Abstract
Background
Endothelial progenitor cells (EPCs) were found to be a potential therapeutic choice for low extremity deep vein thrombosis. The aim of our research was to investigate the effect of resveratrol (RSV) on EPCs that may promote thrombus resolution and its potential pathway.
Material/Methods
EPCs were pretreated with RSV and migration; angiogenesis were evaluated ex vivo. Expression of miR-138 and focal adhesion kinase (FAK) was also tested. A murine model of venous thrombosis was developed as an in vivo model. The effects of RSV treatment on mice with inferior venous thrombosis were evaluated.
Results
We found that RSV increased EPCs migration and tube formation ex vivo. RSV significantly inhibited miR-138 expression. Moreover, we demonstrated that FAK was a target of miR-138 and revealed that FAK knockdown downregulated migration and angiogenesis of RSV-treated EPCs. In addition, RSV-induced EPCs promoted thrombus resolution in a murine model of venous thrombosis.
Conclusions
We found the first evidence that intravenous injection of RSV-treated EPCs enhanced thrombus resolution in vivo. RSV exerted its role by reducing miR-138 expression and therefore upregulated FAK.
MeSH Keywords: Focal Adhesion Protein-Tyrosine Kinases, MicroRNAs, Stem Cells, Stilbenes, Venous Thrombosis
Background
Endothelial progenitor cells (EPCs) are characterized by their ability to self-renew and develop into mature endothelial cells [1]. A number of studies have shown that EPCs possess different functional capacities, including migration, cytokines secretion, and recruitment in response to angiogenesis [2,3]. It has been proposed that the number of EPCs is reduced in patients with cardiovascular risk factors [4]. In addition, there is evidence that EPCs promote new vessel formation and tissue vascularization during ischemia [5].
Resveratrol (trans-3, 4, 5′-trihydroxystilbene) is a type of natural phenol that was identified originally in food such as grapes, blueberries, and some medicinal herbs. It has been designated as the responsible agent of the “French paradox”. Resveratrol (RSV) has been showed to inhibit platelet aggregation and/or adhesion, prevent low density lipoprotein oxidation, suppress proliferation or hypertrophy of smooth muscle cells, and increase high-density lipoproteins [6]. Moreover, previous studies have shown that RSV could markedly raise the migration, proliferation, and adhesive activities of EPCs and accelerate the repair of an injured artery [7,8]. Although RSV increases the number of EPCs and promotes EPCs proliferation, adhesion and migration in a dose- and time-dependent manner, the mechanism is still unclear.
MiRNAs, a class of small non-coding RNA molecules, are found to regulate gene expression at the post-transcriptional level via base-pairing with complementary sequences within mRNA molecules subsequently leading to silence or translational inhibition of the target mRNAs. Research studies have revealed that miRNAs participate in regulating one third of all human genes and are involve in multiple biological functions [9,10]. Several studies have reported that miRNAs regulate the function of EPCs and serve as a novel therapeutic option for venous thromboembolism [11,12]. MiR-138 plays an important role in different types of cancers and functions as a tumor suppressor gene. In addition, it can also regulate hypoxia-induced endothelial cell dysfunction [13,14]. However, to our knowledge, its expression and biological roles in EPCs remain unclear.
In our research we found a novel mechanism of regulation for RSV in regulating the functions of EPCs. We found that RSV could promote migration and angiogenesis of EPCs through miR-138 via targeting focal adhesion kinase (FAK). Furthermore, we tested the efficacy of RSV-induced EPCs in the treatment of vein thrombosis on a murine model.
Material and Methods
Isolation and characterization of EPCs
We collected 80 mL peripheral blood samples from healthy adult volunteers who provided informed consent. The protocols were approved by the Institutional Review Board of the Second Affiliated Hospital of Soochow University. EPCs were isolated and characterized according to previous described methods [5]. Peripheral blood mononuclear cells (PBMCs) were isolated through density gradient centrifugation by adding lymphocyte separation medium (Sigma-Aldrich) and seeded on FN-coated 6-multi-well dishes at a density of 2×107 cells/cm2 with 20% FBS EGM-2 medium (Lonza, Walkersville, MD, USA). After four days in culture, non-adherent cells were removed by washing with phosphate-buffered saline (PBS), and new media was applied. EPCs were identified by their formation of a cobblestone-like morphology, their expression of surface markers including CD31, CD309, and CD34. EPCs were serum depleted before experiments. To evaluate the effect of RSV, EPCs were incubated with 50 μM RSV for 48 hours.
Migration assay
Migration of EPCs was evaluated using a Transwell system. Briefly, isolated EPCs (1×105 cells) were placed on the top compartment pretreated with Matrigel. EBM-2MV medium with 20% FBS was pipetted into the lower reservoir. Following incubation in 5% CO2 cell culture incubator overnight, the membranes were fixed with 70% ethanol and stained with hematoxylin. Cells on the underside of the compartment were examined using three random fields under a light microscope.
