Abstract
Given evidence that eicosapentaenoic acid, docosahexaenoic acid, and anthocyanin-rich blueberries provide neurocognitive benefit, we investigated long-term supplementation in older adults with cognitive complaints. In a 24-week randomized, double blind, placebo-controlled trial, elderly men and women received daily fish oil (FO) or blueberry (BB) or both. Diet records confirmed that participants reduced background consumption of EPA, DHA, and anthocyanins as prescribed. Erythrocyte EPA+DHA composition increased in the FO groups (p = 0.0001). Total urinary anthocyanins did not differ between the groups after supplementation but glycoside and native (food) forms increased only in the blueberry-supplemented groups. The FO (p = 0.03) and BB (p = 0.05) groups reported fewer cognitive symptoms, and the BB group showed improved memory discrimination (p = 0.04), indicating that supplementation improved cognition. Cognitive benefit in the BB group was associated with the presence of urinary anthocyanins reflecting recent BB intake but not with anthocyanin metabolites. However, combined FO+BB treatment was not associated with cognitive enhancement as expected.
Keywords: omega-3 fatty acids, blueberries, anthocyanins, aging, memory, dementia
1. Introduction
Older adults with subjective cognitive complaints have increased risk for future dementia (Jessen et al. 2010), and a subset of these individuals exhibit early neurodegenerative changes, even in the absence of objective cognitive performance deficit (Saykin et al. 2006). Given the extended preclinical phase of Alzheimer’s disease (AD; Sperling et al. 2011), there is increasing interest in intervention strategies to delay or prevent late life dementia by targeting modifiable risks (Imtiaz et al. 2014). Such interventions feature nutrition prominently, including dietary supplementation to increase intake of nutrients that may enhance brain integrity and resilience and neurocognitive function (Ngandu et al. 2015).
There is evidence from prospective epidemiological studies that habitual consumption of fish and higher blood omega-3 polyunsaturated fatty acids (n-3 PUFA) levels may reduce risk for cognitive decline, dementia, and AD (Cunnane et al. 2009). Fish provide the primary dietary source of n-3 PUFA, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3). Rodent studies suggest that dietary n-3 PUFA deficiency reduces brain glucose uptake (Ximenes da Silva et al. 2002) and leads to memory impairment (Moriguchi et al. 2000), whereas n-3 PUFA supplementation is protective against neuropathology in mouse models of AD (Hooijmans et al. 2012). Human neuroimaging evidence suggests that greater fish consumption or higher blood n-3 PUFA level protects cortical structure and function in non-demented elderly subjects (McNamara et al. 2017). Also, the APOE ε4 allele, a genetic risk for AD (Corder et al. 1993), is associated with reduced transfer of n-3 PUFAs to peripheral and central compartments (Plourde et al. 2009; Samieri et al. 2013; Vandal et al. 2014; Yassine et al. 2016a). While the results of controlled n-3 PUFA supplementation trials investigating cognitive outcomes in AD have been negative, cognitive benefit has been observed in non-demented older adults (Mazereeuw et al. 2012). Translational evidence therefore suggests that increasing n-3 PUFA consumption in advance of dementia may represent a promising strategy to mitigate neurodegeneration and associated cognitive decline.
Anthocyanins are flavonoid polyphenolics that impart the red, purple, and blue color to berry fruit and are particularly abundant in blueberries. Ingested anthocyanins are found in various forms in essentially all tissues where they have been sought including the brain (Milbury & Kalt, 2010; Kalt et al. 2008). Preclinical and clinical investigations support a role for anthocyanins in human health (for review see Pojer et al. 2013), and they are well studied in relation to neurocognitive function in aging (Shukitt-Hale et al. 2015). Anthocyanin actions have been associated with multiple benefits that are pertinent to neurodegeneration such as enhanced neuronal signaling (Rendeiro et al. 2013) and resilience (Poulose et al. 2014) and improved glucose disposal (Stull et al. 2010; Seymour et al. 2011). BB supplementation has been shown to ameliorate age-related decline in hippocampus-dependent learning in animals (Joseph et al. 1999; Casadesus et al. 2004). Further, our prior trial involving older adults with Mild Cognitive Impairment (MCI), a prodromal condition involving cognitive decline and greater risk for AD (Albert et al. 2011; Petersen, 2004), showed that daily supplementation with BB juice improved cognitive performance (Krikorian et al. 2010a). More recently, we also observed enhanced regional brain activation with BB supplementation in MCI (Boespflug et al. 2017).
Given such evidence of neurocognitive benefit in the context of aging with n-3 PUFA and BB supplementation, and the importance of developing interventions that are efficacious and compatible with long-term adherence in advance of pathological cognitive decline, we conducted an intervention trial with EPA+DHA from fish oil and whole fruit BB powder. We enrolled older adults who reported cognitive decline but did not exhibit objective impairment in order to intervene before neuropathololgy was clinically evident. The primary outcomes included cognitive symptoms apparent in everyday functioning and neuropsychological performance. We also explored the potential benefit of combined supplementation with both FO and BB given preclinical evidence suggesting that intestinal uptake and blood concentrations of n-3 PUFA can be increased when co-administered with flavonoids (Maestre et al. 2013; Toufektsian et al. 2010). An ancillary goal was to determine whether sustained supplementation was necessary to maintain cognitive benefit by performing an assessment several weeks after termination of the interventions. We expected that any improvement associated with the treatments would be diminished after supplementation was discontinued.
2. Materials and Methods
2.1. Study Design
This was randomized, double blind, parallel groups, placebo-controlled trial. Prospective participants were involved in an initial telephone contact to review inclusion and exclusion criteria and requirements for participation followed by a screening visit in which instruments were administered to characterize level of cognitive impairment. For those who qualified, supplementation and dietary restrictions were initiated after the major assessment at the enrollment visit (week 0) and terminated at the week 24 visit. This was followed by an additional 24 weeks without intervention or dietary restriction before follow up assessment at week 48. We also obtained blood samples after overnight fast and performed anthropometric measures at these study visits. A small breakfast was provided before administration of the neuropsychological protocol. There also was a mid-intervention (week 12) study visit at which participants returned supplement containers, submitted completed diet records, and received supplement supplies and diet diary forms for the remainder of the intervention. We collected urine samples for anthocyanin assays at the enrollment and week 12 visits after overnight fast and before administration of study supplements. We monitored adherence to the protocol in multiple ways including having participants return used and unused fish oil and control capsules and dose packets for the BB and control powders, by collecting diet diaries so that EPA, DHA, and anthocyanin intake outside study supplementation could be calculated, and with red blood cell omega-3 fatty acid levels and urinary anthocyanin measures. We also performed APOE genotyping to examine the impact of the ɛ4 allele on blood n-3 PUFA levels at baseline and following FO supplementation.
Supplemental Figure 1 illustrates the study design and contains enrollment and attrition information. We enrolled 94 participants who were randomized to receive FO (fish oil + placebo powder, n = 21), BB (blueberry powder + placebo oil, n = 24), FO + BB (fish oil + blueberry powder, n = 26) or PL (placebo oil + placebo powder, n = 23). Sixteen participants discontinued participation during the intervention and did not complete the week 24 visit. The non-completers included four from the FO group, five from the BB group, five from the FO + BB group, and two from the PL group. We also excluded two participants (one from the FO + BB group and one from the PL group) because performances on the neuropsychological instruments at enrollment indicated impairment consistent with MCI despite having met inclusion criteria. Data on 76 participants were included in the analyses at week 24. An additional 11 participants, including two FO, three BB, three FO + BB, and three PL participants did not complete the week 48 assessment so that data from 65 participants were included in the post-intervention analyses.
