Summary
Low back pain is the main cause of disability and is associated with intervertebral disc degeneration. Contemporary treatments are limited to palliative therapeutics or aggressive surgical interventions; however, current advancements in cell therapy offer to fill this breach. Clinical data suggest that cell transplantation can accomplish pain relief without any observed adverse effects. Despite a large variety of preclinical studies and preliminary clinical investigations, controversy remains on the optimal cell type and transplantation strategies. The translational potential of this article lies in the aim to update on the current state of intervertebral disc cell therapy and to identify current obstacles.
Keywords: cell therapy, clinical trial, intervertebral disc
Introduction
Low back and neck pain are the most common musculoskeletal conditions worldwide and pose a major global health issue. An approximate 632 million people are affected by low back pain, of which an estimated 5–10% will progress toward a chronic condition [1], [2]. Consequently, low back pain engenders an immense economic burden on society. Annual socioeconomic costs in the United States are estimated to exceed $100 billion [3], [4]. Similar trends are observed in other developed countries [5], [6], [7]. A small reduction in low back pain related health usage or disability could consequently result in a large economic impact [8].
Low back pain is generally associated with intervertebral disc (IVD) degeneration. The IVDs are the fibrocartilage tissue bodies situated between the bony vertebrae, absorbing and distributing complex and substantial loads along the spine [9]. The IVD is composed of a hydrophilic gelatinous core, the nucleus pulposus (NP), which is encompassed by thick layers of tightly radially aligned collagen fibres, forming the annulus fibrosus (AF). Additionally, a thin layer of hyaline cartilage borders the vertebrae and the IVD. This cartilage layer functions as the waste and nutrient exchange for the interior of the IVD [10]. The proteoglycan-rich extracellular matrix (ECM) induces the NP to swell. However, the AF and vertebrae spatially contain the NP, establishing a high hydrostatic pressure. This enables the IVD to absorb extraordinary compressive forces, yet permitting flexible motility [11]. Region-specific cells provide a continuous turnover of ECM that accomplishes these tissue-specific biomechanical features (Figure 1A). AF cells are morphologically similar to collagen-producing fibroblast cells. Endplate cells resemble hyaline chondrocytes. The NP cell population gradually changes up to adulthood, progressively differentiating from large vacuolated notochordal cells to small chondrocyte-like NP cells. The notochord-derived cells are postulated to possess a more potent regenerative capacity compared to the chondrocyte-like NP cells [12].
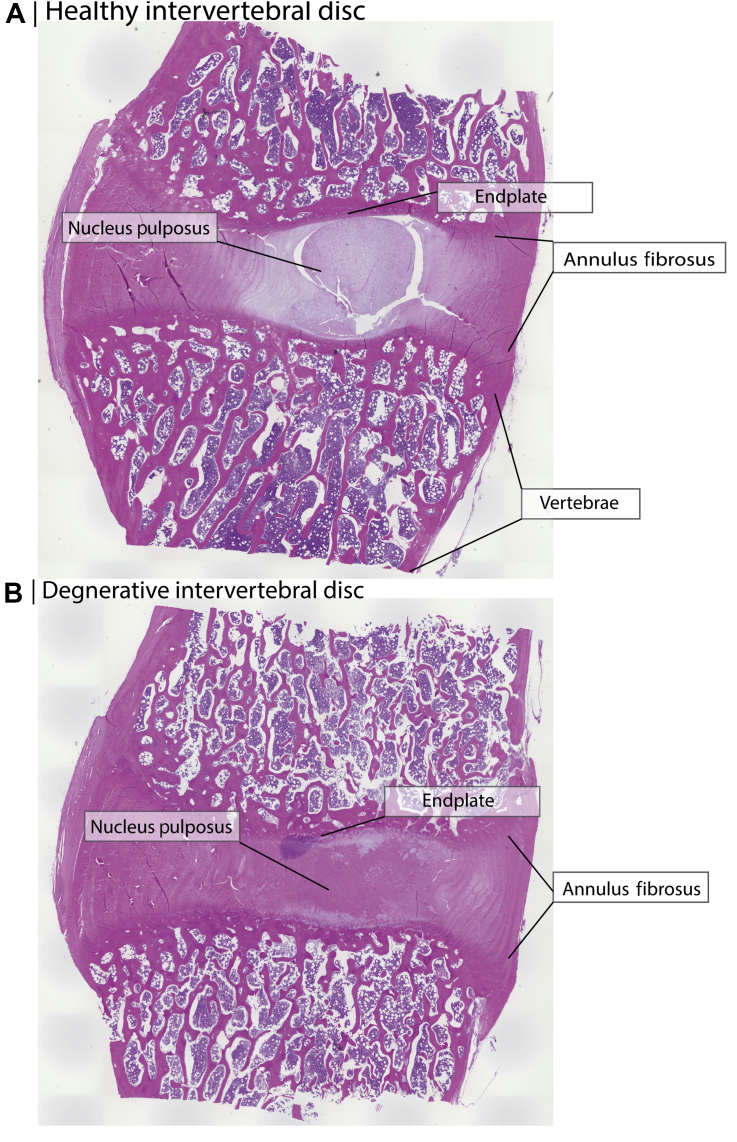
Figure 1.
Canine intervertebral disc and vertebrae sections stained by hematoxylin/eosin staining. (A) A section of a healthy intervertebral disc with a healthy gelatinous nucleus pulposus core. (B) An artificially degenerated disc section with a fibrous nucleus pulposus core. Degeneration was induced by aspirating approximately 25 mg of nucleus pulposus tissue by methods from previously published work [74]. Fourteen weeks after aspiration, the tissue was explanted and stained.