Wound healing assay
Resuspended EPCs (2×106 cells) were seeded into 24-well plates and reached 80% confluence as a monolayer. Gently scratching of the monolayer with a 20 μL pipette tip across the center of the well was followed by a gently washing of the well, twice, with PBS to remove the detached cells and then the wells were replenish with EBM-2 medium. We took photos at 24 hours and 48 hours and the gap distance was examined in three random wells.
In vitro tube formation assay
The angiogenic capacity of EPCs was assessed by using tube formation assays. A total of 5×104 EPCs cells were seeded onto 24-well plates coated with Matrigel. After 24 hours incubation, tube-like structures were monitored under an inverted light microscope. Images were processed using ImageJ software.
RNA extraction and quantitative
Total RNA extraction was performed using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Then cDNA synthesis was carried out using the PrimeScript RT reagent Kit (Takara, Dailan, China). Real-time (RT)-PCR was carried out using SYBR® Green Q-PCR Mix (Thermo Scientific, MBI, USA) and a Roche LightCycler 480 (Roche, Switzerland). Expression of U6 RNA and β-actin were monitored as internal controls. Primers were as follows: FAK, 5′-GTGCTCTTGGTTCAAGCTGGAT-3′ and 5′-ACTTGAGTGAAGTCAGCAAGATGTGT-3′; U6, 5′-GCTTCG GCAGCACATATACTAAAAT-3′ and 5′-CGCTTCACGAATTTGC GTGTCAT-3′; β-actin, 5′-GAGCACAGAGCCTCGCCTTT-3′ and 5′-TCTCCAGGGAGGAGCTGGAA-3′.
Western blot analysis
EPCs were lysed using RIPA buffer and Halt Protease Inhibitor Cocktail kits. Equal load of proteins were separated via SDS-PAGE, and transferred onto NC membranes (Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin, membranes were treated with primary antibody against FAK overnight at 4°C. β-actin was used as the loading control. Labeled bands were detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA) on x-ray films (Kodak, Tokyo, Japan).
Oligonucleotide, vector construction, and transfection
MiR-138 mimics, inhibitor, their negative control, or FAK siRNA were obtained from GenePharma (Shanghai, China) and transfected into cells with Lipofectamine 3000 (Invitrogen, CA, USA). Then 48 hours after incubation, cells were acquired for ex vivo experiments. Expression level of miRNA was tested by quantitative RT-PCR. The mimic and siRNA were as follows: miR-138 mimics, sense: AGCUGGUGUUGUGAAUCAGGCCGTT; anti-sense: CGGCCUGAUUCACAACACCAGCUTT; miR-138 inhibitor, CGGCCUGAUUCACAACACCAGCU; FAK siRNA, sense: GGAGUGGAAAUAUGAAUUGTT; anti-sense: CAAUUCAUAUUUCCACUCCTC.
Luciferase assays
The pMIR-REPORT with wild-type or mutated FAK 3′UTR were constructed by cloning and inserting of a wild-type or mutated sequence into the SpeI and HindIII sites of the pMIR-REPORT Luciferase vector (Ambion, TX, USA). For reporter assays, miRNA mimics and reporter plasmids were co-transfected by using Lipofectamine 3000 reagent into 293T cells. Luciferase activity was analyzed by the measurement of ratio between firefly and renilla.
Animal model and cells transplantation
The study protocol was approved by the Institutional Animal Care and Use Committee of Soochow University. Murine model of venous thrombosis was constructed as we previously described [12]. Briefly, after immuno-deficient male nude rats aged 8–12 weeks old, (Charles River Laboratories Supplier in China, Beijing, China) were anesthetized with 7% pentobarbital, the infarenal inferior vena cava (IVC) was exposed by a midline laparotomy and ligated with 6-0 Prolene sutures. Meanwhile, the blood flow at the confluence of iliac veins was blocked for 15 minutes to induce IVC segment thrombus. Then the animal models were divided into three groups for cell transplantation (n=10 for each group).
Tissue harvesting and histological examination
Either EPCs or control medium was injected via the tail vein on day three after operation. The animals were sacrificed on the seventh day and IVC segments with thrombus were carefully harvested. Before the thrombi were weighed, excessive blood on the thrombi was removed by filter paper. The thrombi were treated with 4% paraformaldehyde overnight. The samples were then dehydrated using a graded ethanol series, treated by dimethybenzene and embedded in paraffin. The 3 μm section was obtained at 200 μm intervals throughout the full length of the thrombus samples, and then stained with H & E staining. Eight micrometer cryosections were acquired for histological analysis.
