Summary
Cell therapy continues to attract growing interest as a promising approach to treat a variety of diseases. Mesenchymal stem cells (MSCs) have been one of the most intensely studied candidates for cell therapy. Since the homing capacity of MSCs is an important determinant of effective MSC-based therapy, the enhancement of homing efficiency is essential for optimizing the therapeutic outcome. Furthermore, trafficking of endogenous MSCs to damaged tissues, also referred to as endogenic stem cell homing, and the subsequent participation of MSCs in tissue regeneration are considered to be a natural self-healing response. Therefore, strategies to stimulate and reinforce the mobilisation and homing of MSCs have become a key point in regenerative medicine. The current review focuses on advances in the mechanisms and factors governing trafficking of MSCs, and the relationship between MSC mobilisation and skeletal diseases, providing insights into strategies for their potential translational implications.
Keywords: cell therapy, homing, mesenchymal stem cells, skeletal diseases
Introduction
Mesenchymal stem cells (MSCs) have been a major research focus in regenerative medicine for several decades. MSC-based translational therapies hold great promise as a novel approach to cure a diverse range of diseases, such as neurological diseases [1], cardiovascular diseases [2], [3], wounds [4], [5], and various musculoskeletal diseases [6], [7], [8]. MSCs are multipotent stromal cells that are capable of differentiating into, and contributing to the regeneration of mesenchymal tissues such as bone, cartilage, fat, tendon, and muscle [9], [10]. MSCs express multiple cell surface antigens, such as CD90, CD105, CD73, and CD44, but lack expression of CD45, CD14, CD11b, CD79a, CD19, and HLA-DR [11], [12]. MSCs have been successfully isolated from various adult tissues, including bone marrow (BM) [11], adipose tissue [13], and peripheral blood (PB) [14]. MSCs possess powerful immunomodulatory properties and ability for tissue repair. In response to adverse stimuli (e.g., bacterial ligands) or injury, the inflammatory response is activated. MSCs sense these potentially damaging events via surface receptors (e.g., toll-like receptors and the inflammasome) and by alterations in local cytokine and chemokine levels, and then migrate locally and systemically to inflammatory sites. MSCs modulate both innate and adaptive immune responses; biological cues in the local microenvironment determine the activation state of MSCs to become immunosuppressive [15], [16]. MSCs not only provide a source of progenitors for cell replacement, but also activate or empower other local cells (such as tissue-resident progenitor or stem cells, endothelial cells, and fibroblasts) to facilitate tissue regeneration via paracrine stimulation [17].
The trafficking of endogenous MSCs to injured tissues, also defined as endogenous stem cell homing, and their subsequent participation in immunomodulation and tissue repair, are considered a natural self-healing response. To take full advantage of the intrinsic regenerative capacity of the body, strategies to stimulate and enhance the mobilisation of endogenous stem cells are of increasing interest. Furthermore, in order to enhance the therapeutic efficiency of exogenous systemically administered stem cells, a clear understanding of the biological concepts underlying stem cell homing is crucial.
It has long been proposed that the cellular and molecular signals of bone injury are highly consistent with embryonic skeletal growth processes, which involve the mobilisation and activation of MSCs. Both tissue-resident and circulating MSCs appear to take part in the processes of bone healing [18]. Immune signals, such as inflammatory mediators and immune cells, trigger the activation and mobilisation of MSCs [19]. Therefore, a better understanding of mechanisms regulating MSC mobilisation and homing may provide novel insights into strategies for successful bone repair. Here, we present a brief summary of the latest findings on the mechanisms and factors regulating MSC trafficking, and the close association between MSC homing and the treatment of musculoskeletal diseases. Our focus is to elucidate the critical role of mobilisation of MSCs in bone healing and provide insights into strategies to accelerate bone healing.
MSCs homing and bone healing
Musculoskeletal diseases remain among the most prevalent and challenging clinical problems, especially for the elderly population. Although simple fractures often heal effectively, the fracture healing process is impaired in 10–20% of fractures, causing nonunion and severe disability [20]. Furthermore, some fractures, such as hip fractures, are threatening injuries with mortality rates of 15–25% [21]. Angiogenesis and osteogenesis are coupled during embryonic skeletal development and bone repair processes, since blood vessels precede the onset of osteogenesis by transporting circulating cells, oxygen, nutrients, and osteogenic signals [22]. Thus, the stimulation of angiogenesis appears to be an important strategy for accelerating fracture healing [23]. Moreover, there is a dynamic homeostatic interplay between bone formation and bone resorption. An imbalance of bone remodelling such that bone formation is not able to compensate for ongoing bone resorption is one of the main mechanisms leading to many bone diseases, such as osteoporosis and nonunion of bone fractures [24], [25] (Figure 1).
