Abstract
Purpose
Despite its many health benefits, moderate exercise can induce joint discomfort when done infrequently or too intensely even in individuals with healthy joints. This study was designed to evaluate whether NEM® (natural eggshell membrane) would reduce exercise-induced cartilage turnover or alleviate joint pain or stiffness, either directly following exercise or 12 hours post exercise, versus placebo.
Patients and methods
Sixty healthy, postmenopausal women were randomly assigned to receive either oral NEM 500 mg (n=30) or placebo (n=30) once daily for two consecutive weeks while performing an exercise regimen (50–100 steps per leg) on alternating days. The primary endpoint was any statistically significant reduction in exercise-induced cartilage turnover via the biomarker C-terminal cross-linked telopeptide of type-II collagen (CTX-II) versus placebo, evaluated at 1 and 2 weeks of treatment. Secondary endpoints were any reductions in either exercise-induced joint pain or stiffness versus placebo, evaluated daily via participant questionnaire. The clinical assessment was performed on the per protocol population.
Results
NEM produced a significant absolute treatment effect (TEabs) versus placebo for CTX-II after both 1 week (TEabs −17.2%, P=0.002) and 2 weeks of exercise (TEabs −9.9%, P=0.042). Immediate pain was not significantly different; however, rapid treatment responses were observed for immediate stiffness (Day 7) and recovery pain (Day 8) and recovery stiffness (Day 4). No serious adverse events occurred and the treatment was reported to be well tolerated by study participants.
Conclusion
NEM rapidly improved recovery from exercise-induced joint pain (Day 8) and stiffness (Day 4) and reduced discomfort immediately following exercise (stiffness, Day 7). Moreover, a substantial chondroprotective effect was demonstrated via a decrease in the cartilage degradation biomarker CTX-II. Clinical Trial Registration number: NCT02751944.
Keywords: chondroprotective, CTX-II, cartilage degradation, breakdown
Introduction
Exercise is an integral component of a healthy lifestyle and is regularly practiced by hundreds of millions of people throughout the world. It is recommended that adults should complete 30 minutes or more of moderate-intensity (aerobic) physical activity every – or nearly every – day.1 Exercise is known to impart a multitude of health benefits, particularly in the prevention of a number of chronic diseases such as cardiovascular disease2 and type II diabetes3 and reduces the incidence and detrimental effects of obesity,4 which is known to contribute to the development of these and other chronic diseases. As people age, physical activity gains even greater importance with the need to maintain articular joint flexibility, lessen frailty, and improve balance to reduce the risk of falls5 that can lead to increased disability and mortality rates in older adults.6,7 Numerous studies have demonstrated the beneficial effects of exercise in relieving osteoarthritis (OA) symptoms,8 particularly for weight-bearing joints such as the knees or hips, and exercise is recommended by the American College of Rheumatology as a nonpharmacologic therapy for OA.9 There is even some clinical evidence to suggest that exercise may play a role in the prevention of OA.10
Despite the fact that articular joints are designed for and intended to move, moderate exercise can still induce discomfort in joints when done infrequently, or too intensely, or with too great a load, or for too long a period. This discomfort is often realized as either pain or stiffness in the joint that was the focus of the exercise. Whether this short-term insult to the joint and connective tissues leads to degenerative disease over the long-term remains to be answered definitively. What is clear is that lack of physical activity (immobility) has significant detrimental effects (morphological, biochemical, and biomechanical) on all joint tissues (eg, ligaments, tendons, cartilage, synovial fluid, joint capsule, and bone). Ligament fibers lose integrity and weaken biomechanically, periarticular muscles and associated tendons atrophy destabilizing the joint, articular cartilage atrophies (loss of cartilage thickness) and lesions form at points of contact, hyaluronic acid content of synovial fluid lessens reducing its lubricating ability, fibro-fatty connective tissue proliferates from the joint capsule (synovium) into the joint space creating adhesions, and periarticular bones become osteoporotic and fragile.11 Fortunately, all of these negative effects are reversible given sufficient time and gradual reloading during the resumption of physical activity.12 Interestingly, many of the detrimental effects seen with joint immobility or lack of physical activity mirror the morphological, biochemical, and biomechanical changes seen in the pathology of OA.12
Cartilage is primarily composed of extracellular matrix (ECM), a composite network of proteins such as type-II collagen interacting with negatively charged polysaccharides such as hyaluronic acid and chondroitin sulfate, all of which are synthesized and secreted by chondrocytes. During normal cartilage turnover (metabolism) in healthy articular joints, ECM production balances ECM breakdown, thereby ensuring the continuous renewal of this critical joint-cushioning tissue. However, pathologic conditions such as OA are characterized by an imbalance in cartilage turnover, in which catabolic processes predominate over anabolic processes. ECM synthesis cannot keep pace with degradation and a loss of structural integrity of articular cartilage results. This cartilage metabolism imbalance coupled with biomechanical stress in the joint leads to chronic inflammation and ultimately irreversible joint destruction. These cartilage degradation products can be found in the blood and urine of both healthy and arthritic subjects.