Digital subtraction angiography (DSA)
Venography was carried out with digital subtraction angiography (DSA) (GE Innova 3100, USA). Under general anesthesia, the tail vein was punctured as the venous access and contrast medium was injected into the IVC in order to determine the recanalization and resolution of thrombus.
Statistical analysis
All values are expressed as mean ±SD. Student’s t-test was applied when there were two groups, while one way ANOVA was used for comparisons of more than two groups. All statistical analyses were performed using SPSS version 21 (Chicago, IL, USA). A two-tailed value of p<0.05 was considered a significant difference.
Results
Characterization of human EPCs
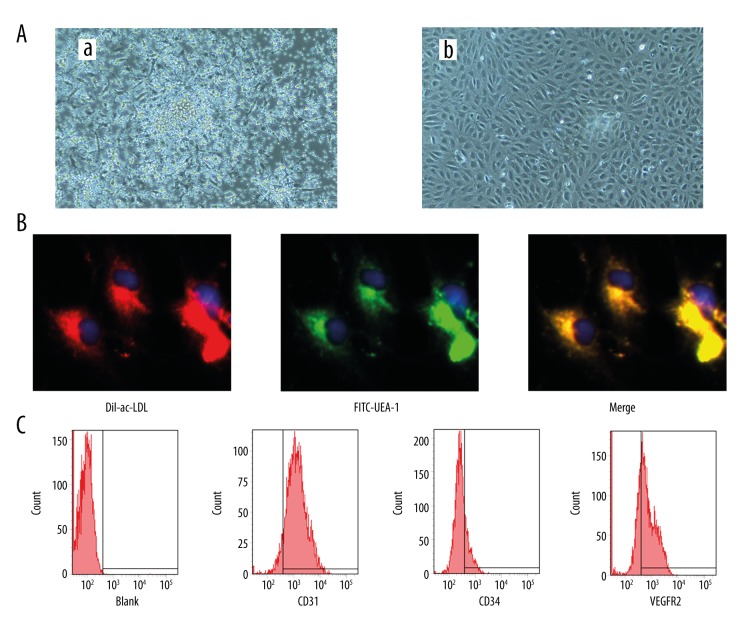
PBMCs isolated and cultured for four days formed a central cluster and exhibited a spindle shaped, endothelial cell-like morphology for 14 days (Figure 1A). Also, EPCs were characterized as adherent cells positive for DiI-labelled acetylated low-density lipoprotein (DiLDL) uptake and lectin binding (Figure 1B). Cells were further identified by demonstrating the expression of CD31 (83.5±4.7%), CD34 (21.9±2.8%), and CD309 (60.3±4.4%) using flow cytometry (Figure 1C).
Figure 1.
Characterization of endothelial progenitor cells (EPCs). (A) EPCs colony exhibited a central cluster on day 4 (a) and formed a spindle shaped, endothelial cell-like morphology on day 14 (b) (100×). (B) Double staining of FITC-UEA-1 and DiI-ac-LDL and merge image (400×). (C) Expression of CD31, CD34 and CD309 of EPCs at day 14.
Effect of RSV on human EPCs migration and tube formation
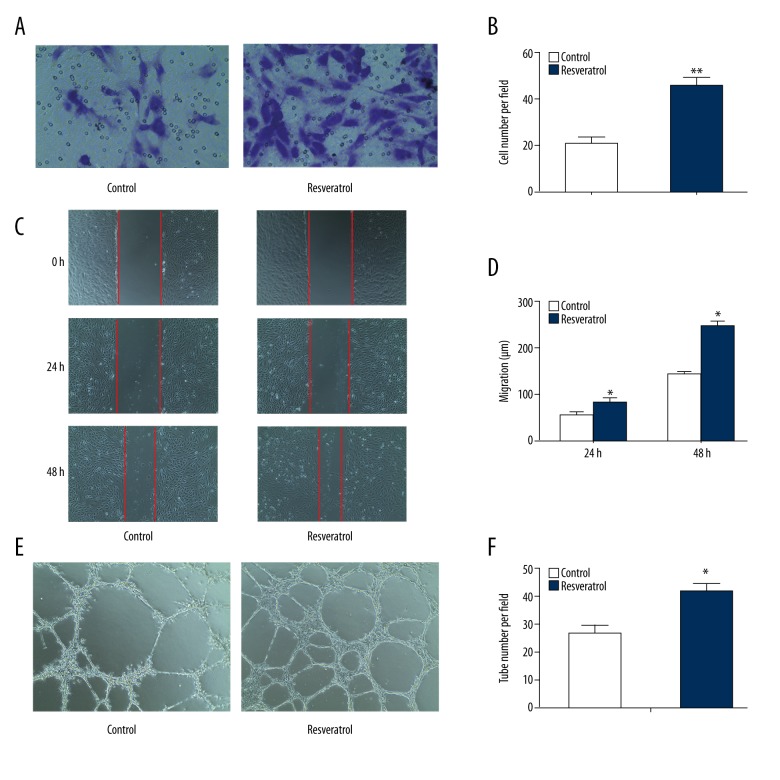
To investigate the role of RSV on EPCs function, EPCs were incubated with 50 μM RSV for 48 hours. Transwell migration assay showed that RSV profoundly promoted migration of EPCs in comparison with the control group (Figure 2A, 2B). Next, the wound healing assay confirmed that culture medium with the addition of RSV increased the migration of EPCs to cross wound space at 24 hours and 48 hours compared with the control group (Figure 2C, 2D).