2.2 Participants
The study protocol was approved by the University of Cincinnati Medical Institutional Review Board and registered with ClinicalTrials Identifier, NCT01746303. Each participant reviewed and signed an informed consent document prior to enrollment. Prospective participants were recruited from the Cincinnati, OH, USA region with print advertising soliciting participation of men and women aged 62- to 80-years-old who had mild, self-perceived cognitive decline with aging. In particular, subjective cognitive decline is differentiated from MCI and prodromal AD. Those with diagnosed or suspected dementia, MCI, diabetes, kidney disease, liver disease, hematological coagulation disorder, allergy to shellfish or seafood, debilitating psychiatric condition, or substance abuse were excluded. We also excluded individuals with regular use of medications or supplements that might affect outcome measures or interact with the study products such as aspirin, anticoagulants, benzodiazepines, and supplements with antioxidant and anti-inflammatory actions.
Research criteria for subjective cognitive decline include self-reported decline in cognitive function and non-impaired age-corrected performance on cognitive tasks (Jessen et al 2014). We administered screening instruments to gather demographic and pertinent medical information and to establish level of cognitive function to confirm the presence of subjective cognitive decline (Jessen et al 2014; Molinuevo et al. 2017). These instruments included the Academic and Medical History Questionnaire (Krikorian et al. 2004), the Clinical Dementia Rating (CDR; Hughes et al. 1982), the California Verbal Learning Test, second edition (CVLT; Delis et al. 2000), Montreal Cognitive Assessment (MOCA; Nesreddine et al. 2005), and the Geriatric Depression Scale (GDS; Yesavage et al. 1983). Inclusion criteria were operationalized as CDR = 0 and MOCA score > 25 or CVLT cumulative acquisition score between 1.0 SD below and 1.0 SD above the age-corrected mean, and GDS < 13. Those with GDS scores greater than 13 were referred for further evaluation of possible depressive disorder and excluded from participation.
2.3. Intervention products
2.3.1. Fish oil
The fish oil and placebo (corn oil) capsules were provided by the Inflammation Research Foundation, Marblehead, MA USA. The capsules were identical in size, shape, and color to protect the blind and were stored at (4° C) until distributed to participants who were instructed to keep the capsules under refrigeration at home. Fish oil was administered in four capsules, each of which contained 400 mg EPA and 200 mg DHA for total daily doses of 1.6 g EPA and 0.8 g DHA. This dose was chosen based on evidence that similar doses were effective in improving cognitive performance in healthy elderly participants (Witte et al. 2014) and in those with mild cognitive dysfunction (Freund-Levi et al. 2006). The placebo oil also was administered in four capsules per day. To minimize potential gastrointestinal discomfort, participants were advised to take two capsules with breakfast and two with dinner. Participants also were instructed to return empty capsule containers at study visits. EPA and DHA contents of the capsules were assayed periodically by gas chromatography to assure consistency across the trial. Neither EPA nor DHA content fell below 400 mg and 200 mg per capsule, respectively, during the course of the intervention. EPA and DHA content did not vary more than 3% and 2.9%, respectively, between the periodic assessments.
2.3.2. Blueberry powder
Whole frozen blueberries (Vaccinium sp.) were freeze dried and powdered to 20 mesh. The blend of Vaccinium berries was provided by the US Highbush Blueberry Council (Folsom, CA USA) and the Wild Blueberry Association of North America (Old Town, ME USA). The dry weight (DW) composition of the BB powder included V. ashei Reade cultivar ‘Tifblue’ (33.3%), V corymbosum L. cultivar “Rubel’ (16.7%), and a mixture of wild phenotypes of V. angustifolium (50%). The berry powder had a phenolic concentration of 20.4 ± 0.31 gallic acid equivalents/g DW, anthocyanin content of 14.5 ± 0.04 mg cyanidin 3-glucoside equivalents/g DW, and an ORAC value of 248 ± 20.6 μmole Trolox equivalents/g DW.
The macronutrient composition of the powder was 95.0% carbohydrate, 0.96% fat, and 2.47% protein and the caloric content was 399 calories per 100 g DW. The placebo powder, which was a proprietary mixture provided by the US Highbush Blueberry Council, was matched for color, taste, and sugar content as closely as possible with food-grade ingredients, and milled similarly. The placebo powder contained 389 calories per 100 g with 97.3% carbohydrate, 0.02% total fat, and <0.781% protein. Fiber was not included in the placebo. We chose a daily dose equivalent of one cup whole blueberry fruit on the basis of our prior blueberry juice study (Krikorian et al. 2010a) and recommendations extrapolated from animal studies (DeFuria et al. 2009). The DW of one cup whole fruit was 25 g. Based on average values for three years, this daily dose provided total phenolic compounds of 417 gallic acid equivalents, 269 mg cyanidin 3-glucoside equivalents of anthocyanins and 6525 μM Trolox equivalents in antioxidant capacity using the ORAC assay (Cao et al. 1993). At the end of three years of storage, the total phenolic concentration of the blueberry powder had declined by 24.6% and the anthocyanin concentration by 41.8%, while there was no loss in the total ORAC antioxidant activity.
BB and placebo powders were packaged in identical, individual dose foil packets containing one-half the daily dose, the BB powder at 12.5 grams per packet and placebo powder at 12 grams per packet. Participants were instructed to take the contents of one packet twice a day with the morning and evening meals. It was recommended that the powder be mixed with water, although consumption with other foods and beverages was not prohibited.
2.4. Diet records and restrictions
All participants were instructed to consume cold-water fish no more than once a week and to avoid fish oil and berry-derived supplements. Participants also were provided with a list of anthocyanin-containing berries and red, purple, or blue plant-based foods such as red wines, juices, and jams and instructed to avoid these foods for the duration of the intervention. To characterize the background diet, three-day diet records were obtained during the week before enrollment, during week 12, and during the final week of the intervention (week 24). Reported intake of food and nutrients, including intake of EPA and DHA and anthocyanins, was analyzed using Nutrition Data System for Research (NDSR) software version 2014, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN USA. NDSR includes an optional module for dietary polyphenols, which was used to estimate intake of anthocyanins. In addition, a validated Omega-3 Dietary Intake Questionnaire (Benisek et al. 2002) was administered prior to the intervention to characterize habitual intake.
2.5. Neuropsychological protocol
We assessed cognitive performance at enrollment, after 24 weeks of treatment, and at post-treatment follow up at week 48. The assessment protocol was designed to evaluate participants’ perceptions of everyday functional capability and objective measures in cognitive domains vulnerable to late life dementia including psychomotor speed, working memory, lexical access, and long-term memory. The Dysexecutive Questionnaire (DEX; Burgess et al. 1998; Chan, 2001) was used to characterize self-assessed change in cognitive effectiveness in everyday activities. This instrument has been shown to be sensitive and a valid indicator of working memory and executive problems (Gerstorf et al. 2008). Working memory failures are characteristic both in the context of neurodegeneration and in non-pathological cognitive aging. Accordingly, we felt that this instrument could be a sensitive index of subjective cognitive inefficiencies and treatment response. We administered the Trail-Making Test, part A (Reitan, 1992) to measure psychomotor speed with a timed task that involved connecting spatially distributed digits in numerical sequence by drawing lines with a pencil. The Trail Making Test, part B (Reitan, 1992) was used to assess set switching aspects of working memory in a similar timed format (Sanchez-Cubillo et al. 2009) and required sequencing digits and letters alternately in numeric and alphabetical order. Controlled Oral Word Production procedures were used to evaluate lexical access under phonological (Miceli et al. 1981) and categorical (Benton, 1968) constraints. These tasks engage executive control processes as well as ability to access semantic knowledge, a characteristic deficiency in cortical dementia such as AD. Alternate forms of the Hopkins Verbal Learning Test were administered at the assessment points to evaluate new learning and long-term memory with scores representing cumulative learning, delayed recall and percentage recalled, and item discrimination in recognition memory, the latter an index of intrusion of extraneous information (Brandt, 1991).
