spinal cord were immersed in RNALater (Ambion, Foster City, CA, USA) and stored for up to 3 days at 4°C. The ipsilateral trigeminal nucleus, trigeminal ganglion, and dura mater were later dissected as previously described (Stucky, et al., 2011).
Multibehavioral Model of Migraine
As previously described, a multibehavioral model of migraine in rat was utilized to study behavioral changes associated with migraine following dural stimulation. This model was based on the International Classification of Headache Disorders (ICHD-II and III) (Society, 2004, Society, 2013) as previously described (Vermeer, 2014). Very briefly, following exposure to estradiol, rats were tested for total locomotor activity, photo- and phonophobia, evoked facial grooming, and acoustic startle reflex.
Locomotor activity was studied utilizing a Force Plate Actimeter (BASi, San Diego, CA, USA), which transmitted data of rat movement during three phases; 0–5 min is the application of IS (“delivery phase”), 6–10 min (“onset phase”), and 11–15 min (“persistence phase”). The total distance traveled and the bouts of low mobility (BLM, a measure of periodic inactivity) were measured.
The Photo- and Phonophobia task was performed in a modified Force Plate Actimeter to study place preference and used externally control speakers (75 dB white noise) and light (250 lux illumination) to examine light and noise sensitivity. The percent of time animals spent in the stimulus chambers while the stimulus was activated and BLM were assessed.
Rats were then examined for facial allodynia with the evoked facial grooming task, which included 3 sprays of water from a metered bottled to the face of the rat. Video recordings of the rats were subsequently analyzed for latency to first groom and percent time spent grooming during the 5 min following stimulus application. Only grooming to their face and heads was quantified, with cleaning swipes defined as the use of one or both forepaws cleaning at or above the eyes.
Finally, rats were placed in Startle Reflex Chambers (Kinder Scientific, Poway, CA, USA). Fourteen randomly mixed pulses of white noise (85 or 90 dB) were emitted at one pulse/min and the maximum startle force (N) was recorded in the first 80 msec after the noise. The average response of 7 trials was calculated.
Gene Expression Analysis
Tissues utilized for these studies were the ipsilateral trigeminal nucleus, trigeminal ganglion, and dura mater. Total RNAs from each sample were extracted, purified, and quantified with a Nanodrop as previously described (Stucky, et al., 2011) and GAPDH expression was measured as the internal control. Gene-specific primers for ERalpha, GPR30, ERK1, ERK2, calcitonin gene-related peptide (CGRP), sodium gated channel 1.8 (Nav1.8), fatty acid amide hydrolase (FAAH), and GAPDH were obtained from Integrated DNA Technologies (Coralville, IA, USA) and qPCR occurred in a 96-well MicroAmp Optical reaction plate.
pERK Analysis
Trigeminal nucleus and ganglion, and dura mater tissue samples were isolated as previously described (Liverman, et al., 2009). Western blot analysis was performed for phosphorylated ERK (the activated form) using a 10% resolving SDS-PAGE gel and 5 micrograms of protein were loaded for each sample as previously described (Vermeer, 2014). Briefly, nitrocellulose membrane was used for transfer and the Odyssey blocking buffer system was applied for 2 hr at room temperature. Antibody dilutions were as follows: primary anti-pERK (1:10000) overnight at 4°C, secondary goat-anti-mouse-Alexa Fluor 680 (1:10000) for 1.5 hr at room temperature. The Odyssey Infrared Fluorescence Imaging System was used to detect and Image J was used to analyze band density. All data were normalized to the internal control β-actin to determine total activated ERK.
Statistical Analysis
Behavioral data were analyzed with a two-way repeated-measures ANOVA and a Bonferroni’s post hoc comparison and confidence intervals using SPSS Statistics 20 (IBM, Chicago, IL, USA). Treatment (estradiol vs. vehicle) was used as the between subjects factor. Data from gene expression and western blot analysis were analyzed using a one-way ANOVA and Tukey’s posttest. For behavioral data, significant differences between estradiol treatment and vehicle (corn oil) treatment are indicated by an asterisk (*). Differences were considered significant if p ≤ 0.05.
