Abstract
Bone metastasis (BM) is the advanced complication of breast cancer, while bone marrow-derived mesenchymal stem cells (BMSCs) in the microenvironment unclearly contribute to cancer metastasis. This study investigated potential roles of transforming growth factor- (TGF-) α in the interaction between breast cancer and BMSCs in BM. Clinical cases of breast cancer with bone metastasis (BMBC), breast cancer without bone metastasis (Non-BM-BC), and benign fibroadenoma (Benign) were enlisted in a retrospective study. TGF-α was found obviously overexpressed in BM lesion of BMBC compared to primary lesion of both BMBC and Non-BM-BC (P < 0.01), and TGF-α was higher in primary lesion of both BMBC and Non-BM-BC (P < 0.01) than Benign group. Interestingly, TGF-α in nontumor tissues of both BMBC and Non-BM-BC was at a higher level than Benign group (P < 0.01), and numbers of macrophages in nontumor tissues of both BMBC and Non-BM-BC (P < 0.01) were higher than Benign group. Furthermore, in cultured human BMSCs, TGF-α stimulated production of procancer cytokines including IL-6, VEGF, FGF10, FGF17, and TGF-β1 in a dose-dependent manner. Thus, TGF-α in BC could potentially be an important signal of carcinogenesis and metastasis. Macrophages in the nontumor tissue of BC may not be protective but could promote cancer metastasis.
1. Introduction
Breast cancer (BC) is the number 1 malignant tumor among females worldwide, and malignant metastasis is the major cause of breast cancer related death. About 70% BC cases are known to develop bone metastasis, and even 20% BC cases have bone metastasis in the early stage of primary lesion [1, 2]. Bone metastasis breast cancer (BMBC) is a complicated and advanced stage that starts in the primary tumor microenvironment. Tumor microenvironment is composed of tumor cells, mesenchymal stem cells (MSCs), fibroblasts, immune cells, vascular endothelial cells, other stromal cells, and extracellular matrix [3]. It has been accepted that the interaction between BC cells and components in the microenvironment could influence the progression of BC [4].
MSCs are multipotent stem cells that are mainly derived from bone marrow, fat tissue, umbilical cord blood, and dental pulp [5–7]. Bone marrow-derived MSCs (BMSCs) are progenitors of fibroblasts, adipocytes, osteoblasts, and cartilage cells in normal human microenvironment and play important roles in tissue regeneration [8, 9]. In the tumor microenvironment, BMSCs produce cytokines and exosomes to promote tumor invasion and metastasis. It has been shown that plasminogen activator inhibitor- (PAI-) 1, interleukin- (IL-) 6, Notch 1, and CD44 produced from BMSCs improve colorectal cancer cell survival and promote its development [10]. IL-6 and vascular endothelial growth factor (VEGF) from BMSCs improve BC metastasis, and the phenomenon was enhanced when BC cells are exposed to IL-6 and VEGF together [11]. In three-dimensional culture, BC cells developed invasion capacity when treated with transforming growth factor- (TGF-) β1 from BMSCs [12]. BC cells have been reported to be able to capture and cannibalize BMSCs in the microenvironment which can enable them to change into a dormant state that can increase drug resistance and immunosuppression and finally increase cancer recurrence [13].
Transforming growth factor- (TGF-) α was reported by bioinformatic analyses to potentially stimulate BMSCs to promote breast cancer [14]. TGF-α is a transforming growth factor as part of a human 160-amino-acid transmembrane precursor, and it is a ligand for the epidermal growth factor receptor which plays roles in tissue regeneration and bone homeostasis and may promote tumorigenesis [15–17]. Elevated TGF-α is associated with the tumorigenesis of the breast and stomach [18, 19]. However, whether TGF-α has a role in stimulating BMSCs during breast cancer bone metastasis still remains unclear.
In this study, as steps for identifying the role of TGF-α in breast cancer bone metastasis, expression of TGF-α in primary lesion and bone metastasis of breast cancer was analyzed and the influence of TGF-α on BMSCs was evaluated.
2. Method and Materials
2.1. Human Cases
Informed consent from all the patients was obtained and the study was approved by Ethics Board of the Third Affiliated Hospital of Southern Medical University.
2.1.1. Breast Cancer with Bone Metastasis (BMBC) Group
Four female breast cancer cases with bone metastasis were enrolled in the retrospective study as the observation group. The 4 cases were diagnosed with breast cancer bone metastasis and received both mastectomy and excision of bone metastasis. The rule-in criteria also included no involvements of central nervous system diseases, gynecological diseases, or autoimmune diseases that might influence breast cancer progression and TGF-α expression. Their primary lesions were in the early stage of the TNM staging system [20]. The clinical data of these 4 BMBC cases were listed in Table 1 and shown in Figure 1.
Table 1.