2.6. Laboratory analyses
2.6.1. Red blood cell fatty acid composition
Whole fasting venous blood (10 ml) was collected to obtain plasma and erythrocytes (red blood cells, RBCs). Plasma and total RBC membrane fatty acid composition were determined with a Shimadzu GC-2010 (Shimadzu Scientific Instruments Inc., Columbia MD USA) using methods described previously (McNamara et al. 2010). Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids).
2.6.2. Urine anthocyanins
Ingested anthocyanins and their in vivo metabolites were measured in urine obtained in a single void collected after overnight fast and before daily powder intake at the enrollment and week 12 visits. Sample and data analyses for urinary anthocyanins were conducted as described by Kalt et al. 2014, except that anthocyanin content was normalized on the basis of urinary creatinine and not urine volume. Total urinary anthocyanin contents were reported in addition to the de-glycosylated glucuronidated anthocyanins (approximately 60% of total), glycosidic forms of anthocyanins (±2% of total), and the native (food) form anthocyanins (±0.2% of total).
2.6.3 Metabolic factors
Blood samples were obtained after overnight fast at the major study visits in order to determine serum glucose and insulin values. Glucose concentration was determined using the glucose-peroxide reaction with normal range defined as 65 to 115 mg/dL. Insulin was measured by radioimmunoassay with reference range 5 to 15 uU/mL.
2.6.4 Anthropometrics
We measured height, body weight, waist circumference at the narrowest waist, and blood pressure.
2.6.5 APOE genotyping
DNA was extracted from whole blood and the three common APOE alleles (ε2, ε3, ε4) were analyzed using a TaqMan assay by the University of Cincinnati genotyping laboratory.
2.7 Statistical analyses
Group differences in demographic variables were evaluated using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for dichotomous variables. The distributions of the neurocognitive and urinary anthocyanin values were assessed for normality with the Shapiro-Wilk test. The scores for Trail-making, parts A and B and the anthocyanin data were found to be not normally distributed and those score distributions were log-transformed for the purposes of statistical analyses. Our a priori hypotheses were concerned with cognitive effects comparing FO treatment against placebo, BB treatment with placebo, and combined FO+BB treatment with placebo. These hypotheses were assessed with separate analysis of covariance (ANCOVA) for each outcome domain in which the final visit measure was compared between groups with age and the corresponding baseline measure as covariates in order to isolate the effect of the intervention (Sheeber, et al. 1996). For the significant ANCOVA effects, we report Cohen’s f effect size (Cohen, 1988), which are characterized as small (f = 0.10), medium (f = 0.0.25), and large (f = 0.40). We assumed moderate effect sizes for the primary outcomes on the basis of our prior supplementation trials. Given Cohen’s f = 0.25 and alpha probability = 0.05, power > 0.90. Our a priori predictions were that that cognitive enhancement would be observed with FO supplementation relative to PL and with BB supplementation relative to PL. In addition, we expected that combined FO and BB treatments would generate greater cognitive benefit relative to placebo. Differences in the content of urinary anthocyanins in the groups at baseline and at week 12 were determined by ANOVA using fitted values after log10 transformation.
3. Results
3.1 Participant sample
Comparison of the sample characteristic of all enrolled participants is shown in Table 1. There was no pre-intervention difference among the groups for age, educational attainment, overall intellectual function, level of depressive symptoms, and anthropometric and metabolic factors. There was no difference between the groups in the proportion of participants diagnosed with hypertension, and all so diagnosed were receiving treatment with blood pressure lowering medications.
Table 1.
Sample characteristics by group
| FO (n = 17) |
BB (n = 19) |
BB+FO (n = 20) |
Placebo (n = 20) |
F(3,72) | p | |
|---|---|---|---|---|---|---|
| Age, y | 69 (5.2) | 68 (3.9) | 68 (4.7) | 67 (4.9) | 0.71 | 0.54 |
| Education, y | 15.6 (2.4) | 15.0 (2.1) | 16.1 (2.0) | 14.7 (1.8) | 1.72 | 0.17 |
| * Gender (M:F) | 10:7 | 8:11 | 7:13 | 10:10 | - | ns |
| MOCA | 27.1 (1.3) | 25.5 (2.2) | 26.1 (2.1) | 26.5 (1.6) | 2.25 | 0.08 |
| GDS | 5.6 (3.5) | 3.6 (3.7) | 3.3 (4.6) | 5.0 (3.6) | 1.51 | 0.21 |
| * HTN, n (%) | 6 (35%) | 9 (47%) | 6 (30%) | 6 (30%) | - | ns |
| BP systolic, mmHg | 130.0 (10.4) | 127.8 (14.6) | 124.0 (18.9) | 132.7 (13.9) | 1.20 | 0.31 |
| BP diastolic, mmHg | 79.6 (7.0) | 74.3 (10.0) | 71.9 (12.1) | 78.4 (10.5) | 2.31 | 0.08 |
| Body weight, kg | 82.6 (15.4) | 76.3 (13.3) | 83.4 (21.2) | 83.7 (14.8) | 0.85 | 0.46 |
| Waist circumference, cm | 100.3 (11.1) | 93.6 (13.4) | 98.1 (16.8) | 97.9 (12.4) | 0.77 | 0.51 |
| Fasting glucose, mg/dl | 101.2 (8.3) | 102.6 (9.2) | 100.4 (8.5) | 103.6 (10.0) | 0.41 | 0.73 |
| Fasting insulin, μU/mL | 14.8 (8.3) | 11.0 (5.3) | 10.3 (4.9) | 11.1 (5.0) | 2.08 | 0.10 |
| HOMA2 IR | 1.9 (1.0) | 1.4 (0.7) | 1.3 (0.65) | 1.4 (0.66) | 1.91 | 0.13 |
| RBC EPA+DHA, % | 5.0 (1.2) | 5.2 (1.3) | 5.1 (1.5) | 4.8 (1.2) | 0.31 | 0.82 |
| Plasma EPA+DHA, % | 2.4 (0.8) | 2.6 (1.2) | 2.1 (0.6) | 2.2 (0.8) | 0.85 | 0.46 |
| * APOE ε4 allele carrier, n (%) | 5 (29%) | 5 (26%) | 5 (25%) | 6 (30%) | - | ns |
Note. Mean (SD) except where percent (%) as indicated. FO = fish oil supplemented group. BB = blueberry powder supplemented group. PL = placebo group. MOCA = Montreal Cognitive Assessment. GDS = Geriatric Depression Scale. HTN = diagnosed hypertension, BP = blood pressure. HOMA2 IR = homeostasis model assessment estimate of insulin resistance. RBC = red blood cell. RBC EPA+DHA values represent percent fatty acid by weight (mg EPA+DHA/total mg fatty acid).
All between group comparisons of proportions were nonsignificant, p > 0.05.
3.2.1. APOE genotyping
APOE ε3/ε3 accounted for 63% of the sample (n = 59), ε3/ε2 for 12% (n = 11), ε4/ε3 for 25% (n = 23), and ε4/ε4 for 1.1% (n = 1). There were 70 APOE ε4 non-carriers. Among APOE ε4 heterozygous carriers (n = 24), 13% were ε4/ε2 carriers and 87% ε4/ε3 carriers. APOE ε4 frequency did not differ between treatment groups. APOE ε4 carriers (n = 24) and APOE ε4 non-carriers (n = 70) were demographically similar (Table 1).
3.2.2. Blood fatty acids
The mean RBC EPA+DHA composition for all participants at baseline was 5.1 (± 1.3) percent of total fatty acids, and there was no group difference in RBC EPA+DHA (p = 0.77) or plasma EPA+DHA (p = 0.81) at baseline (Table 1). RBC and plasma EPA+DHA were positively correlated (r = 0.78, p ≤ 0.0001). Self-reported baseline dietary intake of EPA (p = 0.54) and DHA (p = 0.54) was not different between the groups, and dietary EPA+DHA intake was positively correlated with RBC (r = 0.29, p = 0.005) and with plasma (r = 0.61, p ≤ 0.0001) EPA+DHA composition. Associations between dietary EPA+DHA intake and plasma EPA+DHA levels in APOE ε4 carriers and non-carriers also were not different (p = 0.36), and there was no effect of APOE ε4 carrier status, defined by the presence or absence of at least one ε4 allele, on cognitive outcomes.