Results
Locomotor activity decreased following estradiol exposure
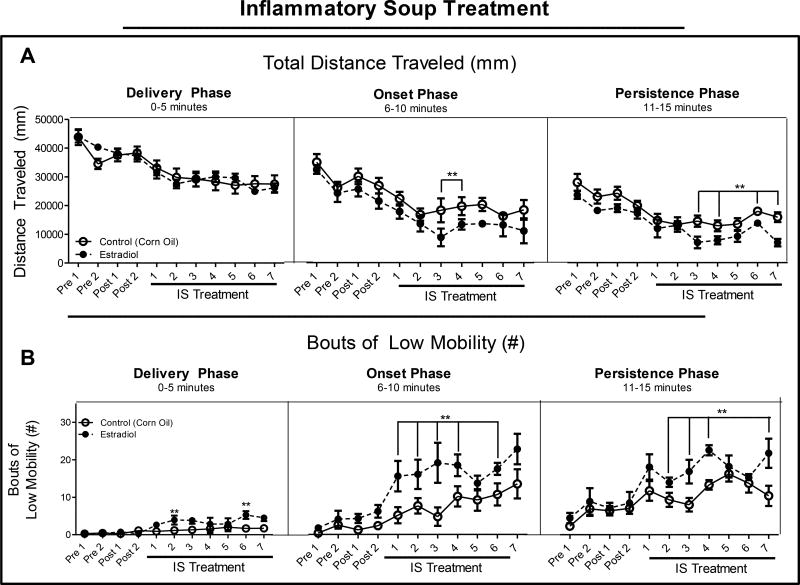
Rats were exposed to 2 µg/kg estradiol or vehicle 30 min prior to dural stimulation by IS and the total locomotor activity was determined. No significant differences were found between the groups during the delivery phase of the IS. Estradiol treatment led to significant decreases in distance traveled on treatment days 3 and 4 in the onset phase and treatment days 3, 4, 6, and 7 in the persistence phase (Figure 1A) in comparison to rats exposed to vehicle (corn oil). Rats treated with estradiol demonstrated significantly increased BLM occurrences on treatment days 2 and 6 of the delivery phase, treatment days 1–4, and 6 during the onset phase, and treatment days 2–4, and 7 of the persistence phase (Figure 1B) compared to treatment with vehicle. These measurements demonstrate reductions in locomotion, consistent with reduced activity and exacerbation of migraine-like behaviors in the presence of estradiol as compared to vehicle treatment.
Figure 1. Total locomotor activity.
(A). Total distance traveled during 15 min open field assessment. Rats were injected with estradiol (2 µg/kg/day) 30 min prior to start of behavioral testing; 0–5 min: delivery of IS to dura mater, 6–10 min: onset of migraine-like behaviors, 11–15 min: persistence of migraine-like behaviors. (B). Bouts of low mobility during each of the three phases. Data are presented as mean ± SEM and in all graphs: Pre = presurgical baseline behavior, Post = postsurgical baseline, and **= p ≤ 0.05. All statistical analysis was performed for estradiol treatment compared to vehicle treatment and n = 7 for estradiol, n = 10 for vehicle.
Estradiol exposure caused significant light and noise avoidance
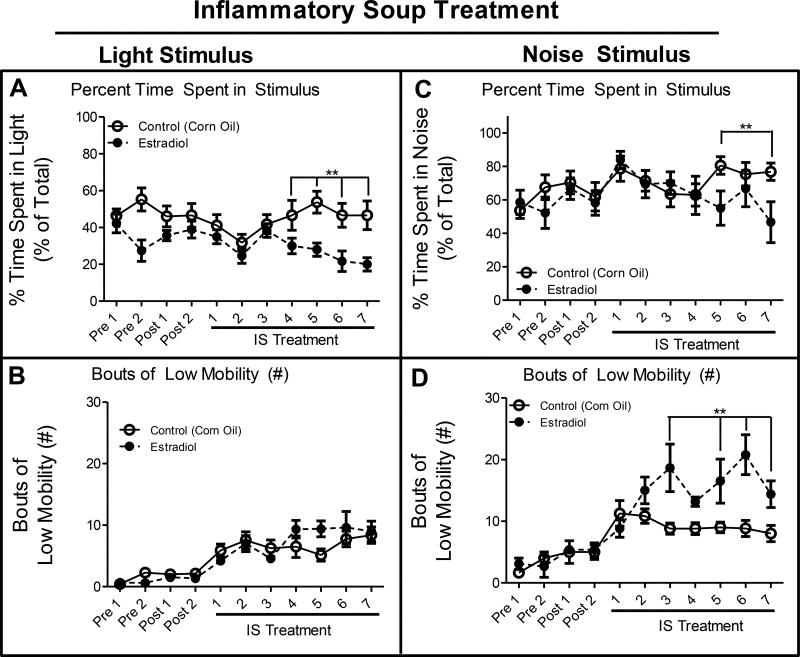
After the total locomotor behavioral analysis, rats were placed in the modified force-plate actimeter which measured locomotion and place preference. Following illumination of a 250 lux lamp, rats treated with estradiol spent significantly less time spent in the light stimulus on IS treatment days 4–7 when compared to treatment with vehicle (Figure 2A). There were no significant differences in BLM in estradiol vs vehicle treatment while the light stimulus was illuminated (Figure 2B). When a 75 dB white noise was emitted from the internal speakers, rats treated with estradiol exhibited significantly elevated noise aversion on treatment days 5 and 7, compared to vehicle treatment spending less time in the stimulus than vehicle-treated rats. Furthermore, estradiol treatment led to significantly elevated BLM while the noise stimulus was activated on treatment days 3, 5, 6, and 7, as compared to vehicle treatment, indicative of elevated noise aversion. These data indicate that estradiol exposure augmented the migraine-associated behaviors of light and noise aversion.
Figure 2. Photo- and Phonophobia.
