Summary
Bone metabolism is tightly regulated by the immune system. Accelerated bone destruction is observed in many bone diseases, such as rheumatoid arthritis, fracture, and particle-induced osteolysis. These pathological conditions are associated with inflammatory responses, suggesting the contribution of inflammation to bone destruction. Macrophages are heterogeneous immune cells and are polarized into the proinflammatory M1 and antiinflammatory M2 phenotypes in different microenvironments. The cytokines produced by macrophages depend on the macrophage activation and polarization. Macrophages and macrophage-derived cytokines are important to bone loss in inflammatory bone disease. Recent studies have shown that macrophages can be detected in bone tissue and interact with bone cells. The interplay between macrophages and bone cells is critical to bone formation and repair. In this article, we focus on the role of macrophages in inflammatory bone diseases, as well as discuss the latest studies about macrophages and bone formation, which will provide new insights into the therapeutic strategy for bone disease.
Keywords: bone metabolism, inflammation, macrophage
The translational potential of this article.
Bone homeostasis is closely relevant to the immune system, which has raised an important field termed osteoimmunology. As one of the first lines of defence of the innate immune response, macrophages play a crucial role in bone integrity in physiological conditions and bone turnover in pathological conditions. During inflammation, macrophages are activated and produce a great amount of cytokines to affect bone formation and bone resorption. In light of this, a better understanding of the mechanisms by which macrophages regulate bone metabolism is essential for targeting macrophages as a therapeutic strategy in inflammatory bone diseases.
Introduction
Bone is a major component that makes up the skeleton of vertebrates. It is the strongest tissue in the body, and supports the body, protects the organs, and stores minerals. Apart from its structural function, bone also possesses metabolic functions. Bone metabolism is a lifetime process which is necessary to preserve structural integrity and maintain mineral homeostasis. This process involves osteoblastic bone formation and osteolytic bone resorption [1]. Bone formation refers to the building of new bone material by osteoblasts. Bone resorption is the process of breaking down bone and releasing the minerals by osteoclasts. The balance between bone formation and bone resorption is tightly controlled by osteocytes, immune cells, and the endocrine system.
Osteocytes, derived from osteoblasts, are the most common cells in bone. They are distributed in the mineralized bone matrix or on the surface of bone, and form an interconnected network with osteoblasts, osteoclasts, and the bone marrow. As a result, osteocytes possess the most potent ability to regulate bone metabolism through direct cell–cell contacts and the release of soluble molecules [2]. Receptor activator of nuclear factor kappa-β ligand (RANKL) is the key regulator for osteoclast differentiation and bone resorption. Osteocytes express higher amounts of RANKL than osteoclasts in vitro [3]. An increasing number of studies have shown that osteocytes can promote osteoclastogenesis and bone resorption through the production of RANKL. Sclerostin, an inhibitor of bone formation, is mainly produced by osteocytes in the bone [2]. Parathyroid hormone or mechanical stimuli can reduce the sclerostin production of osteocytes, leading to increased bone formation. In addition to RANKL and sclerostin, osteocytes also produce other molecules, including nitric oxide, transforming growth factor (TGF), and macrophage chemotactic factor-1, which help to regulate bone metabolism. These findings suggest that osteocytes are the central regulators of bone homeostasis [4].
It is well known that there is a close interrelationship between bone metabolism and the immune system. Bone loss can be seen in some inflammatory diseases, including rheumatoid arthritis (RA) and periodontitis. Osteoimmunology was developed to investigate the impact of the immune system on bone metabolism under physiological and pathological conditions. An increasing number of studies have suggested that osteoimmunology includes the interactions between osteoblasts and osteoclasts, lymphocytes and osteoclasts, and osteoblasts and hematopoietic cells. T cell-deficient mice are osteoporotic and show reduced osteoprotegerin (OPG) production [5]. Depletion of B cells results in bone loss in rats [6]. In addition to immune cells and bone cells, cytokines are important to regulate bone metabolism. Activated T cells can reduce bone resorption and stimulate bone formation through the production of interferon-γ (IFN-γ) or interleukin (IL)-17 [7], [8]. RANKL plays an important role in osteoimmunology. Under physiological conditions, RANKL is necessary for lymph node development. During an inflammatory response, activated T and B lymphocytes can release RANKL, which binds to the receptor activator of nuclear factor kappa-β (RANK) receptor on osteoclast precursors and induces osteoclastogenesis [9]. OPG can inhibit the RANK-RANKL interaction. The increased ratio of RANKL/OPG always indicates osteoclast differentiation and bone resorption [10].