Clinical data of the four bone metastasis breast cancer cases.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Gender | Female | Female | Female | Female |
| Age | 46 | 66 | 71 | 46 |
| Pathology subtypes | Invasive ductal carcinoma | Invasive ductal carcinoma | Invasive lobular carcinoma | Invasive ductal carcinoma |
| Pathological grade | III | III | III | III |
| Molecular subtypes | Luminal B | Luminal B | Luminal B | Luminal B |
| TNM stage, tumor max diameter (cm) | IV, T: 2.2 | IV, T: 2.5 | IV, T: 3.3 | IV, T: 3.7 |
| Location of metastasis | Acetabular bone; axillary lymph node |
Thighbone | Humerus; femur; liver; axillary lymph node |
Blade bone; thighbone; axillary lymph node |
| Remote metastasis organ number | One | One | Three | Two |
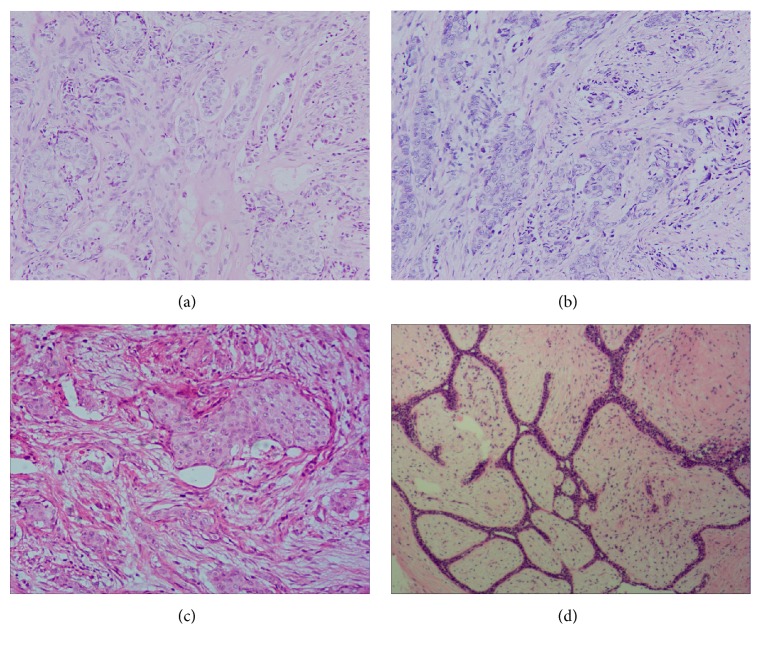
Figure 1.
Histopathological diagnosis (H&E staining) of samples from the 3 groups. (a) Breast cancer bone metastasis (200x). (b) Primary lesion of breast cancer with bone metastasis (200x), showing nonspecific invasive ductal carcinoma, WHO grade III. (c) Primary lesion of breast cancer without bone metastasis (200x), showing nonspecific invasive ductal carcinoma, WHO grade III. (d) Breast fibroadenoma (200x).
2.1.2. Breast Cancer without Bone Metastasis (Non-BM-BC) Control Group
Four female breast cancer cases without bone metastasis were enrolled in the study as Non-BM-BC control group. The 4 cases were diagnosed with resectable breast cancer without bone metastasis (Non-BM-BC) and received mastectomy. The rule-in criteria included age being above 40 years to balance the influence of age, specific pathological diagnosis results (nonspecific invasive ductal carcinoma, WHO grade III; primary lesion, T2; and molecular group: luminal B), and no involvements of central nervous system diseases, gynecological diseases, or autoimmune diseases that might influence breast cancer progression and TGF-α expression. Their primary lesions were in the early stage of TNM staging.
2.1.3. Benign Control Group
Four female breast fibroadenoma cases were enlisted in the study as the negative control group. The 4 cases were diagnosed with potentially malignant breast lump before operation and received lumpectomy. The postoperative pathological results showed breast fibroadenoma. The rule-in criteria included age above 40 years and no involvements of central nervous system diseases, gynecological diseases, or autoimmune diseases. Their primary lesions were in the early stage of TNM staging.
2.2. Immunohistochemical Analyses (IHC)
Samples of primary lesions and bone metastasis were fixed in formalin and embedded in paraffin and analyzed by immunohistochemical analysis. The 4 μm sections were deparaffinized and rehydrated, and their endogenous peroxidase activity was inhibited with 0.3% H2O2 methanol. After being blocked with the 5% normal goat serum for 1 hour at room temperature, the slides were incubated with the following primary antibodies at 4°C overnight: anti-TGF-α polyclonal antibody (ImmunoWay, Plano, TX, 1 : 50), anti-CD68 antibody (Abcam, Cambridge, UK; 1 : 200), anti-IL-6 antibody (ImmunoWay, Plano, TX, 1 : 50), anti-VEGF antibody (ImmunoWay, Plano, TX, 1 : 100), anti-FGF10 antibody (ABclonal, Boston, USA, 1 : 100), anti-FGF17 antibody (ImmunoWay, Plano, TX, 1 : 100), and anti-TGF-β1 antibody (ABclonal, Boston, USA, 1 : 100). Following incubation with biotinylated secondary antibodies, the streptavidin-biotin complex/horseradish peroxidase was applied. Finally, the immunoreaction signal was developed with DAB staining, and the slides were counterstained in hematoxylin.