Figure 2.
Resveratrol regulates migration, tube formation of endothelial progenitor cells. (A, B) Migrated cells counting (200×). ** p<0.01 versus control (n=3). (C, D) Cell migration distance (200×). * p<0.05 versus control (n=3). (E, F) Relative tube number (100×). ** p<0.01 versus control (n=3).
Moreover, we performed an angiogenesis assay to investigate the ability of EPC to participate in neovascularization, which is the most important activity of EPC. Accordingly, we found that EPCs cultured in the presence of RSV showed increased angiogenic activity, as control to normal medium (Figure 2E, 2F). Therefore, these results confirmed that RSV promotes the migration and angiogenesis of EPCs.
RSV reduces miR-138 expression regulating EPCs function
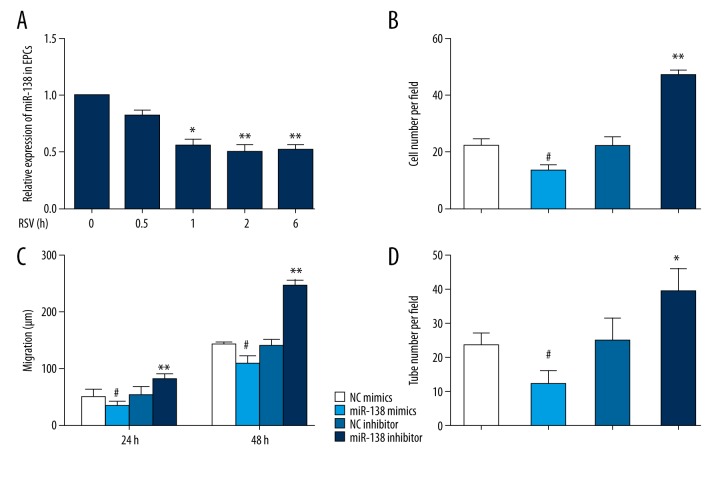
MiR-138 is an essential mediator of endothelial cell (EC) dysfunction and patients with critical limb ischemia (CLI) display increased miR-138 levels [13]. Several studies have reported that RSV could regulate the expression of multiple miRNAs in the context of cancer cell metastasis and invasion [15,16]. To evaluate whether the functional properties of RSV-treated EPCs were affected by miR-138, we performed time-course experiments to study the expression level of miR-138. RT-PCR results revealed that miR-138 was reduced after RSV treatment for up to six hours (Figure 3A). Moreover, to understand the role of miR-138 in regulating EPCs function, miR-138 mimics and inhibitor were applied to overexpress or downregulate the expression of miR-138 in EPCs. Transfected cells were cultured as aforementioned. Results revealed that overexpression of miR-138 reduced the ability of migration and angiogenesis of EPCs. Furthermore, EPCs transfected with miR-138 inhibitor showed opposite behavior on migration and angiogenesis (Figure 3B–3D).
Figure 3.
Resveratrol promotes function of endothelial progenitor cells (EPCs) by regulating expression level of miR-138. (A) Time course of miRNA-138 expression in EPCs treated with RSV. * p<0.05, ** p<0.01. (B–D), EPCs were transfected with miR-138 mimics or inhibitor or negative control. (B) Migrated cells counting (200×). # p<0.05 versus NC mimics (n=3), ** p<0.01 versus NC inhibitor (n=3). (C) Cell migration distance (200×). # p<0.05 versus. NC mimics (n=3), ** p<0.01 versus. NC inhibitor (n=3). (D) Relative tube number (100×). # p<0.05 versus. NC mimics (n=3), * p<0.05 versus. NC inhibitor (n=3).