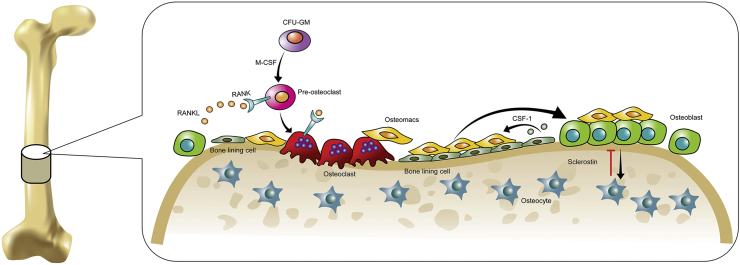
In addition to T and B cells, the role of macrophages in osteoimmunology has received intensive attention. Macrophages are innate immune cells that are responsible for immune surveillance and pathogen removal. Accumulative studies have suggested that macrophages are crucial for bone metabolism and bone tissue engineering. Osteoclasts are derived from the monocyte-macrophage lineage and have been identified as the resident macrophages of the bone (Figure 1). Macrophages can induce matrix mineralization in vitro and induce osteoblast differentiation in vivo [11], [12]. Depletion of macrophages reduces the number of osteoblasts in vivo.
Figure 1.
Macrophages and bone cells in the bone. Osteoclasts are differentiated from colony forming unit-granulocyte macrophage (CFU-GM) precursors in the presence of RANKL and M-CSF. Osteomacs are found in proximity to the bone lining cells, osteoblasts, and osteoclasts. Osteomacs can mediate the proliferation and differentiation of osteoblasts. Osteoblasts can transform into osteocytes, which inhibit bone formation through the production of sclerostin. CSF-1 = colony-stimulating factor 1; M-CSF = macrophage colony-stimulating factor; RANK = receptor activator of nuclear factor kappa-β; RANKL = receptor activator of nuclear factor kappa-β ligand.
The present work will focus on how macrophages interact with bone cells and elucidate how macrophage polarization affects bone formation. Moreover, we will discuss the role of macrophages in inflammatory diseases of the bone.
Macrophages and bone formation
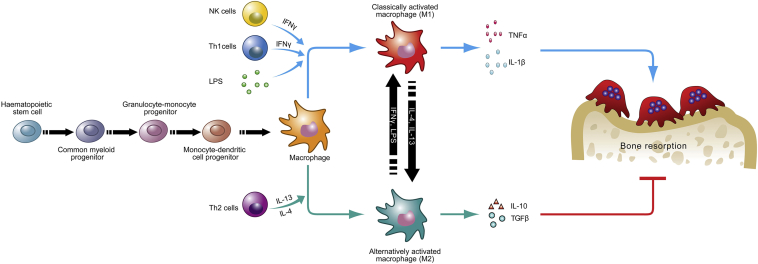
Macrophages are a highly heterogeneous population derived from the myeloid cell lineage. As essential effectors of the innate immune system, macrophages play a critical role in host defence and inflammation. Macrophages can be divided into resident and inflammatory macrophages [13]. Resident macrophages can be found in nearly all tissues, and they participate in tissue repair, immune surveillance, and homeostatic maintenance [14], [15]. Inflammatory macrophages derive from monocytes and traffic via the bloodstream to inflammatory sites. In response to microenvironmental stimuli, macrophages (both resident and inflammatory macrophages) can be activated and acquire distinct functional abilities: proinflammatory M1 (classically activated macrophages) and antiinflammatory M2 (alternatively activated macrophages) (Figure 2) [16]. M1 macrophages have proinflammatory functions and participate in the host defence against pathogens [17]. When activated by IFN-γ, granulocyte macrophage colony-stimulating factor, or other toll-like receptor (TLR) ligands, M1 macrophages can produce proinflammatory cytokines, such as IL-1β, IL-12, tumour necrosis factor-α (TNF-α), and superoxide anions, and induce a Th1 immune response [18]. M2 macrophages contribute to tissue repair and resolution of inflammation. IL-4 and IL-13 can induce M2 macrophages. After activation, M2 macrophages can produce IL-10, IL-1 receptor type α, and TGF-β, which ensue the activation of the Th2 immune response and antiinflammatory functions [19]. Under normal conditions, most macrophages display an M2 phenotype, which helps to maintain tissue homeostasis [20]. In the early stage of inflammation, macrophages are activated and polarized to an M1 phenotype. These M1 macrophages produce nitric oxide and proinflammatory cytokines, which can lead to tissue damage. During the resolution of inflammation, macrophages are predominantly polarized to an M2 phenotype, which can suppress proinflammatory cytokine production, clear debris, and restore tissue homeostasis [21].
Figure 2.