The stained tissue sections were reviewed under a light microscope (Nikon ECLIPSE Ni-U, Tokyo, Japan). The numbers of TGF-α and CD68 positive cells were counted in 5 random fields (400x) of paraneoplastic tissue of each tissue section, respectively.
2.3. Cell Culture
Human bone marrow-derived MSCs (hBMSCs) were purchased from Guangzhou Jenniobio Biotechnology Co., Ltd (Guangzhou, China), and cultured in DMEM medium (GIBCO, Gaithersburg, MD) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 1% penicillin/streptomycin (Invitrogen, Waltham, MA) in a 5% CO2 humidified atmosphere at 37°C. TGF-α exposure experiments were performed using hBMSCs between passages three and seven. Human BMSCs were treated for 8 or 24 hours with recombinant human TGF-α (Sino Biological, Beijing, China) at a concentration of 0 (medium control), 10, or 20 ng/ml.
2.4. Quantitative PCR (q-PCR)
Total RNA was extracted from untreated or TGFα-treated hBMSCs using Trizol reagent (Invitrogen), and cDNA was synthesized using an access RT system (Promega, Madison, WI). Real-time PCR was performed using ABI7500 Real-Time PCR System and SYBR Premix ExTaq II kit (TaKaRa Biotechnology Co., Ltd, Dalian, China). The primer sequences were selected as follows: IL-6, forward primer CATCCTCGACGGCATCTCAG and reverse primer ACCAGGCAAGTCTCCTCATTG; VEGF, forward primer CATCACCATGCAGATTATGCGG and reverse primer GAGGCTCCAGGGCATTAGAC; fibroblast growth factor (FGF) 10, forward primer CCGTACAGCATCCTGGAGATAAC and reverse primer CCTCCCATTATGCTGCCAGTT; FGF 17, forward primer CCCAACCTCACTCTGTGCTTAC and reverse primer TGTAGAGTTGGTACTCGCGG; TGF-β1, forward primer CGACTCGCCAGAGTGGTTAT and reverse primer GGTAGTGAACCCTGCGTTGAT. The PCR condition was 95°C for 30 s, followed by 40 cycles of amplification (95°C for 5 s, 60°C for 34 s, and 72°C for 34 s). The relative quantification was determined using the ΔΔ−Ct method and mRNA expression levels were normalized to internal control gene GAPDH. Each sample was tested three times.
2.5. Statistics
SPSS 19.0 (IBM SPSS Inc., Chicago, IL) was used to evaluate data. One-way analysis of variance followed by LSD t-test was used to analyze differences between groups, and 2-tailed significance was determined. Results are presented as the mean ± standard deviation (SD) for all parameters measured. P < 0.05 was considered statistically significant.
3. Results
3.1. TGF-α in Bone Metastasis and Primary Lesion of Breast Cancer and Benign Control
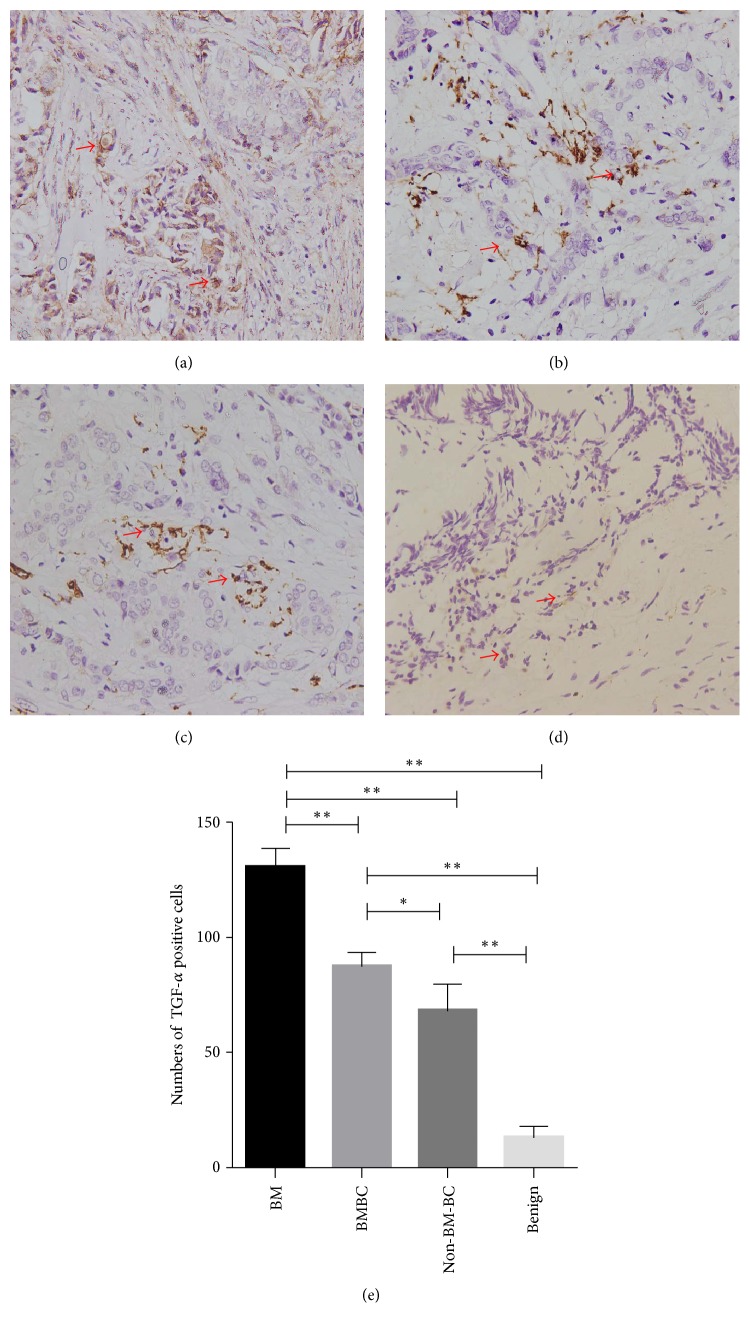
As analyzed by immunohistochemistry, bone metastases had a higher level TGF-α (more positive cells) than their own primary lesion (P < 0.01). TGF-α level in primary lesion of BMBC group was higher than that in primary lesion of BC without bone metastasis (P < 0.05) (Figure 2). Benign control had a much lower level of TGF-α than the bone metastasis (P < 0.01) and all primary lesions (P < 0.01) (Figure 2).