Following FO supplementation significant group by visit (baseline, week 24, week 48) interactions were observed for RBC EPA, F(6,120) = 10.92, p ≤ 0.0001, DHA F(6,120) = 5.31, p ≤ 0.0001, and EPA+DHA F(6,120) = 9.10, p ≤ 0.0001, and the ratio of arachidonic acid (AA; 20:4n-6) to EPA+DHA, F(6,120) = 6.42, p ≤ 0.0001, but not for AA, F(6,120) = 1.53, p = 0.18 (Supplemental Figure 2). At 24 weeks, RBC EPA+DHA composition increased significantly from baseline in the FO (32%; p ≤ 0.0001) and FO+BB (33%; p ≤ 0.0001) groups, but not in the BB (−12%; p = 0.13) or PL (−11%; p = 0.14) groups. The increases in RBC EPA+DHA levels observed at the week 24 visit in the FO and FO+BB groups returned to baseline levels following the 24-week discontinuation phase (Supplemental Figure 2). With respect to plasma fatty acid levels, significant group by visit (baseline, week 24) interactions also were observed for EPA, F(3,63) = 16.25, p ≤ 0.0001, DHA, F(3,72) = 3.77, p = 0.01, and EPA+DHA, F(3,72) = 11.61, p ≤ 0.0001, and the AA/EPA+DHA ratio, F(3,72) = 6.69, p = 0.0005, but not for AA, F(3,72) = 0.67, p = 0.57 (data not shown). In APOE ε4 carriers (n = `10) and non-carriers (n = 37) receiving FO supplementation, the group by visit interaction for the baseline to week 24 increase in RBC DHA (p = 0.51), RBC EPA (p = 0.96), plasma DHA (p = 0.42), and plasma EPA (p =0.80) were not significant.
3.2.3. Urinary anthocyanins
Anthocyanins, including native forms and phase 2 metabolites, were measured in urine collected after overnight fast before morning intake of the supplement. Samples were obtained at enrollment and after 12 weeks’ daily intake. Total urinary anthocyanin excretion among the four groups was not different between enrollment and week 12, nor was the content of anthocyanin aglycone glucuronide conjugates, which made up 65% of the total (Table 2). Glycoside forms of anthocyanins, which comprised 2% of the total, declined over 12 weeks in the BB placebo groups but did not change in the two BB-supplemented groups, p = 0.03 (Table 2). Washout of food form anthocyanins was evident in participants that received BB placebo, p = 0.03 (Table 2), while food form anthocyanins, which comprised just 0.2%, were greater in the BB-supplemented groups after 12 weeks’ daily intake.
Table 2.
Total urinary anthocyanin subgroups
| Total anthocyanin | Major aglycone conjugates | Glycoside anthocyanin | Native anthocyanin | ||
|---|---|---|---|---|---|
|
| |||||
| 100% | 65% | 2% | 0.2% | ||
|
| |||||
| Group | week | log10 ng/mg creatinine
|
|||
| FO | 0 | 2.614 (411) |
2.427 (267) |
1.058 (11.43) |
−0.4059 (0.390) |
| FO | 12 | 2.671 (469) |
2.464 (291) |
0.8184 (6.58) |
−0.1569 (0.70) |
| BB | 0 | 2.729 (535)1 |
2.548 (353) |
0.9856 (9.67) |
−0.03587 (0.920) |
| BB | 12 | 2.671 (468) |
2.491 (310) |
0.9729 (9.40) |
0.1383 (1.37) |
| BB + FO | 0 | 2.605 (403) |
2.408 (265) |
0.8719 (7.45) |
−0.01097 (0.980) |
| BB + FO | 12 | 2.598 (396) |
2.463 (290) |
0.973 (9.40) |
0.2699 (1.86) |
| PL | 0 | 2.756 (570) |
2.571 (372) |
1.228 (16.90) |
−0.6388 (0.230) |
| PL | 12 | 2.534 (342) |
2.315 (207) |
0.709 (5.12) |
−0.3386 (0.460) |
|
| |||||
| grand mean, log10 | 2.647 | 2.461 | 0.9521 | −0.1423 | |
| SEM | 0.05906 | 0.06287 | 0.1083 | 0.159 | |
| Time effect probability | ns | ns | 0.035 | 0.030 | |
Note. Values = log10 transformed mean, ng/mg creatinine. Values in parentheses = mean ng/mg, back-transformed from log10. FO = fish oil group. BB = blueberry group. PL = placebo group. Urine collected after overnight fast and before morning supplement dose at enrollment and after 12 weeks’ daily supplementation. Total anthocyanins = all anthocyanin compounds including native and metabolite forms. Native (food form) anthocyanins are unmetabolized forms present in blueberries.
3.2.4. Omega-3 fatty acid and anthocyanin consumption in the background diet
Daily intake of EPA and DHA and of each of six major anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin) were derived from diet records obtained prior to enrollment, at study mid-point, and at the end of the intervention using the polyphenol module of the NDSR program. Separate repeated measures analyses indicated that there was significant change over time with respect to EPA, F(2.,136) = 8.62, p < 0.0001, Cohen’s f = 0.38, DHA, F(2.,136) = 11.99, p < 0.0001, Cohen’s f = 0.46, and EPA + DHA F(2.,136) = 11.34, p < 0.0001, Cohen’s f = 0.45, indicating reduced consumption external to the study during the intervention (Figure 1). There was no treatment group by time interaction for external consumption of these n-3 PUFA. Similarly, there were significant time effects indicating reduced external consumption of anthocyanins including cyanidin, F(2,136) = 3.46, p = 0.03, Cohen’s f = 0.22, pelargonidin, F(2,136) = 5.07, p = 0.04, Cohen’s f = 0.27, malvidin, F(2,136) = 21.33, p > 0.0001, Cohen’s f = 0.55, peonidin, F(2,136) = 16.82, p < 0.0001, Cohen’s f = 0.49, and petunidin, F(2,136) = 13.17, p < 0.0001, Cohen’s f = 0.43. There was a time by group interaction effect for pelargonidin, F(2,136) = 2.23, p = 0.04, Cohen’s f = 0.32, but for no other anothcyanin (Figure 2). Notably, pelargonidin is a major anthocyanin not found in blueberries.
Figure 1.
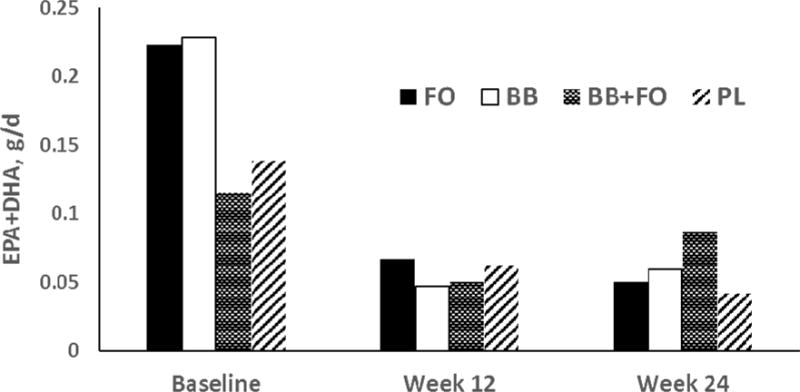
Mean daily intake (g/d) of EPA+DHA in the background diet by group determined from three-day diet records obtained during the week before enrollment (Baseline), the week before the interim visit (week 12), and during the final week of the intervention (week 24). Repeated measures ANCOVA indicated effect for time, F = 11.53, p < 0.000.