A number of biomarkers of cartilage turnover have been investigated for their diagnostic and prognostic properties.13 Of these biomarkers, C-terminal cross-linked telopeptide of type-II collagen (CTX-II), a marker of cartilage degradation, has shown the most potential. It has been associated with both the incidence and progression of OA in multiple clinical trials14,15 and is predictive of the progression of OA both radiographically,16 including two ≥5 year longitudinal studies,17,18 and by MRI.19 Urinary CTX-II (uCTX-II) levels are known to be substantially elevated in those afflicted with articular joint diseases like OA and rheumatoid arthritis (RA), but levels are also known to be elevated in a variety of healthy subsets of the population, as well. For example, uCTX-II levels in growing children are about 50-fold higher than that of adults.20 uCTX-II levels have been shown to be elevated because of high-impact, strenuous exercise in healthy college-aged endurance athletes such as cross-country runners by about 85% over age- and weight-matched controls, but were not significantly elevated in lower-impact endurance athletes like swimmers and rowers.21 uCTX-II has also been shown to be about twofold higher in postmenopausal women versus age-matched premenopausal women and moderately elevated (~25%) in overweight persons (body mass index [BMI], ≥25 kg/m2) versus normal weight controls (BMI <25 kg/m2).22
Eggshell membrane (ESM), found between the calcified shell and the albumin in chicken eggs, is primarily composed of fibrous proteins such as collagen Type I,23 which form the mesh-like structure of the bilayered material. ESMs have also been shown to contain other bioactive components, namely, glycosaminoglycans (ie, dermatan sulfate, chondroitin sulfate, hyaluronic acid, etc).24 ESM is known to reduce the expression of various pro-inflammatory cytokines, including the key mediators of inflammation interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) both in vitro25 and in vivo.26 A proprietary form of ESM, commercially available as the branded product NEM® (natural eggshell membrane); ESM Technologies, LLC, Carthage, MO, USA), has demonstrated safety and efficacy in multiple clinical trials in relieving joint pain and stiffness in humans with OA27–29 and has been shown to reduce CTX-II levels in rat models of both OA30 and RA31 and in naturally occurring joint disease in dogs.32
Because of the importance of exercise to overall health and disease prevention particularly as it relates to the long-term health of joints and joint disease, there is a need to evaluate therapeutics that can not only protect joints from the potential detrimental effects from exercise but also reduce the resultant pain and stiffness from exercise thereby encouraging adherence to a regular exercise regimen. Taking into account the apparent sensitivity of articular cartilage to increased joint strain in postmenopausal women, a 2-week randomized controlled trial was conducted to evaluate the effects of NEM brand ESM on exercise-induced joint pain, stiffness, and cartilage turnover in healthy, postmenopausal women. The results are presented herein.
Patients and methods
Study design
This study was conducted utilizing the services of a clinical contract research organization (QPS Bio-Kinetic, Springfield, MO, USA) according to a single-center, randomized, double-blind, placebo-controlled, parallel-group design in accordance with the US Food and Drug Administration (FDA)’s principles of Good Clinical Practice (Title 21, Code of Federal Regulations, Parts 50 and 56 and ICH E6) and the Declaration of Helsinki (1996 version) (Clinical Trial Registration Number NCT02751944). The study protocol was approved by a registered institutional review board (IRB) (Bio-Kinetic Clinical Applications IRB #1; Reg No IRB00002771) and subjects provided their written informed consent in order to participate. After meeting all inclusion/exclusion criteria at screening, eligible subjects were then randomized (1:1) to receive either NEM or placebo in the order in which they were enrolled in the study using a permuted block randomization table consisting of four subjects per block. Treatment consisted once-daily orally of either 500 mg of NEM or placebo. Treatment compliance was checked at clinic visits by participant interview and by counting the number of unused doses of the study capsules. Acetaminophen was allowed for pain relief rescue, if necessary. Subjects recorded the time and amount of acetaminophen taken in subject diaries. All participants, clinical staff, and study management staff remained blinded to treatment assignment throughout the study.
The intent of this study was to evaluate whether NEM brand ESM would reduce cartilage turnover (via the CTX-II biomarker) or alleviate joint pain or stiffness, either directly following exercise or 12 hours post exercise (recovery), versus placebo. Participants performed 50–100 steps per leg utilizing a 6″-tall aerobics step at the clinical site. At screening, subjects performed 50 steps per leg, increasing by 10 at a time per leg up to 100 steps per leg, until they experienced at least a 1 unit change in discomfort (either pain or stiffness) from their resting rating. This number of steps was then assigned to that subject for the remainder of the study. Participants then followed their assigned exercise regimen on alternating days for 2 consecutive weeks (ie, Sunday, Tuesday, Thursday, Saturday, Monday, Wednesday, and Friday). Additionally, participants were required to provide blood and urine samples approximately 24 hours after the final weekly exercise visit, that is, on Friday of Week 1 and Saturday of Week 2. Changes in pain and stiffness (both immediate and 12-hour) and changes in CTX-II levels were compared to those of the placebo group.
Patients
All healthy postmenopausal women, 40–75 years of age, who had not been diagnosed with a joint or connective tissue disorder of the lower extremities (ie, ankles, knees, or hips), were considered for enrollment in the study. To be eligible to participate, subjects must have been amenorrheic (postmenopausal) for at least 12 months prior to baseline evaluation, either naturally or surgically. Additionally, subjects must not have had persistent lower-extremity joint pain at rest; however, subjects could have experienced mild periodic lower-extremity joint pain rated ≤3 on a 10-point ordinal scale. Subjects must have been sufficiently healthy to perform moderate exercise as judged by a medical examination including vital signs (ie, resting heart rate, blood pressure, respiration) and an electrocardiogram (ECG). Subjects were required to suspend all current prescription or over-the-counter pain relief medications (ie, nonsteroidal anti-inflammatory drugs [NSAIDs], analgesics, opioids, anti-depressants prescribed for painful conditions such as fibromyalgia, or joint supplements) at least 30 days prior in order to participate in the study. Subjects who were currently taking analgesic or related medications were eligible to participate in the study following a 14-day washout period for NSAIDs, a 7-day washout period for opioids, and a 90-day washout period for injected steroids or antidepressants. Subjects currently taking joint supplements such as glucosamine, chondroitin sulfate, curcumin, and Boswellia were only eligible after a 3-month washout period. Subjects could not meet any of the classification criteria (other than age >50 years) according to the American College of Rheumatology for either OA33 or RA.34 Subjects were also excluded if they were currently receiving remission-inducing drugs such as methotrexate or immunosuppressive medications or had received them within the past 3 months. They were further excluded if they had a confounding inflammatory disease or condition (gout, pseudo gout, lupus, Paget’s disease, chronic pain syndrome, etc) that would interfere with assessment of lower-extremity joints. Moreover, subjects were excluded if they participated in activities involving intensive use of the lower extremities (ie, running/jogging, sports, bicycling, dancing, etc) two or more days per week or participated in activities that involved moderate use of the lower extremities (ie, walking, golfing, yoga, etc) three or more days per week. Other exclusionary criteria were body weight 275 pounds (125 kg) or greater, a known allergy to eggs or egg products, or pregnant or breastfeeding women. Subjects previously enrolled in a research study involving an investigational product (drug, device, or biologic) or a new application of an approved product, within 30 days of screening, were also excluded from participating in the trial.
