Summary
In vitro and in vivo studies evidenced that mesenchymal stem cells (MSCs) contribute to intervertebral disc (IVD) regeneration by differentiation towards the disc phenotype and matrix synthesis and/or by paracrine signalling to endogenous cells, thereby promoting a healthier disc phenotype in degenerative discs. The aim of this study was to investigate IVD response to human MSC (hMSC) treatment based on the disc degenerative state and hMSC carrier. Bovine caudal IVDs with endplates were cultured in a bioreactor under simulated physiological (0.1 Hz load and sufficient glucose) or degenerative (10 Hz load and limited glucose) conditions for 7 days. Discs were partially nucleotomised, restored with hMSCs in either fibrin gel or saline solution and cultured under physiological conditions for 7 days. Controls included fibrin and saline without hMSCs. Cell viability, histology, disc height, and gene expression analyses were performed to evaluate regeneration. hMSCs in fibrin were viable and homogenously distributed following 7 days of culture under dynamic loading in partially nucleotomised discs. IVD response to hMSCs was conditioned by both disc degenerative state and hMSC carrier. The effect of the regenerative treatment was stronger on simulated-degenerative discs than on simulated-physiological discs. hMSCs in fibrin induced a superior anabolic response in degenerative IVDs compared with fibrin alone, thus suggesting an added value of the cellular therapy compared with an acellular solution. When comparing fibrin and saline as a hMSC carrier, a significantly higher anabolic response was observed in IVDs treated with hMSCs in fibrin. Moreover, it was found that the degenerative state of the disc influenced hMSC differentiation. Indeed, a significantly higher expression of specific discogenic markers (ACAN and CA12) was observed in hMSCs implanted into physiological discs than in those implanted into degenerative discs. In conclusion, host disc cells and donor hMSC response depend on the degenerative state of the host disc and carrier used for hMSC delivery, and these two aspects need to be considered for a successful translation of hMSC therapies for the treatment of IVD degeneration.
Keywords: anabolism, bioreactor, degeneration, fibrin, nucleotomy, saline
Introduction
An important hallmark of intervertebral disc (IVD) degeneration is the marked decrease in the number of viable and functionally active endogenous cell populations [1]. Therefore, cell transplantation therapies have widely been investigated for IVD regeneration. In particular, regenerative effects of bone marrow-derived mesenchymal stem cells (MSCs) have been reported in vitro in preclinical studies and in some clinical trials [2], [3]. Compared with differentiated IVD cells, the main advantages of MSCs include their almost unlimited availability, the one-step isolation procedure, and their high proliferation and multilineage differentiation capability [4].
While promising results were reported from preclinical and clinical studies, recent work has focused on the mechanisms underlying MSCs effects on IVD cells and tissues. Thus, two complementary roles have been attributed to the MSCs that may contribute to tissue regeneration [5]. First, MSCs can acquire an IVD-like phenotype under appropriate culture conditions or in co-culture with IVD cells, thereby directly contributing to new matrix synthesis. Factors that may promote a chondrocytic or IVD-like phenotype include low oxygen conditions, hydrostatic pressure or mechanical compressive loading, growth factor supplementation, and co-culture with native IVD cells [6], [7], [8], [9]. Moreover, in vitro and in vivo studies demonstrated an increased expression of chondrocytic/IVD markers in MSCs injected into the IVD in rat tail and rabbit disc degeneration models [10], [11], [12]. Second, anabolic, anti-inflammatory, and anti-apoptotic properties have been attributed to the implanted MSCs [13]. In vitro coculture experiments demonstrated stimulatory effects of MSCs on the proliferation and extracellular matrix synthesis of nucleus pulposus (NP) and annulus fibrous (AF) cells [14], [15]. Furthermore, MSCs supplementation was effective in suppressing pro-inflammatory markers in degenerative and inflammatory cell and tissue culture models [16], [17]. These findings strongly suggest a prominent role of trophic factors provided by the therapeutically applied MSCs [18], [19], [20].
Nevertheless, the harsh microenvironment within a degenerative IVD may compromise the viability and activity of externally delivered cell populations. In particular, several in vitro studies indicate that low pH conditions (similar to the situation within a degenerative IVD) severely impair the survival of exposed MSCs [21], [22]. This suggests that the degenerative state of an IVD may play a critical role with respect to the effectiveness of a MSC transplantation therapy. Indeed, results from a previous rabbit disc degeneration model suggest that the severity of disc degeneration may influence the therapeutic effect of MSCs [23]. However, this has not been verified in a large animal model. Evaluation of new therapies in a bioreactor model is an important intermediate step for their translation.