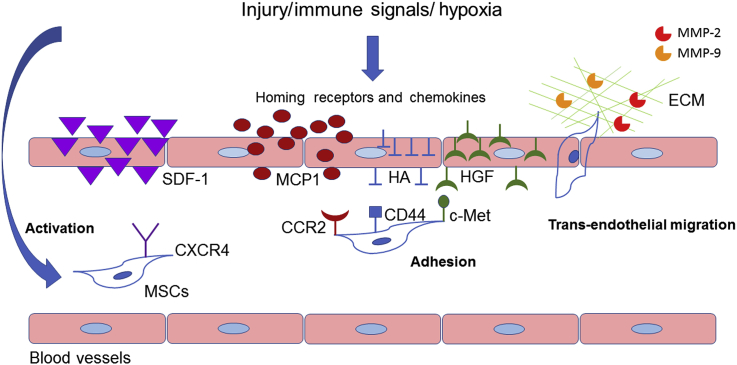
Figure 1.
Coordinated multistep process of mesenchymal stem cell (MSC) migration. The trafficking of MSCs is mainly composed of activation, adhesion, and transendothelial migration. In response to injury, immune signals, or hypoxia, homing receptors and chemokines are stimulated and released. Consequently, MSC activation occurs. Activated MSCs are mobilised into the peripheral blood circulation. The subsequent adhesion step is mainly achieved by the specific interaction between many chemokines and homing receptors, such as stromal cell-derived factor (SDF-1), CXC chemokine receptor (CXCR) 4, hepatocyte growth factor (HGF), c-Met, hyaluronic acid (HA), CD44, monocyte chemoattractant proteins (MCPs), and CC chemokine receptor (CCR)2 interaction. Matrix metalloproteinases (MMPs) contribute to MSC transendothelial migration via degradation of extracellular matrix (ECM).
Healing of fractures is a complex process involving the interplay of osteogenesis and angiogenesis. Natural repair of fractures comprises inflammatory, repair, and remodelling phases. The mobilisation and recruitment of circulating or resident stem cells, and systemically mobilised and recruited MSCs are involved in the fracture healing [19]. The recruitment of BM-MSCs to fracture sites is mainly mediated by the stromal cell-derived factor (SDF)1/CXC chemokine receptor (CXCR) 4 signalling axis [26]. Moreover, MSCs play critical roles in mediating the coupling of bone resorption and formation. In response to osteoclastic bone resorption, active transforming growth factor (TGF)-β released by the bone matrices induces migration and mobilisation of MSCs to the local site of repair, which is essential for coordinating bone remodelling [27]. In addition, transplanted MSCs have been found to stimulate angiogenesis, thereby leading to enhanced bone healing [28]. Conversely, impaired BM-MSCs mobilisation may lead to delayed osteoporotic fracture healing. As the numbers of BM-MSCs and PB-MSCs of ovariectomised mice are significantly lower than those of the mice with sham surgery at 12 hours, 24 hours, and 72 hours after fracture, ovariectomised mice have lower intrinsic capacity for bone regeneration [29]. Therefore, MSC homing augments bone healing mainly by regulating the bone remodelling and angiogenesis processes.
Potential indications of MSCs promoting bone healing
MSCs have been shown to enhance bone regeneration in several preclinical and clinical studies by differentiating directly into bone forming cells and modulating the biological environment by secreting growth factors and anti-inflammatory cytokines [30], [31]. Using MSCs expressing firefly luciferase, Granero-Molto et al [32] demonstrated that MSCs migrated (via the CXCR4 receptor) to the fracture site and improved healing by affecting the biomechanical properties and increasing the cartilage and bone content of the callus. MSCs are also a promising tool to treat critical size bone defects. One clinical study with 6–7-years follow up reported the successful treatment of defects of long bones [33]. Another large study for the treatment of nonunion with in vitro expanded autologous BM-MSCs is currently registered (https://www.clinicaltrials.gov/). While migration of MSCs towards a critical size bone defect is mainly directed by the SDF-1/CXCR4 axis, that there is a cumulative effect on stem cell migration to the defect site when SDF-1 is released combined with the growth factor bone morphogenetic protein-2 [30]. While the bone-regenerative potential of MSCs only recruited by SDF-1 is low, the combination of SDF-1 and bone morphogenetic protein-2 significantly improves bone regeneration. Therefore, it seems that migrated MSCs need an osteoinductive stimulus to significantly increase bone formation. Since MSC treatment increases vascularisation and bone formation, it is also a promising tool to treat osteonecrosis. In one study, core decompression of femoral heads with avascular necrosis was combined with MSC injection and bisphosphonate therapy [34]. There was a trend to lower risk for total joint replacement in the group with MSCs, however the results were not significant. Another large clinical multicentre study addressing this question is also currently registered (https://www.clinicaltrials.gov/). Wear-particle-related osteolysis is one of the main reasons for aseptic loosening of total joint replacements [35]. Wear-particle disease is characterised by a macrophage-driven inflammatory process that leads to bone destruction (osteolysis) [36]. Macrophages release the cytokine macrophage inflammatory protein-1α after having contact with wear particles. Macrophage inflammatory protein-1α is ligand of the chemokine CC receptor (CCR)1 of MSCs and recruits MSCs towards the site of wear-particle-related osteolysis [37]. Gibon et al [31] found that MSCs recruited by CCR1 due to wear particles reduce the osteolytic process and increase bone mineral density. Therefore, MSCs appear to differentiate into bone-forming cells as well as modulate the inflammatory process towards more regenerative conditions [38]. A systemic bone disease that concerns most of the geriatric patients is osteoporosis. Preclinical studies show that systemic administration of allogenic MSCs promotes osteoblastogenesis and prevents glucocorticoid-induced osteoporosis in rats [39].