The polarization of macrophages can affect bone destruction. Macrophages can be activated and polarized into the proinflammatory M1 and antiinflammatory M2 phenotypes. M1 macrophages can secrete TNFα and IL-1β, inducing bone resorption. M2 macrophages can secrete IL-10 and TGFβ, inhibiting bone resorption. IFN = interferon; IL = interleukin; LPS = lipopolysaccharide; NK = natural killer; TGFβ = transforming growth factor β; TNFα = tumour necrosis factor α.
A mountain of evidence has suggested that both resident and inflammatory macrophages can influence bone formation. Osteoclasts have been traditionally viewed as the resident macrophages in the bone. In recent years, a large population of bone-resident macrophages has been identified in the periosteal and endosteal tissues. These macrophages are termed osteomacs, and comprise about one-sixth of all cells in the bone marrow. Interestingly, osteomacs are closely adjacent to osteoblasts in the endosteal surface of bone, suggesting that osteomacs may provide proanabolic support to osteoblasts and promote bone formation [22]. The positive role of osteomacs on bone formation has been demonstrated in an in vitro culture system. Chang et al [11] reported that the mineralization of osteoblasts was decreased 23-fold when osteomacs were removed from primary calvarial osteoblast culture. On the contrary, the addition of osteomacs to purified osteoblasts can increase mineralization. Osteomacs can also maintain the maturation of osteoblasts in vivo. Depletion of osteomacs inhibits osteoblast maturation and reduces bone formation. It has been suggested that osteomacs can detect and respond to bone damage by promoting type I collagen production and bone mineralization in a tibial injury model [23], [24]. Moreover, using a MAFIA mouse model, Cho et al [25] reported that parathyroid hormone treatment can augment osteomacs in bone. Depletion of early lineage macrophages causes osteopenia, while depletion of differentiated macrophages can increase osteogenic differentiation and parathyroid hormone anabolism.
Like resident macrophages, inflammatory macrophages also possess the ability to influence bone formation. Classically activated M1 macrophages can produce oncostatin M, which promotes osteogenesis and the mineralization of mesenchymal stem cells (MSCs) in vitro [26]. However, Fernandes et al [27] reported that conditioned medium from alternatively activated M2 macrophages can promote the osteoblastic differentiation of MSCs in an oncostatin M-dependent manner. The study from Horwood [28] supported the notion that alternatively activated macrophages remain the potent inducers of osteoblast formation. In recent years, the roles of inflammatory macrophages in bone formation have been widely investigated in disease states. Using a mouse osteonecrosis model, Wu et al [29] found that a large number of M1 macrophages was observed in the early stage of osteonecrosis. Predominantly M2 macrophages were identified in the late stage, accompanied by appositional new bone formation. Bone tissue engineering is an ideal field to investigate the role of inflammatory macrophages on bone formation. Macrophages mediate both bone fracture healing and the inflammatory response to implanted biomaterials [30]. Biomaterial implants induce the polarization of M1 macrophages, which leads to an inflammatory foreign body reaction, granuloma formation, fibrous encapsulation, and, finally, biomaterial integration failure [31]. On the contrary, M2 macrophages play a crucial role in reducing fibrous encapsulation, promoting vascularization, and improving biomaterial integration. Cobalt incorporated with β-tricalcium phosphate can promote macrophage polarization towards an M1 phenotype, which is associated with bone destruction and fibrous encapsulation [32]. Many “immuno-informed” biomaterials have been designed to induce the M2 polarization of macrophages to fulfil a regeneration function [33]. High surface wettability materials can promote macrophage polarization towards an M2 phenotype, which can increase osteointegration [34]. Nanostructured titanium surfaces and surface bioactive ion chemistry can markedly increase M2 macrophages, which results in increased osteogenesis [35]. It has been reported that M2 macrophages can promote the osteoblast differentiation of MSCs, as evidenced by increased alkaline phosphatase and bone mineralization [36]. β-tricalcium phosphate-stimulated macrophages can promote osteoblast differentiation of bone marrow MSCs [37]. All of these results suggest that macrophages have crucial effects on bone formation during bone inflammation.