In the present study, we used a bovine whole IVD organ culture model in a mechanical loading bioreactor to analyse the effects of implanted MSCs. The well-established IVD bioreactor systems allow the culture of whole IVDs under defined mechanical and nutritional conditions [24]. Since it is difficult to obtain whole human IVDs in large numbers (and with similar degenerative conditions), IVDs from large animals (bovine, ovine, caprine) have been widely used as a substitute for the human IVDs. IVDs from large animals are the closest IVD models of the human IVD in terms of dimensions, diffusion properties (large diffusion distances), cell types (loss of notochordal cells by adulthood), and extracellular matrix (the nucleus cannot be removed by simple aspiration like in the smaller animal models) [25]. The bovine and ovine caudal IVD models have been widely used as they can be easily obtained from slaughterhouses. Because these species do not present a naturally-occurring degeneration (unlike chondrodystrophic dogs), several methods have been used to induce degeneration as a prelude to study regeneration, including detrimental, high-frequency loading, injection of inflammatory molecules, and injection of enzymes [24]. Specifically, Illien-Junger et al [26] have shown that bioreactors can be used to maintain IVDs in a healthy (physiological) state as well as to induce early stages of degeneration, which were associated with increased cell death and catabolism. Discs could be maintained in a physiological condition under low-frequency loading and sufficient nutrition (0.6 ± 0.2 MPa, 8 h/d at 0.2 Hz, medium containing 4.5 g/L glucose), while degeneration was initiated by applying high-frequency loading in combination with limited nutrition (0.6 ± 0.2 MPa, 8 h/d at 10 Hz, medium containing 2 g/L glucose) [26]. In another study, Mwale et al [27] used a bovine whole IVD organ culture model to investigate the effect of MSCs and Link N on early stages of IVD degeneration. They found that both MSCs and Link N can restore glycosaminoglycan content in degenerated discs.
Here, we hypothesised that the state of the IVD at the time of MSC implantation and the type of MSC carrier will have an impact on the response to the treatment. To avoid damaging the AF, a previously established nucleotomy model through the endplate was used to investigate NP restoration [28]. A transpedicular approach to access the nucleus pulposus through the endplate has already been described in a sheep model [29]. Fibrin hydrogel and saline solution were compared as carriers for MSCs. Our data indicate significantly different reactions of both host IVD cells and graft MSCs depending on the IVD state and the MSC carrier, improving our understanding of the efficiency of MSC transplantation therapy.
Materials and methods
Whole IVD culture under simulated physiological and degenerative conditions
Bovine IVDs including endplates (n = 36; mean height = 8.86 ± 1.18 mm, mean diameter = 13.64 ± 1.27 mm) were excised from the tails of 6–8-month-old calves obtained from a local abattoir, prepared for organ culture as previously described, and randomly assigned to either physiological or degenerative culture conditions (Figure 1) [26].
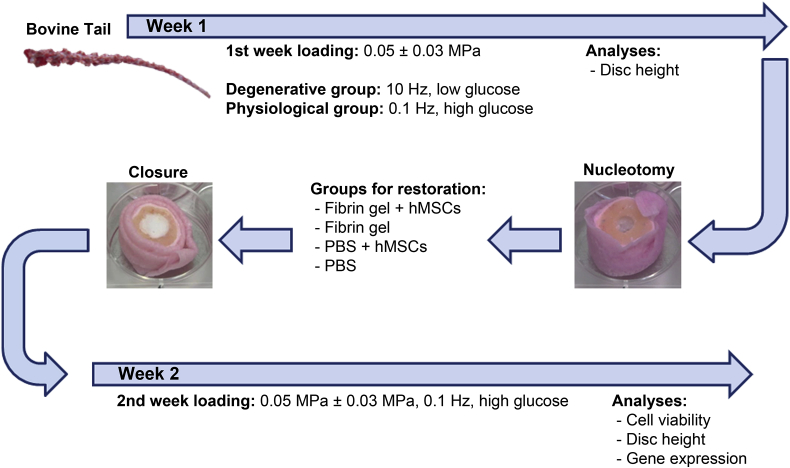
Figure 1.
Schematic overview of the experimental design. hMSCs = human mesenchymal stem cells; PBS = phosphate-buffered saline.
Discs were cultured in either simulated physiological or simulated degenerative conditions [26]. Physiological culture medium was composed of Dulbecco's modified Eagle medium (DMEM) containing 4.5 g/L glucose and supplied with 100 U/mL penicillin and 100 μg/mL streptomycin (all products from Gibco, Invitrogen, Basel, Switzerland), 0.1% Primocin (Invivogen, LabForce, Muttenz, Switzerland), 2% screened foetal bovine serum (FBS; Pan Biotech, Aidenbach, Germany), and 1% insulin-transferrin-selenium (ITS+; Corning, ThermoFischer Scientific, Reinach, Switzerland). Physiological loading was performed at a low frequency (0.1 Hz, 0.05 ± 0.03 MPa, 3 h/d, 7 days) in an IVD bioreactor system. The degenerative group was cultured in a degenerative culture medium composed of DMEM containing 2 g/L glucose and the same components as above; discs were loaded at a high frequency (10 Hz, 0.05 ± 0.03 MPa, 3 h/d, 7 days). After each loading period, discs were moved to six-well plates and kept in free swelling conditions (recovery period). For all discs, culture medium was replaced every day after loading.