MSCs trafficking
When potentially injurious situations occur, MSCs will be recruited and mobilised into damaged bone via local mechanisms and the peripheral circulation. The specific factors that lead to tissue-specific homing of MSCs are still under debate. MSC homing is defined as the arrest of MSCs within the vasculature of a tissue followed by transmigration across the endothelium. MSC migration appears to be a multistep process, which is mainly mediated by homing receptors, endothelial co-receptors, and chemotactic cytokines. Among these, SDF-1/ CXCR4 signalling axis has been demonstrated to be vital for MSC homing [40]. In addition, the monocyte chemoattractant proteins (MCPs) have also been demonstrated to regulate MSC migration. MCPs attract cells by activating their cognate receptor, CCR2, which is expressed on monocyte surfaces [41], [42]. Thus, the MCP/CCR2 pathway is also involved in recruiting MSCs to inflammatory sites [43]. Shinohara et al [44] used a parabiosis model with green fluorescent protein (GFP)+ MSCs [44]. MSCs were also engineered to express SDF-1 or MCP-3 or remained naïve. Parabiosis mice were allocated into five different groups. A fibular osteotomy was performed on the GFP− mouse 4 weeks after parabiosis and the homing of GFP+ MSCs investigated. Consistently, the authors found more GFP+ cells in SDF-1 and MCP-3 groups. Furthermore, in order to prove the contribution of recruited GFP+ MSCs to the fracture callus, the authors colocalised GFP expression and alkaline-phosphatase-positive (AP+) cells using immunohistochemistry. They showed that the fraction of AP+ and GFP+ was significantly higher in the callus of both the SDF-1 and MCP-3 groups. Using the same parabiosis model, Otsuru et al [45] found similar results. In addition, hepatocyte growth factor (HGF)/c-met signalling has also been found taking part in mobilising human MSCs [46]. Takai et al [47] first reported the expression of HGF and the cognate receptor c-met in human BM stromal cells, which is required for haematopoiesis. HGF is a multifunctional cytokine involved in many biological processes [48], [49], [50]. Studies have further demonstrated that HGF also functions as a strong chemotactic signal to mobilise and attract MSCs for tissue repair by interacting with c-met [46], [48].
There may exist various subpopulations of MSCs with varying homing capacities as MSCs are heterogeneous. A subset of MSCs strongly express active CXCR4, thus they possess higher homing capacity [51]. Moreover, freshly isolated MSCs have been shown to display enhanced homing ability compared to their culture-expanded counterparts [52], [53]. Homing receptors, such as CXCR4, which have been upregulated in the BM and in ischaemic tissues, are usually absent on the surface of culture-expanded MSCs [52], [53]. As MSCs have been proven to gain or lose certain surface markers during culture [54], which might influence their homing capability, the passage number of MSCs used for cellular therapy is an important determinant. Furthermore, MSCs treated with a cocktail of cytokines [HGF, stem cell factor, Flt-3 ligand, interleukin (IL)-3, and IL-6] in culture expressed higher levels of CXCR4 and possessed enhanced homing capacity [52]. Three-dimensional culture mimicking the in vivo niche may be an important research direction for maintaining the homing ability of MSCs during long-term ex vivo culture (e.g., culture in hydrogels under hypoxic conditions).