Macrophages and fracture healing
Bone fracture is a common medical condition resulting from disease, trauma, or age-related bone fragility. In most cases, fracture healing is a complex process known as indirect (secondary) fracture healing, involving inflammation, callus formation, and remodelling [38]. Macrophages have been reported to participate in the inflammatory stage of fracture healing [39]. During the early stage of fracture healing, hematoma formation and inflammation trigger the healing process. The infiltration of inflammatory cells to the fracture site is a prerequisite for fracture healing. Neutrophils are the first inflammatory cells to arrive at the injury site. They can produce proinflammatory cytokines which help to recruit macrophages to the injury site in the early stage of inflammation. C-C chemokine receptor 2-deficient mice displayed reduced macrophage recruitment and delayed fracture healing [40]. Macrophages can clear dead cells and produce a series of cytokines, including TNF-α, IL-1β, IL-6, and C-C chemokine ligand 2. TNF-α is involved in cell recruitment and secondary inflammatory signalling induction. IL-1 produced by macrophages can promote angiogenesis, induce the production of IL-6, and promote the formation of primary cartilaginous calluses [41]. IL-6 can recruit MSCs to the injury site and stimulate angiogenesis and osteoblast differentiation [42]. Recent studies have shown that macrophages are involved in the entire process of fracture healing. Resident macrophages (osteomacs) are predominately in the maturing hard callus. Both inflammatory and resident macrophages can increase anabolic mechanisms during endochondral callus formation. Proinflammatory cytokines secreted by macrophages can regulate both osteoblast and osteoclast differentiation. Macrophages can enhance osteogenesis through the production of cytokines such as bone morphogenetic protein 2, bone morphogenetic protein 4, and TGF-β1 [43]. Oncostatin is a cytokine of the IL-6 family. Activated macrophages can secrete oncostatin and induce the osteogenic differentiation of MSCs in vitro. In the initial inflammatory stage, macrophages produce a large amount of oncostatin M, which serves to promote intramembranous bone healing. Depletion of macrophages results in the reduction of oncostatin M, collagen type 1, and bone mineralization [44]. In a tibial fracture mouse model, the osteogenic differentiation of MSCs was also reduced after depletion of macrophages, followed by reduced callus formation and bone deposition. Interestingly, IFN-γ secreted by macrophages can suppress osteoclastogenesis through the degradation of TNF receptor associated factor 6.
Macrophages and rheumatoid arthritis
RA is a chronic inflammatory disease characterized by joint inflammation and subsequent cartilage destruction and bone erosion. The typical symptoms of RA are synovial hypertrophy and inflammation caused by the accumulation of fibroblasts, lymphocytes, neutrophils, and monocytes/macrophages. Among these cells, activated macrophages are the main source of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, C-X-C motif chemokine ligand 4 (CXCL4), and CXCL7. These cytokines can activate endothelial cells, induce synovial inflammation, increase osteoclastogenesis, and, finally, lead to joint damage [45], [46]. It is well accepted that macrophages play an important role in the pathogenesis of RA. An increased number of sublining macrophages in the synovium has been accepted as an early biomarker of RA [47]. Moreover, the degree of joint destruction is correlated with the accumulation of CD68+ sublining macrophages [48]. Many factors have been reported to regulate the polarization of macrophages in the pathogenesis of RA. The concentrations of oestrogens are increased in the synovial fluid of RA patients. Oestrogens can increase the proliferation and M1 polarization of macrophages, suggesting that oestrogens can promote RA through regulating macrophage polarization [49], [50]. A recent study shows that succinate receptor 1+ macrophages can secrete succinate into the synovial fluid. These fluids can induce the production of IL-1β from macrophages, which promotes the pathogenesis of RA [51].
Recent studies have shown that the interaction between macrophages and T cells may contribute to the pathogenesis of RA [52], [53]. T cells are thought to be another contributor to RA. Macrophages possess the ability to regulate T cell migration and polarization. Synovial effector memory T cells express C-X-C chemokine receptor 6. Synovial macrophages highly express CXCL16, which can promote the recruitment of C-X-C chemokine receptor 6+ T cells to the rheumatoid joints [52]. After that, macrophages can secrete cytokines, including IL-2, IL-1β, IL-6, IL-12, and IL-23. IL-2 can induce Th1 polarization, while IL-1β, IL-6, and IL-23 can induce Th17 polarization [53]. Th1 and Th17 cells accumulate in the tissue and fluid of the joints in patients with RA, and participate in the pathogenesis and development of RA [54].
The differentiation of macrophages into osteoclasts contributes to bone erosion. Proinflammatory cytokines, including TNF-α, IL-1, IL-6, and IL-17, can induce the expression of RANKL in synovial fibroblasts. RANKL then promotes the osteoclastic differentiation of macrophages. It has been suggested that macrophages can induce bone erosion and damage in the pathogenesis of RA.
Macrophages and osteoarthritis
Osteoarthritis (OA) is a common joint disease characterized by cartilage breakdown, synovial fibrosis, and osteophyte formation. It has long been known that OA is a noninflammatory arthritis and mechanical factors play an important role in the initiation of OA. However, increasing studies have demonstrated that synovial inflammation can be found in both early and late stages in most OA patients, and is recognized as a characteristic of OA [55]. Abundant proinflammatory cytokines, including TNF-α, matrix metalloproteinase-3, IL-1β, and IL-29, have been found in the synovium of patients with OA. These cytokines are responsible for inflammation induction and cartilage degradation in OA [56].