(A). Percent of time (percent of total) that rats spent in the light stimulus side of the box when the light was activated. (B). Bouts of low mobility while the light stimulus was activated. (C). Percent of time (percent of total) animals spend in the noise stimulus side of the box when the noise stimulus was activated. (D). Bouts of low mobility while the noise stimulus was activated. Data are presented as mean ± SEM and in all graphs: Pre = presurgical baseline behavior, Post = postsurgical baseline, and **= p ≤ 0.05. All statistical analysis was performed for estradiol treatment compared to vehicle treatment and n = 7 for estradiol, n = 10 for vehicle.
Estradiol treatment led to allodynia-associated behaviors
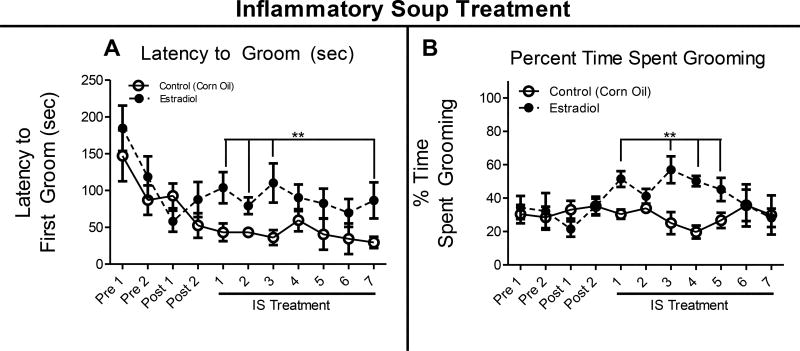
Immediately following the light and noise aversion task, rats were placed in a clean cage and video recorded for evoked grooming habits. They were sprayed in the face 3 times with a metered bottle of water and filmed for 5–6 min. Exposure to estradiol led to a significant increase in latency to groom on treatment days 1–3, and 7 as compared to vehicle treatment (Figure 3A). Furthermore, animals treated with estradiol groomed for a longer period of time on treatment days 1, and 3–5 compared to vehicle exposure (Figure 3B). These results indicate an increase in facial allodynia related behaviors, including the avoidance of grooming following a water spray to the face.
Figure 3. Evoked Grooming.
(A). Latency to groom after rat was sprayed three times with a metered water bottle. (B). Percent time spent grooming (percent of total) following sprays of water to the face. Data are presented as mean ± SEM and in all graphs: Pre = presurgical baseline behavior, Post = postsurgical baseline, and **= p ≤ 0.05. All statistical analysis was performed for estradiol treatment compared to vehicle treatment and n = 7 for estradiol, n = 10 for vehicle.
Exposure to estradiol caused enhanced acoustic startle
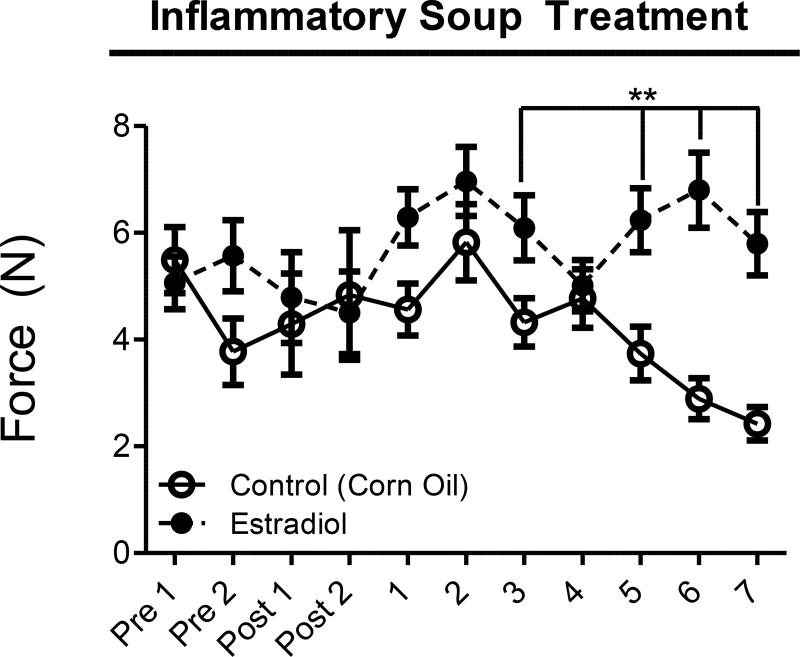
Finally, rats were placed in Kinder Scientific Startle Reflex boxes to determine if estradiol treatment led to an altered startle response. The startle force was measured in animals with two different intensities, 85 and 90 dB. Treatment with estradiol produced no significant differences in startle force at 85 dB (data not shown). When exposed to a 90 dB noise, Figure 4 shows that rats treated with estradiol exhibited significantly elevated startle responses on treatment days 3, and 5–7 compared to vehicle treatment. These results indicate elevated startle response following estradiol exposure.
Figure 4. Acoustic Startle Reflex.