However, the signals that regulate stem cell mobilisation are often weakened or impaired because the function of SDF-1 is short lived. A recent study has demonstrated that low-intensity pulsed ultrasound promotes fracture healing by stimulating MSCs homing via upregulation of local and serum SDF-1 levels [55]. Prolongation of the expression of SDF-1 may be an important strategy for improving MSC homing and bone healing. Therefore, in pathological settings, particularly during the late phases of certain musculoskeletal diseases, restoration of the impaired SDF-1/CXCR4 signalling axis may be crucial for restoring and maintaining MSC homing capacity.
Inflammation and MSCs homing
Inflammation is a cellular response that occurs during tissue injury, which is characterised by increased vascular permeability, recruitment of inflammatory cells, release of inflammatory mediators, and turnover of matrices. It is generally believed that inflammation is an important regulator for bone regeneration, which initiates the repair cascade [56]. Following bone injury, initial inflammatory response occurs, macrophages infiltrate into sites of injury, which is vital for endochondral ossification [57]. After macrophage recruitment, lymphocytes (e.g., T lymphocytes) migrate into the fracture callus and initiate the adaptive immune response [58]. Concomitantly, large numbers of proinflammatory cytokines [such as IL-1β, IL-6, and tumour necrosis factor (TNF)-α] are released [17], [56], [59]. When MSCs sense the immune signals, they will be activated, mobilised, and recruited into inflammatory sites, thereby facilitating tissue regeneration.
Immune cells and MSCs may share common signalling pathways regulating cell migration. The recruitment of inflammatory cells and MSCs requires interactions of multiple adhesion molecules expressed on the migrating cells and their cognate ligands expressed on vascular endothelium. One of the most important adhesion molecules is monocyte chemoattractant protein (MCP)-1, which is produced predominantly by macrophages and endothelial cells. Increased expression level of MCP-1 stimulates macrophage infiltration [60]. MCP-1/CCR2 interaction also enhances MSCs adhesion and migration [43]. Moreover, CD44 appears to be another important adhesion molecule [61]. The CD44–hyaluronic acid (HA) interaction is crucial for activated T-cell extravasation into sites of inflammation. Furthermore, CD44–HA interaction also enhances MSC adhesion and motility. Platelet-derived growth factor facilitates MSC migration by elevating CD44 expression level [62].
Recent studies have suggested that immune signals have a direct influence on MSC migration. Some proinflammatory cytokines, such as interferon-γ and TNF-α, increased the production of matrix metalloproteinases (MMPs) in MSCs, thereby enhancing the capacity of MSCs to migrate through the extracellular matrix [63]. Preincubation with TNF-α has been shown to enhance the sensitivity and migration of MSCs toward chemokines. These chemokines include SDF-1, RANTES, and macrophage-derived chemokine [64]. Some anti-inflammatory mediators themselves are chemotactic cytokines that attract MSCs. For example, IL-6 secreted by active contractile muscle cells during short intensive exercise, which is associated with an anti-inflammatory response, stimulates migration and recruitment of MSCs [65], [66]. Therefore, the local and systemic inflammatory state may have an important role in triggering the migration and homing of MSCs.
Hypoxia and MSC homing
The local oxygen level is another important factor governing MSC mobilisation and migration. MSCs reside in a complex microenvironment or so-called niche in vivo. The components of the niche include local oxygen tension, extracellular matrices (ECMs), and other stromal cells [67], [68], [69]. Bone regeneration attempts to recapitulate the normal skeletal development during embryogenesis. In pathological situations such as fracture, blood supply is usually disrupted and hypoxic microenvironments occur. The hypoxia-inducible factors (HIFs) are key regulators of cellular adaptive response to hypoxia for adult and embryonic organisms, regulating the expression of numerous genes affecting cell survival and trafficking, angiogenesis, and cell metabolism in adverse conditions [70], [71], [72]. Both HIF-α and HIF-β subunits exist as isoforms. HIF-α subunits are regulated by a multistep process, including changes in activity, abundance, mitochondrial RNA splicing, and subcellular localisation. HIFs mainly consist of HIF-1, HIF-2, and HIF-3 [73]. HIF-1α levels are regulated by proteolysis through an oxygen-sensitive mechanism. Under normoxic conditions, HIF-1α undergoes prolyl hydroxylation and is ligated by von Hippel–Lindau protein, an E3 ubiquitin ligase, and degraded by the proteasome finally. Under hypoxia, HIF-1α prolyl hydroxylation and degradation is suppressed, and HIF-1α accumulates in the nucleus where it forms a dimer with the HIF-1β subunit. The dimer then forms a transcriptional complex with coactivator p300, regulating the expression of > 60 downstream target genes, including VEGF, SDF-1, and CXCR4 [71], [72], [74], [75], [76], [77]. The HIF pathway plays important roles in skeletal development. The HIF-1α pathway is also activated during the process of bone repair, which is required for angiogenesis and bone healing [78]. Therefore, the HIF/vascular endothelial growth factor signalling pathway may be another important therapeutic target for successful bone healing (Figure 2).