The accumulation of macrophages in the synovial lining can be recognized as the main morphological characteristic of synovitis [57]. Macrophages are the main source of TNF-α and IL-1β. The number of macrophages is increased in the synovial tissue in small animal models of OA [58]. Increased macrophages are also found in the synovium and subchondral bone of OA patients, and are associated with joint severity [59]. These macrophages can be identified by cell surface markers, including CD163, CD68, CD14, and F4/80. When the knees of patients with OA were examined, it was determined that an increase in CD14 and CD163 indicates inflammatory phenotypes, OA severity, and progression risk [60]. When macrophages were depleted, synovial cells did not produce significant amounts of macrophage-derived cytokines, such as TNF-α and IL-1β [61]. Moreover, synovial fibroblast-derived IL-6, IL-8, and matrix metalloproteinase-1 were also reduced after macrophage depletion. Thus, macrophages not only mediate synovial inflammation directly, but also promote inflammation through activating synovial fibroblasts.
The polarization of macrophages also affects the progression of OA. M1 cytokines, including IL-1α, IL-1β, and TNF-α, are increased in the synovium of OA patients. By contrast, the M2 cytokine IL-1 receptor type α is reduced [62]. The latest in vitro study has shown that M1 macrophages are responsible for inflammation and the degeneration of cartilage in OA. However, M2 macrophages cannot suppress the inflammatory and degenerative effects of M1 macrophages [63].
Macrophages and peri-implant osteolysis
Peri-implant osteolysis is the most common complication after total joint arthroplasty. It has been commonly thought that peri-implant osteolysis is a chronic inflammatory response to wear particles [64]. Wear particles are biologically active and can initiate an innate inflammatory reaction, leading to bone osteolysis and implant loosening [65]. Macrophages and wear particles are abundant in the granulomatous periprosthetic membrane. Wear particles can be recognized and phagocytosed by macrophages, which triggers the proliferation, differentiation, and activation of macrophages [66]. Adsorbed proteins and receptors on the cell surface are involved in the interaction between macrophages and wear particles. Wear particle-mediated macrophage activation is partially induced by TLRs [67]. TLRs, including TLR2, TLR4, TLR5, and TLR9, are increased on macrophages in periprosthetic tissues. Integrins such as macrophage-1 antigen can modulate macrophage adhesion and activation and the phagocytosis of wear particles [68]. Macrophage-1 antigen and arginine-glycine-aspartate-binding integrins are critical to macrophage-induced inflammatory responses to ultra high molecular weight polyethylene wear debris [69].
A series of proinflammatory factors, including IL-1, IL-6, TNF-α, and osteopontin, can be produced by wear particle-activated macrophages. These cytokines can induce the expression of RANKL, which activates osteoclasts and leads to bone resorption and osteolysis. Wear particles can also stimulate macrophages to produce chemokines, such as macrophage inflammatory protein-1 and monocyte chemotactic protein-1. These chemokines can recruit peripheral macrophages, which promote osteoclastogenesis and bone resorption [70].
Recent studies have shown that the response of macrophages to wear particles is dependent on the state of macrophage polarization [71], [72]. Titanium particles can induce the M1 polarization of macrophages, which leads to the production of IFN-γ [72]. Contrarily, the M2 polarization of macrophages can ameliorate debris-induced osteolysis [73].
Macrophages as therapeutic targets in inflammatory bone diseases
As they are responsible for driving inflammatory and destructive damage in bones, macrophages appear to be promising therapeutic targets in inflammatory bone diseases. Sirtuin 1 can inhibit synovial inflammation in RA through reducing the number of macrophages [74]. IL-1 receptor type 2 can inhibit the action of IL-1 on macrophages, leading to the alleviation of collagen-induced arthritis [75]. Recently, Shin et al [76] found that human stem cells can polarize macrophages towards an M2 phenotype and alleviate RA. Local IL-4 or FTY720 (agonist for sphingosine 1-phosphate receptors) delivery can also polarize macrophages towards an M2 phenotype, which helps to reduce wear particle-induced osteolysis and enhance bone regeneration in cranial defects [77]. As a macrophage-derived proinflammatory cytokine, TNF-α is an ideal therapeutic target in arthritis. Many anti-TNF-α drugs (infliximab, adalimumab, certolizumab, and golimumab) are highly effective in RA [78]. Recently, adalimumab and infliximab have been reported to alleviate knee pain, synovitis, and bone marrow oedema in OA [79], [80].