Figure 2.
TGF-α in different tumor tissue. (a) TGF-α in bone metastasis (BM) lesion of breast cancer (400x), with arrows pointing to TGF-α positive cells; (b) TGF-α in primary lesion of breast cancer with bone metastasis (BMBC) (400x); (c) TGF-α in primary lesion of breast cancer without bone metastasis (Non-BM-BC) (400x); (d) TGF-α in benign breast fibroadenoma (400x); (e) comparison of TGF-α positive cells in tumor lesion of different groups (total positive cells in 5 random fields). ∗P < 0.05; ∗∗P < 0.01.
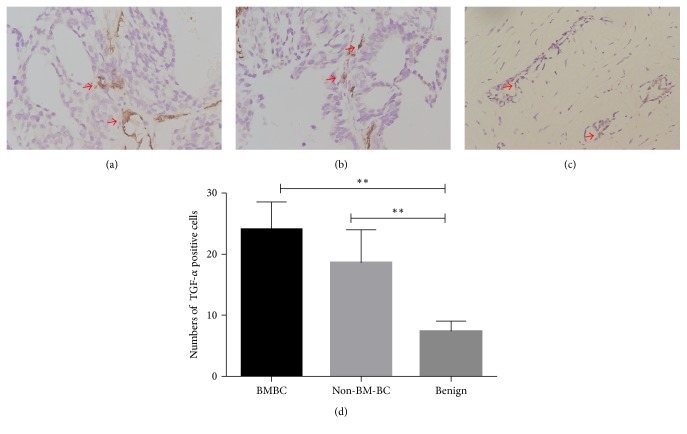
Interestingly, TGF-α is expressed at higher levels in the nontumor tissues of both BMBC and Non-BM-BC while it was seldom found in nontumor tissues of the benign control (P < 0.01) (Figure 3). No significant differences were found in TGF-α levels in the nontumor tissues between BMBC and Non-BM-BC groups (P > 0.05) (Figure 3).
Figure 3.
TGF-α expression in different nontumor tissues around tumor. (a) TGF-α in nontumor tissues around primary lesion of breast cancer with bone metastasis (BMBC) (400x, arrows pointing to TGF-α positive cells); (b) TGF-α in nontumor tissues around primary lesion of breast cancer without bone metastasis (Non-BM-BC) (400x); (c) TGF-α in nontumor tissues around benign breast fibroadenoma (400x); (d) comparison of TGF-α positive cells in nontumor tissues of different groups (total positive cells within 5 random fields). ∗∗P < 0.01.
3.2. Macrophages (CD68+ Cells) in BC Lesion and Nontumor Tissues
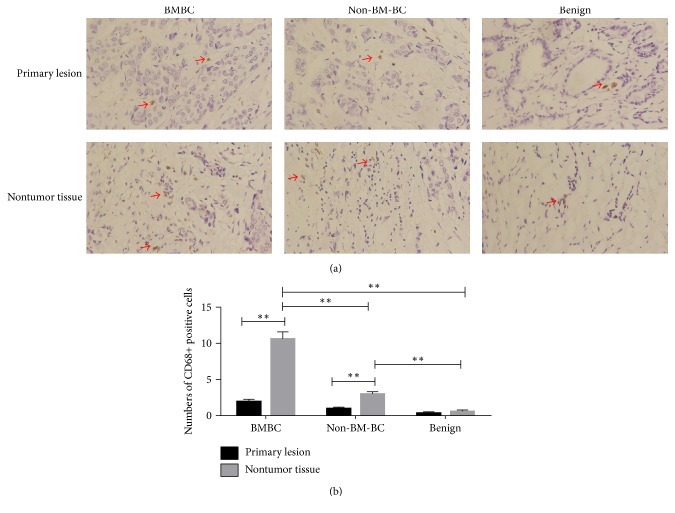
Since macrophages are a known source of TGF-β1 in breast cancer, macrophages were detected by CD68 immunohistochemistry and positive cells were counted in BC lesion and nontumor tissues. Macrophages in BC lesion were found to be fewer than the nontumor tissue (P < 0.01) (Figure 4). Numbers of macrophages in BC lesion were not different from those in benign lesion. Numbers of macrophages in nontumor tissue of BMBC and Non-BM-BC were higher than those in nontumor tissue of benign control (P < 0.01).