The exact origin of IVD degeneration remains to be identified; however, the NP is believed to be the place of onset, as this tissue displays the severest change during the early stages of IVD degeneration [13], [14]. The progressive degeneration is defined by a decrease in production of proteoglycans and an increase in matrix degrading proteins [15], [16]. Moreover, a larger portion of NP cells become senescent, and cell viability declines [17]. The overall loss of proteoglycans is followed by a decrease in IVD water content and a disorganisation of collagen fibres. Consequently, the disc loses its hydrostatic features and shrinks in size [18]. Morphologically, the NP changes from a soft fibrocartilage tissue towards a stiffer fibrotic structure [19] (Figure 1B). These structural changes alter the biomechanical features along the spine, affecting adjacent vertebrae and potentially inducing facet joints arthritis, bone spur formation, and promoting spine diseases such as canal stenosis, spondylolisthesis, and kyphoscoliosis.
Despite the well-known ensuing pathologies of IVD degeneration, preventative and therapeutic approaches remain primarily palliative. Conservative treatment modalities, such as physical therapy and pain medication are used, whereas for more severe cases surgical resection or immobilisation of the disc is applied. The effects of these treatment options are limited in reversing and restoring the IVD homeostasis [20]. In fact, the surgical procedures often further disrupt the biomechanical disbalance, resulting in accelerated degeneration in adjacent segments [21]. Therefore, a great interest has developed for the application of cell transplantation to restore IVD homeostasis and reverse the degeneration process. In this narrative review, we will discuss the rationale for cell transplantation-mediated therapies. Finally, we will review published and ongoing clinical trials to elucidate current obstacles and potential solutions.
Innate regeneration
Disc degeneration is characterised as a disease of ageing, hallmarked by a loss of viable cells and an increase in cell senescence [17], [22]. Moreover, reports have demonstrated that IVD progenitor populations diminish with age [23]. For most tissues, new and active cells can be attracted to damaged or distressed sites to promote repair and regain homeostasis. Studies have explored the potential of in situ cell recruitment into the IVD. In a bovine whole IVD ex vivo culture, exogenous human mesenchymal stem cells (MSCs) were able to infiltrate the disc via the endplates. This study found that infiltration increases for IVDs in a degenerated state [24]. Additionally, an in vivo rabbit study revealed a possible migratory pathway into the outer AF layers from a stem cell niche seen in the AF adjoining the perichondrium- and outer ligament zone [25]. Both studies indicate that cell recruitment into the IVD is possible; however, it remains limited to the vascularised tissues, such as the endplate and most outer AF. Because of the avascular nature of the IVD, cell migration into the NP and inner AF appears limited [26], [27].
A second innate regeneration source is endemic progenitor cell populations. A previous study identified an MSC-like CD105+CD73+CD90+CD45−CD34−CD14−CD11b−CD79−CD19−HLADR− plastic-adherent cell population from a complete IVD extract to possess in vitro self-renewal and multilineage differentiation potential [28]. However, their in vivo characteristics and localisation remain undetermined. A later study identified a NP-specific Tie2+ and GD2+ progenitor cell population [23]. This particular population had a superior ECM production and proliferation, and showed multipotent differentiation capacity both in vivo and in vitro. The identified progenitor cell population might pose a promising target to stimulate and induce tissue repair. In an in vitro assessment, Tie2+ cells were stimulated by ANG-1, which resulted in an increased colony formation and decreased apoptosis rate [23]. The same group showed that Tie2+ GD2+ populations decrease with age. This could pose an obstacle in using these progenitor cell populations as a target for endemic cell-mediated repair, in particular, for older patients.
Clinical reports
Owing to the lack of active cell populations in the IVD and limited cell recruiting potential, a large variety of investigations have aimed to introduce cell populations into the IVD. These introduced cells can thereafter be incorporated in situ and contribute to de novo matrix production or stimulate endemic cells through trophic factors. In total, eight published and 12 unpublished clinical trials assessing cellular transplantations for IVD regeneration were identified as of November 2016 (Table 1). The first-in-human trial was a prospective study of 10 discogenic low back pain patients treated with autologous hematopoietic stem cells followed by 2 weeks of hyperbaric oxygen therapy [29]. One year after transplantation, no improvement in visual analog scores were observed. No additional radiographic or magnetic resonance imaging (MRI)-established hydration values were reported.
Table 1.
Reported clinical trial overview.
| Study | Year | Location | Mode | Cell type | Concentration | Volume | Carrier | Indication | n | Period (y) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Haufe and Mork [29] | 2006 | USA | Autologous | Haematopoietic stem cells | Unspecified | Unspecified | Suspension | Discogenic low back pain | 10 | 1 | No improvement |
| Meisel et al [30], [31] | 2007 | Germany/Austria | Autologous | Intervertebral disc cells | Unspecified | Unspecified | Suspension | Single level lumbar disc herniation discectomy | 112 | 4 | Reduced low back pain, improved hydration in treated an adjacent IVDs, no change in disc height |
| Yoshikawa et al [32] | 2010 | Japan | Autologous | Bone marrow mesenchymal stem cells | Unclear | 540 mm3 | Pelnac Collagen sponge | Lumbar spinal canal stenosis | 2 | 2 | Improvement hydration and reduced low back pain |
| Orozco et al [33] | 2011 | Spain | Autologous | Bone marrow mesenchymal stem cells | 10(±5) × 106 | 1 mL | Suspension | Lumbar disc degeneration and chronic low back pain | 10 | 1 | No adverse effects, reduced pain and disability, improved hydration, no enhanced disc height |
| Coric et al [35] | 2013 | USA | Allogenic | Juvenile articular chondrocytes | 1–2 × 107 | 1–2 mL | Fibrin | Single level degeneration disc disease with low back pain | 15 | 1 | Improvement in low back pain and on MRI |
| Pettine et al [34] | 2014 | USA | Autologous | Bone marrow concentrate | 121(±11) × 106 | 2–3 mL | Not applicable | Discogenic low back pain | 26 | 1 | Reduced low back pain, sporadic hydration increase |
| Mochida et al [38] | 2015 | Japan | Autologous | Reactivated nucleus pulposus cells | 1 × 106 | 702 μL | Suspension | Disc degeneration adjacent to fused disc | 9 | 3 | No adverse effects, no progression of degeneration detected |
| Elabd et al [39] | 2016 | USA | Autologous | Bone marrow mesenchymal stem cells | 31(±14) × 106 | 0.25–1 mL | Suspension | Chronic low back pain | 5 | 4–6 | No adverse effects, reduced IVD protrusion, improved mobility & strength |
IVD = intervertebral disc; MRI = magnetic resonance imaging.