Effect of RSV on EPCs function is mediated via upregulation of FAK
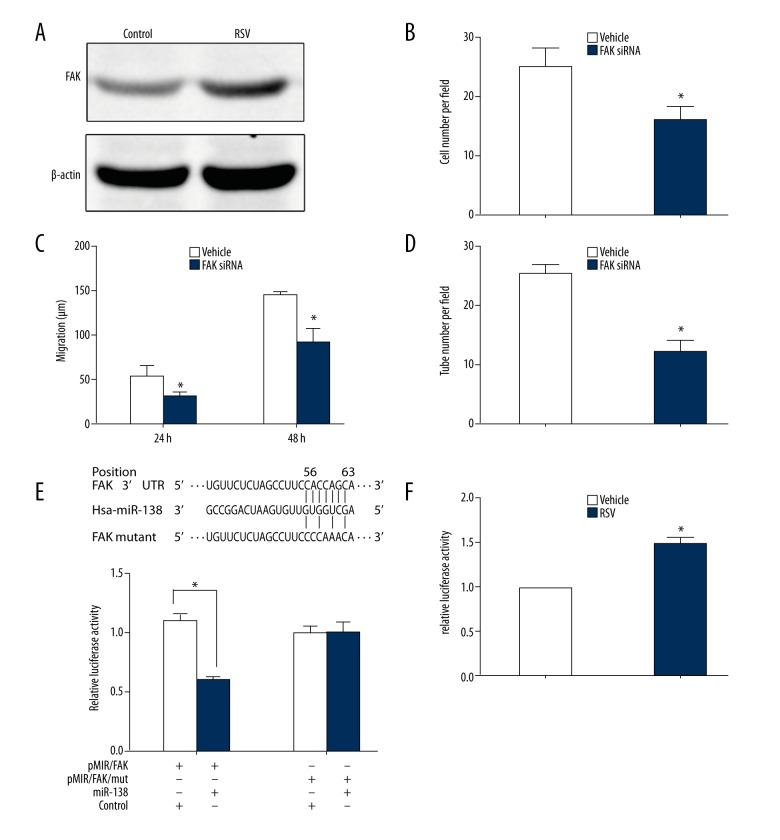
Previous studies have shown that FAK could act as mediator of RSV in the regulation of migration of vascular smooth muscle cells [17]. We proposed to study the effect of FAK in the context of functional regulation of EPCs. Western blotting analysis revealed that RSV increased the level of FAK (Figure 4A). To further investigate the role of FAK in RSV-induced EPCs function, we employed FAK siRNA that thus allowed for downregulation of FAK. After transfected with FAK siRNA, RSV-induced EPCs exhibited decreased cell migration and angiogenesis when compared to the vehicle controls group (Figure 4B–4D).
Figure 4.
Upregulation of focal adhesion kinase (FAK) by resveratrol (RSV) enhances migration and tube formation of endothelial progenitor cells (EPCs). (A) The protein level of FAK in EPCs treated by RSV or vehicle. (B–D) RSV-induced EPCs were transfected with FAK siRNA or vehicle. (B) Decreased migrated cells in EPCs transfected with FAK siRNA. (n=3, * p<0.05 versus vehicle group). (C) Decreased migration distance in EPCs transfected with FAK siRNA at 24 hours and 48 hours. (n=3, * p<0.05 versus vehicle group). (D) Decreased tube number in EPCs transfected with FAK siRNA (n=3, * p<0.05 versus vehicle group). (E) A schematic representation showing the putative target site of FAK and mutated target site for miR-138 with the seed region and base substitutions (upper). Luciferase report assays were performed on HEK 293 T cells with co-transfection of FAK-UTR-WT or FAK-UTR-MUT. (F) Luciferase assay of the FAK 3′UTR showed increased activity when EPCs were treated with RSV, as compared to control (vehicle).
Based on multiple databases (TargetScan, RNA22-HAS and MicroCosm Targets), FAK has been regarded as a potential target of miR-138 because of the presence of potential binding site located at 3′ UTR. To confirm that FAK is directly regulated by miR-138, we performed luciferase assay by using a luciferase reporter vector with a region of 3′ UTR FAK. Our results revealed that miR-138 mimics led to decreased luciferase activity compared with that of pMIR/FAK/mut (Figure 4E). This indicates a direct interaction between miR-138 and the 3′ UTR of FAK. In addition, we compared the luciferase activity of EPCs with or without RSV treatment. Analysis showed that after RSV treatment, EPCs transfected with reporter vector increased the luciferase activity compared to that of the vehicle control (Figure 4F). Taken together, these results demonstrated that RSV and miR-138 influence FAK expression, subsequently regulating EPCs function.