Acoustic startle reflex force (N) following a 90 dB pulse measured in the 80 msec after the pulse. Data are presented as mean ± SEM and pre = presurgical baseline behavior, post = postsurgical baseline, and **= p ≤ 0.05. All statistical analysis was performed for estradiol treatment compared to vehicle treatment and n = 7 for estradiol, n = 10 for vehicle.
Estradiol treatment altered expression of estrogen-, nociception- and vasodilationrelated genes
Animals were sacrificed and ipsilateral trigeminal nucleus (TGN), trigeminal ganglion (TGG), and dura mater were collected and qPCR was performed. There were significant gene expression differences between the estradiol- and vehicle-treated rats, as displayed in Table 1A (TGN), Table 1B (dura mater), and Table 1C (TGG). Importantly, there was a significant increase in GPR30 mRNA levels in estradiol treated TGN samples. Furthermore, estradiol treatment led to significant elevation in mRNA levels of ERalpha, GPR30, ERK1/2, FAAH, and CGRP in dura mater tissues when compared to ovariectomized controls. Similar results were found in TGG samples, with significant elevation in ERalpha, GPR30, ERK1, FAAH and CGRP demonstrated in estradiol treated animals as compared to ovariectomized controls. These results indicate that estradiol treatment led to elevation in the expression of genes known to be associated with estradiol signaling, inflammation, vasodilation, and endogenous cannabinoid metabolism indicative of mechanisms thought to be involved in migraine pathogenesis.
Table 1.
Effect of estradiol treatment on estrogen- and nociception-related gene regulation
| A: TGNa | |||
|
| |||
| Gene | Control | IS Alone | IS + E |
|
| |||
| ERα | 1.133 ± 0.65 | 0.1233 ± 0.21 | 1.604 ± 0.47 |
| GPR30 | 1.047 ± 0.38 | 0.9396 ± 0.79 | 2.831 ± 0.91**,# |
| ERK1 | 1.024 ± 0.25 | 1.740 ± 1.20 | 0.439 ± 0.38# |
| ERK2 | 1.079 ± 0.45 | 1.649 ± 1.08 | 0.333 ± 0.16# |
| Nav1.8 | NA | NA | NA |
| FAAH | 1.102 ± 0.52 | 0.1063 ± 0.09 | 1.218 ± 0.48 |
| CGRP | 1.001 ± 0.14 | 0.708 ± 0.42 | 0.447 ± 0.28# |
|
| |||
| B: Dura Matera | Control | IS Alone | IS + E |
|
| |||
| ERα | 1.020 ± 0.23 | 0.03919 ± 0.02 | 3.894 ± 1.53**,# |
| GPR30 | 1.123 ± 0.55 | 0.06115 ± 0.07 | 2.671 ± 0.54**,# |
| ERK1 | 1.026 ± 0.26 | 9.670 ±5.15** | 107.93 ± 23.75**,# |
| ERK2 | 1.019 ± 0.20 | 4.664 ± 1.19 | 253.425 ± 43.86**,# |
| Nav1.8 | NA | NA | NA |
| FAAH | 1.124 ± 0.56 | 1.049 ± 0.48 | 2.796 ± 0.86**,# |
| CGRP | 1.120 ± 0.54 | 7.599 ± 1.11** | 7.857 ± 1.27** |
|
| |||
| C: Trigeminal Gangliona | Control | IS Alone | IS + E |
|
| |||
| ERα | 1.005 ± 0.77 | 1.369 ± 0.13 | 1.860 ± 0.62**,# |
| GPR30 | 0.877 ±0.26 | 1.595 ± 0.22** | 2.149 ± 0.44**,# |
| ERK1 | 1.778 ± 2.18 | 4.595 ± 1.15** | 3.323 ± 1.05** |
| ERK2 | 1.318 ± 1.44 | 3.570 ± 2.74 | 3.923 ± 0.93 |
| Nav1.8 | 1.102 ± 0.48 | 2.018 ± 0.78 | 1.849 ± 3.31 |
| FAAH | 1.061 ± 0.35 | 2.737 ± 1.26** | 4.224 ± 1.04** |
| CGRP | 1.134 ± 0.58 | 2.111 ± 0.59 | 2.552 ± 0.42**,# |
Data are reported as fold-increase in specific mRNA levels in trigeminal nucleus, dura mater, and trigeminal ganglion (mean ± SEM). Gene expression was normalized to internal controlGAPDH and compared to ovariectomized control rats (**) or comparison of IS+E treatment to IS alone (#).