On the contrary, a German- and Austrian-based prospective controlled randomised multicentre trial assessed transplantation of IVD cells combined with discectomy. This resulted in a clinically significant decrease in pain score compared to a discectomy only group [30], [31]. Additionally, improved hydration was observed in both treated and adjacent discs at 2 years follow-up. No significant difference in disc height index was observed between the groups.
A Japanese group investigated the potential of autologous bone marrow derived MSC in two female patients suffering from lumbar spinal stenosis with insatiability and presence of air in the IVD space [32]. The MSCs were infused into a dermal-derived collagen sponge. The stenosed spinal cord was fenestrated and via percutaneous procedure, the grafted collagen sponge was inserted into the afflicted disc. Radiographic imaging revealed enhancement in stability and reduction of air in the IVD 2 years after the operation. Moreover, improved hydration was confirmed in both patients. Finally, self-reported low back pain and leg numbness improved.
Subsequently, an autologous bone marrow-derived MSC pilot study was performed in Spain [33]. Ten patients who had chronic low back pain with disc degeneration in the lumbar area received MSC suspension directly injected into the NP. Three months after transplantation, a clinical and significant reduction was observed in lumbar pain scores. Rapid improvement was also observed in decreasing disability score. One-year follow-up MRI evaluations showed a significant increase in water content; however, no improvement was observed in disc height index.
An open-label nonrandomised pilot study reported the effects of bone marrow concentrate therapy [34]. Twenty-six patients received autologous bone marrow concentrate, treated bone marrow aspirate containing a variety of MSCs and unspecified growth factors, anti-inflammatory factors, and chemoattractants. Patients were included with discogenic low back pain. After 3 months, 6 months, and 12 months, a continuous significant reduction in 21 of 26 patients was observed by reported pain and disability scores. Eight of the 20 evaluated patients showed a one-level improvement in Pfirrmann grading after 1 year. Additionally, the data suggest that the effectiveness of the transplantation is dependent on implanted cell concentration. Patient age was an indicator for in vitro MSC expansion capacity and therefore the effectiveness of the treatment.
In a prospective study, 15 patients diagnosed with single-level lumbar spondylosis paired with mechanical low back pain were treated via a single percutaneous transplantation of allogenic juvenile chondrocytes encapsulated in a fibrin matrix [35]. Six months follow-up revealed that 10 of the 13 evaluated patients had enhanced MRI hydration values. No immunological, neurological deterioration, or other severe adverse effects were reported. Pain and disability as surveyed via the Oswestry disability index improved for all recipients during the 12 months follow-up. Disc height index status was not reported. A new clinical trial [36] has been submitted; however, this has been terminated because of a change in clinical strategy [37].
Subsequently, a Japanese group has reported a prospective clinical study of nine patients with Pfirrmann Grade III disc degeneration in levels adjacent to discs scheduled for lumbar intervertebral fusion [38]. Extruded discs were recovered, and viable NP cells were isolated via enzymatic digestion. NP cells were thereafter cocultured with autologous bone marrow derived MSC for 3 days. The reactivated autologous NP cells were transplanted into the IVDs adjacent to the fused IVD 7 days after the first fusion surgery. MRI revealed a slight improvement in one patient. All patients reported being relieved of low back pain 3 years after transplantation. Finally, disc height index did not deteriorate during the 3-year follow-up. No adverse effects were reported.
The final published clinical report describes a feasibility study of autologous bone marrow derived MSC transplantation in five patients with degenerative disc disease [39]. The MSC cells were preconditioned by expanding them under 5% O2 hypoxic conditions prior to transplantation. After a 4- to 6-year follow-up, all patients reported improvement in pain, and four out of five reported improvement in mobility. Four patients showed a reduction in posterior protrusion, and for one patient the protrusion increased. Nevertheless, MRI assessment revealed a mild reduction of disc height in four out of five patients. No neoplasms or other abnormalities were observed. Hydration changes of the discs were not reported.