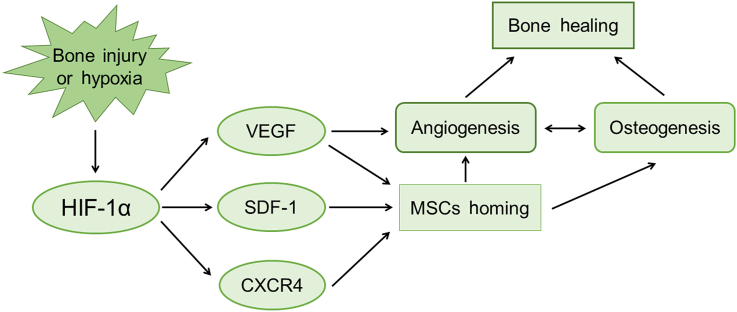
Figure 2.
Hypoxia-inducible factor (HIF)-1-dependent signalling pathways regulating bone healing. Once bone injury or hypoxia happens, HIF-1α activation and stabilisation occur. Vascular endothelial growth factor (VEGF), stromal cell-derived factor (SDF)-1, and CXC chemokine receptor (CXCR) 4 are directly positively regulated by HIF-1α. Increased expression of VEGF, SDF-1, and CXCR4 stimulates mesenchymal stem cell (MSC) homing. VEGF is critical for angiogenesis. Improved MSC homing is involved in both osteogenesis and proangiogenesis, which is vital for bone healing.
Recent studies have indicated that hypoxia contributes to MSC mobilisation and homing. Transiently hypoxic microenvironments (such as injured tissue or tumour) may represent the stem cell niche to some extent, in which HIF-1α stabilisation and activation of SDF-1 and CXCR4 occur, thereby facilitating the recruitment and homing of CXCR4-positive stem cells to damaged tissues. MSCs were found in circulating blood of nonstimulated rats; the circulating MSC pool was consistently and dramatically increased by almost 15-fold when the rats were exposed to chronic hypoxia [79]. Furthermore, hypoxic preconditioning enhances the migration ability and therapeutic efficacy of human MSCs [80]. Moreover, the state of tumour-induced hypoxia, which often perpetuates the inflammatory state, induces numerous angiogenic and inflammatory mediators that can stimulate MSC migration towards tumours [81]. Both SDF-1 and CXCR4 have been implicated in tumour cell metastasis [82]. Goldstein has shown endogenous human BM-MSC migration from a physiological bone environment to tumours based on tumour-derived TGF-β1, increasing their bone metastasis frequency consequently [83]. Thus, hypoxia and inflammation attract MSCs to tumours. In conclusion, HIF1α-induced SDF-1 expression stimulates the migration and homing of circulating CXCR4-positive MSCs to injured tissues [71].
MMPs also play critical roles in the transendothelial migration of MSCs. MMPs function mainly by stimulating the degradation of ECMs around MSCs. In particular, MMP-2 and MMP-9 participate in MSC migration through degradation of collagen and gelatin [84], [85]. Furthermore, the expression level of MMPs in MSCs is increased by hypoxia [86].
Many cytokines or growth factors, such as vascular endothelial growth factor-A [87] and basic fibroblast growth factor [88], increase MSC migration. Mobilising MSCs can also be achieved by administering cytokines such as granulocyte-colony stimulating factor (G-CSF), SDF-1, and stem cell factor [89], [90]. G-CSF is the most commonly used mobilising agent. However, a minority of healthy donors could hardly respond to administration of G-CSF [91]. Therefore, a careful search for more general and effective stem-cell-mobilising agent is imperative (Figure 3).
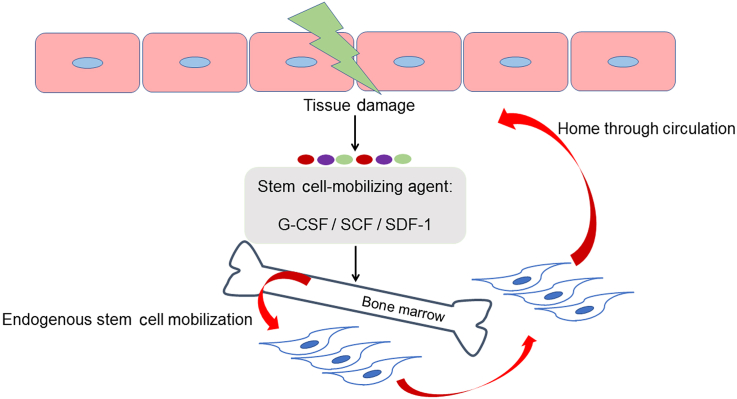
Figure 3.