** or # = p≤ 0.05 with Tukey post-test following one-way ANOVA.
ERK activation was augmented by estradiol exposure
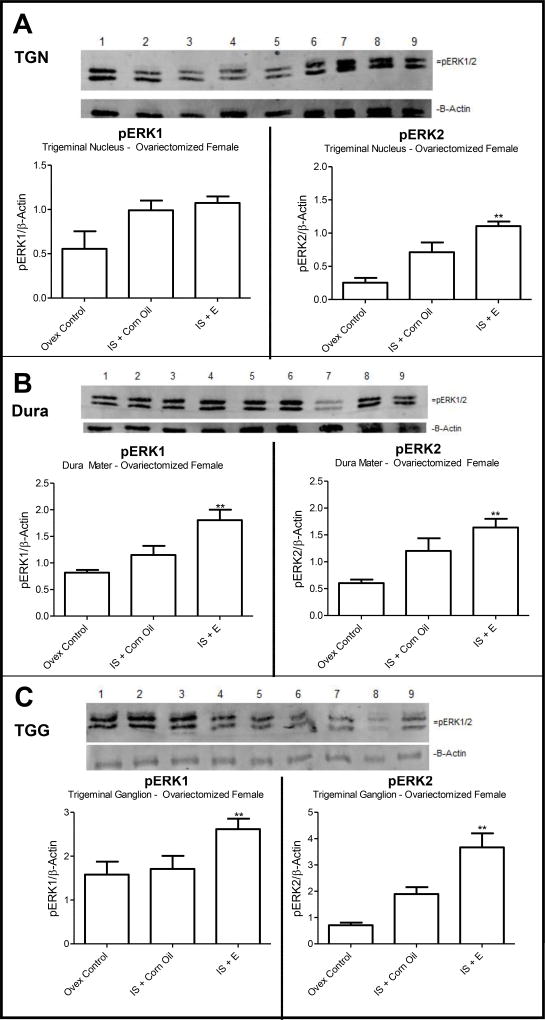
Utilizing western blotting analysis for ERK phosphorylation, activation of ERK was determined. The data in Figure 5A demonstrate representative gels for pERK1 and 2 analyses in samples from the TGN. Analysis using ImageJ demonstrates a significant 2.71-fold increase in pERK2 levels in rats exposed to estradiol as compared to ovariectomized controls. Dura samples analyzed for pERK1 and 2 also demonstrated significant increases in ERK activation (Figure 5B) in rats treated with estradiol; pERK1 exhibited a 2.21-fold increase and pERK2 a 2.7-fold increase in phosphorylation as compared to ovariectomized controls. TGG samples also showed significant increases in pERK levels when estradiol was present as compared to ovariectomized controls and to IS dural stimulation alone: pERK1 demonstrated a 1.66-fold increase; pERK2 a 5.19-fold increase (Figure 5C). These results demonstrate the ability of estradiol treatment to lead to activation of the nociception-related ERK.
Figure 5. pERK Levels in TGN, Dura Mater, and TGG.
(A). Representative gel of TGN stained for pERK; (A) show average levels of pERK1 and pERK2 (n=3 gels). (B) Representative gel of dura mata stained for pERK; (B) show average levels of pERK1 and pERK2 (n=3 gels).(C). Representative gel of TGG stained for pERK with following graphs depicting average levels of toal pERK1 and 2 (n=3 gels). Lane 1–2: IS alone, Lanes 3–9: IS + estradiol. Data are presented as the mean ± SEMs (n = 3 gels) when normalized to β-actin control lane. Image J analysis values were averaged (graphical data) **p < 0.05 when estradiol was compared to ovariectomized control animals and #p < 0.05 when estradiol was compared with IS alone and n = 7 for estradiol, n = 10 for vehicle.
Discussion
The effect of estradiol exposure on behavioral outcomes related to migraine was examined in a multibehavioral model of migraine in rat. These experiments investigated a number of behaviors and genes related to migraine. The results demonstrate several important findings. One, estradiol exposure leads to significantly augmented migraine-like behaviors. Second, treatment with estradiol increases gene expression of estrogen receptors, and markers of inflammation, vasodilation, and cannabinoid-related proteins which explain the downstream mechanisms behind sex differences found in migraine. Finally, experiments showed similarities to previously published results regarding BPA exposure (Vermeer, 2014), and demonstrate that estradiol and BPA may be signaling through similar pathways to exacerbate migraine.
Estradiol treatment demonstrated significantly decreased locomotor activity (Figure 1) and significantly increased light and noise aversion (Figure 2). In both activities, rats exposed to estradiol exhibited significantly increased BLM, which corresponds to observation that humans frequently avoid routine activity during a migraine attack.
Furthermore, evoked facial grooming studies showed rats treated with estradiol had significantly increased latency to groom and time spent grooming as compared to vehicle-treated rats following a dural stimulation with IS (Figure 3). As previously mentioned, migraineurs will commonly describe facial allodynia during an attack; including, pain during hair brushing or face shaving (Bernstein and Burstein, 2012, Wieseler, et al., 2010). Furthermore, several previous studies have established the ability of estradiol exposure to produce facial and peripheral allodynia in animal models. Importantly, our model demonstrates recruitment of similar downstream mechanisms of inflammation (Liverman, 2007), and vasodilation (Sarajari and Oblinger, 2010) exhibited in other work, indicating that our model causes similar results of facial allodynia following treatment with estradiol.