Twelve additional unpublished clinical trials were registered at the United States (US) National Health Institutes or the European Union Clinical Trials Register (Table 2). Two registered trials from Spain have been altered from an autologous transplantation study to a new allogenic bone marrow derived MSC clinical trial [40], [41], [42]. This, prospective, randomised, blinded, and Phase I–II controlled clinical trial aims to compare allogenic MSC to 1% mepivacaine treatment. Another study from Australia and the US finished a mesenchymal precursor cell transplantation trial in 2015 [43]. Within this study, they applied a specific STRO-3 positive bone marrow derived MSC population that has been suggested to possess a higher proliferation capacity and potency [44], [45]. A continuation in the form of a prospective, multicentre, randomised, double-blind, placebo-controlled Phase III trial has been accepted and aims to enrol 360 patients, making it the largest human IVD cell therapy study [46]. A pilot Phase I/II study, from Austria and Germany, aims to assess the safety and efficacy of autologous disc chondrocyte transplantation for nucleotimised and degenerative lumbar discs. The study set out to compare a placebo group of transplantation IVD chondrocytes suspension and a control group treated with only surgery to the transplantation of nucleotimised disc cells in a modified albumin hyaluronic acid gel (NOVOCART® Basic; TETEC, Reutlingen, Germany) [47], [48], [49]. The study aims to follow patients for a period of 5 years, making it the longest follow-up study on human IVD cell transplantation registered. Next, a clinical trial assessed juvenile chondrocyte transplantation in a fibrin gel [36]. However, the trial has been terminated because of a change in clinical strategies [37]. A US study aims to assess bone marrow derived MSC in either a hyaluronic acid gel or in decellularised bone matrix in 100 lumbar disc degeneration patients [50]. A study from Denmark aims to assess the clinical benefit from bone marrow derived MSC in 34 lumbosacral spondylosis without myelopathy patients [51]. A small study from South Korea aims to assess adipose tissue-derived MSC transplantation encapsulated in a hyaluronic acid derived gel [52]. It appears to be a redesigned trial from the 2012 study proposal [53]. Finally, a study within the US aims to assess adipose derived MSC [54]. This study, however, will transplant the MSCs in suspension, with a follow-up of 6 months.
Table 2.
Ongoing clinical trial overview.
| Sponsor | Start | Completion | Location | Mode | Cell type | Concentration | Volume (mL) | Carrier | Indication | n | Period | Status | GOV ID | EudraCT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red de Terapia Celular [40] | 2010 | Unspecified | Spain | Autologous | Bone marrow mesenchymal stem cells | 0.5–1.5 × 106/kg bodyweight | Unspecified | Suspension | Lumbar degenerative disc disease | 15 | 6 mo | Unknown | NCT01513694 | — |
| Mesoblast, Ltd. [43] | 2011 | 2015 | United States/Australia | Allogenic | Messenchymal precursor cells | 6 × 106 or 18 × 106 | Unspecified | Hyaluronic acid | Lumbar disc degeneration and chronic low back pain | 100 | 6 mo | Completed | NCT01290367 | — |
| Red de Terapia Celular [41] | 2011 | 2012 | Spain | Autologous | Bone marrow mesenchymal stem cells | Unspecified | Unspecified | Unspecified | Lumbar disc degeneration and chronic low back pain | 0 | 1 y | Withdrawn | NCT02440074 | — |
| Biostar [53] | 2012 | 2014 | South Korea | Autologous | Adipose derived mesenchymal stem cells | 4 × 107 | 1 | Unspecified | Lumbar disc degeneration and chronic low back pain | 8 | 6 mo | Unknown | NCT01643681 | — |
| Tetec AG [48], [49] | 2012 | 2021 | Austria/Germany | Autologous | Disc chondrocytes | Unclear | 0.5–2 | NOVOCART Disc plus (modified albumin hyaluronic acid gel) | Nucleotomised and degenerative lumbar disc | 120 | 5 y | Ongoing (not recruiting) | NCT01640457 | 2010-023830-22 |
| ISTO Technologies, Inc. [36] | 2012 | 2016 | United States | Allogenic | Juvenile chondrocytes | Unspecified | Unspecified | Fibrin | Lumbar disc degeneration and chronic low back pain | 44 | 2 y | Terminated | NCT01771471 | — |
| The Foundation for Spinal Research, Education and Humanitarian Care, Inc. [50] | 2013 | 2018 | United States | Autologous or Allogenic | Bone marrow mesenchymal stem cells | Unspecified | Unspecified | Hyaluronic acid/decellularised bone matrix | Lumbar disc degeneration and chronic low back pain | 100 | 2 y | Enrolling | NCT02529566 | — |
| Århus University Hospital [51] | 2013 | Unspecified | Denmark | Autologous | Bone marrow mesenchymal stem cells | Unspecified | Unspecified | Unspecified | Lumbar disc degeneration and chronic low back pain | 34 | 2 y | Ongoing | — | 2012-003160-44 |
| Red de Terapia Celular [42] | 2013 | 2016 | Spain | Allogenic | Bone marrow mesenchymal stem cells | 10 (±5) × 106 | 1 | Suspension | Lumbar disc degeneration and chronic low back pain | 24 | 1 y | Ongoing (not recruiting) | NCT01860417 | — |
| Bioheart, Inc. [54] | 2014 | 2017 | United States | Autologous | Adipose derived stem cells | Patient-specific | Patient-specific | Suspension | Lumbar disc degeneration and chronic low back pain | 100 | 6 mo | Recruiting | NCT02097862 | — |
| Inbo Han [52] | 2015 | 2017 | South Korea | Autologous | Adipose derived stem cells | 2 or 4 × 107 | 2 | Tissuefill Hyaluronic acid derivatives | Lumbar disc degeneration and chronic low back pain | 10 | 1 y | Recruiting | NCT02338271 | — |
| Mesoblast, Ltd. [46] | 2015 | 2020 | United States/Australia | Allogenic | Mesenchymal precursor cells | 6 × 106 | 2 | Hyaluronic acid | Lumbar disc degeneration and chronic low back pain | 360 | 2 y | Recruiting | NCT02412735 | — |
IVD = intervertebral disc.
Notably, 10 out of the 12 unpublished study proposals aim to assess MSCs, either derived from bone marrow [40], [41], [42], [43], [46], [50], [51] or adipose tissue [52], [53], [54]. One terminated study aimed to assess juvenile chondrocytes transplantation [36]. Additionally, disc-derived chondrocyte transplantations are ongoing with a finalisation date of 2021 [48], [49].