Human MSC isolation and expansion
Human MSCs (hMSCs) were obtained from bone marrow aspirates with written consent of the patient (ethical approval KEK 2016-00141, Bern). hMSCs isolated by Ficoll gradient and adherence to tissue culture plastic were expanded in α-minimum essential medium (Gibco, Invitrogen) containing 100 U/mL penicillin, 100 μg/mL streptomycin, 10% FBS (Pan Biotech), and 5 ng/mL basic fibroblast growth factor (Peprotech, Rocky Hill, CT, USA). Early passage (P2–P4) hMSCs from six different donors were used in this study (48.7 ± 19.1 years, mean age and standard deviation).
Application of hMSCs to IVDs
Following physiological or degenerative culture, discs were partially nucleotomised (defect diameter = 4 mm) using an endplate approach, which allowed the supplementation of defined volumes of cell suspensions while preserving an intact AF [28], [29]. Discs were randomly assigned to one of the following groups (n = 6/group; approximately 150 μL/defect): (1) hMSCs in fibrin (Baxter Biosurgery, Vienna, Austria); (2) fibrin alone; (3) hMSCs in phosphate-buffered saline (PBS); and (4) PBS alone. A fibrinogen concentration of 60 mg/mL was used to allow sufficient resistance to dynamic loading, while preserving adequate cell viability [30], thrombin/fibrinogen ratio was set at 0.03 U/mg and a cell density close to native IVD was used (6 × 106 cells/mL) for both fibrin and PBS groups. After 30 minutes, the endplate core was repositioned and sealed with polymethylmethacrylate (PMMA) cement (Vertecem Mixing Kit; Synthes, Teknimed S.A., Bigorre, France; Figure 1) [28]. Discs were further cultured under physiological conditions (physiological medium with 0.1% ε-aminocaproic acid [31]; low-frequency loading) for 7 days. Fibrin gels seeded with hMSCs and cultured in well plates for the same period of time were included as controls.
Cell viability, distribution and disc height
For cell viability experiments, hMSCs were labelled with PKH26 (Sigma-Aldrich, Buchs, Switzerland) before application to IVDs. At the end of the culture period, the endplates were removed with a scalpel and the fibrin gel was collected with a spatula. Fibrin gels were stained with 10 μM Calcein AM (3 hours at 4°C and 1 hour at 37°C). Small samples (3 mm × 3 mm × 3 mm) of inner AF (AFi) and outer AF (AFo) and NP were stained with 10 μM Calcein AM/2 μM ethidium homodimer (both from Sigma-Aldrich) (3 hours at 4°C and 1 hour at 37°C) and visualised as previously described [32].
For sagittal histological sections, discs were fixed in 4% buffered formalin, decalcified, and embedded in PMMA [28]. For transversal sections, the intact endplate was removed prior to embedding in cryocompound. Sections (10 μm thick) were postfixed in 4% buffered formalin and stained with Safranin O/Fast Green, as previously described [33].
Disc height was measured using a calliper at selected time points after loading and after free-swelling recovery. Each disc was measured at four positions, and the average value was used to calculate the percentage of disc height change normalised to height at dissection (Day 0).
Gene expression
Fibrin-hMSC samples were lysed with 1 mL TRI reagent and 5 μL polyacryl carrier (Molecular Research Center, Cincinnati, OH, USA). IVD was separated into AFo, AFi, and NP; tissues (100–150 mg) were finely chopped and treated with 2 mg/mL pronase (Roche, Sigma-Aldrich) in DMEM on an orbital shaker (1 hour at 37°C, 5 mL pronase solution/100 mg tissue). Following addition of 1 mL FBS and two washes in PBS, samples were frozen in liquid nitrogen and pulverised in a custom-made pestle device. The resulting powder was homogenised in TRI Reagent, mechanically disrupted with a Tissue Lyzer (Qiagen, Hombrechtikon, Switzerland; 25 Hz, 6 minutes), and RNA was isolated according to the manufacturer’s instructions. For reverse transcription, TaqMan reagents (ThermoFischer Scientific) were used for bovine RNA, and SuperScript Vilo Synthesis kit (Invitrogen, ThermoFischer Scientific) was used for hMSC RNA. Expression levels of genes of interest (Table 1, Table 2) were measured by real-time PCR (QuantStudio 6 Flex: Applied Biosystems, Rotkreuz, Switzerland) using the ddCt method [32], [34], [35]. For IVDs receiving hMSC treatment, the corresponding tissue (NP, AFi, AFo) from IVDs without hMSCs (receiving fibrin or saline alone) was used as the reference. Human COL1 and COL2 could not be analysed due to the bovine RNA presence in fibrin and lack of primers without cross-reactivity with bovine RNA.
Table 1.
| Gene | Abbreviation |
|---|---|
| Collagen type I | COL1 |
| Collagen type II | COL2 |
| Aggrecan | ACAN |
| Matrix-metalloproteinase-3 | MMP3 |
| A disintegrin- like and a metallopeptidase with thrombospondin type 1 motif 4 | ADAMTS4 |
| Ribosomal protein, large, P0 (endogenous control) | RPLP0 |
PCR = polymerase chain reaction.