Conclusion and future perspectives
Bone and the immune system are closely linked both in physiological and pathological conditions. Inflammation is responsible for bone loss in many clinical diseases. As an important population of immune cells, macrophages play a critical role in bone formation and destruction. During inflammation, macrophages (both resident and inflammatory macrophages) are activated and produce a large amount of cytokines. These cytokines can promote osteoblast or osteoclast differentiation, ultimately affecting bone formation. Harnessing macrophage-mediated inflammation is a promising strategy for bone regeneration. The correct understanding of the mechanisms by which macrophages regulate bone metabolism is essential for identifying useful therapeutic targets in inflammatory bone diseases.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding/support
We acknowledge the support from the National Natural Science Foundation of China (81572131, 81301341), Natural Science Foundation of Jiangsu Province (BK20151210).
References
- 1.Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien C.A., Nakashima T., Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 4.Delgado-Calle J., Bellido T., Roodman G.D. Role of osteocytes in multiple myeloma bone disease. Curr Opin Support Palliat Care. 2014;8:407–413. doi: 10.1097/SPC.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Toraldo G., Li A., Yang X., Zhang H., Qian W.P. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klausen B., Hougen H.P., Fiehn N.E. Increased periodontal bone loss in temporarily B lymphocyte-deficient rats. J Periodontal Res. 1989;24:384–390. doi: 10.1111/j.1600-0765.1989.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 7.Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 8.Croes M., Oner F.C., van Neerven D., Sabir E., Kruyt M.C., Blokhuis T.J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 9.El-Jawhari J.J., Jones E., Giannoudis P.V. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury. 2016;47:2399–2406. doi: 10.1016/j.injury.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 10.van Tuyl L.H., Voskuyl A.E., Boers M., Geusens P., Landewe R.B., Dijkmans B.A. Baseline RANKL: OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1623–1628. doi: 10.1136/ard.2009.121764. [DOI] [PubMed] [Google Scholar]
- 11.Chang M.K., Raggatt L.J., Alexander K.A., Kuliwaba J.S., Fazzalari N.L., Schroder K. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 12.Vi L., Baht G.S., Whetstone H., Ng A., Wei Q., Poon R. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30:1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 13.Raggatt L.J., Wullschleger M.E., Alexander K.A., Wu A.C., Millard S.M., Kaur S. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184:3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S., Pluddemann A., Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X.Q., Dai Y., Yang Y., Huang C., Meng X.M., Wu B.M. Emerging role of microRNAs in regulating macrophage activation and polarization in immune response and inflammation. Immunology. 2016;148:237–248. doi: 10.1111/imm.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan H.Y., Wang N., Li S., Hong M., Wang X., Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016:2795090. doi: 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 20.Dey A., Allen J., Hankey-Giblin P.A. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2015;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills C.D., Thomas A.C., Lenz L.L., Munder M. Macrophage: SHIP of immunity. Front Immunol. 2014;5:620. doi: 10.3389/fimmu.2014.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hume D.A., Loutit J.F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- 23.Jilka R.L., Weinstein R.S., Parfitt A.M., Manolagas S.C. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res. 2007;22:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 24.Alexander K.A., Chang M.K., Maylin E.R., Kohler T., Muller R., Wu A.C. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 25.Cho S.W., Soki F.N., Koh A.J., Eber M.R., Entezami P., Park S.I. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A. 2014;111:1545–1550. doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guihard P., Danger Y., Brounais B., David E., Brion R., Delecrin J. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes T.J., Hodge J.M., Singh P.P., Eeles D.G., Collier F.M., Holten I. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLoS One. 2013;8:e73266. doi: 10.1371/journal.pone.0073266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwood N.J. Macrophage polarization and bone formation: a review. Clin Rev Allergy Immunol. 2016;51:79–86. doi: 10.1007/s12016-015-8519-2. [DOI] [PubMed] [Google Scholar]
- 29.Wu X., Xu W., Feng X., He Y., Liu X., Gao Y. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int J Immunopathol Pharmacol. 2015;28:351–361. doi: 10.1177/0394632015593228. [DOI] [PubMed] [Google Scholar]