Cell sources
The selection of a proper cell type and source is crucial for successful regeneration of the IVD, as indicated by Haufe and Mork [29]. The choice of cell types is dependent on practical issues such as accessibility, abundance, and safety concerns, such as tumourigenesis and immunogenicity. The capacity to differentiate and contribute to IVD homeostasis and ECM production under stringent biomechanical forces should be considered. The avascular nature establishes a hypertonic, acidic, hypoglycemic, and hypoxic microenvironment, forming an obstacle for exogenous cell to survive and thrive. Furthermore, introducing a new population of cells increases nutrient demand and might, therefore, promote further imbalance, resulting in additional cell death and tissue degeneration [55], especially considering that end-plate perfusion is also negatively affected by disc degeneration [56]. Despite these harsh conditions, innate NP cells have the unique ability to survive within this avascular niche. Unlike most other cells, NP cells express hypoxia-inducible factor 1-alpha (HIF1α) continuously, regardless of oxygen concentrations, advocating the NP cells regulation system to adapt towards glycolysis, making NP cells well equipped to manage with limited nutrient accessibility [57], [58]. Additionally, HIF1α enhances NP-specific ECM matrix production [59]. It remains unclear if transplanted cells are able to acquire the functional properties similar to NP cells to survive within this unique niche.
A variety of cells has been explored preclinically as a cell source for disc regeneration. Roughly, these cell types can be divided into five categories: (1) IVD-derived cells, (2) chondrocyte-like cells; (3) MSCs; (4) induced pluripotent stem cells (iPSCs), and (5) embryonic stem (ES) cells. No published human studies explored ES or iPSC cell transplantation. Recent investigations have revealed that iPSCs can be successfully induced to chondrocyte-like cell [60], [61]. ES cell and iPSC application hold great potential; nevertheless, their inherent risk of tumourigenesis and ethical concerns demand cautiousness.
IVD-derived cells and chondrocytes are inherently predisposed to create NP-like ECM and survive under hypoxic conditions. However, their accessibility and low cell yield are considered a disadvantage. Additionally, the available tissue sources are commonly compromised or diseased. For this reason, Mochida et al [38] assessed an MSC coculture system to reactivate the NP cell derived from the degenerated discs. The reactivation resulted in a significant increase in cell proliferation compared to the cells cultured without coculture. NP cells without coculture did not show an increase in cell number after 7 days of culture. Reactivation presents a promising option to circumvent the cell senesce obstacle from degenerated IVD tissue. The study of Meisel et al [30] did not implement a reactivation method to their IVD-derived chondrocytes. However, their patient pool was limited to herniated discs and therefore the senescence concern might not pose an issue. Therefore, excised herniated IVD tissue might offer a promising source for IVD-derived cells. In both cases, improvements in pain and MRI assessments were observed. Similarly, the transplantation of juvenile articular chondrocytes obtained relatively young and healthy cells as a cell source for their treatment [35]. Therefore, it appears that IVD-derived cells or chondrocyte are able to improve IVD conditions. It will be interesting to see the result from the large multicentre NOVOCART clinical trial, which is set to finish in 2021. This long-term follow-up study will most likely reveal new insights into the potential of using IVD as a cell source for safe and effective cell therapy.
The most commonly clinically evaluated cell types are MSCs. MSCs are multipotent cells and have the advantage of being efficiently expandable and easily accessible from a variety of tissue source (e.g., umbilical cord, bone marrow, and adipose tissue). MSCs from different sources are, however, predisposed to certain differentiation pathways. The most potent source for NP differentiation remains to be determined. Hematopoietic stem cells, however, might not be the optimal cell source, as was suggested by the first IVD cell therapy clinical trial [29]. Other studies evaluating bone marrow derived MSC were able to decrease pain scores, and three out of the four trials reported improvement in hydration values. No adverse effects were reported in any of the studies; however, preclinical studies have shown that leakage of MSCs from degenerated discs can result in osteophyte formation [62]. It remains to be elucidated if preconditioning MSCs towards a chondrogenic state would be beneficial.
Additionally, the mode of transplantation should be taken into account. Autologous cell transplantation presents the lowest risk of immunogenic responses; however, this approach commonly requires timely and costly cell expansion, differentiation, and selection procedures. Moreover, ex vivo culturing increases the chance of infectious agents to be introduced. On the contrary, allogenic or xenogenic transplantation support an off-the-shelf product. Nevertheless, the introduction of xenogenic and allogenic agents pose a risk of triggering an immunogenic reaction. The immunogenicity of allogenic or xenogenic cells in the IVD space has not been well documented; however, the interior of the IVD is considered an immune-protected tissue, owing to its avascular nature [63]. The clinical trials cited support this notion as none reported any immunogenicity-related effects in their allogenic cell transplantations.
Transplantation methodology
Because of the avascular characteristics of the IVD, transplantation via injection currently remains the only option of administration. Nevertheless, injection into the IVD requires puncturing of the AF, which has been shown to accelerate degeneration [64]. Another essential consideration of cell transplantation is the carrier or medium in which cells are implanted. The majority of studies focused on transplanted cells in suspension; however, a selection of trials encapsulated their cell products. Carriers used within these clinic trials are atelocollagen [32], fibrin [35], hyaluronic acid [43], [46], [50], and hyaluronic acid derivative gel [48], [49], [52]. Gels and scaffold can enhance in situ incorporation and guide differentiation [65]. Encapsulation is also a potential method to limit cell leakage. Cell leakage might occur either during the transplantation procedure or in situ through AF fissures, which could lead to osteophyte formation [62]. Finally, the carriers can be modified and loaded with bioactive factors or drugs to enhance the desired regeneration process. Another issue to consider is the number of cells implanted and the volume of the transplantation. Work from Serigano et al [66] demonstrated in a canine disc degeneration model that the efficacy of a cell therapy is dependent on the number of transplanted cells. All studies mentioned here seem to consistently administer 106 or 107 cells per disc. One exception is the bone marrow concentrate study, which implanted 1.2 × 108 cells on average [34]. In effect, it appears that current cell dosages can promote restoration of low back pain; nevertheless, current work does not reveal an optimal cell dose.