Figure 4.
Numbers of macrophages (CD68+ cells) in different tissues. (a) Macrophages in primary lesion and nontumor tissues (400x, arrows indicating CD68+ positive cells); (b) comparison of macrophage numbers in different tissues. ∗∗P < 0.01.
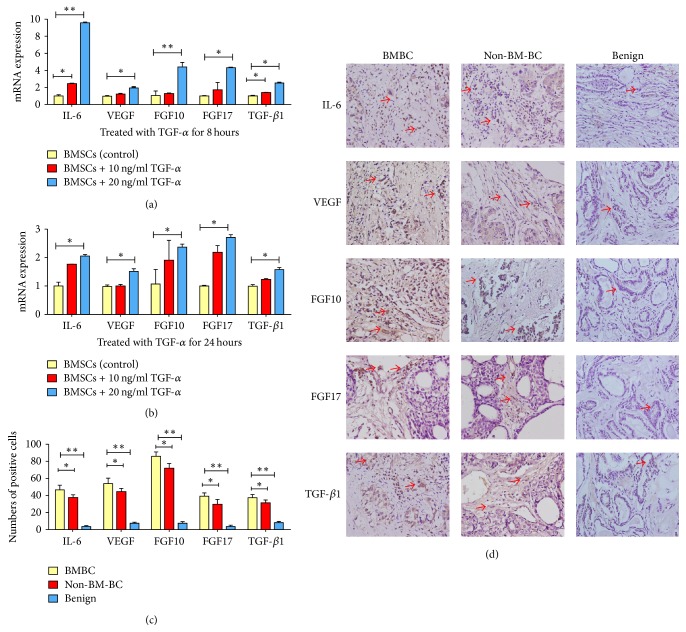
3.3. Induction of Cytokines and Growth Factors from Human BMSCs under TGF-α Stimulation and Their Increased Expression Levels in Different Nontumor Tissues around Tumor
Effects of TGF-α stimulation on expression of known genes involved in breast cancer metastasis were examined in human BMSCs. As analyzed by q-PCR, within 8 hours of TGF-α stimulation, expression of these procancer cytokines/growth factors was found to increase in a dose-dependent manner (Figure 5(a)). Within 24 hours, BMSCs with 20 ng/ml TGF-α stimulation produced significantly higher levels of IL-6 (P < 0.05), VEGF (P < 0.05), FGF10 (P < 0.05), FGF17 (P < 0.05), and TGF-β1 (P < 0.05), compared with BMSCs with 0 or 10 ng/ml TGF-α treatment (Figure 5(b)). These cytokines are expressed at higher levels in the nontumor tissues of both BMBC and Non-BM-BC while they were seldom found in the benign control (P < 0.01) (Figures 5(c) and 5(d)). The level in the nontumor tissues of BMBC is higher than that of the Non-BM-BC group (P < 0.05) (Figures 5(c) and 5(d)).
Figure 5.
Changes in mRNA expression of cytokines and growth factors in BMSCs treated with different concentrations of TGF-α. (a) 8 hours after treatment; (b) 24 hours after treatment; (c) comparison of cytokines and growth factors expression in different nontumor tissues around tumor. ∗P < 0.05; ∗∗P < 0.01 compared with no treatment control; (d) cytokines and growth factors expression in different nontumor tissues around tumor (400x, arrows indicating positive cells).
4. Discussions
Breast cancer bone metastasis in the early stage of primary lesion is threatening the patients at the rate of 20%. The BMBC patients with removable bone metastasis like the 4 cases in the study were rare. Most of them developed unresectable multiple bone metastases [21–23]. While the interaction between BC cells and BMSCs has been reported to contribute to tumor angiogenesis, invasion, and thus metastasis [24, 25]; the signals (such as cytokines/growth factors) responsible for or underlying this interaction remain largely unclear. In the current study, our results showed that, in breast cancer bone metastasis, TGF-α might play a signaling role bridging BC cells and BMSCs and promote the bone metastasis.