Table 2.
| Gene | Abbreviation |
|---|---|
| Aggrecan | ACAN |
| Cytokeratin-19 | KRT19 |
| Carbonic anhydrase XII | CA12 |
| Ribosomal protein, large, P0 (endogenous control) | RPLP0 |
PCR = polymerase chain reaction.
Statistical analysis
Statistical analyses were performed using Graph Pad Prism version 7.0 (GraphPad Software; San Diego, CA, USA). After confirming normal distribution, paired t-tests were performed (p < 0.1 trend, p < 0.05 significant difference). For gene expression analyses, 2−ddCt data were log2 transformed prior statistical analysis. Comparisons of a single carrier type with and without hMSCs was performed using −dCt values.
Results
Cell viability and distribution in nucleotomised IVDs
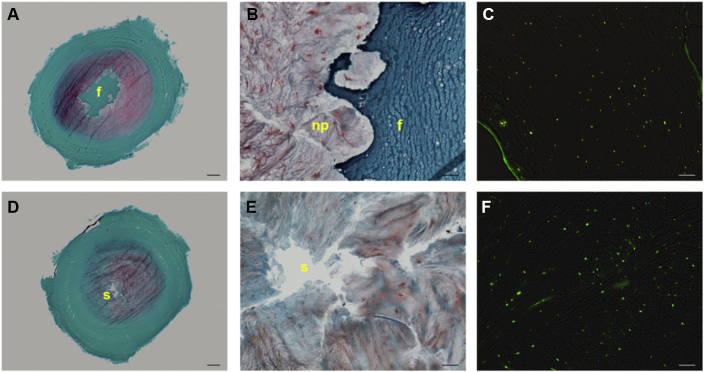
Fibrin gels effectively filled the nucleotomised space and prevented swelling of the surrounding tissue (Figure 2A). There was a good integration with the native tissue (Figure 2B). hMSCs were > 95% viable and homogenously distributed in the fibrin carrier (Figure 2C). When hMSCs were supplied in PBS, NP showed an intrinsic capacity to swell and partially refill the defect (Figure 2D), whereas hMSCs were not observed in the IVD tissue surrounding the defect (Figure 2E). The cells in the tissue surrounding the defect were > 80% viable (Figure 2F), and the disc tissue viability was not affected by the treatment used (fibrin or PBS, with or without hMSCs).
Figure 2.
Degenerative-loaded bovine intervertebral discs (IVDs) restored with human mesenchymal stem cells (hMSCs) in (A) and (B) fibrin (D) and (E) and saline solution following 7 days of dynamic culture. (A) and (D) Safranin O/Fast green stained transversal sections overviews (scale bar = 1 mm); (B) and (E) defect/tissue interface magnified views (scale bar = 100 μm); (C) combined phase contrast and fluorescent images of fibrin gel with PKH26-labelled hMSCs stained with calcein AM (yellow = viable hMSC, red = dead hMSC) and (D) nucleus pulposus tissue stained with calcein AM/ethidium homodimer (scale bar = 100 μm) (green = viable disc cell; red/yellow = dead disc cell). Note that fibrin prevents tissue swelling into the nucleotomised space and limits proteoglycan loss, as attested by the stronger Safranin O stain (A vs. D); fibrin can fill irregularly shaped defects (B). Note the homogeneous distribution of viable hMSCs in fibrin (C) and a majority of viable NP cells inside degenerated tissue (F). f = fibrin gel; np = nucleus pulposus s = saline.
Disc height changes
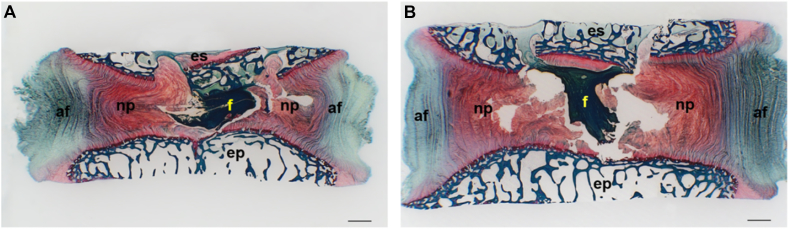
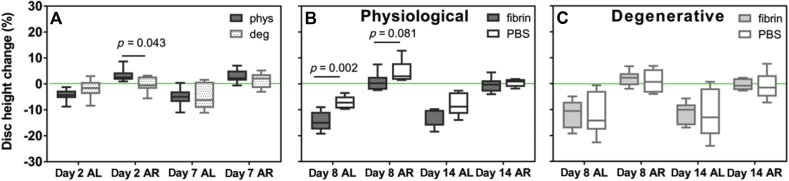
Fibrin gels were able to deform following cyclic loading (Figure 3A) and recovery (Figure 3B). During pre-conditioning, degenerative-loaded discs showed a lower disc height recovery after loading than physiologically-loaded discs that was significant at Day 2 (Figure 4A). Following treatment, physiologically-loaded discs showed an initial significantly higher disc height loss and trend for lower recovery when treated with fibrin than with saline; however, the differences became less strong with culture time (Figure 4B). No differences were observed in the presence or absence of hMSCs. Disc height changes of degenerative-loaded discs following loading and recovery were not significantly affected by the filler (Figure 4C).