Figure 2.
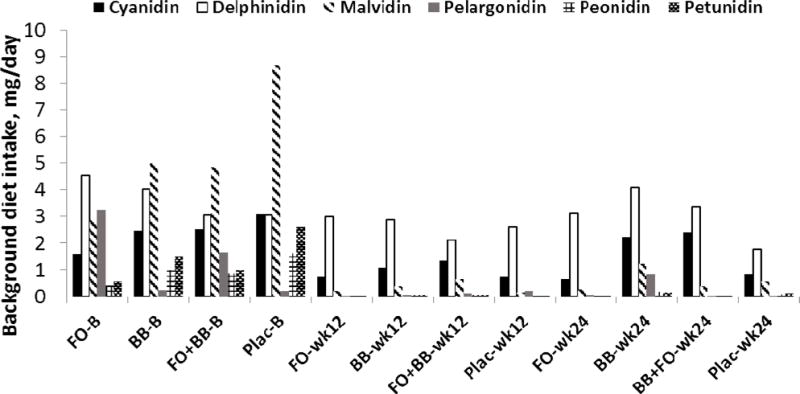
Anthocyanin consumption (mean daily intake, mg) in the background diet by group determined from three-day diet records obtained during the week before enrollment (B), before the interim visit (week 12), and during the final week of the intervention (week 24). Separate repeated measures analyses indicated reduced external consumption of cyanidins, p = 0.03, pelargonidin, p = 0.04, malvidin, p = 0.00, peonidin, p = 0.00, and petunidin, p < 0.0001. A significant group by time interaction was evident for pelargonidin only, p = 0.04.
3.2.5. Serum glucose, insulin, and estimated insulin resistance
ANCOVA was used to assess between group differences in week 24 values for fasting glucose, fasting insulin, and estimated insulin resistance from calculated HOMA2-IR. Age and corresponding baseline values were included as covariates measures. There was no effect of the FO intervention on glucose (103.8 vs 105.7, p = 0.96), insulin (14.1 vs 12.3, p = 0.38), or HOMA2-IR (1.8 vs 1.64, p = 0.77); no effect of the BB intervention on glucose (104.6 vs 105.7, p = 0.83), insulin (10.8 vs 12.3, p = 0.49),or HOMA2-IR (1.4 vs 1.6, p = 0.46); and no effect of combined FO and BB intervention on glucose (102.5 vs 105.7, p = 0.90), insulin (10.8 vs 12.3, p = 0.77), or HOMA2-IR (1.4 vs 1.6, p = 0.72).
3.3. Neuropsychological outcomes
Summary data by group for the week 24 symptom and cognitive performance measures are contained in Table 3. Comparing FO supplementation against PL, there was a significant effect indicating reduced cognitive symptoms in everyday activities as measured by the DEX, F(1,32) = 4.94, p = 0.03, Cohen’s f = 0.39 (Figure 3). However, we found no effect for motor speed, working memory, learning and retention, and lexical access. The BB group also reported fewer cognitive symptoms, F(1,35) = 3.99, p = 0.05, Cohen’s f = 0.34 (Figure 3). In addition, there was an effect indicating improved discrimination in recognition memory on the HVLT for the BB-treated group, F(1,35) = 4.24, p = 0.04, Cohen’s f = 0.34 (Figure 4). There was no effect in any cognitive domain for the combined FO and BB powder group.
Table 3.
Neurocognitive performance by group
| FO (n = 17) |
BB (n = 19) |
FO + BB (n = 20) |
PL (n = 20) |
|||||
| wk 0 | wk 24 | wk 0 | wk 24 | wk 0 | wk 24 | wk 0 | wk 24 | |
| Trail-making A, s | 34.2 | 30.1 | 36.4 | 29.7 | 32.9 | 29.5 | 32.3 | 28.7 |
| Trail-making B, s | 65.7 | 57.7 | 79.7 | 75.6 | 74.6 | 64.2 | 74.3 | 67.4 |
| HVLT learning | 26.0 | 27.2 | 25.5 | 28.3 | 27.4 | 28.2 | 27.4 | 28.0 |
| HVLT recall | 8.9 | 9.6 | 9.6 | 10.0 | 10.4 | 9.7 | 10.1 | 10.0 |
| HVLT discrimination | 10.2 | 10.7 | 10.3 | 11.2* | 10.8 | 10.5 | 10.8 | 10.8 |
| HVLT retained, % | 88.3 | 93.6 | 96.2 | 91.1 | 95.4 | 89.1 | 91.3 | 94.1 |
| Phonological access | 36.8 | 41.7 | 33.4 | 36.3 | 39.0 | 43.4 | 40.2 | 42.7 |
| Semantic access | 17.9 | 19.3 | 18.3 | 18.6 | 19.7 | 22.3 | 18.7 | 19.1 |
| DEX | 13.9 | 11.1* | 15.0 | 11.9* | 12.6 | 11.7 | 14.2 | 15.9 |
Note. Mean values at week 0 (baseline) and week 24 visits. wk = study week. FO = fish oil supplemented group. BB = blueberry powder supplemented group. PL = placebo group.
= significant effect vs placebo at p < 0.05. Scores for Trail-making parts A and B are time on task, lower scores reflecting better performance, no minimum or maximum score. HVLT = Hopkins Verbal Learning Test. HVLT learning, maximum score = 36. HVLT recall, maximum score = 12. HVLT discrimination, maximum score = 12. HVLT retained %, maximum score = 100 (higher rarely). Phonological access = controlled oral word production under phonological constraint, no maximum score. Semantic access = controlled oral word production, no maximum score. DEX = Dysexecutive Questionnaire, lower score represents less perceived cognitive inefficiency, maximum score = 80.
Figure 3.
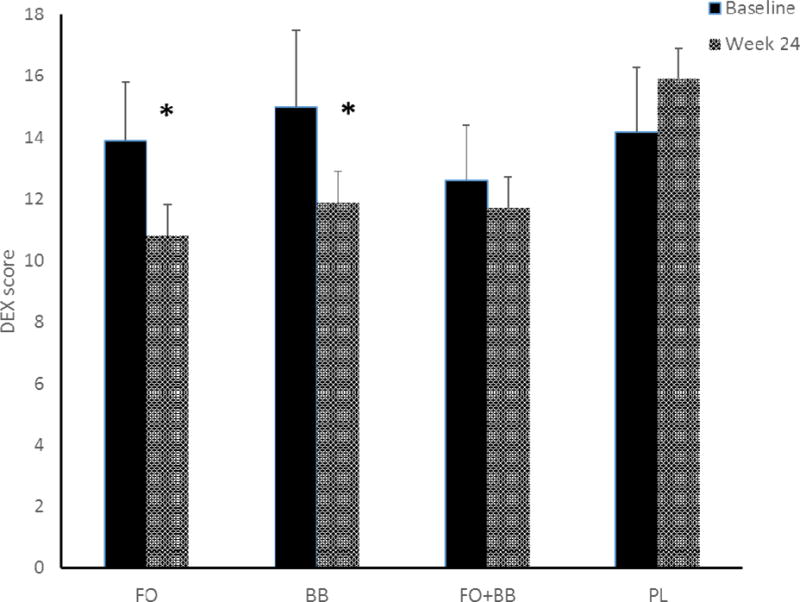
Cognitive symptoms in everyday activities as measured by the Dysexecutive Questionnaire (DEX) were reduced after 24 weeks’ treatment for participants receiving fish oil (FO), F(1,32) = 4.94, p = 0.03 and those receiving blueberry powder (BB), F(1,35) = 3.99, p = 0.05. Lower scores indicate less symptom disturbance. Error bars = SEM.
Figure 4.