In the human BMBC, TGF-α was found to be expressed in the bone metastasis lesion, at a higher level than that in primary lesion of BMBC and Non-BM-BC (Figure 2), and TGF-α level in primary lesion of BMBC and Non-BM-BC was found to be higher than that in Benign control. These findings suggest that TGF-α might be a special signal for breast cancer and its bone metastasis. In the malignant lesion, cancer cells could be the source of TGF-α. In this study, TGF-α was found to promote BMSCs to increase their transcription of IL-6, VEGF, FGF10, FGF 17, and TGF-β1 that could promote BC metastasis obviously (Figure 5). IL-6, VEGF, and TGF-β1 have been proved to be commonly overexpressed in human breast cancer cases [26–28], FGF10 played the important role in type III epithelial-mesenchymal transition (EMT) on breast cancer cells and initiation of metastasis [29], and high expression of FGF17 causes tamoxifen resistance [30]. It could be inferred that overexpressed TGF-α in BC is an important signal that may bridge bone metastasis, BC, and BMSCs.
Interestingly, in the human BMBC samples, TGF-α was also overexpressed in the nontumor tissue around the primary lesion when compared to those in benign control, but it was not expressed at a higher level than that of the nontumor tissue of Non-BM-BC (Figure 3). Our analyses on TGF-α expression in the “soil” of BC suggest that TGF-α in the microenvironment might not be single signal in breast cancer bone metastasis. However, TGF-α in the microenvironment might play important roles in primary breast cancer development, and thus TGF-α in normal breast tissue might be a prognostic marker for predicting the risk of BC.
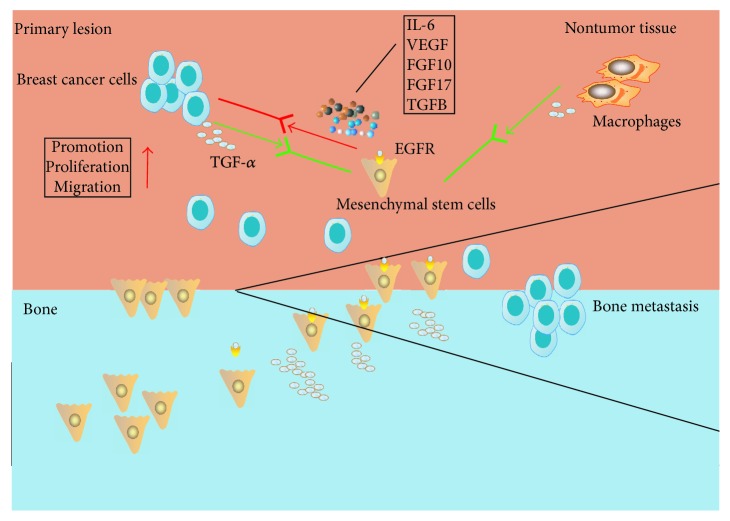
In the breast microenvironment, macrophages are an important component and known as a major cellular source of TGF-α [31, 32]. In the current study, macrophages were found at higher numbers in the nontumor tissue of BC than benign control (Figure 4). This could potentially explain the higher level of TGF-α in the nontumor tissue of BC. In the physiological situation, the interaction of macrophages and BMSCs has been recognized to be important in tissue regeneration during tissue repair, and TGF-α is the signal for interaction between macrophages and BMSCs in wound healing [33–36]. Bone metastasis frequently happens to the human loaded bones like the cases presented in the study, and many risk factors could cause damage and subsequent repair in the human loaded bones [37, 38]. With the above known roles of TGF-α and our finding of overexpressed TGF-α being found mostly in the bone metastasis lesion in the study (Figure 2), it is possible that BC with elevated expression of TGF-α might mimic the macrophages in tissue regeneration to influence BMSCs to promote BC to metastasis (Figure 6). On the other hand, as the source of TGF-α, macrophages in the nontumor tissue of BC may play a more procancer role than an anticancer role.
Figure 6.
The hypothesis that TGF-α stimulates bone marrow-derived mesenchymal stem cells (BMSCs) in breast cancer bone metastasis. Breast cancer influences BMSCs to promote BC metastasis to bone. In the process, breast cancer cells and nontumor tissue macrophages produce TGF-α to stimulate BMSCs that express the receptor EGFR, and activated BMSCs produce procancer cytokines/growth factors to promote BC.
In summary, TGF-α in BMBC lesion was found to be overexpressed and could be an important signal. TGF-α in BC lesion and from nontumor tissue could stimulate BMSCs to promote cancer metastasis. In the further study, more operable BMBC cases would be enrolled. Protein analyses would be performed to define the precise role of TGF-α in breast cancer secretion. BMSCs transfected by no-load virus red fluorescent protein would be used in in vivo models to evaluate the action mechanism of TGF-α locally expressed in BC lesion in influencing the BMSCs and in promoting bone metastasis, information from which could lead to therapeutic targets by controlling TGF-α signaling in both primary lesion and the surrounding microenvironment.
Acknowledgments
The authors would like to thank Professor Li Liang of the Key Laboratory of Molecular Tumor Pathology in Guangdong Province for her instructions on pathological analyses. This work was supported by the Science and Technology Plan Project of Tianhe District (201604KW011). Cory J. Xian is funded by NHMRC Senior Research Fellowship (1042105).
Contributor Information
Xiaolong Liu, Email: lxl1979@i.smu.edu.cn.
Lixin Liu, Email: dr.lixin.liu@hotmail.com.