Clinical endpoints/treatment response
The primary endpoint for the study was any statistically significant reduction in exercise-induced cartilage turnover (via the CTX-II biomarker) versus placebo evaluated at 1 and 2 weeks of treatment. Treatment response (reduction) was defined as either a lesser magnitude increase or a decrease from baseline in mean uCTX-II (corrected for creatinine [Cr]) induced by exercise in the NEM group compared to the placebo group. Urine samples were collected from the second void of the morning within 24 hours of completing the final exercise period for each week. Secondary endpoints were any statistically significant reductions in either exercise-induced joint pain or stiffness versus placebo evaluated daily via participant questionnaire. The questionnaire consisted of an immediate assessment (during or up to 1 hour post exercise) and a 12-hour assessment. Each assessment asked the participants to rate their joint pain and stiffness on a 10-point ordinal scale with zero equating to no pain (or stiffness) and 10 equating to severe pain (or stiffness). A similar 10-point ordinal scale was also used to evaluate participant joint pain and stiffness at rest (average from the prior 7 days) and during screening assessment, deemed baseline.
Assessment of uCTX-II
Urine samples were collected from the second void of the morning. Fifteen milliliters from each urine specimen were centrifuged at 3,000 rpm for 10 minutes at 12°C–25°C. Following centrifuging, two 5 mL aliquots were removed by pipette and were immediately placed in a −20°C freezer. Samples were stored frozen (−20°C) until analysis. Only a single aliquot of the pair was subsequently thawed for initial analysis, to avoid repeated freeze/thaw cycles that might result in aberrant repeat assay values. Urinary concentrations of CTX-II were measured via enzyme-linked immunosorbent assay using a commercial immunoassay (Urine CartiLaps EIA; Immunodiagnostic Systems, Inc, Gaithersburg, MD, USA) and urinary Cr was measured via a colorimetric assay (Cr (urinary) Colorimetric Assay Kit; Cayman Chemical Company, Inc, Ann Arbor, MI, USA) according to the manufacturers’ instructions using a SpectraMax Plus 384 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). uCTX-II concentrations were subsequently normalized by dividing uCTX-II expressed in micrograms per liter (µg/L) by urinary Cr expressed in millimoles per liter (mmol/L), the results of which were reported as nanograms of CTX-II per millimole of creatinine (ng/mmol Cr). Samples were assayed in duplicate and all assays were repeated (n=4). Duplicate assay values that were within 90% agreement were subsequently averaged. Repeated assay means that were within 90% agreement were further averaged. If repeat assay means differed by more than 10%, an additional assay was performed in duplicate with the remaining frozen aliquot of urine. The additional repeat assay mean was either substituted for the original outlier, or if insufficient agreement was reached with either original repeat assay mean, then all three were averaged. About 36% of the uCTX-II assays were repeated three times, primarily due to interassay variability. Overall intra-assay coefficients of variation were 6.83 and 2.03 for uCTX-II and Cr, respectively.
Investigational product
The investigational product, NEM, is derived from ESM and is manufactured via a patented process. During the manufacture of NEM, the ESM is partially hydrolyzed utilizing a gentle enzymatic process, as opposed to using harsh indiscriminate chemicals, to enhance gastrointestinal absorption while preserving the membrane’s natural biological activity. For this clinical study, 500 mg of NEM (Lot #8012980) or placebo (500 mg of excipients) was provided in #0 vegetarian capsules by ESM Technologies, LLC (Carthage, MO, USA). Treatment and placebo capsules were identical in appearance, odor, and taste and were stored in closed containers at ambient temperature. Participants were instructed to take one capsule daily with water, approximately the same time each morning before eating breakfast.
Safety/adverse events (AEs)
Secondary objectives of the study were to evaluate tolerability and safety or any adverse reactions associated with ingestion of NEM. Subjects underwent a thorough medical examination at screening and upon study completion by a licensed physician including a complete medical history, physical examination, vital signs, ECG, clinical chemistry, hematology, and urinalysis. Vital signs included resting heart rate, blood pressure, respirations, and temperature. Clinical chemistry included alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, protein (total), bilirubin (total), blood urea nitrogen, Cr, potassium, sodium, chloride, calcium, and bicarbonate. Hematology included complete blood count consisting of red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, and white blood cell count with differential (neutrophils, eosinophils, basophils, lymphocytes, and monocytes). Urinalysis included bilirubin, blood, glucose, ketones, nitrites, pH, protein, specific gravity, urobilinogen, and leukocyte esterase. Additionally, participants’ self-assessment diaries were reviewed at each clinic visit and any discomfort beyond what would be expected with exercise or any other AEs were recorded and reported in accordance with applicable FDA regulations. AEs and serious AEs were assessed by the clinical investigator and were followed until resolution, as necessary. Serious AEs were required to be reported to the clinical monitor immediately.