- 30.Ma Q.L., Zhao L.Z., Liu R.R., Jin B.Q., Song W., Wang Y. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials. 2014;35:9853–9867. doi: 10.1016/j.biomaterials.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Graney P.L., Roohani-Esfahani S.I., Zreiqat H., Spiller K.L. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. J R Soc Interface. 2016;13 doi: 10.1098/rsif.2016.0346. pii: 20160346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Yuen J., Crawford R., Chang J., Wu C., Xiao Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated beta-tricalcium phosphate. Biomaterials. 2015;61:126–138. doi: 10.1016/j.biomaterials.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 33.Sridharan R., Cameron A.R., Kelly D.J., Kearney C.J., O'Brien F.J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015;18:313–325. [Google Scholar]
- 34.Hotchkiss K.M., Reddy G.B., Hyzy S.L., Schwartz Z., Boyan B.D., Olivares-Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–434. doi: 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C.H., Kim Y.J., Jang J.H., Park J.W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology. 2016;27:085101. doi: 10.1088/0957-4484/27/8/085101. [DOI] [PubMed] [Google Scholar]
- 36.Gong L., Zhao Y., Zhang Y., Ruan Z. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016;46:65–71. [PubMed] [Google Scholar]
- 37.Chen Z., Wu C., Gu W., Klein T., Crawford R., Xiao Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35:1507–1518. doi: 10.1016/j.biomaterials.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 39.Oni O.O. The early stages of the repair of adult human diaphyseal fractures. Injury. 1997;28:521–525. doi: 10.1016/s0020-1383(97)00062-4. [DOI] [PubMed] [Google Scholar]
- 40.Xing Z., Lu C., Hu D., Yu Y.Y., Wang X., Colnot C. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S.K., Lorenzo J. Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol. 2006;18:411–418. doi: 10.1097/01.bor.0000231911.42666.78. [DOI] [PubMed] [Google Scholar]
- 42.Yang X., Ricciardi B.F., Hernandez-Soria A., Shi Y., Pleshko Camacho N., Bostrom M.P. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champagne C.M., Takebe J., Offenbacher S., Cooper L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- 44.Guihard P., Boutet M.A., Brounais-Le Royer B., Gamblin A.L., Amiaud J., Renaud A. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am J Pathol. 2015;185:765–775. doi: 10.1016/j.ajpath.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Dimitroulas T., Nikas S.N., Trontzas P., Kitas G.D. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev. 2013;12:958–966. doi: 10.1016/j.autrev.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Yeo L., Adlard N., Biehl M., Juarez M., Smallie T., Snow M. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis. 2016;75:763–771. doi: 10.1136/annrheumdis-2014-206921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wijbrandts C.A., Vergunst C.E., Haringman J.J., Gerlag D.M., Smeets T.J., Tak P.P. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56:3869–3871. doi: 10.1002/art.22964. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton J.A., Tak P.P. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 2009;60:1210–1221. doi: 10.1002/art.24505. [DOI] [PubMed] [Google Scholar]
- 49.Kou X.X., Li C.S., He D.Q., Wang X.D., Hao T., Meng Z. Estradiol promotes M1-like macrophage activation through cadherin-11 to aggravate temporomandibular joint inflammation in rats. J Immunol. 2015;194:2810–2818. doi: 10.4049/jimmunol.1303188. [DOI] [PubMed] [Google Scholar]
- 50.Cutolo M., Capellino S., Sulli A., Serioli B., Secchi M.E., Villaggio B. Estrogens and autoimmune diseases. Ann N Y Acad Sci. 2006;1089:538–547. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 51.Littlewood-Evans A., Sarret S., Apfel V., Loesle P., Dawson J., Zhang J. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213:1655–1662. doi: 10.1084/jem.20160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruth J.H., Haas C.S., Park C.C., Amin M.A., Martinez R.J., Haines G.K., 3rd CXCL16-mediated cell recruitment to rheumatoid arthritis synovial tissue and murine lymph nodes is dependent upon the MAPK pathway. Arthritis Rheum. 2006;54:765–778. doi: 10.1002/art.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egan P.J., van Nieuwenhuijze A., Campbell I.K., Wicks I.P. Promotion of the local differentiation of murine Th17 cells by synovial macrophages during acute inflammatory arthritis. Arthritis Rheum. 2008;58:3720–3729. doi: 10.1002/art.24075. [DOI] [PubMed] [Google Scholar]
- 54.Leipe J., Grunke M., Dechant C., Reindl C., Kerzendorf U., Schulze-Koops H. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 55.Pelletier J.P., Martel-Pelletier J., Abramson S.B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 56.Takano S., Uchida K., Miyagi M., Inoue G., Aikawa J., Fujimaki H. Synovial macrophage-derived IL-1beta regulates the calcitonin receptor in osteoarthritic mice. Clin Exp Immunol. 2016;183:143–149. doi: 10.1111/cei.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun A.R., Friis T., Sekar S., Crawford R., Xiao Y., Prasadam I. Is synovial macrophage activation the inflammatory link between obesity and osteoarthritis? Curr Rheumatol Rep. 2016;18:57. doi: 10.1007/s11926-016-0605-9. [DOI] [PubMed] [Google Scholar]