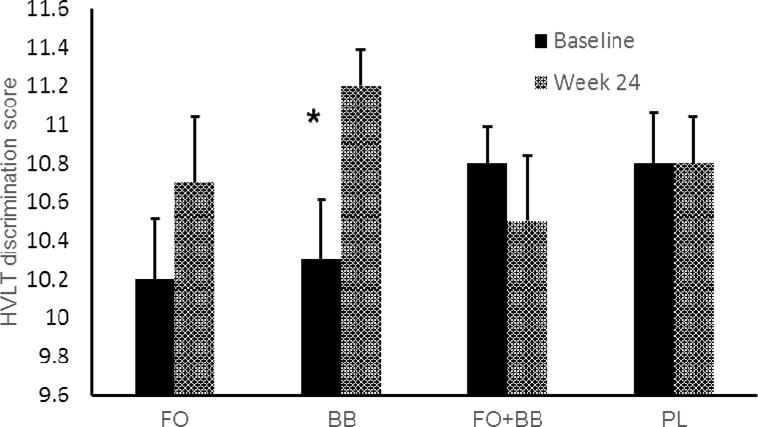
Recognition memory discrimination performance for the Hopkins Verbal Learning Test (HVLT). The BB-treated group exhibited greater accuracy indicating improved discrimination of target from non-target items, F(1,35) = 4.24, p = 0.04. Error bars = SEM.
The effect for improved memory discrimination in the BB group was not maintained at week 48, which occurred 24 weeks after discontinuation of the supplement protocol. However, the reduction of cognitive symptoms was maintained at week 48 for both the FO group, F(1,27) = 5.70, p = 0.02, Cohen’s f = 0.46, and the BB group, F(1,29) = 4.85, p = 0.03, Cohen’s f = 0.41.
3.5. Safety and tolerability
Among the 16 individuals who discontinued participation during the intervention phase, flu, psoriasis, vertigo, and constipation symptoms were reported by three. There was no indication that these symptoms were related to the study supplements. The small number of minor adverse responses suggests that the duration of the study was the major cause of non-completion. Life events such as vacations and unexpected family obligations and decreasing tolerance for the burden of participation over time likely reduced motivation to maintain participation.
4. Discussion
The primary objectives of this study were to determine the effects of treatment with FO and with BB on everyday cognitive inefficiencies and on neuropsychological performance in cognitive domains vulnerable to dementia in older adults with subjective complaints but without clinically evident cognitive decline. We also investigated whether combined FO and BB supplementation might produce enhanced benefit as compared with either supplement alone.
Following 24 weeks’ treatment we found that both the FO and BB groups reported lower levels of cognitive inefficiency in activities of everyday life. These effects are of interest in the context of a sample of cognitively unimpaired older adults with subjective cognitive complaints and indicate that meaningful cognitive benefit contributed to improved functional capability. There was no change in level of depressive symptoms with either the FO or BB intervention suggesting that mood enhancement did not influence the perception of improved everyday functioning.
We also observed improved discrimination in memory for the BB group on the HVLT recognition memory task. This finding indicates that BB supplementation was associated with improved resistance to interference of extraneous material in memory. Such interference, reflected as false positive or intrusion errors, are prominent in neurodegenerative conditions such as AD, which involves impaired semantic processing. However, memory interference also is evident in non-pathological cognitive aging and has been related to diminished inhibitory control in working memory (Robert et al 2009). The DEX also assesses several aspects of behavior that are dependent on inhibition, so that enhanced cognitive inhibitory control likely contributed to improvements in both DEX and memory discrimination performances for the BB-treated group (Robert et al. 2009). It is interesting that the effect for improved function in everyday activities was apparent in both of the individual treatment groups, although perhaps mediated by different mechanisms. That is, FO supplementation may have benefited participants through mechanisms such as improved brain glucose uptake (Moriguchi et al. 2000; Ximenes da Silva et al. 2002), while the BB treatment may have produced improved circulation and neural signaling (Boespflug et al 2017; Rendeiro et al. 2013; Shukitt-Hale, 2015). We did not, however, obtain evidence in this study that would bear directly on whether such mechanisms mediated perceived functional improvement.
The observed benefits for the BB-treated group is consistent with findings in studies in aged animals demonstrating enhanced working memory (Casadesus et al. 2004; Shukitt-Hale et al. 2015) and with our prior human trial involving older adults with MCI (Krikorian et al 2010a). However, we did not find performance benefits on other objective measures, including tasks assessing speed of processing, working memory, and lexical access with BB treatment. In contrast, our prior berry supplementation studies in older samples with MCI, a risk condition for dementia involving significant cognitive impairment, demonstrated enhancements with respect to new learning and retention as well as reduced memory interference (Krikorian et al 2010a; 2010b; 2012). It is possible that benefit related to nutritional supplementation is more amenable to measurement in older, more impaired individuals because of the higher ceiling for measured performance enhancement inherent in samples with greater impairment. In addition, the neurobiological response to supplementation may be diminished in the absence of pathology. Notably, non-pathological aging is associated with decline in working memory (Fabiani, 2012), and to that extent, our findings of behavioral and cognitive performance enhancement on working memory related tasks may reflect improvement in that domain because of expectable age-related decline. Whereas, it would be more difficult to demonstrate improved performance in relatively non-impaired domains such as lexical access.
The maintenance of the reduction of everyday cognitive inefficiency beyond the 24-week discontinuation phase was unexpected. This also is noteworthy given the failure to maintain the other major effect involving reduced memory interference in the BB-supplemented group. We do not have data on dietary intake after discontinuation of the intervention that would allow investigation of the extent to which these effects might be associated with intake of omega-3 fatty acids and BB.
We found that 24 weeks’ FO supplementation was sufficient to increase EPA+DHA biostatus robustly and that neither APOE ε4 genotype nor concomitant BB supplementation affected n-3 PUFA metabolism. We also observed increased excretion of native form anthocyanins in the BB supplemented groups as expected. However, combining FO with BB supplementation did not result in comparatively greater EPA+DHA absorption, as was suggested by preliminary preclinical studies. The diet records confirmed that participants were able to curtail consumption of n-3 PUFA- and anthocyanin-containing foods as prescribed by the study protocol. Reported adverse responses were few and minor, and none was related to the intervention. It appeared that the greater impediment to study completion was the extended period of the intervention and follow up interval.
The absence of a beneficial effect of combined FO and BB supplementation is surprising, particularly in the context of benefit associated with each of the individual treatments. It is unclear what factors might have contributed to this lack of effect, although one consideration is that daily, long-term supplementation with the combined treatments may have subverted a beneficial response in some way. Both n-3 PUFA (Anderson & Taylor, 2012) and flavonoid (Son et al. 2008) effects are mediated through transcription factor NF-E-2 related factor 2 (Nrf2; Anderson et al. 2012; Scapagnini et al. 2010). Nrf2 activity is upregulated by acute oxidative stress and toxicity, although generally tightly regulated by a suppressor protein (Keap1; Maher & Yamamoto, 2010), suggesting that sustained and excessive (as opposed to acute) upregulation may not be beneficial. As observed in other contexts involving chronic consumption of direct antioxidant vitamins, excessive activation of Nrf2 is possible and may contribute to adverse effects such as a net pro-oxidant environment (Zhang et al. 2010).