Statistical analysis
The hypothesis for this study was that the treatment group would be superior to that of the placebo group in limiting the increase in uCTX-II levels resulting from moderate exercise. A 16% absolute change in the mean treatment response (uCTX-II would increase by an average of at least 16% more in the placebo group than in the treatment group) was expected based upon the prior proof-of-concept trial (unpublished). We estimated that a sample size of 60 subjects would need to be enrolled to provide the study with a statistical power of 80% to detect a clinically meaningful difference between the treatment group and the placebo group, assuming a rate of response of −4% for the treatment group and a rate of response of +12% for the placebo group, with no dropouts. As the actual enrollment for the study was 60 subjects, this should be sufficient to provide adequate safety and comparative effectiveness information. Descriptive statistics were calculated including mean age, height, weight, BMI, and number of steps per leg, and comparisons of this demographic data were made with a Kruskal–Wallis test for multiple independent samples at baseline to validate randomization. Following evaluation for normality, postbaseline statistical analyses were done utilizing either an independent-group t-test (CTX-II) or repeated-measures univariate analysis of variance (rm-ANOVA) (pain and stiffness). Items found to have statistical significance with rm-ANOVA were then compared using a Kruskal–Wallis test for multiple independent samples. In all cases, statistical significance was accepted at P<0.05. Analysis of the primary endpoint, as well as all secondary endpoints, was conducted on the per-protocol population. SYSTAT software (version 13) (Systat Software, Inc, San Jose, CA, USA) was used for all statistical analyses.
Results
Recruitment began in November 2015 at a single clinical site in Missouri and the final follow-up was conducted in January 2016. One hundred seventy-two (female) subjects were screened and a total of 60 (female) subjects between the ages of 44 and 74 years were enrolled in the trial and underwent randomization. Thirty subjects (50.0%) were randomized to the placebo group and 30 subjects (50.0%) were randomized to the NEM treatment group. All subjects completed the study per the protocol and there were no dropouts (0%). Compliance with the study treatment regimen was good in both treatment groups, as judged by capsule count at clinic visits and all subjects attended every assigned exercise period.
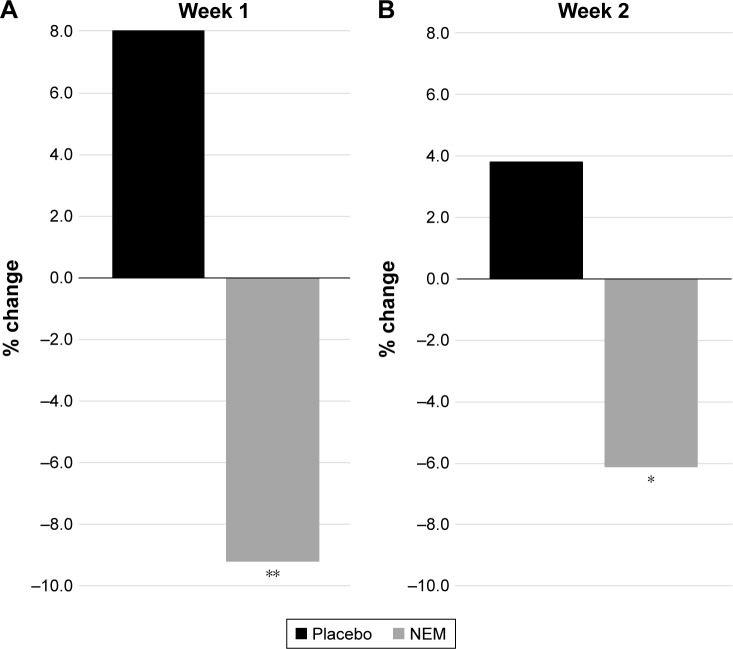
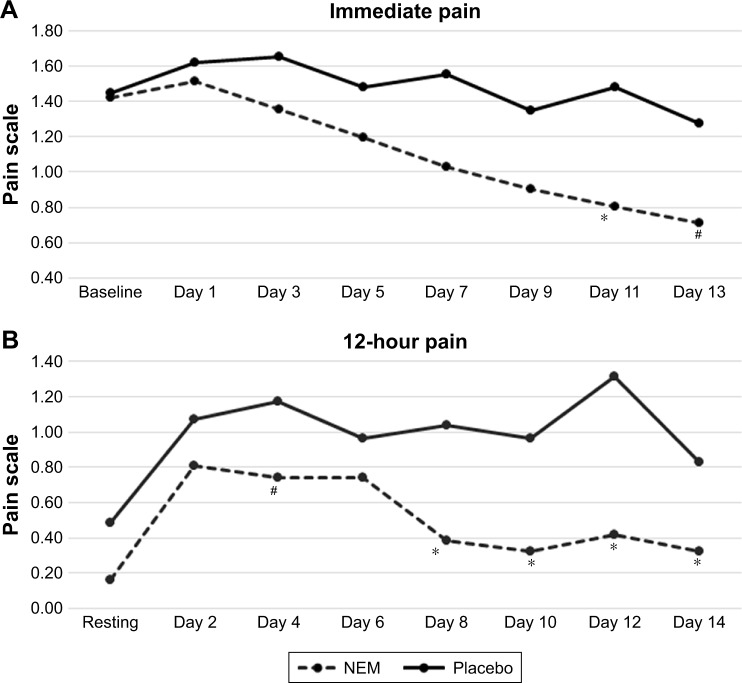
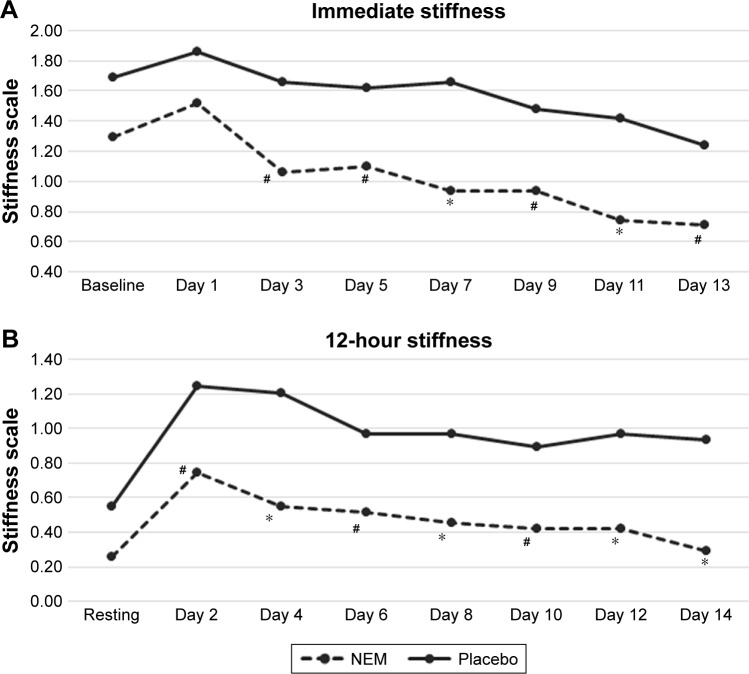
Participant demographic data (Table 1) was initially evaluated to validate randomization. There were no statistical differences between treatment groups in any of the parameters listed (not shown). Importantly, parameters expected to have a bearing on endpoint differences (ie, BMI, steps per leg, uCTX-II, baseline pain, and stiffness) were evenly distributed between treatment groups. A clinical comparison of valid subjects was carried out to obtain mean scores for each of the outcome measures (CTX-II, pain, and stiffness) after 1 week and 2 weeks of exercise (Table 2). Absolute treatment effects (TEabs) for all outcome measures were calculated as the net difference of treatment versus placebo for the mean change in treatment effect from baseline (or resting) for each group expressed as a percent. Negative values represent superior results in the treatment group. Statistical analysis of the primary outcome measure (CTX-II) revealed that supplementation with NEM produced a significant treatment response versus placebo after both 1 week (TEabs −17.2%, P=0.002) and 2 weeks of exercise (TEabs −9.9%, P=0.042) (Figure 1). The overall trend for