Figure 3.
Safranin O/Fast green stained methacrylate sagittal section of nucleotomised intervertebral discs treated with human mesenchymal stem cells in fibrin gel collected immediately (A) after completion of the last dynamic loading cycle and (B) following free swelling. Note the ability of fibrin to recover its initial shape following loading release. af = annulus fibrosus; ep = endplate; es = endplate stopper; f = fibrin gel with hMSCs; hMSCs = human mesenchymal stem cells; np = nucleus pulposus, Scale bar = 1 mm.
Figure 4.
Evolution of disc height relative to the initial dimensions after dissection: (A) during pre-conditioning under physiological (phys) or degenerative (deg) conditions (n = 12) following treatment with fibrin gel or saline (PBS) in (B) physiological discs and (C) degenerative discs (n = 6). Note that the loading protocol induced a higher disc height recovery in physiologically-loaded discs than in degenerative-loaded discs only in the initial phase of the loading (Day 2) and that these were levelled out in long term (Day 7). In physiologically-loaded discs, treatment with saline (PBS) initially promoted a better preservation of disc height following loading (Day 8 AL) and a higher swelling potential (Day 8 AR) than fibrin, but these differences were evened out in long term (Day 14). In degenerative-loaded discs, saline was ineffective in preserving disc height following loading both initially and in long term. AL = disc height after loading, AR = disc height after free-swelling recovery. PBS = phosphate-buffered saline.
Effects of MSCs in physiological and degenerative discs
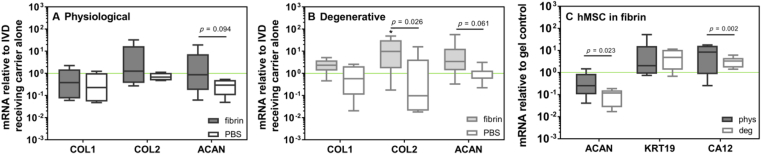
The hMSC impact on physiologically-loaded discs was minimal and independent of the carrier (Figure 5A). In degenerative-loaded discs, hMSC application using fibrin significantly increased the expression of matrix-associated genes (COL2 and ACAN) in IVD cells compared with the hMSC delivery in saline (Figure 5B). The delivery of hMSCs in combination with fibrin induced a stronger upregulation of anabolic markers (COL1, COL2, ACAN) than fibrin alone (baseline in Figure 5B). Furthermore, the differentiation of hMSCs was affected by the loading history of the discs whereby physiological discs induced a significantly higher expression of ACAN and CA12 in implanted hMSCs than the degenerative ones (Figure 5C).
Figure 5.
Anabolic gene expression of intervertebral discs (IVDs) treated with human mesenchymal stem cells (hMSCs) in fibrin or saline with a (A) physiological and (B) degenerative background. Data from NP, AFi, and AFo are combined. Data are expressed as 2−ddCt relative to IVDs receiving the carrier alone (fibrin or PBS) (n = 12); * indicates p < 0.05 relative to fibrin alone. (C) Expression of NP cell markers in hMSCs cultured for 1 week in discs with a physiological or degenerative background. Data are expressed as 2−ddCt relative hMSC-fibrin gel cultured in a well plate (n = 12). KRT19: cytokeratin-19, CA12: carbonic anhydrase 12. Note that IVD response to hMSCs was conditioned by both disc degenerative state and hMSC carrier. The effect of the regenerative treatment with hMSCs in fibrin was stronger on simulated-degenerative discs than on simulated-physiological discs (B vs. A). hMSCs in fibrin induced a superior anabolic response in degenerative IVDs compared with fibrin alone (*, B), suggesting an added value of the cellular therapy compared with an acellular solution (B). When comparing fibrin and saline as hMSC carrier, a significantly higher anabolic response was observed in IVDs treated with hMSCs in fibrin (A and B, COL2 and ACAN). The degenerative state of the disc influenced hMSC differentiation with a significantly higher expression of specific discogenic markers (ACAN and CA12) in hMSCs implanted into physiological discs than in those implanted into degenerative discs. AFi = inner annulus fibrosus; AFo = outer annulus fibrosus; NP = nucleus pulposus.
Discussion
Our hypothesis was that IVD response to hMSC treatment would be conditioned by: (1) the state of the disc and (2) the type of carrier used for hMSC administration. Our results confirmed these assumptions: hMSC influence on physiological discs was minimal, independent of carrier type (Figure 5A); in contrast, hMSC delivery was beneficial in degenerative discs when a fibrin carrier was used, whereas hMSC contribution was limited when cells were applied with a saline solution (Figure 5B). Our results confirm in vitro findings of Strassburg et al [36] who showed that hMSCs enhanced the expression of matrix-associated genes in degenerate NP cells but had no effect on healthy NP cells as well as in vivo findings by Ho et al [23] who illustrated in a small-animal model that hMSC regenerative effect was dependent on the severity of disc degeneration. By providing a physical environment close to human IVD (e.g., in terms of size and diffusion distances) [25], the whole organ culture bovine caudal model used in this study represents an important intermediate step for the translation of new therapies from bench to bedside. To the best of our knowledge, this is the first organ culture study that shows that disc response to stem cells is influenced by the state of the disc and hMSC carrier.