After 12 weeks, there was no significant decline in total anthocyanin excretion in the BB placebo groups, reflecting an extremely long persistence of these metabolites in vivo. Anthocyanin persistence in the gastrointestinal tract was documented previously in a human study after a five-day anthocyanin-free run-in and seven-day washout (Kalt et al. 2017a; 2017b). However, the 12-week washout interval in the BB placebo groups in this trial shows, for the first time, how well retained are the in vivo anthocyanin-related intermediates. Such remarkable retention is due at least in part to their propensity for flow in enterohepatic recirculation (EHR; Roberts et al. 2002). Anthocyanins, and other flavonoids, are of a molecular weight and polarity range that allows them to be dissolved and readily associate with bile phospholipids (Kalt et al. 2014; Roberts et al. 2002; Xia et al. 2012). Once absorbed, anthocyanins, along with bile acids and other bile components, are circulated via portal vein flow to the liver where they are subject to phase 2 metabolism (e.g., glucouronidation) and then released from the liver into bile, re-concentrated in the gall bladder, and released into the duodenum with bile in response to gastric and GI signaling. Prolonged excretion of anthocyanin into urine may also relate to the membrane solubility of deglycosylated anthocyanins, a notion that is supported by results from a pig feeding study where anthocyanins were found in all body tissues including tissues of low perfusion like brain regions and eyes (Kalt et al. 2008).
The very long-term retention of anthocyanins complicates the conduct of clinical versus animal studies. While human participants will enter studies with a substantial reservoir of anthocyanins from habitual consumption, animals used in anthocyanin research will have had no such dietary history and, potentially, a much greater capacity for absorption. Furthermore, this implies that the observed cognitive benefit in the BB-treated group was associated with consumption of the native form anthocyanins and not with anthocyanin metabolites that also were present in the BB placebo group. This was evident by the two fold higher native form anthocyanins in the BB group after 12 weeks. Also, because urine was collected before intake of the first blueberry powder dose of the day, native anthocyanin values would be expected to be at their lowest levels.
Among the limitations of this study was the absence of a run-in period with dietary restrictions against berry and fish oil consumption. Such a run-in period would have provided a period of abstinence from the supplemented products for all the groups. However, data from a prior human study (Kalt et al 2014) and this trial indicate that a run-in period of several weeks would not have influenced the presence of anthocyanin metabolites. Measurement limitations with respect to sensitivity to modest cognitive enhancement in a sample of cognitively healthy older adults might have contributed to the more limited benefits of the intervention products in comparison with our prior studies in MCI. The inclusion of more challenging cognitive tasks along with subjective cognitive scales in future studies in this population would be an important consideration.
In conclusion, this randomized, double-blind placebo-controlled trial demonstrated that 24 weeks’ supplementation with FO and with BB was associated with reduced self-reported inefficiencies in everyday functioning as well as reduced interference in memory for those receiving BB supplementation. Enhancement of perceived functional capability suggests that FO- and BB-treated participants experienced meaningful improvement of cognitive capability, a notable finding given that subjective cognitive complaints were an inclusion criterion for study participation and a symptom reflecting potentially greater risk for decline with aging (Jessen et al. 2010). Combined FO and BB therapy was not associated with cognitive or functional enhancement, an unexpected result. Nor was combined treatment associated with increased red blood cell n-3 PUFA concentration. We also derived important and novel data concerning the long-term persistence of anthocyanin metabolites, even after 12 weeks with little anthocyanin intake external to the study. Further, the fact that cognitive benefits were observed in the BB-treated group despite the extended persistence of anthocyanin metabolites in both BB and PL groups supports the notion that intake of the native anthocyanin compounds generated an acute response associated with cognitive benefit, such as vaso-modulating effects. However, a beneficial effect of the longer-term metabolism of these compounds cannot be ruled out.
Supplementary Material
Highlights.
Fish oil or blueberry (BB) supplementation improved cognitive function in older adults
Fish oil (FO) supplementation increased red blood cell EPA+DHA content
Anthocyanin metabolite levels did not differ between blueberry (BB) and placebo groups
Food form anthocyanins occurred only in the BB group in association with cognitive benefit
Lack of cognitive benefit with combined FO+BB raises the question of pro-oxidant effect
Acknowledgments
This study was supported by a National Institute of Health grant AG034617 to RK and RKM (Co-PIs). The NIH did not have a role in the analysis or interpretation of the research. This trial was registered at clinicaltrials.gov with identifier NCT01746303. The authors thank the Inflammation Research Foundation, Marblehead, MA USA for providing the fish oil and placebo capsules, the US Highbush Blueberry Council, Folsom, CA USA and the Wild Blueberry Association of North America, Old Town, ME USA for providing BB and placebo powder, the Kentville Research and Development Centre for analyzing urinary anthocyanins, the Bionutrition Core of the Clinical Translational Research Center at the University of Cincinnati for assistance with dietary intake analysis and the University of Cincinnati Genotyping Laboratory for APOE allele determinations.
RKM has received research support from Martek Biosciences Inc, DSM Nutritional Products, LLC, Ortho-McNeil Janssen, NARSAD, and NIH, served on the Inflammation Research Foundation scientific advisory board, and was a paid consultant for VAYA Pharma Inc., and Vifor Pharma Inc. RK has received research support from the NIH, the U.S. Highbush Blueberry Council, The Wild Blueberry Association of North America, and served on the Ketone Advisory Board of Accera, Inc. WK has received research support from the Wild Blueberry Association of North America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cognitive response to omega-3 fatty acid and blueberry supplementation in older adults
Disclosure Statement
None of the other authors has actual or potential conflict of interest, including financial, personal or other relationships with individuals or organizations that could inappropriately influence or be perceived as influencing their work.
The data contained in this manuscript has not been previously published nor submitted for publication elsewhere. This manuscript will not be submitted elsewhere while under consideration at Neurobiology of Aging.
This human trial was conducted according to extant human subject guidelines and approved by the medical institutional review board of the University of Cincinnati.
All authors have reviewed the manuscript and approve its content and accuracy.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman JJ, Fox NC, Gamst A, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Taylor DA. Stressing the heart of the matter: re-thinking the mechanisms underlying therapeutic effects of n-3 polyunsaturated fatty acids. F1000Reports Medicine. 2012;4:13. doi: 10.3410/M4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Thayne K, Harris M, Carraway K, Shaikh SR. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem J. 2012;441:359–366. doi: 10.1042/BJ20110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benisek D, Bailey-Hall E, Oken H, Masayesva S, Arterburn L. Validation of a simple food frequency questionnaire as an indicator of long chain omega-3 intake. 93rd Annual AOCS Meeting; Montreal, Quebec, Canada. 2002. p. S96. [Google Scholar]
- Benton AL. Differential behavioral effects in frontal disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Boespflug EL, Eliassen JC, Dudley JA, Shidler MD, Kalt W, Summer SS, Stein AS, Stover AN, Krikorian R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutritional Neuroscience. 2017 doi: 10.1080/1028415X.2017.1287833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Evans N, Emslie H, Wilson BA. The ecological validity of tests of executive function. J International Neuropsychological Society. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Rad Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, STellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- Chan RC. Dysexecutive symptoms amoung a non-clinical sample: A study with the use of the Dysexecutive Questionnaire. Br J Psychology. 2001;92:551–565. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences, second edition. Lawrence Erlbaum Associates. 1988 [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cornish IM. Factor structure of the everyday memory questionnaire. Br J Psychology. 2000;91:427–438. doi: 10.1348/000712600161916. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Plourde M, Pifferi F, Bégin M, Féart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res. 2009;48:239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- DeFuria J, Bennett G, Strissel KJ, Perfield JW, II, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequaelae. J Nutrition: Nutrition and Disease. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Adult version Manual. San Antonio TX: Psychological Corporation; 2000. California verbal learning test – second edition. [Google Scholar]