immediate pain was not significantly different from placebo (P=0.209) (Figure 2A), despite a statistically significant decrease from baseline for Days 7–13 in the NEM treatment group (Day 13 TEabs −38.1%), whereas the placebo group was not significantly different from baseline at any time point. The overall trend for recovery (12-hour) pain was significantly different from placebo (P=0.016), and this difference occurred at Day 8 and continued through Day 14 (end of study) (Figure 2B). By the end of the 2-week evaluation period, recovery pain had nearly returned to resting levels for the NEM treatment group (Day 14 TEabs −11.8%), while placebo group recovery pain levels remained substantially elevated. The overall trend for immediate stiffness was significantly different from placebo (P=0.042) and this difference occurred at Days 7 and 11, with Days 3, 5, 9, and 13 showing a positive trend (P<0.10) for being different from placebo (Figure 3A). Both groups experienced less immediate stiffness from performing the exercise regimen as the study progressed; however, the NEM treatment group felt a greater benefit (Day 13 TEabs −18.5%). The overall trend for recovery (12-hour) stiffness was also significantly different from placebo (P=0.014) and this difference occurred at Days 4, 8, 12, and 14, with Days 2, 6, and 10 showing a positive trend (P<0.10) for being different from placebo (Figure 3B). Similar to recovery pain, recovery stiffness had nearly returned to resting levels for the NEM treatment group (Day 14 TEabs −56.3%), while the placebo group recovery stiffness levels remained substantially elevated.
Table 1.
Participant baseline demographic data
| Demographic parameters | NEM | Placebo |
|---|---|---|
| Age, years | 56.1±7.4 | 57.7±5.9 |
| Sex | ||
| Female (%) | 30 (100) | 30 (100) |
| Height, cm | 164±5 | 164±7 |
| Weight, kg | 75.7±13.6 | 78.1±14.5 |
| BMI, kg/m2 | 28.2±5.4 | 28.8±4.4 |
| uCTX-II, ng/mmol Cr | 258±106 | 236±175 |
| Resting pain score | 0.2±0.4 | 0.5±0.8 |
| Resting stiffness score | 0.3±0.6 | 0.6±0.9 |
| Number of steps per leg | 61.9±16.2 | 59.3±11.9 |
| Baseline pain score | 1.4±1.1 | 1.4±1.4 |
| Baseline stiffness score | 1.3±1.0 | 1.7±1.3 |
Notes: Except where indicated otherwise, values are reported as mean ± SD (n=30 per group). There were no statistical differences between treatment groups in any of the listed parameters. Baseline pain and stiffness scores were obtained from performing the number of steps per leg determined at screening.
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by the square of height in meters; NEM, natural eggshell membrane; uCTX-II, urinary C-terminal cross-linked telopeptide of type-II collagen, reported as nanograms per millimole of creatinine (ng/mmol Cr).
Table 2.
Mean scores for CTX-II and (immediate and 12-hour) pain and stiffness in NEM-supplemented and placebo groups at baseline (or resting) and after 1 and 2 weeks of exercise
| Parameter | Weeks
|
Treatment
|
|
|---|---|---|---|
| Post treatment | NEM | Placebo | |
| uCTX-II | Resting (n=30, 30) | 258±106 | 236±175 |
| 1 (n=30, 30) | 234±83* | 255±172* | |
| 2 (n=30, 30) | 242±101# | 245±184 | |
| Immediate pain | Baseline (n=30, 30) | 1.4±1.1 | 1.4±1.4 |
| 1 (n=30, 30) | 1.0±1.1* | 1.6±1.5 | |
| 2 (n=30, 30) | 0.7±0.9* | 1.3±1.5 | |
| 12-hour pain | Resting (n=30, 30) | 0.2±0.4 | 0.5±0.8 |
| 1 (n=30, 30) | 0.4±0.6* | 1.0±1.3* | |
| 2 (n=30, 30) | 0.3±0.6# | 0.8±1.2# | |
| Immediate stiffness | Baseline (n=30, 30) | 1.3±1.0 | 1.7±1.3 |
| 1 (n=30, 30) | 0.9±0.9# | 1.7±1.5 | |
| 2 (n=30, 30) | 0.7±0.9* | 1.2±1.5# | |
| 12-hour stiffness | Resting (n=30, 30) | 0.3±0.6 | 0.6±0.9 |
| 1 (n=30, 30) | 0.5±0.7* | 1.0±1.2* | |
| 2 (n=30, 30) | 0.3±0.6 | 0.9±1.4# | |
Notes: Values are reported as mean ± SD.
P<0.05;
P<0.10 within group from baseline or resting.