Strategies to maximise innate regenerative capacity via stimulation of endogenous stem cell mobilisation. After tissue damage, stem cell-mobilising agent (e.g., G-CSF, SCF, SDF-1) is administered to further reinforce endogenous stem cell mobilization. Mobilised mesenchymal stem cells are recruited into damaged tissue via circulation, leading to accelerated recovery. G-CSF = granulocyte colony-stimulating factor; SCF = stem cell factor; SDF-1 = stromal cell-derived factor-1.
Safety concerns of systemic MSC therapy
The main cytokine that has proven to be important to the recruitment of MSCs is SDF-1. In addition to its critical role in facilitating tissue regeneration, SDF-1 is known to be secreted by tumours and is in clinical use as tumour marker [92]. No previous reports have described SDF-1-induced malignancy, but this potential adverse event cannot be excluded yet. Additionally there is some evidence that MSC therapy might promote cancer recurrence in tumour-bearing animals [93], but there is no report that MSCs lead to tumour formation in humans. Due to the potential tumour-promoting risk in tumour patients, it would be prudent to screen patients for the presence of pre-existing malignancy before they receive MSC therapy or a therapy with systemic MSC mobilisation.
Conclusion
The recruitment and homing of MSCs are essential for bone healing. MSC mobilisation accelerates bone healing mainly by stimulating angiogenesis and coordinating bone remodelling. SDF-1/CXCR4 and HIF-1α signalling pathways play critical roles in MSC homing and bone healing. Furthermore, factors such as CD44, hypoxia, immune signals, and different cytokines are crucial for MSC migration. In pathological settings, MSC homing is often impaired due to decreased expression of SDF-1. Therefore, the restoration and normalization of signalling pathways of impaired tissue due to injury may be an important strategy for augmenting bone regeneration. To stimulate and reinforce MSC homing is promising for the future translational medicine.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
The work was partially supported by grants from National Natural Science Foundation of China (81371946 and 81374568); Hong Kong Government Research Grant Council, General Research Fund (470813, 14119115 and 9054014) and grants from China Shenzhen City Science and Technology Bureau (GJHZ20140419120051680 and JCYJ20150630165236960). This study was also supported in part by SMART program, Lui Che Woo Institute of Innovative Medicine, The Chinese University of Hong Kong and the research was made possible by resources donated by Lui Che Woo Foundation Limited.
Contributor Information
Stuart B. Goodman, Email: goodbone@stanford.edu.
Gang Li, Email: gangli@cuhk.edu.hk.
References
- 1.Deng J., Petersen B.E., Steindler D.A., Jorgensen M.L., Laywell E.D. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 2.Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 3.Duran J.M., Makarewich C.A., Sharp T.E., Starosta T., Zhu F., Hoffman N.E. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113:539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y., Chen L., Scott P.G., Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Sun Y., Yang X.Y., Ji S.Z., Han S., Xia Z.F. Mobilised bone marrow-derived cells accelerate wound healing. Int Wound J. 2013;10:473–479. doi: 10.1111/j.1742-481X.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendicino M., Bailey A.M., Wonnacott K., Puri R.K., Bauer S.R. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Pountos I., Jones E., Tzioupis C., McGonagle D., Giannoudis P.V. Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surgery Br. 2006;88:421–426. doi: 10.1302/0301-620X.88B4.17060. [DOI] [PubMed] [Google Scholar]
- 8.Harris M.T., Butler D.L., Boivin G.P., Florer J.B., Schantz E.J., Wenstrup R.J. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Yuehua Jiang B.N.J., Reinhardt R.L., Schwartz R.E., Keenek C.D., Ortiz-Gonzalezk X.R., Morayma Reyes T.L. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 12.Lv F.J., Tuan R.S., Cheung K.M., Leung V.Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 13.Eirin A., Zhu X.Y., Krier J.D., Tang H., Jordan K.L., Grande J.P. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan C., He Q., Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J orthop Res. 2006;24:610–618. doi: 10.1002/jor.20119. [DOI] [PubMed] [Google Scholar]
- 15.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Chen X., Armstrong M.A., Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 18.Bruder S.P., Fink D.J., Caplan A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirley D., Marsh D., Jordan G., McQuaid S., Li G. Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res. 2005;23:1013–1021. doi: 10.1016/j.orthres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res. 1998:S22–S30. doi: 10.1097/00003086-199810001-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lyles K.W., Colon-Emeric C.S., Magaziner J.S., Adachi J.D., Pieper C.F., Mautalen C. Zoledronic acid and clinical fractures and mortality after hip fracture. New Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schipani E., Maes C., Carmeliet G., Semenza G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Mineral Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glowacki J. Angiogenesis in fracture repair. Clin Orthop Relat Res. 1998:S82–S89. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 24.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 25.Hill P.A. Bone remodelling. Br J Orthodont. 1998;25:101–107. doi: 10.1093/ortho/25.2.101. [DOI] [PubMed] [Google Scholar]