EPCs mediated by RSV promotes recanalization of venous thrombosis in vivo
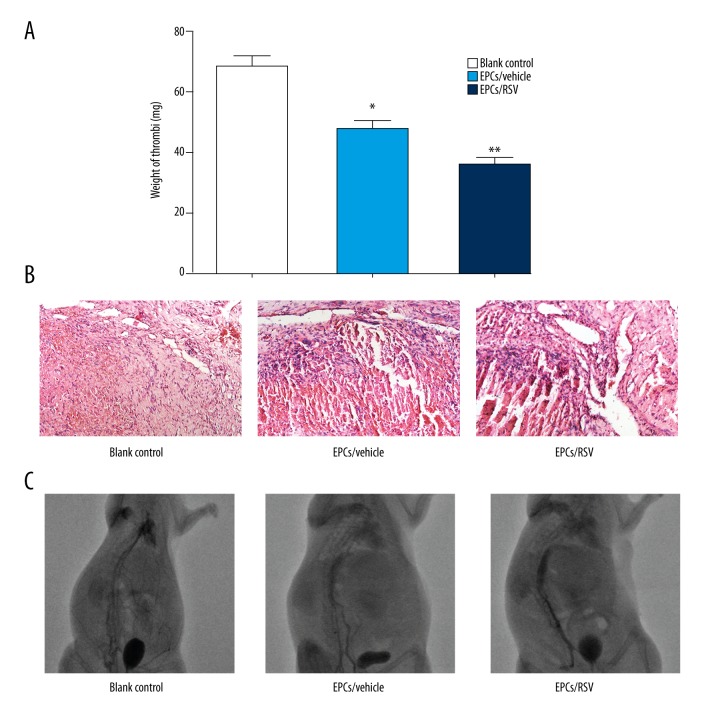
Murine models of venous thrombosis were constructed by IVC ligation. EPCs pre-treated by RSV were intravenously injected into the rats. As shown in Figure 5A, the weight of thrombus significantly decreased in RSV-induced EPCs group at day 7 after transplantation. This was in sharp contrast to the control group in which the mean weight of thrombus was 68.5 mg.
Figure 5.
Resveratrol (RSV)-induced endothelial progenitor cells (EPCs) promotes recanalization of venous thrombosis in vivo. (A) Weight of the venous thrombi at day 7 post the transplantation. Data are expressed as mean ±SEM (n=10, * p<0.05 versus blank control; ** p<0.005 versus blank control). (B) Hematoxylin and eosin (H & E) staining of thrombus sects acquired on 7 day after transplantation (200×). (C) DSA image revealed significantly increase of thrombus recanalization and resolution in rats transplanted with RSV-induced EPCs compared to blank control on day 7.
The sections were also subjected to H & E staining analysis. In parallel, histological analysis revealed the presence of nucleated cells within the thrombus on day 7. Also, erythrocyte, platelets, and fibrin were found in the center of the thrombus. There were more nucleated cells and channels in the RSV-induced EPCs group than in the control group (Figure 5B). DSA was performed as the reference standard for evaluation of venous thrombosis. We observed significantly increased contrast agent in rats injected with RSV-induced EPCs, suggesting better thrombus recanalization and resolution on day 7, compared with that of the control group (Figure 5C).
Discussion
The most important finding in our study was the demonstration of a positive effect of RSV-treatment EPCs leading to increased migration and tube formation. Moreover, we confirmed that effect of RSV on EPCs function was regulated by reduction of miR-138 and upregulation of FAK. Furthermore, we constructed the murine model of venous thrombosis and found that administration of RSV-treatment EPCs contributed to promotion of thrombus resolution and revascularization in vivo.
It has been shown that RSV exerts many pharmacological functions including antitumor, antiaging, anti-cardiovascular disease and anti-oxidation [18–20]. It has been shown that RSV may prevent endothelial cells from ultrastructural damage and apoptosis in high-dose interleukin-2 treatment against melanoma [21]. Furthermore, Huang et al. revealed that RSV played an important role in increasing the number, viability, and function of bone marrow EPCs via upregulating phosphorylation of NO synthase and Akt [22]. However, few studies have reported the effect of RSV on peripheral blood derived EPCs and the potential mechanism.
MicroRNA are short non-coding RNAs which negatively regulate the translational efficacy and stability of target mRNA. Also, they have been reported to be involved in multiple biological processes including vascular development, homeostasis, and differentiation [23]. Recent papers have elucidated that miRNAs influence angiogenesis and modulate the behavior of EPCs [24,25]. In this study, we proposed a novel mechanism underlying the RSV-dependent regulation of EPCs function, thus improving the efficacy of EPCs in the revascularization of vein thrombus.