Abbreviations: NEM, natural eggshell membrane; uCTX-II, urinary C-terminal cross-linked telopeptide of type-II collagen, reported as nanograms per millimole of creatinine (ng/mmol Cr).
Figure 1.
Percent change from resting in urinary CTX-II in NEM-supplemented and placebo groups after 1 week (A) and 2 weeks (B) of exercise.
Notes: *P<0.05; **P<0.01 versus placebo.
Abbreviations: CTX-II, C-terminal cross-linked telopeptide of type-II collagen, reported as nanograms per millimole of creatinine (ng/mmol Cr); NEM, natural eggshell membrane.
Figure 2.
A plot of immediate pain (A) and 12-hour pain (B) in NEM-supplemented and placebo groups across 2 weeks of exercise.
Notes: Overall trend differences were determined via rm-ANOVA. Trend differences found to have statistical significance with rm-ANOVA were then compared using a Kruskal–Wallis test for multiple independent samples to identify specific time points that differed between treatment and placebo. *P<0.05; #P<0.10 versus placebo.
Abbreviations: NEM, natural eggshell membrane; rm-ANOVA, repeated-measures analysis of variance.
Figure 3.
A plot of immediate stiffness (A) and 12-hour stiffness (B) in NEM-supplemented and placebo groups across 2 weeks of exercise.
Notes: Overall trend differences were determined via rm-ANOVA. Trend differences found to have statistical significance with rm-ANOVA were then compared using a Kruskal–Wallis test for multiple independent samples to identify specific time points that differed between treatment and placebo. *P<0.05; #P<0.10 versus placebo.
Abbreviations: NEM, natural eggshell membrane; rm-ANOVA, repeated-measures analysis of variance.
There were 25 non-exercise-related AEs reported by the treatment group, the most common of which were eight instances of headache, three instances of nausea, and three instances of cold/flu/sinus congestion. None of the treatment group AEs were judged by the clinical investigator to be associated with treatment. There were 32 non-exercise-related AEs reported by the placebo group, the most common of which were 12 instances of headache, three instances of nausea, and five instances of cold/flu/sinus congestion. None of the placebo group AEs were judged by the clinical investigator to be associated with treatment. There were no serious AEs reported during the study in either treatment group. Although there were a greater number of AEs in the placebo group (56%) versus the NEM group (44%), the overall use of acetaminophen was similar between the groups. There were seven instances of acetaminophen use in the placebo group (avg 18.6 mg/subject/day) and eight instances in the NEM treatment group (avg 18.0 mg/subject/day) with the vast majority of these instances being related to either headache or cold/flu/sinus congestion. The treatment was reported to be well tolerated by study participants.
Discussion
Discomfort during and resulting from exercise is extremely common, particularly for those 45 years and older. It is important to minimize this discomfort and to mitigate any long-term consequences that may result in order to realize the myriad health benefits of regular exercise, and in the elderly to prevent the detrimental musculoskeletal changes that are a consequence of immobility. This trial was designed to evaluate the effects of NEM brand ESM in relieving exercise-induced pain, stiffness, and cartilage turnover in healthy, postmenopausal women. NEM demonstrated meaningful beneficial effects for all three of these clinical endpoints.
NEM-treated subjects experienced 38.1% less exercise-induced (immediate) pain than placebo subjects by the end of the 2-week evaluation period. Although this difference failed to reach statistical significance in the overall trend, Day 11 differed statistically from placebo and Day 13 fell just shy of significance. This substantial decrease from baseline for the NEM-treated group was also statistically significant (within group) for Days 7–13, whereas it was not different from baseline at any time point for the placebo group. Combined, these two statistical comparisons suggest that the overall trend may have reached significance had the study had slightly greater enrollment or had the evaluation period continued for an additional few days to a week. The overall trend for recovery from exercise-induced pain was statistically significant for NEM-treated subjects compared to the placebo group. This improved pain recovery occurred just after 1 week (Day 8) of supplementation with NEM and persisted through the remainder of the study. Importantly, recovery pain had nearly returned to resting levels for the NEM-treated subjects by the end of the 2-week evaluation period while placebo group recovery pain levels remained substantially elevated.
NEM-treated subjects experienced 18.5% less exercise-induced (immediate) stiffness and recovered 56.3% better than placebo subjects by the end of the 2-week evaluation period. Both of these substantial improvements were statistically different from placebo overall, with the difference in immediate stiffness occurring after 1 week (Day 7) of supplementation with NEM and recovery from stiffness differing after only 4 days of supplementation. While these differences were not continuously significant across the entire evaluation period, the discontinuous timepoints fell just shy of significance (P<0.10). Again, this issue likely would have been resolved had enrollment been slightly greater. Similar to recovery pain, recovery stiffness had nearly returned to resting levels for the NEM-treated group while placebo group recovery stiffness levels again remained substantially elevated.