Outcome parameters
All identified studies limited their outcome parameters to radiography and MRI combined with self-reported pain and disability scores. Although reduction in pain and disability scores is the primary intention, they do not directly present improvements in IVD features. Moreover, none of the identified studies have corrected for a placebo effect, and therefore results should be interpreted with caution (Table 1). MRI modalities remain the primary outcome to evaluate repair of IVD structures by assessing the disc height index, Pfirrmann classification, and intensity values. However, reports have indicated the lack of relationship between MRI classification and histological features [67]. Hydration loss is not a unique aspect of disc degeneration, and features such as cell clustering and AF fissures are not observable by MRI. New advancements in and clinical standardisation of imaging techniques are impending [68]. In particular, we would like to highlight MR spectroscopy, a developing technique that would allow direct assessment of in situ matrix composition [69], [70]. In addition, progress in the field of biomarkers, such as the discovery of CCL5 and CXCL6 [71], might also offer new, easy, and reliable outcome parameters to assess cell therapy efficacy.
Identifying the right patient
The clinical trials presented in this review have mainly focused their efforts on alleviating IVD degeneration associated pain and disability. However, the role of disc degeneration in the presentation of pain remains controversial. Moreover, disc degeneration is promoted by a multitude of factors, such as genetics, lifestyle, mechanical factors, and ageing. Additionally, cell therapy might provide a more preventive opportunity for patients with mild degeneration, to impede progression towards secondary spinal diseases. Therefore, selection criteria to identify which patient population that will benefit from IVD cell therapy remain to be determined [70], [72]. This might be hindered, however, by an inadequacy of imaging modalities to detect small alteration in IVD matrix on a clinical scale. Furthermore, new insights in the genetic predispositions of disc degeneration are emerging as an encouraging method to select the right patients for cell transplantation [73]. Finally, identifying and normalising biomarkers will provide new opportunities to subdivide patient populations.
Conclusion
Preclinical and clinical results demonstrate the potential of IVD cell therapies. In the presented overview, all studies implementing bone marrow derived MSC or chondrocyte-like cells resulted in reported pain relief, disability reduction, and improved imaging features. Nevertheless, it remains uncertain if the transplanted cells are able to induce or contribute to de novo matrix production. At present, diagnostic methods are limited in detecting potential matrix regeneration. New innovations and standardisation are therefore highly needed. Moreover, a placebo-controlled study is necessary to confirm the effectiveness of pain and disability reduction. Still, the absence of adverse side effects, in particular immunogenic reactions, indicates that cell transplantations (including allogenic transplantation) are a safe approach, supporting the notion that the IVD is an immune privileged tissue. The questions of optimal cell source and optimal administration method remain to be determined. In addition, current investigations have focused predominantly on repairing the NP. Investigations on repairing AF and endplate could offer new opportunities and insights. Cell therapies pose a novel opportunity to bridge the gap between contemporary palliative therapies and aggressive surgical treatment. Well-designed clinical trials will determine the potential of cell therapies as a novel tool against IVD degeneration and low back pain.
Conflicts of interest
Author J. Schol receives personal financing from Regience K.K., Tokyo, Japan, and DiscGenics Inc., Salt Lake City, United States.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
References
- 1.Meucci R.D., Fassa A.G., Faria N.M. Prevalence of chronic low back pain: systematic review. Rev Saude Publ. 2015;49 doi: 10.1590/S0034-8910.2015049005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz J.N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 4.Martin B.I., Deyo R.A., Mirza S.K., Turner J.A., Comstock B.A., Hollingworth W. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 5.van Tulder M.W., Koes B.W., Bouter L.M. A cost-of-illness study of back pain in The Netherlands. Pain. 1995;62:233–240. doi: 10.1016/0304-3959(94)00272-G. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T., Matsudaira K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur Spine J. 2013;22:432–438. doi: 10.1007/s00586-012-2439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossschadl F., Stolz E., Mayerl H., Rasky E., Freidl W., Stronegger W.J. Rising prevalence of back pain in Austria: considering regional disparities. Wien Klin Wochenschr. 2016;128:6–13. doi: 10.1007/s00508-015-0857-9. [DOI] [PubMed] [Google Scholar]
- 8.Loisel P., Lemaire J., Poitras S., Durand M.J., Champagne F., Stock S. Cost–benefit and cost-effectiveness analysis of a disability prevention model for back pain management: a six year follow up study. Occup Environ Med. 2002;59:807–815. doi: 10.1136/oem.59.12.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connell G.D., Newman I.B., Carapezza M.A. Effect of long-term osmotic loading culture on matrix synthesis from intervertebral disc cells. BioResearch Open Access. 2014;3:242–249. doi: 10.1089/biores.2014.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alini M., Eisenstein S.M., Ito K., Little C., Kettler A.A., Masuda K. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panjabi M.M., Oxland T.R., Yamamoto I., Crisco J.J. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load–displacement curves. J Bone Joint Surg Am. 1994;76:413–424. doi: 10.2106/00004623-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Gantenbein B., Calandriello E., Wuertz-Kozak K., Benneker L.M., Keel M.J., Chan S.C. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskelet Disord. 2014;15:422. doi: 10.1186/1471-2474-15-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Halloran D.M., Pandit A.S. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13:1927–1954. doi: 10.1089/ten.2005.0608. [DOI] [PubMed] [Google Scholar]