- Fabiani M. It was the best of times; it was the worst of times: A psychophysiologist’s view of cognitive aging. Psychophysio. 2012;49:283–304. doi: 10.1111/j.1469-8986.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Siedlecki KL, Tucker-Drob EM, Salthouse TA. Executive dysfunctions across adulthood: measurement properties and correlates of the DEX self-report questionnaire. Neuropsychol Dev Cogn B Aging. 2008;15:424–445. doi: 10.1080/13825580701640374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Pasker-de Jong PC, de Vries RB, Ritskes-Hoitinga M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimer’s pathology in animal models of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2012;28:191–209. doi: 10.3233/JAD-2011-111217. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Ivey LI, Hodgson JM, Croft KD, Lewis JR, Prince RL. Flavonoid intake and all cause mortality. Am J Clin Nutr. 2015;101:1012–20. doi: 10.3945/ajcn.113.073106. [DOI] [PubMed] [Google Scholar]
- Imtiaz B, Tolppanen AM, Kivipelto M, Soininen H. Future directions in Alzheimer’s disease from risk factors to prevention. Bioch Pharmacol. 2014;88:661–670. doi: 10.1016/j.bcp.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kolsch H, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychia. 2010;76:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Bostel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, et al. A conceptual framework fro research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dementia. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplements. J Neruosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SA, Graf BA, O’Leary JM, Milbury PE. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- Kalt W, Liu Y, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SA. Anthocyanin metabolites are abundant and persistent in human urine. J Agric Food Chem. 2014;62:3886–3903. doi: 10.1021/jf500107j. [DOI] [PubMed] [Google Scholar]
- Kalt W, McDonald JE, Liu Y, Fillmore SAE. Flavonoid metabolites in human urine during blueberry anthocyanin intake. J Agri Food Chem. 2017a;65:1582–1591. doi: 10.1021/acs.jafc.6b05455. [DOI] [PubMed] [Google Scholar]
- Kalt W, McDonald JE, Liu Y, Fillmore SE. Human anthocyanin bioavailability: effect of intake duration and dosing. 2017b doi: 10.1039/c7fo01074e. manuscript under review. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Zimmerman ME, Fleck DE. Inhibitory control in obsessive-compulsive disorder. Brain Cognit. 2004;54:257–259. doi: 10.1016/j.bandc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Nash T, Shidler MD, Kalt W, Vinqvist-Tymchuk M, Shukitt-Hale B, Joseph JA. Blueberry Supplementation Improves Memory in Older Adults. J Agri Food Chem. 2010a;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord Grape Juice Supplementation Improves Memory Function In Older Adults with Mild Cognitive Impairment. Brit J Nutri. 2010b;103:730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Boespflug EL, Fleck DE, Stein AL, Wightman JD, Shidler MD, Sadat-Hossieny S. Concord Grape Juice Supplementation and Neurocognitive Function in Human Aging. J Agri Food Chem. 2012;60:5736–5742. doi: 10.1021/jf300277g. [DOI] [PubMed] [Google Scholar]
- Maestre R, Douglass JD, Kodukula S, Medina I, Storch J. Alterations in the intestinal assimilation of oxidized PUFAs are ameliorated by a polyphenol-rich grape seed extract in an in vitro model and Caco-2 cells. J Nutr. 2013;143:295–301. doi: 10.3945/jn.112.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J, Yamamoto M. The rise of antioxidant signaling – the evolution and hormetic actions of Nrf2. Toxicology and Applied Pharmacology. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Mazereeuw G, Lanctôt KL, Chau SA, Swardfager W, Herrmann N. Effects of ω-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012;33:1482. doi: 10.1016/j.neurobiolaging.2011.12.014. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Asch RH, Lindquist DM, Krikorian R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot Essent Fatty Acids. 2017 doi: 10.1016/j.plefa.2017.05.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek RJ, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Caltagirone C, Gainotti G, Masullo C, Silveri MC, Villa G. Influence of age, sex, literacy and pathologic lesion on incidence, severity and type of aphasia. Acta Neruol Scand. 1981;64:370–382. doi: 10.1111/j.1600-0404.1981.tb04416.x. [DOI] [PubMed] [Google Scholar]
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacological Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J Agri Food Chem. 2010;58:3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, et al. Implementation of subjective cognitive decline criteria in research studies. Alzhiemer’s Dementia. 2017;13:296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Ngandu T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomized controlled trial. The Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Plourde M, Vohl MC, Vandal M, Couture P, Lemieux S, Cunnane SC. Plasma n-3 fatty acid response to an n-3 fatty acid supplement is modulated by apoE epsilon4 but not by the common PPAR-alpha L162V polymorphism in men. Br J Nutr. 2009;102:1121–1124. doi: 10.1017/S000711450938215X. [DOI] [PubMed] [Google Scholar]
- Pojer E, Mattivit F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: A review. Compre Rev. 2012;12:483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Bielinski D, Carrihill-Knoll KL, Rabin BM, Shukitt-Hale B. Protective effects of blueberry and strawberry diets on neuronal stress following exposure to 56Fe particles. Brain Research. 2014;1593:9–18. doi: 10.1016/j.brainres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Rendeiro C, Vauzour D, Rattray M, Waffo-Téguo P, Mérillon JM, Butler LT, Williams CM, Spencer JP. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLos One. 2013;28:8. doi: 10.1371/journal.pone.0063535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Borella E, Fagot D, Lecerf T, de Ribaupiere A. Working memory and inhibitory control across the life span: Intrusion errors in the Reading Span Test. Mem Cognition. 2009;37:336–345. doi: 10.3758/MC.37.3.336. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Magnasson BM, Frank J, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41:751–790. doi: 10.2165/00003088-200241100-00005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Inter Neuropsycholo Soc. 2009;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Samieri C, Lorrain S, Buaud B, Vaysse C, Berr C, Peuchant E, Cunnane SC, Barberger-Gateau P. Relationship between diet and plasma long-chain n-3 PUFAs in older people: impact of apolipoprotein E genotype. J Lipid Res. 2013;54:2559–2567. doi: 10.1194/jlr.P036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapagnini G, Sony V, Nader AG, Calogero C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour EM, Tanone II, Urcuyo-Llanes DE, Lewis SK, Kirakosyan A, Kondoleon MG, Kaufman PB, Bolling SF. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J Med Food. 2011;14:1511–1518. doi: 10.1089/jmf.2010.0292. [DOI] [PubMed] [Google Scholar]
- Sheeber LB, Sorensen ED, Howe SR. Data analytic techniques for treatment outcome studies with pretest/posttest measurements: an extensive primer. J Psychiatr Res. 1996;30:185–199. doi: 10.1016/0022-3956(96)00012-X. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Bielinski DF, Lau FC, Willis LM, Carey A, Joseph JA. The beneficial effects of berries on cognition, motor behavior and neuronal function in ageing. Brit J Nutri. 2015;114:1542–1549. doi: 10.1017/S0007114515003451. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutrition. 2010;140:1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufektsian MC, Salen P, Laporte F, Tonelli C, de Lorgeril M. Dietary flavonoids increase plasma very long-chain (n-3) fatty acids in rats. J Nutr. 2011;141:37–41. doi: 10.3945/jn.110.127225. [DOI] [PubMed] [Google Scholar]
- Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem N, Jr, Calon F, Plourde M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem. 2014;129:516–526. doi: 10.1111/jnc.12640. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Flöel A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014;24:3059–3068. doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- Xia B, Zhou Q, Zheng Z, Ye L, Hu M, Liu Z. A novel local recycling mechanism that enhances enteric bioavilability of flavonoids and prolongs their residence time in the gut. Mol Pharmaceutics. 2012;9:3246–3258. doi: 10.1021/mp300315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. J Neurochem. 2002;81:1328–1337. doi: 10.1046/j.1471-4159.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- Yassine HN, Rawat V, Mack WJ, Quinn JF, Yurko-Mauro K, Bailey-Hall E, Aisen PS, Chui HC, Schneider LS. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer’s disease. Alzheimers Res Ther. 2016a;8:25. doi: 10.1186/s13195-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine HN, Feng Q, Azizkhanian I, Rawat V, Castor K, Fonteh AN, Harrington MG, Zheng L, Reed BR, DeCarli C, Jagust WJ, Chui HC. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016b;73:1208–1216. doi: 10.1001/jamaneurol.2016.1924. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jingbo P, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicology and Applied Pharmacology. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