- 58.Piscaer T.M., Muller C., Mindt T.L., Lubberts E., Verhaar J.A., Krenning E.P. Imaging of activated macrophages in experimental osteoarthritis using folate-targeted animal single-photon-emission computed tomography/computed tomography. Arthritis Rheum. 2011;63:1898–1907. doi: 10.1002/art.30363. [DOI] [PubMed] [Google Scholar]
- 59.Geurts J., Patel A., Hirschmann M.T., Pagenstert G.I., Muller-Gerbl M., Valderrabano V. Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J Orthop Res. 2016;34:262–269. doi: 10.1002/jor.23009. [DOI] [PubMed] [Google Scholar]
- 60.Daghestani H.N., Pieper C.F., Kraus V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bondeson J., Wainwright S.D., Lauder S., Amos N., Hughes C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith M.D., Triantafillou S., Parker A., Youssef P.P., Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 63.Utomo L., Bastiaansen-Jenniskens Y.M., Verhaar J.A., van Osch G.J. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis Cartilage. 2016;24:2162–2170. doi: 10.1016/j.joca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Yang H., Xu Y., Zhu M., Gu Y., Zhang W., Shao H. Inhibition of titanium-particle-induced inflammatory osteolysis after local administration of dopamine and suppression of osteoclastogenesis via D2-like receptor signaling pathway. Biomaterials. 2016;80:1–10. doi: 10.1016/j.biomaterials.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 65.Shao H., Shen J., Wang M., Cui J., Wang Y., Zhu S. Icariin protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials. 2015;60:92–99. doi: 10.1016/j.biomaterials.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 66.Rao A.J., Gibon E., Ma T., Yao Z., Smith R.L., Goodman S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8:2815–2823. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenfield E.M. Do genetic susceptibility, Toll-like receptors, and pathogen-associated molecular patterns modulate the effects of wear? Clin Orthop Relat Res. 2014;472:3709–3717. doi: 10.1007/s11999-014-3786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakshit D.S., Lim J.T., Ly K., Ivashkiv L.B., Nestor B.J., Sculco T.P. Involvement of complement receptor 3 (CR3) and scavenger receptor in macrophage responses to wear debris. J Orthop Res. 2006;24:2036–2044. doi: 10.1002/jor.20275. [DOI] [PubMed] [Google Scholar]
- 69.Zaveri T.D., Dolgova N.V., Lewis J.S., Hamaker K., Clare-Salzler M.J., Keselowsky B.G. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials. 2017;115:128–140. doi: 10.1016/j.biomaterials.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bitar D., Parvizi J. Biological response to prosthetic debris. World J Orthop. 2015;6:172–189. doi: 10.5312/wjo.v6.i2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jamsen E., Kouri V.P., Ainola M., Goodman S.B., Nordstrom D.C., Eklund K.K. Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants. J Biomed Mater Res A. 2017;105:454–463. doi: 10.1002/jbm.a.35913. [DOI] [PubMed] [Google Scholar]
- 72.Pajarinen J., Kouri V.P., Jamsen E., Li T.F., Mandelin J., Konttinen Y.T. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013;9:9229–9240. doi: 10.1016/j.actbio.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 73.Rao A.J., Nich C., Dhulipala L.S., Gibon E., Valladares R., Zwingenberger S. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res A. 2013;101:1926–1934. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S.Y., Lee S.W., Kim H.Y., Lee S.Y., Lee W.S., Hong K.W. SIRT1 inhibits differentiation of monocytes to macrophages: amelioration of synovial inflammation in rheumatoid arthritis. J Mol Med (Berl) 2016;94:921–931. doi: 10.1007/s00109-016-1402-7. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu K., Nakajima A., Sudo K., Liu Y., Mizoroki A., Ikarashi T. IL-1 receptor type 2 suppresses collagen-induced arthritis by inhibiting IL-1 signal on macrophages. J Immunol. 2015;194:3156–3168. doi: 10.4049/jimmunol.1402155. [DOI] [PubMed] [Google Scholar]
- 76.Shin T.H., Kim H.S., Kang T.W., Lee B.C., Lee H.Y., Kim Y.J. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7:e2524. doi: 10.1038/cddis.2016.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Das A., Tanner S., Barker D.A., Green D., Botchwey E.A. Delivery of S1P receptor-targeted drugs via biodegradable polymer scaffolds enhances bone regeneration in a critical size cranial defect. J Biomed Mater Res A. 2014;102:1210–1218. doi: 10.1002/jbm.a.34779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atzeni F., Benucci M., Salli S., Bongiovanni S., Boccassini L., Sarzi-Puttini P. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev. 2013;12:575–579. doi: 10.1016/j.autrev.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 79.Grunke M., Schulze-Koops H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann Rheum Dis. 2006;65:555–556. doi: 10.1136/ard.2006.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Gaudio P.A. Infliximab therapy for 2 patients with Vogt-Koyanagi-Harada syndrome. Ocul Immunol Inflamm. 2008;16:167–171. doi: 10.1080/09273940802204527. [DOI] [PubMed] [Google Scholar]