The choice of an appropriate carrier for stem cell delivery was found to be important. Our results suggest that the fibrin gels used in this study effectively filled the nucleotomised space, prevented disorganisation of the surrounding IVD tissue, supported stem cell viability (Figure 2), and allowed paracrine signalling between hMSCs and disc cells (Figure 5). Similar to the in vitro indirect co-culture study by Yang et al [19], we found in our organ cultures an upregulation of anabolic gene expression in degenerative discs following exposure to hMSCs. Moreover, appropriate carriers could avoid possible shortcomings associated with hMSC delivery with saline solutions as the latter have been associated with cell leakage and osteophyte formation [37]. The use of a carrier that is capable of withstanding dynamic loading, such as the fibrin gel used in this study (Figure 3), has two advantages: first, hMSCs can sense the dynamic loading, which has been shown to trigger hMSC chondrogenesis [7], and second, the exchange of paracrine signals between hMSCs and disc cells could be facilitated by dynamic loading conditions. Saline was an inefficient carrier for hMSCs, possibly due to less precise localisation of hMSCs in the IVD space and reduced sensitivity to loading. In this respect, IVD bioreactors provide a favourable setting for such studies.
Previous in vivo studies have shown a positive effect of stem cells on disc height preservation [38]. In our organ culture model, stem cells did not influence disc height (Figure 4); thus, longer cultures or an in vivo follow-up study would be required to assess this point. We found that saline solutions had an initial positive effect on the height of IVDs with a physiological background, which could be explained by a higher swelling potential than degenerative discs.
The response to hMSC treatment was stronger in degenerative discs. Conversely, MSCs showed a better differentiation potential towards IVD phenotype in physiological discs. Therefore, we may speculate that hMSCs delivered to healthy discs may differentiate towards the disc-like phenotype, whereas hMSCs delivered to early degenerative discs will release trophic factors that will stimulate IVD cells to regain a healthy phenotype. Indeed, recent in vivo work suggests that the inflammatory environment associated with early stages of degeneration may play a key role in promoting MSC paracrine effects [39]. The disc native environment has specific characteristics (hypoxic, avascular, low glucose, hydrostatic pressure) that have a strong impact on MSC behaviour (survival, proliferation, differentiation, matrix synthesis) as reviewed by Huang et al [40]. It has been shown that hypoxia can promote stem cell proliferation and differentiation towards the disc-like/ chondrogenic phenotype. However, the combination of hypoxia and serum deprivation has a negative impact on MSCs and can lead to cell death. It has been repeatedly shown that cyclic mechanical loading can stimulate hMSC chondrogenic differentiation through transforming growth factor-β signalling, but excessive load may be detrimental. The disc environment changes with degeneration (acidic pH, vasculature infiltration, loss of hydrostatic pressure, presence of inflammatory cytokines, and matrix proteinases). Studies with rat bone marrow MSCs have shown negative effects in vitro that were proportional to pH acidity (ranging from 7.4 to 6.8), but effects of pH on human MSCs in IVDs are still unknown. Vasculature infiltration may favour metabolic exchanges and thus play a positive role on hMSC survival. On the other hand, vasculature infiltration makes the discs accessible to the immune cells, which can further accelerate the degenerative cascade; however, the exact role of IVD vascularisation on exogenous MSC behaviour in human IVDs has not yet been clarified. It should be noted that from a cellular point of view, co-cultures of MSCs with degenerated IVD cells have shown both the ability of MSCs to differentiate towards the disc phenotype and the capacity of IVD cells to regain a healthier phenotype.
Limitations of the study are linked to the fact that only early stages of degeneration were investigated. Indeed, it is still an open challenge to reproduce ex vivo late stages of disc degeneration that are representative of the human condition. The chosen loading regime was relatively low, and further studies will be needed to assess MSC therapies in a more mechanically-challenging environment. Finally, the trans-endplate model may be less close to the clinical scenario than an approach through the AF. Nonetheless, since AF closure remains an open challenge, the trans-endplate approach [41] allows for the separate investigation of NP and AF repair.
The present study has several clinical implications. First, pilot clinical studies have suggested that stem cell injection (with saline as a carrier) contributes to reduce chronic back pain [42], [43]. Other studies have suggested a positive role of saline injections in reducing radicular pain [44]; however, a large clinical study has not been performed yet. Our results suggest that the chosen fibrin composition may be a better carrier than saline to induce regenerative changes in the disc tissue. Second, with organ cultures, it is possible to assess regenerative therapies not only at the macroscopic level but also at the molecular level. For instance, in addition to clinically-accessible parameters such as disc height (which can be clinically measured by magnetic resonance), it is possible to study changes in the expression of genes of both the donor stem cells and host disc cells (which to date cannot be investigated in the patients). Third, organ cultures can provide a useful screening tool to optimise stem cell carriers and help identify the most promising approaches for translation in in vivo models and later in humans. Towards this aim, modern imaging techniques can nowadays detect the degenerative grade with great precision and contribute to identify the patient groups that can best benefit from the MSC treatment.