- 26.Kitaori T., Ito H., Schwarz E.M., Tsutsumi R., Yoshitomi H., Oishi S. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arth Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todeschi M.R., El Backly R., Capelli C., Daga A., Patrone E., Introna M. Transplanted umbilical cord mesenchymal stem cells modify the in vivo microenvironment enhancing angiogenesis and leading to bone regeneration. Stem Cell Dev. 2015;24:1570–1581. doi: 10.1089/scd.2014.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang J., Guo H.L., Ding D.F., Wu Y.Y., Zhao Y.F., Gu X.F. Changes of mesenchymal stromal cells mobilization and bone turnover in an experimental bone fracture model in ovariectomized mice. Int J Clin Exp Pathol. 2015;8:10228–10238. [PMC free article] [PubMed] [Google Scholar]
- 30.Zwingenberger S., Yao Z., Jacobi A., Vater C., Valladares R.D., Li C. Enhancement of BMP-2 induced bone regeneration by SDF-1alpha mediated stem cell recruitment. Tissue Eng A. 2014;20:810–818. doi: 10.1089/ten.tea.2013.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibon E., Yao Z., Rao A.J., Zwingenberger S., Batke B., Valladares R. Effect of a CCR1 receptor antagonist on systemic trafficking of MSCs and polyethylene particle-associated bone loss. Biomaterials. 2012;33:3632–3638. doi: 10.1016/j.biomaterials.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granero-Molto F., Weis J.A., Miga M.I., Landis B., Myers T.J., O'Rear L. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcacci M., Kon E., Moukhachev V., Lavroukov A., Kutepov S., Quarto R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 34.Gianakos A.L., Moya-Angeler J., Duggal S., Zambrana L., Fields K.G., Mintz D.N. The efficacy of bisphosphonates with core decompression and mesenchymal stem cells compared with bisphosphonates alone in the treatment of osteonecrosis of the hip: a retrospective study. HSS J. 2016;12:137–144. doi: 10.1007/s11420-016-9487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kandahari A.M., Yang X., Laroche K.A., Dighe A.S., Pan D., Cui Q. A review of UHMWPE wear-induced osteolysis: the role for early detection of the immune response. Bone Res. 2016;4:16014. doi: 10.1038/boneres.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman S.B., Huie P., Song Y., Schurman D., Maloney W., Woolson S. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80:531–539. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 37.Huang Z., Ma T., Ren P.G., Smith R.L., Goodman S.B. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J Biomed Mater Res A. 2010;94:1264–1269. doi: 10.1002/jbm.a.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pajarinen J., Lin T.H., Nabeshima A., Jamsen E., Lu L., Nathan K. Mesenchymal stem cells in the aseptic loosening of total joint replacements. J Biomed Mater Res A. 2017;105:1195–1207. doi: 10.1002/jbm.a.35978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sui B., Hu C., Zhang X., Zhao P., He T., Zhou C. Allogeneic mesenchymal stem cell therapy promotes osteoblastogenesis and prevents glucocorticoid-induced osteoporosis. Stem Cells Translat Med. 2016;5:1238–1246. doi: 10.5966/sctm.2015-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moll N.M., Ransohoff R.M. CXCL12 and CXCR4 in bone marrow physiology. Expert Rev Hematol. 2010;3:315–322. doi: 10.1586/ehm.10.16. [DOI] [PubMed] [Google Scholar]
- 41.Charo I.F., Myers S.J., Herman A., Franci C., Connolly A.J., Coughlin S.R. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belema-Bedada F., Uchida S., Martire A., Kostin S., Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2:566–575. doi: 10.1016/j.stem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Wang L., Li Y., Chen J., Gautam S.C., Zhang Z., Lu M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- 44.Shinohara K., Greenfield S., Pan H., Vasanji A., Kumagai K., Midura R.J. Stromal cell-derived factor-1 and monocyte chemotactic protein-3 improve recruitment of osteogenic cells into sites of musculoskeletal repair. J Orthop Res. 2011;29:1064–1069. doi: 10.1002/jor.21374. [DOI] [PubMed] [Google Scholar]
- 45.Otsuru S., Tamai K., Yamazaki T., Yoshikawa H., Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 46.Neuss S., Becher E., Woltje M., Tietze L., Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 47.Takai K., Hara J., Matsumoto K., Hosoi G., Osugi Y., Tawa A. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood. 1997;89:1560–1565. [PubMed] [Google Scholar]