MiR-138 is involved in regulating tumorigenesis of various malignancies by acting as an oncogene or tumor suppressor gene, depending on the cell type. For instance, Jiang et al. [26] reported that overexpression of miR-138 in TSCC cells decreased proliferation, cell cycle arrest, and apoptosis via targeting GNAI2. It was also observed that a decreased aberrant expression of miR-138 was associated with an inhibition of cell proliferation and an enhancement of cell apoptosis in gallbladder carcinoma [27]. In addition, upregulation of miR-138 played a protective role in myocardial adaptation to chronic hypoxia, which was mediated by the MLK3/JNK/c-jun signaling pathway [28]. However, the function of miR-138 is little known. In this study, we observed that overexpression of miR-138 inhibited migration and angiogenesis of EPCs. Interestingly, these findings were in accordance with previously research on cancer cells. We also demonstrated an inverse correlation between RSV and miR-138. Moreover, functional analysis revealed that forced expression of miR-138 counterbalanced the positive effect of RSV. Therefore, our findings revealed that miR-138 plays an important role in EPCs function.
To elaborate the potential mechanism by which miR-138 regulates RSV-treated EPCs function, we used the bioinformatics tool method to identify the target gene. We analyzed, using several software programs, the potential target gene of miR-138 and found the 3′ untranslated region of FAK existed at the binding sites. Furthermore, the site of miR-138 is reported to be highly conserved among different species, suggesting its important role in regulation of FAK in multiple species. FAK, an intracellular non-receptor tyrosine kinase and a major mediator of signal transduction, has critical functions in proliferation and differentiation of many cell types. Inhibition of FAK has been shown to cause decreased growth in human breast cancer cells [29]. Luciferase assay ascertained that miR-138 directly bound to 3′ UTR of FAK. In addition, FAK protein expression decreased in EPCs transfected with miR-138 mimics. More importantly, we observed that treatment of RSV caused increased luciferase activity. In addition, RSV strongly downregulated miR-138 expression, thus increasing FAK expression, which indicated an interaction of RSV and miR-138 in regulating the expression of FAK.
The resolution of deep venous thrombus is a complex process of organization and formation of neovascular channels within the thrombus, which requires the orchestration of different cells [30]. Monocytic inflammatory infiltrates contributed to the appearance of vascular channels, leading to resolution by encouraging angiogenesis. Significant numbers of bone marrow-derived EPCs have also been found in naturally resolving thrombi [31], but their role in thrombus recanalization and the potential mechanism is unclear. Several studies have found EPCs migrated to thrombi and released proangiogenic cytokines and growth factors such as VEFG, SDF-1, and IGF [3]. In addition, growing evidence revealed impaired endothelial lining can be restored by circulating EPCs [32]. Li et al. reported that bone marrow-derived EPCs increased blood flow in a murine model of chronic thrombosis [8]. However, reduced number and impaired function of EPCs in various diseases are potential difficulties in clinical success of EPCs. Therefore, several studies focused on substance and biological methods that may protect EPCs from dysfunction. Kong et al. reported that decreased miR-483-3p expression promoted EPCs migration and tube formation by targeting SRF [12].
Henrich et al. showed that high dose simvastatin reduced TNF-α-induced apoptosis of EPCs, suggesting simvastatin has protective effects on EPCs survival and differentiation in a hyperinflammation condition [33]. In this study, we found that intravenous injection of RSV-treatment EPCs could promote recanalization of venous thrombosis in vivo. These data suggest that application of RSV as a supplement may play an important role in regulating EPCs function and improve outcomes of deep vein thrombosis.
Conclusions
In conclusion, we established that RSV plays an important role in regulating EPCs function via reducing miR-138 expression and therefore increasing FAK. Furthermore, the application of RSV-treated EPCs improved thrombus recanalization and resolution in a vein thrombosis rat model. Therefore, these data suggest new clinical application in vascular diseases.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No.81400345, No.30972941), Jiangsu Provincial Health Department’s Medical Science Program (H201211), Suzhou Science and Technology Development Plan (SYSD-2015152), Graduate Research and Innovation Program in Colleges and Universities of Jiangsu Province (NO.KYCX17_1997)
References
- 1.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–28. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 2.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14(8):318–22. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 5.Hill JM, Zalos G, Halcox J, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. Obstetrical & Gynecological Survey. 2003;58(7):467–68. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 6.Delmas D, Jannin B, Latruffe N. Resveratrol: Preventing properties against vascular alterations and ageing. Mol Nutr Food Res. 2005;49(5):377–95. doi: 10.1002/mnfr.200400098. [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Wang C, Fan HH, et al. Effects of resveratrol on endothelial progenitor cells and their contributions to reendothelialization in intima-injured rats. J Cardiovasc Pharmacol. 2006;47(5):711–21. doi: 10.1097/01.fjc.0000211764.52012.e3. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Huang J, Zou J, et al. Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol. 2007;34(11):1109–15. doi: 10.1111/j.1440-1681.2007.04667.x. [DOI] [PubMed] [Google Scholar]