During acoustic startle testing, which measured enhanced phonophobic startle response in the rats, estradiol treatment produced significantly enhanced startle force at 90 dB compared to vehicle treatment (Figure 4). There are previous literature reports demonstrating increased hearing sensitivity in the menstrual cycle during periods of high estrogen (i.e. proestrus) (Al-Mana, et al., 2010), which are analogous to the results shown here.
Dural stimulation by inflammatory soup has been shown to elevate mRNA levels of genes related to migraine (Stucky, et al., 2011), and these results demonstrate that estradiol enhances this. There was significant elevation of mRNA levels of several migraine-related genes (Table 1A–C). It is important to note that the major cranial vessels and the dura mater receive innervation from the trigeminal ganglion, which also sends sensory information to the brainstem trigeminal nucleus (Levy and Strassman, 2002, Olesen, et al., 2009, Panneton, et al., 2011, Strassman and Levy, 2006). The current results demonstrate significant elevations in the expression of estrogen- and nociception-related genes consistently in the dura mater and trigeminal ganglion following estradiol exposure and stimulation by IS (Tables 1B and 1C, respectively), and a significant increase in trigeminal neuron GPR30 mRNA levels following estradiol treatment (Table 1A). Furthermore, protein analysis of activated ERK also showed significant elevation following estradiol exposure compared to ovariectomized controls and dural stimulation alone. These results suggest estradiol augments migraine through increases in downstream nociceptive and inflammation pathway activation, leading to elevated pain signaling within the trigeminal system.
Previous studies in human and animal models have shown that mRNA levels of many nociceptive and inflammatory genes are regulated by estradiol. Specifically, several studies have demonstrated the ability of estradiol exposure to lead to activation of ERK (Filardo, et al., 2000, Liverman, et al., 2009), increases in sodium gated channel activity (Kow, et al., 2006), as well as increased mRNA levels of FAAH (Cupini, et al., 2006, Greco, et al., 2010, Grimaldi, et al., 2012), the major metabolic regulator of anandamide, an endogenously produced cannabinoid. Furthermore, studies have shown that estradiol treatment leads to elevation in release of CGRP in the dura mater and trigeminal ganglion (Gupta, et al., 2007, Stucky, et al., 2011), key to vasodilation which plays a role in migraine onset and progression (Bigal and Walter, 2014, Goadsby, et al., 1990, Raddant and Russo, 2011). It is important to note that although there were no significant changes to CGRP mRNA levels in the trigeminal nucleus or ganglion of rats given a dural stimulation of IS following exposure to vehicle (corn oil). Previous studies support these results (Stucky, et al., 2011); demonstrating that baseline levels of CGRP in the trigeminal ganglion are similar between male and female rats, but following IS dural stimulation, only male rats exhibited a significant increase in mRNA levels of CGRP. These results indicate that expression of CGRP may not reflect behavioral differences between IS and vehicle dural stimulation. Overall, these results demonstrate the ability of estradiol to cause amplification of downstream upregulation of several pathways associated with nociception leading to significantly exacerbated migraine-like behaviors.
It is important to note that published literature regarding the effects of estradiol in migraine pathogenesis contains conflicting data. Some studies have demonstrated that estradiol withdrawal, during the perimenstrual period for example, is associated with menstrual migraine without aura (MacGregor, et al., 2006), and that during times of high estradiol in pregnancy, women will experience a decrease in their migraine frequency (Maggioni, et al., 1997). Furthermore, while studies have demonstrated that estradiol withdrawal can lead to a migraine attack, it requires estradiol priming beforehand (Lichten, et al., 1996). Conversely, there are many studies also demonstrating that an increase in estrogen leads to sensitization of the trigeminal system (Martin, et al., 2007), and a significant increase in migraine prevalence in females at the onset of puberty (Bousser, 2004). Combined, these reports demonstrate the complexity of migraine pathogenesis. The proestrus level chosen for this study was examined in order to further investigate reports of elevated migraine sensitivity during periods of high estradiol as described above, and in order to compare directly with previously published results demonstrating exacerbated migraine-like behavior in the presence of bisphenol A (BPA), (Vermeer, 2014) the most prevalent xenoestrogen in our environment.
The mechanisms behind migraine are not well understood. It is important to note that estradiol is not the only factor thought to play a role in migraine onset and progression (Andress-Rothrock, et al., 2010, Kelman, 2007, Pietrobon and Moskowitz, 2013). Previous data have demonstrated the ability of bisphenol A (BPA) exposure to alter pain behavior and inflammatory signaling; including, altered peripheral allodynia (Aloisi, et al., 2002, Ceccarelli, et al., 2009) and increased ERK activation in cell culture (Dong, et al., 2011). Until recently, no studies have addressed the effect of environmental estrogens on migraine pathogenesis. Due to the ability of BPA and other xenoestrogens to bind to and activate estrogen receptors, exposure to BPA has the potential to increase migraine severity. Comparisons between the data presented here and previously published work in our laboratory where animals were exposed to the environmental estrogen BPA exhibit many similarities. Following BPA exposure, rats exhibited similar behavioral responses; decreased locomotor activity, increase light and noise sensitivity, altered grooming, and enhanced acoustic startle (Vermeer, 2014). Furthermore, BPA treatment led to elevation of mRNA levels of nociception- and estradiol-related genes, including ERK activation (Vermeer, 2014). These results indicate that BPA and estradiol may be signaling through similar pathways to cause exacerbated migraine-like behaviors. Studies have demonstrated that BPA has the ability to interact with and activate ERalpha, ERβ, and GPR30 (Katchy, et al., 2014, Montes-Grajales and Olivero-Verbel, 2013). Interestingly, a recently published study demonstrated BPA-mediated activation of ERK in a cell model, and the use of a GPR30 antagonist in conjunction with BPA exposure led to suppression of ERK activation (Ge, et al., 2014). These results suggest that a major route of BPA signaling and downstream pathway activation may occur through the G protein-coupled estrogen receptor, GPR30.