- 14.Antoniou J., Steffen T., Nelson F., Winterbottom N., Hollander A.P., Poole R.A. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiler C., Nerlich A.G., Zipperer J., Bachmeier B.E., Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Maitre C.L., Freemont A.J., Hoyland J.A. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 17.Gruber H.E., Ingram J.A., Norton H.J., Hanley E.N., Jr. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- 18.Raj P.P. Intervertebral disc: anatomy–physiology–pathophysiology–treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Chee A., Thonar E.J., An H.S. Intervertebral disk repair by protein, gene, or cell injection: a framework for rehabilitation-focused biologics in the spine. PM R. 2011;3:S88–S94. doi: 10.1016/j.pmrj.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Siepe C.J., Heider F., Wiechert K., Hitzl W., Ishak B., Mayer M.H. Mid- to long-term results of total lumbar disc replacement: a prospective analysis with 5- to 10-year follow-up. Spine J. 2014;14:1417–1431. doi: 10.1016/j.spinee.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Hilibrand A.S., Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190s–194s. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Gruber H.E., Ingram J.A., Davis D.E., Hanley E.N., Jr. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J. 2009;9:210–215. doi: 10.1016/j.spinee.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Sakai D., Nakamura Y., Nakai T., Mishima T., Kato S., Grad S. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Illien-Junger S., Pattappa G., Peroglio M., Benneker L.M., Stoddart M.J., Sakai D. Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine. 2012;37:1865–1873. doi: 10.1097/BRS.0b013e3182544a8a. [DOI] [PubMed] [Google Scholar]
- 25.Henriksson H.B., Svala E., Skioldebrand E., Lindahl A., Brisby H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine. 2012;37:722–732. doi: 10.1097/BRS.0b013e318231c2f7. [DOI] [PubMed] [Google Scholar]
- 26.Sakai D., Nishimura K., Tanaka M., Nakajima D., Grad S., Alini M. Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study. Spine J. 2015;15:1356–1365. doi: 10.1016/j.spinee.2013.07.491. [DOI] [PubMed] [Google Scholar]
- 27.Tzaan W.C., Chen H.C. Investigating the possibility of intervertebral disc regeneration induced by granulocyte colony stimulating factor-stimulated stem cells in rats. Adv Orthop. 2011;2011:602089. doi: 10.4061/2011/602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco J.F., Graciani I.F., Sanchez-Guijo F.M., Muntion S., Hernandez-Campo P., Santamaria C. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine. 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 29.Haufe S.M., Mork A.R. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15:136–137. doi: 10.1089/scd.2006.15.136. [DOI] [PubMed] [Google Scholar]
- 30.Meisel H.J., Siodla V., Ganey T., Minkus Y., Hutton W.C., Alasevic O.J. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Meisel H.J., Ganey T., Hutton W.C., Libera J., Minkus Y., Alasevic O. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15:S397–S405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshikawa T., Ueda Y., Miyazaki K., Koizumi M., Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine. 2010;35:E475–E480. doi: 10.1097/BRS.0b013e3181cd2cf4. [DOI] [PubMed] [Google Scholar]
- 33.Orozco L., Soler R., Morera C., Alberca M., Sanchez A., Garcia-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822–828. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 34.Pettine K.A., Murphy M.B., Suzuki R.K., Sand T.T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33:146–156. doi: 10.1002/stem.1845. [DOI] [PubMed] [Google Scholar]
- 35.Coric D., Pettine K., Sumich A., Boltes M.O. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine. 2013;18:85–95. doi: 10.3171/2012.10.SPINE12512. [DOI] [PubMed] [Google Scholar]
- 36.US National Library of Medicine—ClinicalTrials.gov 2013 Available at: https://clinicaltrials.gov/ct2/show/NCT01771471. [Accessed 25 November 2016].
- 37.Adis Insight – Chondrocyte disc regeneration therapy – Isto Biologics. Available at: http://adisinsight.springer.com/drugs/800029145 [Accessed 26 November 2016]
- 38.Mochida J., Sakai D., Nakamura Y., Watanabe T., Yamamoto Y., Kato S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cells Mater. 2015;29:202–212. doi: 10.22203/ecm.v029a15. discussion 12. [DOI] [PubMed] [Google Scholar]
- 39.Elabd C., Centeno C.J., Schultz J.R., Lutz G., Ichim T., Silva F.J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016;14:253. doi: 10.1186/s12967-016-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US National Library of Medicine—ClinicalTrials.gov 2012 Available at: https://clinicaltrials.gov/ct2/show/study/NCT01513694. [Accessed 25 November 2016].
- 41.US National Library of Medicine—ClinicalTrials.gov 2015 Available at: https://clinicaltrials.gov/ct2/show/NCT02440074. [Accessed 25 November 2016].
- 42.US National Library of Medicine—ClinicalTrials.gov 2013 Available at: https://clinicaltrials.gov/ct2/show/NCT01860417. [Accessed 25 November 2016].
- 43.US National Library of Medicine—ClinicalTrials.gov 2011 Available at: https://clinicaltrials.gov/ct2/show/NCT01290367. [Accessed 25 November 2016].
- 44.Ghosh P., Moore R., Vernon-Roberts B., Goldschlager T., Pascoe D., Zannettino A. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16:479–488. doi: 10.3171/2012.1.SPINE11852. [DOI] [PubMed] [Google Scholar]
- 45.Gronthos S., Fitter S., Diamond P., Simmons P.J., Itescu S., Zannettino A.C. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007;16:953–963. doi: 10.1089/scd.2007.0069. [DOI] [PubMed] [Google Scholar]
- 46.US National Library of Medicine—ClinicalTrials.gov 2015 Available at: https://clinicaltrials.gov/ct2/show/NCT02412735. [Accessed 25 November 2016].
- 47.Tschugg A., Michnacs F., Strowitzki M., Meisel H.J., Thome C. A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART Disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: study protocol for a randomized controlled trial. Trials. 2016;17:108. doi: 10.1186/s13063-016-1239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.EU Clinical Trials Register—clinicaltrialregister.eu, 2012 Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-023830-22/AT. [Accessed 25 November 2016].