- 9.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: Tiny players in a big field. Immunity. 2007;26(2):133–37. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Mattes J, Collison A, Foster PS. Emerging role of microRNAs in disease pathogenesis and strategies for therapeutic modulation. Curr Opin Mol Ther. 2008;10(2):150–57. [PubMed] [Google Scholar]
- 11.Kong L, Du X, Hu N, et al. Downregulation of let-7e-5p contributes to endothelial progenitor cell dysfunction in deep vein thrombosis via targeting FASLG. Thromb Res. 2016;138(138):30–36. doi: 10.1016/j.thromres.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Kong L, Hu N, Du X, et al. Upregulation of miR-483-3p contributes to endothelial progenitor cells dysfunction in deep vein thrombosis patients via SRF. J Transl Med. 2016;14(1):23. doi: 10.1186/s12967-016-0775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen A, Ren S, Lerchenmuller C, et al. MicroRNA-138 regulates hypoxia-induced endothelial cell dysfunction by targeting S100A1. PLoS One. 2013;8(11):78684. doi: 10.1371/journal.pone.0078684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Lv XB, Wang XP, et al. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle (Georgetown, Tex) 2012;11(13):2495–506. doi: 10.4161/cc.20898. [DOI] [PubMed] [Google Scholar]
- 15.Bae S, Lee E, Cha HJ, et al. Resveratrol alters microRNA expression profiles in A549 human non-small cell lung cancer cells. Mol Cells. 2011;32(3):243–49. doi: 10.1007/s10059-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar S, Kumar A, Rimando AM, et al. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget. 2015;6(29):27214–26. doi: 10.18632/oncotarget.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Chen L, Varadharajan TS, et al. Resveratrol inhibits glucose-induced migration of vascular smooth muscle cells mediated by focal adhesion kinase. Mol Nutr Food Res. 2014;58(7):1389–401. doi: 10.1002/mnfr.201300698. [DOI] [PubMed] [Google Scholar]
- 18.Gupta SC, Kannappan R, Reuter S, et al. Chemosensitization of tumors by resveratrol. Ann NY Acad Sci. 2011;1215(1):150–60. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Ahmad F, Philp A, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–33. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnefontrousselot D. Resveratrol and cardiovascular diseases. Nutrients. 2016;8(5):250. doi: 10.3390/nu8050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan H, Singh NP, Singh UP, et al. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS One. 2012;7(4):e35650. doi: 10.1371/journal.pone.0035650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang P, Chen Y, Tsai H, et al. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol. 2010;30(4):869–77. doi: 10.1161/ATVBAHA.109.200618. [DOI] [PubMed] [Google Scholar]
- 23.Kane NM, Thrasher AJ, Angelini GD, Emanueli C. Concise review: MicroRNAs as modulators of stem cells and angiogenesis. Stem Cells (Dayton, Ohio) 2014;32(5):1059–66. doi: 10.1002/stem.1629. [DOI] [PubMed] [Google Scholar]
- 24.Zuo K, Li M, Zhang X, et al. MiR-21 suppresses endothelial progenitor cell proliferation by activating the TGFβ signaling pathway via downregulation of WWP1. Int J Clin Exp Pathol. 2015;8(1):414–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Ong S, Lee WH, Huang M, et al. Cross talk of combined gene and cell therapy in ischemic heart disease role of exosomal microRNA transfer. Circulation. 2014;130(11 Suppl 1):S60–69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang L, Dai Y, Liu X, et al. Identification and experimental validation of G protein alpha inhibiting activity polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous cell carcinoma. Hum Genet. 2011;129(2):189–97. doi: 10.1007/s00439-010-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma F, Zhang M, Gong W, et al. MiR-138 suppresses cell proliferation by targeting Bag-1 in gallbladder carcinoma. PLoS One. 2015;10(5):e0126499. doi: 10.1371/journal.pone.0126499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He S, Liu P, Jian Z, et al. miR-138 protects cardiomyocytes from hypoxia-induced apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun. 2013;441(4):763–69. doi: 10.1016/j.bbrc.2013.10.151. [DOI] [PubMed] [Google Scholar]
- 29.Golubovskaya VM, Beviglia L, Xu L, et al. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor – mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277(41):38978–87. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 30.Modarai B, Burnand KG, Humphries J, et al. The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost. 2005;93(5):801–9. doi: 10.1160/TH04-09-0596. [DOI] [PubMed] [Google Scholar]
- 31.Modarai B, Burnand KG, Sawyer B, Smith A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation. 2005;111(20):2645–53. doi: 10.1161/CIRCULATIONAHA.104.492678. [DOI] [PubMed] [Google Scholar]
- 32.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58(2):148–51. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Henrich D, Seebach C, Wilhelm K, et al. High dosage of simvastatin reduces TNF-α-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1β in vitro. J Surg Res. 2007;142(1):13–19. doi: 10.1016/j.jss.2006.04.011. [DOI] [PubMed] [Google Scholar]