Conclusions
Overall, the study presented here demonstrates that estradiol exposure leads to augmented migraine-like behaviors and amplified expression of genes involved in estrogen and nociception signaling. These results implicate underlying mechanisms behind the role estrogen plays in migraine pathogenesis and could explain how estrogen and environmental estrogens alter migraine severity and duration. Selected gene targeting of pathways associated with migraine onset and progression, such as downregulation of FAAH or inhibition of sodium gated channel activity, may reveal new strategies and treatments to help increase the quality of life of migraineurs.
Highlights.
Migraine is one of the most common neurological disorders in the US.
Women experience migraine three times more frequently than men.
Estradiol is implicated to play a role in migraine sex differences.
Estradiol exposure in a rat migraine model leads to exacerbated migraine behaviors.
Genes related to inflammation and vasodilation are increased with estradiol treatment.
Acknowledgments
This work was supported by an institutional training grant from the National Institute of Environmental Health Sciences [ES007079] and the University of Kansas Medical Center’s Lied Foundation, and the Biobehavioral Measurement Core of the Kansas Intellectual and Developmental Disabilities Research Center [HD02528].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akerman S, Holland PR, Hoffmann J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia. 2013;33:577–592. doi: 10.1177/0333102412472071. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Alteration in auditory function during the ovarian cycle. Hear Res. 2010;268:114–122. doi: 10.1016/j.heares.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi AM, Della Seta D, Rendo C, Ceccarelli I, Scaramuzzino A, Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002;937:1–7. doi: 10.1016/s0006-8993(02)02446-0. [DOI] [PubMed] [Google Scholar]
- 4.Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache. 2010;50:1366–1370. doi: 10.1111/j.1526-4610.2010.01753.x. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol. 2012;8:89–99. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigal ME, Walter S. Monoclonal Antibodies for Migraine: Preventing Calcitonin Gene-Related Peptide Activity. CNS Drugs. 2014 doi: 10.1007/s40263-014-0156-4. [DOI] [PubMed] [Google Scholar]
- 7.Bousser MG. Estrogens, migraine, and stroke. Stroke. 2004;35:2652–2656. doi: 10.1161/01.STR.0000143223.25843.36. [DOI] [PubMed] [Google Scholar]
- 8.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 9.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 10.Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB. Sex Differences in the Prevalence, Symptoms, and Associated Features of Migraine, Probable Migraine and Other Severe Headache: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- 11.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 12.Cairns BE. The influence of gender and sex steroids on craniofacial nociception. Headache. 2007;47:319–324. doi: 10.1111/j.1526-4610.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.Cairns BE, Sessle BJ, Hu JW. Characteristics of glutamate-evoked temporomandibular joint afferent activity in the rat. J Neurophysiol. 2001;85:2446–2454. doi: 10.1152/jn.2001.85.6.2446. [DOI] [PubMed] [Google Scholar]
- 14.Cairns BE, Sim Y, Bereiter DA, Sessle BJ, Hu JW. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Res. 2002;957:338–344. doi: 10.1016/s0006-8993(02)03671-5. [DOI] [PubMed] [Google Scholar]
- 15.Ceccarelli I, Fiorenzani P, Della Seta D, Massafra C, Cinci G, Bocci A, Aloisi AM. Perinatal exposure to xenoestrogens affects pain in adult female rats. Neurotoxicol Teratol. 2009;31:203–209. doi: 10.1016/j.ntt.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Cupini LM, Bari M, Battista N, Argiro G, Finazzi-Agro A, Calabresi P, Maccarrone M. Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia. 2006;26:277–281. doi: 10.1111/j.1468-2982.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 17.Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut. 2011;159:212–218. doi: 10.1016/j.envpol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 19.Ge LC, Chen ZJ, Liu HY, Zhang KS, Liu H, Huang HB, Zhang G, Wong CK, Giesy JP, Du J, Wang HS. Involvement of activating ERK1/2 through G protein coupled receptor 30 and estrogen receptor alpha/beta in low doses of bisphenol A promoting growth of Sertoli TM4 cells. Toxicol Lett. 2014;226:81–89. doi: 10.1016/j.toxlet.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 21.Greco R, Gasperi V, Sandrini G, Bagetta G, Nappi G, Maccarrone M, Tassorelli C. Alterations of the endocannabinoid system in an animal model of migraine: evaluation in cerebral areas of rat. Cephalalgia. 2010;30:296–302. doi: 10.1111/j.1468-2982.2009.01924.x. [DOI] [PubMed] [Google Scholar]