Disclosure
The first corresponding author is Xiaolong Liu and co-corresponding author is Lixin Liu.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Authors' Contributions
Jingbo Sun and Haiyan Cui contributed equally to the work. Xiaolong Liu, Lixin Liu, Jingbo Sun, and Haiyan Cui were responsible for study design. Jingbo Sun and Yanxin Gao conducted the study. Jingbo Sun, Xiaolong Liu, Yanxin Gao, and Haiyan Cui collected the data. Xiaolong Liu, Jingbo Sun, Yanxin Gao, and Haiyan Cui analyzed the data. Cory J. Xian, Xiaolong Liu, Jingbo Sun, Yanxin Gao, Yangjian Pan, Kun Zhou, Jingzhan Huang, Jin Lan, and Haiyan Cui were responsible for data interpretation. Xiaolong Liu, Jingbo Sun, Haiyan Cui, Yanxin Gao, Yangjian Pan, Kun Zhou, Jingzhan Huang, Jin Lan, and Lixin Liu drafted the manuscript. Cory J. Xian, Xiaolong Liu, and Lixin Liu revised the manuscript. All authors have read and approved the final submitted manuscript.
References
- 1.Manders K., van de Poll-Franse L. V., Creemers G.-J., et al. Clinical management of women with metastatic breast cancer: A descriptive study according to age group. BMC Cancer. 2006;6:179. doi: 10.1186/1471-2407-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Madar S., Goldstein I., Rotter V. 'Cancer associated fibroblasts'—more than meets the eye. Trends in Molecular Medicine. 2013;19(8):447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.velaei K., Samadi N., Barazvan B., Soleimani Rad J. Tumor microenvironment-mediated chemoresistance in breast cancer. The Breast. 2016;30:92–100. doi: 10.1016/j.breast.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Alge D. L., Zhou D., Adams L. L., et al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. Journal of Tissue Engineering & Regenerative Medicine. 2010;4(1):73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erices A., Conget P., Minguell J. J. Mesenchymal progenitor cells in human umbilical cord blood. British Journal of Haematology. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 7.Zuk P. A., Zhu M., Ashjian P., et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell (MBoC) 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y., Cai W., Zhou S., Xu L., Jiang C. Protective effect of bone marrow derived mesenchymal stem cells in lipopolysaccharide-induced acute lung injury mediated by claudin-4 in a rat model. American Journal of Translational Research. 2016;8(9):3769–3779. [PMC free article] [PubMed] [Google Scholar]
- 9.Gao F., Chiu S. M., Motan D. A., et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death & Disease. 2016;7(1, article e2062) doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Malley G., Heijltjes M., Houston A. M., et al. Mesenchymal stromal cells (MSCs) and colorectal cancer - A troublesome twosome for the anti-tumour immune response? Oncotarget . 2016;7(37):60752–60774. doi: 10.18632/oncotarget.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca A., Lamura L., Gallo M., Maffia V., Normanno N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. Journal of Cellular Biochemistry. 2012;113(11):3363–3370. doi: 10.1002/jcb.24212. [DOI] [PubMed] [Google Scholar]
- 12.McAndrews K. M., McGrail D. J., Ravikumar N., Dawson M. R. Mesenchymal Stem Cells Induce Directional Migration of Invasive Breast Cancer Cells through TGF-β. Scientific Reports. 2015;5 doi: 10.1038/srep16941.16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartosh T. J., Ullah M., Zeitouni S., Beaver J., Prockop D. J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs) Proceedings of the National Acadamy of Sciences of the United States of America. 2016;113(42):E6447–E6456. doi: 10.1073/pnas.1612290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Luca A., Roma C., Gallo M., et al. RNA-seq analysis reveals significant effects of EGFR signalling on the secretome of mesenchymal stem cells. Oncotarget . 2014;5(21):10518–10528. doi: 10.18632/oncotarget.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer I., Alcántara S., Ballabriga J., et al. Transforming growth factor-α (TGF-α) and epidermal growth factor-receptor (EGF-R) immunoreactivity in normal and pathologic brain. Progress in Neurobiology. 1996;49(2):99–123. doi: 10.1016/0301-0082(96)00009-3. doi: 10.1016/0301-0082(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 16.Raja S., Byakod G., Pudakalkatti P. Growth factors in periodontal regeneration. International Journal of Dental Hygiene. 2009;7(2):82–89. doi: 10.1111/j.1601-5037.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 17.Xian C. J. Roles of epidermal growth factor family in the regulation of postnatal somatic growth. Endocrine Reviews. 2007;28(3):284–296. doi: 10.1210/er.2006-0049. [DOI] [PubMed] [Google Scholar]