In conclusion, we found that the response of large animal nucleotomised IVDs to hMSC treatment was conditioned by the IVD degenerative state and hMSC carrier. Administration of hMSCs with fibrin through the endplate stimulated the anabolic response of degenerative but not healthy discs. Saline solution proved to be an inefficient carrier for hMSCs in nucleotomised IVDs, highlighting the importance of a careful selection of the cell carrier. Whole organ culture bioreactors can provide an important investigational tool for the assessment and translation of stem cell-based therapies. Further studies will focus on hMSC carrier optimisation and development of whole organ culture models simulating different degeneration stages.
Conflicts of interest
The material contained in the manuscript has not been previously published and is not being concurrently submitted elsewhere. The authors have no conflicts of interest relevant to this article.
Acknowledgements
The authors gratefully acknowledge AO Spine International and North American Spine Society Research Grant for funding this study, and Nora Goudsouzian, Christoph Sprecher, and Robert Peter for excellent technical support. Luzia Douma was supported by an AO Foundation Fellowship in Health Sciences and Technology from ETH Zürich Foundation.
References
- 1.Sakai D., Nakamura Y., Nakai T., Mishima T., Kato S., Grad S. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai D., Andersson G.B. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–256. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- 3.Mochida J., Sakai D., Nakamura Y., Watanabe T., Yamamoto Y., Kato S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater. 2015;29:202–212. doi: 10.22203/ecm.v029a15. discussion 212. [DOI] [PubMed] [Google Scholar]
- 4.Vadala G., Russo F., Ambrosio L., Loppini M., Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8:185–201. doi: 10.4252/wjsc.v8.i5.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F., Leung V.Y., Luk K.D., Chan D., Cheung K.M. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther. 2009;17:1959–1966. doi: 10.1038/mt.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoyanov J.V., Gantenbein-Ritter B., Bertolo A., Aebli N., Baur M., Alini M. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–547. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- 7.Dai J., Wang H., Liu G., Xu Z., Li F., Fang H. Dynamic compression and co-culture with nucleus pulposus cells promotes proliferation and differentiation of adipose-derived mesenchymal stem cells. J Biomech. 2014;47:966–972. doi: 10.1016/j.jbiomech.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Y., Feng S., Liu W., Fu Q., Li Y., Li X. Preconditioning of mesenchymal stromal cells toward nucleus pulposus-like cells by microcryogels-based 3D cell culture and syringe-based pressure loading system. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33509. [DOI] [PubMed] [Google Scholar]
- 9.Strassburg S., Richardson S.M., Freemont A.J., Hoyland J.A. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5:701–711. doi: 10.2217/rme.10.59. [DOI] [PubMed] [Google Scholar]
- 10.Henriksson H.B., Svanvik T., Jonsson M., Hagman M., Horn M., Lindahl A. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009;34:141–148. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 11.Sakai D., Mochida J., Iwashina T., Watanabe T., Nakai T., Ando K. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 12.Le Maitre C.L., Baird P., Freemont A.J., Hoyland J.A. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res Ther. 2009;11:R20. doi: 10.1186/ar2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J., Deng G., Tian Y., Pu Y., Cao P., Yuan W. An in vitro investigation into the role of bone marrowderived mesenchymal stem cells in the control of disc degeneration. Mol Med Rep. 2015;12:5701–5708. doi: 10.3892/mmr.2015.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T., Sakai D., Yamamoto Y., Iwashina T., Serigano K., Tamura F. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28:623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y., Zhang X.J., Fang L., Zhao T.B. Co-culture of annulus fibrosus cells and bone marrow mesenchymal stem cells. Genet Mol Res. 2015;14:3932–3938. doi: 10.4238/2015.April.27.7. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z., Luo B., Liu Z.H., Samartzis D., Liu Z., Gao B. Adipose-derived stromal cells protect intervertebral disc cells in compression: implications for stem cell regenerative disc therapy. Int J Biol Sci. 2015;11:133–143. doi: 10.7150/ijbs.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allon A.A., Butcher K., Schneider R.A., Lotz J.C. Structured bilaminar coculture outperforms stem cells and disc cells in a simulated degenerate disc environment. Spine (Phila Pa 1976) 2012;37:813–818. doi: 10.1097/BRS.0b013e31823b055f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim E.K., Lee J.S., Kim D.E., Kim S.K., Jung B.J., Choi E.Y. Autogenous mesenchymal stem cells from the vertebral body enhance intervertebral disc regeneration by paracrine interaction: an in vitro pilot study. Cell Transplant. 2016;25:1819–1832. doi: 10.3727/096368916X691420. [DOI] [PubMed] [Google Scholar]