Interestingly, recovery from stiffness was more rapid (Day 4 vs Day 8) and exhibited a greater treatment response (−56% vs −12%) than recovery from pain. This is generally consistent with what has been observed in prior clinical studies with NEM that were conducted in OA subjects,27–29 particularly in relation to the magnitude of the treatment responses. This observed clinical effect is also consistent with the proposed mechanism of action of NEM. That is, the reduction in proinflammatory cytokines demonstrated in previous mechanistic investigations25,26,30 would be expected to have a direct correlation to joint stiffness, which is a likely result of localized inflammation. Although NEM has also been shown to affect pain signal transduction via reducing prostaglandin E2 (PGE2) in an OA rat model,30 the corresponding clinical effect would be expected to lag behind any anti-inflammatory effect as PGE2 synthesis is amplified by the inducible enzyme, cyclooxygenase-2 (COX-2), that is induced by and responsive to proinflammatory cytokines.35 Moreover, the perception of pain (nociception) has been shown to be hypersensitized by proinflammatory cytokines36 wherein normally painful stimuli are perceived as more painful. This hyperalgesia is thought to contribute substantially to the nature of chronic pain perception in joint diseases like OA.37 A hydrolysate of NEM was also found to moderately inhibit the enzyme 5-lipoxygenase (5-LOX) (IC50 11,188 µg/mL) in vitro while not inhibiting COX-1 or COX-2 to any appreciable extent (unpublished). Leukotrienes are produced via the 5-LOX conversion of cell-surface arachidonic acid and have been shown to play a significant role in both nociception and chronic and inflammatory pain pathways.38 One of these lipid proinflammatory mediators, leukotriene B4, was found to be significantly reduced by NEM in an OA rat model.30
NEM-treated subjects also experienced markedly less cartilage degradation than the placebo group. uCTX-II levels were 17.2% lower after 1 week of supplementation with NEM while this reduction moderated slightly to −9.9% after 2 weeks of supplementation. When the trial was initially conceived, it was expected that NEM would simply mitigate the increase in urinary outflux of CTX-II resulting from exercise-induced cartilage degradation. It is noteworthy that NEM not only prevented such an increase, but actually reduced uCTX-II below baseline levels (Figure 1). This remarkable chondroprotective effect resulting from treatment with a natural health product is the first instance, to our knowledge, demonstrated in healthy subjects. Similarly, a profound chondroprotective effect from NEM treatment was recently demonstrated for the first time in dogs with naturally occurring joint disease.32 NEM was initially shown to affect CTX-II in a rat model of OA30 wherein this effect was found to correlate with chondroprotection via histopathology and micro-CT-arthography. In that study, NEM treatment suppressed cartilage deformation and preserved cartilage volume compared to untreated OA controls. At least part of this chondroprotection may result from the anti-inflammatory properties of NEM noted previously; however, serum levels of matrix metalloproteinases (MMP-2 and -9) known to degrade cartilage, were also substantially reduced.30
NEM’s effect on CTX-II production may be further evidence of a second immunomodulatory mechanism of action outside of the typical direct interaction with immune cells in the blood or inflamed tissues. That is, 50% of OA patients were found to have autoantibodies to type II collagen39 and these autologous fragments as well as other cartilage-derived fragments are thought to be a major driver of the immune-mediated cartilage destruction that typifies OA progression. The immune component of OA includes antigen-specific proliferation of T cells, synovial membrane infiltration, and the subsequent localized production of proinflammatory cytokines including IL-1β and TNF-α among others.39 Similarly, collagen-induced arthritis (CIA), a well-accepted animal model of RA, is initiated by subdural injection of type II collagen in combination with an immunogenic adjuvant. The adjuvant functions via the activation of the cytoplasmic transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the subsequent antigen-specific T-cell proliferation pathway that closely mirrors that described above for OA. This CIA rat model has been used to validate an immune regulatory process known as “oral tolerance”. Oral tolerance refers to the phenomenon of a reduced peripheral immune response (tolerance) that results from the repeated exposure of the mucosal immune system in the gut to ingested protein antigens. Oral tolerance to immunogenic peptides that are repeatedly ingested is believed to result from immune surveillance within the gut-associated lymphoid tissue as a way for the body to prevent an inappropriate or unnecessary immune response to proteins normally consumed in the diet. A hydrolysate of NEM was found to activate NF-κB in vitro,40 and in a subsequent study employing the CIA rat model, oral supplementation with NEM substantially suppressed swelling due to inflammation and markedly lessened cartilage damage, pannus formation, and periarticular bone resorption histologically.31 Immune tolerance to cartilage components, particularly type II collagen, from oral supplementation with NEM (which contains types I, V, and X collagen) is a more likely explanation for the longer-term reduction in CTX-II formation demonstrated here than is direct suppression of localized inflammation in the joints experiencing strain from exercise.
There was a moderate number of AEs in the trial, all of which were deemed unrelated to treatment, and no serious AEs occurred. Although there were a greater number of AEs in the placebo group (56%) versus the NEM group (44%), the overall use of acetaminophen was similar between the groups with seven instances and eight instances, respectively, with the vast majority of these instances being related to either headache or cold/flu/sinus congestion. No side effects were noted in this trial, nor in any of the five prior trials published to date.27–29 Food-derived natural products such as NEM would be expected to have a robust safety profile, and this has been confirmed throughout its clinical research experience, excluding the obvious egg allergy concern.
Conclusion
NEM brand ESM, 500 mg once daily, rapidly improved recovery from exercise-induced joint pain (Day 8) and stiffness (Day 4) and also significantly reduced the discomfort from stiffness immediately following exercise (Day 7). Moreover, a substantial chondroprotective effect was demonstrated from supplementation with NEM through a lasting decrease in the cartilage degradation biomarker CTX-II. There were no dropouts in the study, and treatment with NEM was reported to be well tolerated. The beneficial effects of NEM versus placebo in exercise-induced joint pain, stiffness, and cartilage turnover described here for the first time in healthy, postmenopausal women should help women of this age group to stay active and maintain healthy joints as they age. The ability to recover quickly from exercise-induced discomfort (both pain and stiffness) could lead to more frequent exercise and may ultimately reduce the rate of exercise discontinuation. And although not a sensory response, the knowledge that one’s joint cartilage is also being protected from damage due to exercise should further improve one’s inclination to exercise and continue exercising.
Acknowledgments
The study sponsor ESM Technologies, LLC would like to thank all of the study participants.
Footnotes
Disclosure
KJR and MB are employees of ESM Technologies, LLC. CA is an independent distributor for ESM’s product. JT is a paid consultant for ESM Technologies, LLC. The authors report no other conflicts of interest in this work.