- 18.Coffey R. J., Washington M. K., Corless C. L., Heinrich M. C. Ménétrier disease and gastrointestinal stromal tumors: Hyperproliferative disorders of the stomach. The Journal of Clinical Investigation. 2007;117(1):70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder J. A., Troyer K. L., Lee D. C. Cooperative induction of mammary tumorigenesis by TGFα and Wnts. Oncogene. 2000;19(28):3193–3199. doi: 10.1038/sj.onc.1203652. [DOI] [PubMed] [Google Scholar]
- 20.Orucevic A., Chen J., McLoughlin J. M., Heidel R. E., Panella T., Bell J. Is the TNM staging system for breast cancer still relevant in the era of biomarkers and emerging personalized medicine for breast cancer - An institution's 10-year experience. The Breast Journal. 2015;21(2):147–154. doi: 10.1111/tbj.12367. [DOI] [PubMed] [Google Scholar]
- 21.Lu X., Kang Y. Organotropism of breast cancer metastasis. Journal of Mammary Gland Biology and Neoplasia. 2007;12(2-3):153–162. doi: 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- 22.Hess K. R., Varadhachary G. R., Taylor S. H., et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 23.Guise T. A., Kozlow W. M., Heras-Herzig A., Padalecki S. S., Yin J. J., Chirgwin J. M. Molecular mechanisms of breast cancer metastases to bone. Clinical Breast Cancer. 2005;5(Supplement 2):S46–S53. doi: 10.3816/CBC.2005.s.004. [DOI] [PubMed] [Google Scholar]
- 24.Karnoub A. E., Dash A. B., Vo A. P., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 25.Martin F. T., Dwyer R. M., Kelly J., et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT) Breast Cancer Research and Treatment. 2010;124(2):317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 26.Noman A. S., Uddin M., Chowdhury A. A., et al. Serum sonic hedgehog (SHH) and interleukin-(IL-6) as dual prognostic biomarkers in progressive metastatic breast cancer. Scientific Reports. 2017;7(1):1796. doi: 10.1038/s41598-017-01268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linardou H., Kalogeras K. T., Kronenwett R., et al. The prognostic and predictive value of mRNA expression of vascular endothelial growth factor family members in breast cancer: A study in primary tumors of high-risk early breast cancer patients participating in a randomized Hellenic Cooperative Oncology Group trial. Breast Cancer Research. 2012;14(6, article no. R145) doi: 10.1186/bcr3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Kruijf E. M., et al. The prognostic role of TGF-beta signaling pathway in breast cancer patients. Ann Oncol. 2013;24(2):384–390. doi: 10.1093/annonc/mds333. [DOI] [PubMed] [Google Scholar]
- 29.Abolhassani A., Riazi G. H., Azizi E., et al. FGF10: Type III epithelial mesenchymal transition and invasion in breast cancer cell lines. Journal of Cancer. 2014;5(7):537–547. doi: 10.7150/jca.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer D., Sieuwerts A. M., Look M. P., Van Agthoven T., Foekens J. A., Dorssers L. C. J. Fibroblast growth factor receptor 4 predicts failure on tamoxifen therapy in patients with recurrent breast cancer. Endocrine-Related Cancer. 2008;15(1):101–111. doi: 10.1677/ERC-07-0080. [DOI] [PubMed] [Google Scholar]
- 31.Su S., Liu Q., Chen J., et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Madtes D. K., Raines E. W., Sakariassen K. S., et al. Induction of transforming growth factor-α in activated human alveolar macrophages. Cell. 1988;53(2):285–293. doi: 10.1016/0092-8674(88)90390-X. [DOI] [PubMed] [Google Scholar]
- 33.Lee M. J., Kim M. Y., Heo S. C., et al. Macrophages regulate smooth muscle differentiation of mesenchymal stem cells via a prostaglandin F2α-mediated paracrine mechanism. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(11):2733–2740. doi: 10.1161/atvbaha.112.300230. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y.-H., Tabata Y. Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell-derived factor-1 and a macrophage recruitment agent enhances wound closure. Journal of Biomedical Materials Research Part A. 2016;104(4):942–956. doi: 10.1002/jbm.a.35635. [DOI] [PubMed] [Google Scholar]
- 35.Di G., Du X., Qi X., et al. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Investigative Ophthalmology & Visual Science. 2017;58(10):4344–4354. doi: 10.1167/iovs.17-21506. [DOI] [PubMed] [Google Scholar]
- 36.Cho S.-Y., Kwon E.-Y., Choi S.-M., et al. Immunomodulatory effect of mesenchymal stem cells on the immune response of macrophages stimulated by Aspergillus fumigatus conidia. Medical Mycology. 2016;54(4):377–383. doi: 10.1093/mmy/myv110. [DOI] [PubMed] [Google Scholar]
- 37.Hassanshahi M., Hassanshahi A., Khabbazi S., Su Y.-W., Xian C. J. Bone marrow sinusoidal endothelium: damage and potential regeneration following cancer radiotherapy or chemotherapy. Angiogenesis. 2017:1–16. doi: 10.1007/s10456-017-9577-2. [DOI] [PubMed] [Google Scholar]
- 38.Fan C., Georgiou K. R., Morris H. A., et al. Combination breast cancer chemotherapy with doxorubicin and cyclophosphamide damages bone and bone marrow in a female rat model. Breast Cancer Research and Treatment. 2017;165(1):41–51. doi: 10.1007/s10549-017-4308-3. [DOI] [PubMed] [Google Scholar]