- 22.Grimaldi P, Pucci M, Di Siena S, Di Giacomo D, Pirazzi V, Geremia R, Maccarrone M. The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cell Mol Life Sci. 2012;69:4177–4190. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache. 2011;51:905–922. doi: 10.1111/j.1526-4610.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Villalon CM, Mehrotra S, de Vries R, Garrelds IM, Saxena PR, MaassenVanDenbrink A. Female sex hormones and rat dural vasodilatation to CGRP, periarterial electrical stimulation and capsaicin. Headache. 2007;47:225–235. doi: 10.1111/j.1526-4610.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 25.He Z, He YJ, Day AL, Simpkins JW. Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and-independent mechanisms. J Neurol Sci. 2002;193:79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- 26.Katchy A, Pinto C, Jonsson P, Nguyen-Vu T, Pandelova M, Riu A, Schramm KW, Samarov D, Gustafsson JA, Bondesson M, Williams C. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol Sci. 2014;138:21–35. doi: 10.1093/toxsci/kft271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 28.Kow LM, Devidze N, Pataky S, Shibuya I, Pfaff DW. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2006;1116:1–11. doi: 10.1016/j.brainres.2006.07.104. [DOI] [PubMed] [Google Scholar]
- 29.Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;10:CD008541. doi: 10.1002/14651858.CD008541.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Levy D, Burstein R. The vascular theory of migraine: leave it or love it? Ann Neurol. 2011;69:600–601. doi: 10.1002/ana.22422. [DOI] [PubMed] [Google Scholar]
- 31.Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002;88:3021–3031. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- 32.Lichten EM, Lichten JB, Whitty A, Pieper D. The confirmation of a biochemical marker for women's hormonal migraine: the depo-estradiol challenge test. Headache. 1996;36:367–371. doi: 10.1046/j.1526-4610.1996.3606367.x. [DOI] [PubMed] [Google Scholar]
- 33.Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29:729–741. doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liverman CS, Brown J, Klein RM, Berman NE. Evidence of peripheral mechanisms for regulation of facial allodynia. Headache. 2007;47:789-. [Google Scholar]
- 36.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67:2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 37.Maggioni F, Alessi C, Maggino T, Zanchin G. Headache during pregnancy. Cephalalgia. 1997;17:765–769. doi: 10.1046/j.1468-2982.1997.1707765.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache. 2007;47:552–563. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 39.Montes-Grajales D, Olivero-Verbel J. Computer-aided identification of novel protein targets of bisphenol A. Toxicol Lett. 2013;222:312–320. doi: 10.1016/j.toxlet.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- 41.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 42.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panneton WM, Gan Q, Livergood RS. A trigeminoreticular pathway: implications in pain. PLoS One. 2011;6:e24499. doi: 10.1371/journal.pone.0024499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 45.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol. 2010;224:163–169. doi: 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwedt TJ, Shapiro RE. Funding of research on headache disorders by the National Institutes of Health. Headache. 2009;49:162–169. doi: 10.1111/j.1526-4610.2008.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro RE, Goadsby PJ. The long drought: the dearth of public funding for headache research. Cephalalgia. 2007;27:991–994. doi: 10.1111/j.1468-2982.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 49.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 50.Society IH. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 51.Society IH. The International Classification of Headache Disorders: 3rd Edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 52.Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology. 1975;25:239–244. doi: 10.1212/wnl.25.3.239. [DOI] [PubMed] [Google Scholar]
- 53.Somerville BW. Estrogen-withdrawal migraine. II. Attempted prophylaxis by continuous estradiol administration. Neurology. 1975;25:245–250. doi: 10.1212/wnl.25.3.245. [DOI] [PubMed] [Google Scholar]
- 54.Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- 55.Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache. 2011;51:674–692. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vermeer LMM, Gregory E, Winter ML, McCarson KE, Berman NEJ. Exposure to Bisphenol A Exacerbates Migraine-Like Behaviors in a Multibehavior Model of Rat Migraine. Toxicol Sci. 2014;137:416–427. doi: 10.1093/toxsci/kft245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q, Cao J, Hu F, Lu R, Wang J, Ding H, Gao R, Xiao H. Effects of estradiol on voltage-gated sodium channels in mouse dorsal root ganglion neurons. Brain Res. 2013;1512:1–8. doi: 10.1016/j.brainres.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 58.Wieseler J, Ellis A, Sprunger D, Brown K, McFadden A, Mahoney J, Rezvani N, Maier SF, Watkins LR. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2010;185:236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci. 2010;30:8710–8719. doi: 10.1523/JNEUROSCI.6323-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]