References
- 1.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 2.Sofia F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15(3):247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 3.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 4.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 6.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57(3):492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 8.Pisters MF, Veenhof C, van Meeteren NL, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review. Arthritis Rheum. 2007;57(7):1245–1253. doi: 10.1002/art.23009. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 10.Valderrabano V, Steiger C. Treatment and prevention of osteoarthritis through exercise and sports. J Aging Res. 2010;2011:374653. doi: 10.4061/2011/374653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop Relat Res. 1987;219:28–37. [PubMed] [Google Scholar]
- 12.Brandt KD. Response of joint structures to inactivity and to reloading after immobilization. Arthritis Rheum. 2003;49(2):267–271. doi: 10.1002/art.11009. [DOI] [PubMed] [Google Scholar]
- 13.Lotz M, Martel-Pelletier J, Christiansen C, et al. Value of biomarkers in osteoarthritis: current status perspectives. Ann Rheum Dis. 2013;72(11):1756–1763. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valdes AM, Meulenbelt I, Chassaing E, et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage. 2014;22(5):683–689. doi: 10.1016/j.joca.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 15.van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FP. Serum and urinary biochemical markers for knee and hiposteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. 2010;18(5):605–612. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Sowersy MF, Karvonen-Gutierrezy CA, Yosefy M, et al. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage. 2009;17(12):1609–1614. doi: 10.1016/j.joca.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharif M, Kirwan J, Charni N, Sandell LJ, Whittles C, Garnero P. A 5-year longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis-association with disease progression. Rheumatol (Oxford) 2007;46(6):938–943. doi: 10.1093/rheumatology/kel409. [DOI] [PubMed] [Google Scholar]
- 18.Reijman M, Hazes JM, Bierma-Zeinstra SM, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50(8):2471–2478. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 19.Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17(3):384–389. doi: 10.1016/j.joca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Eyre DR, Shao P, Vosberg-Smith K, Weis M, Shaffer K, Yoshihara P. Cross-linked telopeptides from collagen types I, II and III in human urine. J Bone Miner Res. 1996;11(S1):S413. [Google Scholar]
- 21.O’Kane JW, Hutchinson E, Atley LM, Eyre DR. Sport-related differences in biomarkers of bone resorption and cartilage degradation in endurance athletes. Osteoarthritis Cartilage. 2006;14(1):71–76. doi: 10.1016/j.joca.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Mouritzen U, Christgau S, Lehmann HJ, Tankó LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62(4):332–336. doi: 10.1136/ard.62.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong M, Hendrix MJ, von der Mark K, Little C, Stern R. Collagen in the egg shell membranes of the hen. Dev Biol. 1984;104(1):28–36. doi: 10.1016/0012-1606(84)90033-2. [DOI] [PubMed] [Google Scholar]
- 24.Baker JR, Balch DA. A study of the organic material of hen’s-egg shell. Biochem J. 1962;82:352–361. doi: 10.1042/bj0820352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson KF, Ruff KJ, Jensen GS. Effects of Natural Eggshell Membrane (NEM) on cytokine production in cultures of peripheral blood mononuclear cells: increased suppression of tumor necrosis factor-α levels after in vitro digestion. J Med Food. 2012;15(4):360–368. doi: 10.1089/jmf.2011.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruff KJ, DeVore DP. Reduction of pro-inflammatory cytokines in rats following 7-day oral supplementation with a proprietary eggshell membrane-derived product. Mod Res Inflamm. 2014;3(1):19–25. [Google Scholar]
- 27.Ruff KJ, Winkler A, Jackson RW, DeVore DP, Ritz BW. Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: a randomized, multicenter, double-blind, placebo-controlled clinical study. Clin Rheumatol. 2009;28(8):907–914. doi: 10.1007/s10067-009-1173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danesch U, Seybold M, Rittinghausen R, Treibel W, Bitterlich N. NEM® brand eggshell membrane effective in the treatment of pain associated with knee and hip osteoarthritis: results from a six-center, open-label German Clinical Study. J Arthritis. 2014;3(3):136. [Google Scholar]
- 29.Brunello E, Masini A. NEM® brand eggshell membrane effective in the treatment of pain and stiffness associated with osteoarthritis of the knee in an Italian Study Population. Int J Clin Med. 2016;7(1):169–175. [Google Scholar]
- 30.Sim BY, Bak JW, Lee HJ, et al. Effects of natural eggshell membrane (NEM) on monosodium iodoacetate-induced arthritis in rats. J Nutr Health. 2015;48(4):310–318. [Google Scholar]
- 31.Wedekind KJ, Ruff KJ, Atwell CA, Evans JL, Bendele AM. Beneficial effects of natural eggshell membrane (NEM) on multiple indices of arthritis in collagen-induced arthritic rats. Modern Rheumatol. 2017;27(5):838–848. doi: 10.1080/14397595.2016.1259729. [DOI] [PubMed] [Google Scholar]
- 32.Ruff KJ, Kopp KJ, Von Behrens P, Lux M, Mahn M, Back M. Effectiveness of NEM® brand eggshell membrane in the treatment of suboptimal joint function in dogs: a multi-center, randomized, double-blind, placebo-controlled study. Vet Med Res Rep. 2016;7:113–121. doi: 10.2147/VMRR.S101842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 34.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria – an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 35.Kawabata A. Lipid mediators and pain signaling: prostaglandin E2 and pain – an update. Biol Pharm Bull. 2011;34(8):1170–1173. doi: 10.1248/bpb.34.1153. [DOI] [PubMed] [Google Scholar]
- 36.Kress M. Nociceptor sensitization by proinflammatory cytokines and chemokines. Open Pain J. 2010;3:97–107. [Google Scholar]
- 37.Schiable HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–556. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi K, Okubo M. Lipid mediators and pain signaling: leukotrienes in nociceptive pathway and neuropathic/inflammatory pain. Biol Pharm Bull. 2011;34(8):1163–1169. doi: 10.1248/bpb.34.1163. [DOI] [PubMed] [Google Scholar]
- 39.Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56(2):409–424. doi: 10.1002/art.22369. [DOI] [PubMed] [Google Scholar]
- 40.Ruff KJ, Durham PL, O’Reilly A, Long FD. Eggshell membrane hydrolyzates activate NF-κB in vitro: possible implications for in vivo efficacy. J Inflamm Res. 2015;8:49–57. doi: 10.2147/JIR.S78118. [DOI] [PMC free article] [PubMed] [Google Scholar]