- 48.Forte G., Minieri M., Cossa P., Antenucci D., Sala M., Gnocchi V. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto K., Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 50.Okunishi K., Dohi M., Nakagome K., Tanaka R., Mizuno S., Matsumoto K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 51.Wynn R.F., Hart C.A., Corradi-Perini C., O'Neill L., Evans C.A., Wraith J.E. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 52.Shi M., Li J., Liao L., Chen B., Li B., Chen L. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 53.Rombouts W.J., Ploemacher R.E. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 54.Jones E., McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 55.Wei F.Y., Leung K.S., Li G., Qin J., Chow S.K., Huang S. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106722. e106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 57.Xing Z., Lu C., Hu D., Yu Y.Y., Wang X., Colnot C. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrew J.G., Andrew S.M., Freemont A.J., Marsh D.R. Inflammatory cells in normal human fracture healing. Acta Orthopaed Scand. 1994;65:462–466. doi: 10.3109/17453679408995493. [DOI] [PubMed] [Google Scholar]
- 59.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 60.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pure E., Cuff C.A. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 62.Zhu H., Mitsuhashi N., Klein A., Barsky L.W., Weinberg K., Barr M.L. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 63.Hemeda H., Jakob M., Ludwig A.K., Giebel B., Lang S., Brandau S. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 64.Ponte A.L., Marais E., Gallay N., Langonne A., Delorme B., Herault O. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt A., Bierwirth S., Weber S., Platen P., Schinkothe T., Bloch W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br J Sports Med. 2009;43:195–198. doi: 10.1136/bjsm.2007.043208. [DOI] [PubMed] [Google Scholar]
- 66.Wong M.M., Fish E.N. Chemokines: attractive mediators of the immune response. Semin Immunol. 2003;15:5–14. doi: 10.1016/s1044-5323(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 67.Fuchs E., Tumbar T., Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 68.Estrada J.C., Albo C., Benguria A., Dopazo A., Lopez-Romero P., Carrera-Quintanar L. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19:743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohyeldin A., Garzon-Muvdi T., Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 72.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 73.Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 74.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kallio P.J., Okamoto K., O'Brien S., Carrero P., Makino Y., Tanaka H. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 77.Liu H., Xue W., Ge G., Luo X., Li Y., Xiang H. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Comm. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 78.Wan C., Gilbert S.R., Wang Y., Cao X., Shen X., Ramaswamy G. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rochefort G.Y., Delorme B., Lopez A., Herault O., Bonnet P., Charbord P. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 80.Rosova I., Dao M., Capoccia B., Link D., Nolta J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spaeth E., Klopp A., Dembinski J., Andreeff M., Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 82.Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein R.H., Reagan M.R., Anderson K., Kaplan D.L., Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010;70:10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steingen C., Brenig F., Baumgartner L., Schmidt J., Schmidt A., Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 85.De Becker A., Van Hummelen P., Bakkus M., Vande Broek I., De Wever J., De Waele M. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. doi: 10.3324/haematol.10475. [DOI] [PubMed] [Google Scholar]
- 86.Ben-Yosef Y., Lahat N., Shapiro S., Bitterman H., Miller A. Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res. 2002;90:784–791. doi: 10.1161/01.res.0000015588.70132.dc. [DOI] [PubMed] [Google Scholar]
- 87.Schichor C., Birnbaum T., Etminan N., Schnell O., Grau S., Miebach S. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt A., Ladage D., Schinkothe T., Klausmann U., Ulrichs C., Klinz F.J. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 89.Deng J., Zou Z.M., Zhou T.L., Su Y.P., Ai G.P., Wang J.P. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32:641–651. doi: 10.1007/s10072-011-0608-2. [DOI] [PubMed] [Google Scholar]
- 90.Lapidot T., Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 91.Fu S., Liesveld J. Mobilization of hematopoietic stem cells. Blood Rev. 2000;14:205–218. doi: 10.1054/blre.2000.0138. [DOI] [PubMed] [Google Scholar]
- 92.Sun X., Cheng G., Hao M., Zheng J., Zhou X., Zhang J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metast Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paino F., La Noce M., Di Nucci D., Nicoletti G.F., Salzillo R., De Rosa A. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: implication for autologous fat grafting. Cell Death Dis. 2017;8:e2568. doi: 10.1038/cddis.2016.308. [DOI] [PMC free article] [PubMed] [Google Scholar]