- 19.Yang S.H., Wu C.C., Shih T.T., Sun Y.H., Lin F.H. In vitro study on interaction between human nucleus pulposus cells and mesenchymal stem cells through paracrine stimulation. Spine (Phila Pa 1976) 2008;33:1951–1957. doi: 10.1097/BRS.0b013e31817e6974. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang A., Cerchiari A.E., Tang X., Liebenberg E., Alliston T., Gartner Z.J. Effects of cell type and configuration on anabolic and catabolic activity in 3D co-culture of mesenchymal stem cells and nucleus pulposus cells. J Orthop Res. 2017;35:61–73. doi: 10.1002/jor.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naqvi S.M., Buckley C.T. Bone marrow stem cells in response to intervertebral disc-like matrix acidity and oxygen concentration: implications for cell-based regenerative therapy. Spine (Phila Pa 1976) 2016;41:743–750. doi: 10.1097/BRS.0000000000001314. [DOI] [PubMed] [Google Scholar]
- 22.Wuertz K., Godburn K., Neidlinger-Wilke C., Urban J., Iatridis J.C. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa 1976) 2008;33:1843–1849. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho G., Leung V.Y., Cheung K.M., Chan D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res. 2008;49:15–21. doi: 10.1080/03008200701818595. [DOI] [PubMed] [Google Scholar]
- 24.Gantenbein B., Illien-Junger S., Chan S.C., Walser J., Haglund L., Ferguson S.J. Organ culture bioreactors—platforms to study human intervertebral disc degeneration and regenerative therapy. Curr Stem Cell Res Ther. 2015;10:339–352. doi: 10.2174/1574888x10666150312102948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alini M., Eisenstein S.M., Ito K., Little C., Kettler A.A., Masuda K. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illien-Junger S., Gantenbein-Ritter B., Grad S., Lezuo P., Ferguson S.J., Alini M. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 2010;35:1744–1752. doi: 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]
- 27.Mwale F., Wang H.T., Roughley P., Antoniou J., Haglund L. Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng Part A. 2014;20:2942–2949. doi: 10.1089/ten.tea.2013.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z., Lezuo P., Pattappa G., Collin E., Alini M., Grad S. Development of an ex vivo cavity model to study repair strategies in loaded intervertebral discs. Eur Spine J. 2016;25:2898–2908. doi: 10.1007/s00586-016-4542-0. [DOI] [PubMed] [Google Scholar]
- 29.Vadala G., De Strobel F., Bernardini M., Denaro L., D'Avella D., Denaro V. The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J. 2013;22:S972–S978. doi: 10.1007/s00586-013-3007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelaez D., Huang C.Y., Cheung H.S. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009;18:93–102. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 31.Kupcsik L., Alini M., Stoddart M.J. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2309–2313. doi: 10.1089/ten.tea.2008.0400. [DOI] [PubMed] [Google Scholar]
- 32.Peroglio M., Grad S., Mortisen D., Sprecher C.M., Illien-Jünger S., Alini M. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2012;21:S839–S849. doi: 10.1007/s00586-011-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vadala G., Russo F., Pattappa G., Peroglio M., Stadelmann V.A., Roughley P. A Nucleotomy model with intact annulus fibrosus to test intervertebral disc regeneration strategies. Tissue Eng Part C Methods. 2015;21:1117–1124. doi: 10.1089/ten.TEC.2015.0086. [DOI] [PubMed] [Google Scholar]
- 34.Peroglio M., Eglin D., Benneker L.M., Alini M., Grad S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013;13:1627–1639. doi: 10.1016/j.spinee.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Risbud M.V., Schoepflin Z.R., Mwale F., Kandel R.A., Grad S., Iatridis J.C. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–293. doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strassburg S., Hodson N.W., Hill P.I., Richardson S.M., Hoyland J.A. Bi-directional exchange of membrane components occurs during co-culture of mesenchymal stem cells and nucleus pulposus cells. PLoS One. 2012;7:e33739. doi: 10.1371/journal.pone.0033739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vadala G., Sowa G., Hubert M., Gilbertson L.G., Denaro V., Kang J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–355. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 38.Sakai D., Mochida J., Iwashina T., Hiyama A., Omi H., Imai M. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 39.Maidhof R., Rafiuddin A., Chowdhury F., Jacobsen T., Chahine N.O. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res. 2016 doi: 10.1002/jor.23350. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y.C., Leung V.Y., Lu W.W., Luk K.D. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J. 2013;13:352–362. doi: 10.1016/j.spinee.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Vadala G., Russo F., Pattappa G., Schiuma D., Peroglio M., Benneker L.M. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976) 2013;38:E319–E324. doi: 10.1097/BRS.0b013e318285bc4a. [DOI] [PubMed] [Google Scholar]
- 42.Orozco L., Soler R., Morera C., Alberca M., Sanchez A., Garcia-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822–828. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 43.Pettine K.A., Murphy M.B., Suzuki R.K., Sand T.T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33:146–156. doi: 10.1002/stem.1845. [DOI] [PubMed] [Google Scholar]
- 44.Fukui S., Iwashita N., Nitta K., Tomie H., Nosaka S. The results of percutaneous intradiscal high-pressure injection of saline in patients with extruded lumbar herniated disc: comparison with microendoscopic discectomy. Pain Med. 2012;13:762–768. doi: 10.1111/j.1526-4637.2012.01400.x. [DOI] [PubMed] [Google Scholar]