- 49.US National Library of Medicine—ClinicalTrials.gov 2012 Available at: https://clinicaltrials.gov/ct2/show/NCT01640457. [Accessed 25 November 2016].
- 50.US National Library of Medicine—ClinicalTrials.gov 2015 Available at: https://clinicaltrials.gov/ct2/show/NCT02529566. [Accessed 25 November 2016].
- 51.EU Clinical Trials Register—clinicaltrialregister.eu, 2013 Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-003160-44/DK. [Accessed 25 November 2016].
- 52.US National Library of Medicine—ClinicalTrials.gov 2015 Available at: https://clinicaltrials.gov/ct2/show/NCT02338271. [Accessed 25 November 2016].
- 53.US National Library of Medicine—ClinicalTrials.gov 2012 Available at: https://clinicaltrials.gov/ct2/show/record/NCT01643681. [Accessed 25 November 2016].
- 54.US National Library of Medicine—ClinicalTrials.gov, 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02097862. [Accessed 25 November 2016].
- 55.Huang Y.C., Urban J.P., Luk K.D. Intervertebral disc regeneration: do nutrients lead the way? Nat Rev Rheumatol. 2014;10:561–566. doi: 10.1038/nrrheum.2014.91. [DOI] [PubMed] [Google Scholar]
- 56.Muftuler L.T., Jarman J.P., Yu H.J., Gardner V.O., Maiman D.J., Arpinar V.E. Association between intervertebral disc degeneration and endplate perfusion studied by DCE-MRI. Eur Spine J. 2015;24:679–685. doi: 10.1007/s00586-014-3690-3. [DOI] [PubMed] [Google Scholar]
- 57.Risbud M.V., Guttapalli A., Stokes D.G., Hawkins D., Danielson K.G., Schaer T.P. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 58.Risbud M.V., Schipani E., Shapiro I.M. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–1583. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gogate S.S., Nasser R., Shapiro I.M., Risbud M.V. Hypoxic regulation of beta-1,3-glucuronyltransferase 1 expression in nucleus pulposus cells of the rat intervertebral disc: role of hypoxia-inducible factor proteins. Arthritis Rheum. 2011;63:1950–1960. doi: 10.1002/art.30342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oldershaw R.A., Baxter M.A., Lowe E.T., Bates N., Grady L.M., Soncin F. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 61.Chen J., Lee E.J., Jing L., Christoforou N., Leong K.W., Setton L.A. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One. 2013;8:e75548. doi: 10.1371/journal.pone.0075548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vadala G., Sowa G., Hubert M., Gilbertson L.G., Denaro V., Kang J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–355. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 63.Nomura T., Mochida J., Okuma M., Nishimura K., Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;(389):94–101. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Michalek A.J., Buckley M.R., Bonassar L.J., Cohen I., Iatridis J.C. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010;10:1098–1105. doi: 10.1016/j.spinee.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pereira D.R., Silva-Correia J., Oliveira J.M., Reis R.L. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J Tissue Eng Regen Med. 2013;7:85–98. doi: 10.1002/term.500. [DOI] [PubMed] [Google Scholar]
- 66.Serigano K., Sakai D., Hiyama A., Tamura F., Tanaka M., Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267–1275. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 67.Davies B.M., Atkinson R.A., Ludwinski F., Freemont A.J., Hoyland J.A., Gnanalingham K.K. Qualitative grading of disc degeneration by magnetic resonance in the lumbar and cervical spine: lack of correlation with histology in surgical cases. Br J Neurosurg. 2016;30:414–421. doi: 10.3109/02688697.2016.1161174. [DOI] [PubMed] [Google Scholar]
- 68.Brayda-Bruno M., Tibiletti M., Ito K., Fairbank J., Galbusera F., Zerbi A. Advances in the diagnosis of degenerated lumbar discs and their possible clinical application. Eur Spine J. 2014;23(Suppl. 3):S315–S323. doi: 10.1007/s00586-013-2960-9. [DOI] [PubMed] [Google Scholar]
- 69.Mader K.T., Peeters M., Detiger S.E., Helder M.N., Smit T.H., Le Maitre C.L. Investigation of intervertebral disc degeneration using multivariate FTIR spectroscopic imaging. Faraday Discuss. 2016;187:393–414. doi: 10.1039/c5fd00160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang A.M., Cao P., Yee A., Chan D., Wu E.X. Detection of extracellular matrix degradation in intervertebral disc degeneration by diffusion magnetic resonance spectroscopy. Magn Reson Med. 2015;73:1703–1712. doi: 10.1002/mrm.25289. [DOI] [PubMed] [Google Scholar]
- 71.Grad S., Bow C., Karppinen J., Luk K.D., Cheung K.M., Alini M. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cells Mater. 2016;31:1–10. doi: 10.22203/ecm.v031a01. [DOI] [PubMed] [Google Scholar]
- 72.Malik K.M., Cohen S.P., Walega D.R., Benzon H.T. Diagnostic criteria and treatment of discogenic pain: a systematic review of recent clinical literature. Spine J. 2013;13:1675–1689. doi: 10.1016/j.spinee.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 73.Huijnen I.P., Rusu A.C., Scholich S., Meloto C.B., Diatchenko L. Subgrouping of low back pain patients for targeting treatments: evidence from genetic, psychological, and activity-related behavioral approaches. Clin J Pain. 2015;31:123–132. doi: 10.1097/AJP.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 74.Nukaga T., Sakai D., Tanaka M., Hiyama A., Nakai T., Mochida J. Transplantation of activated nucleus pulposus cells after cryopreservation: efficacy study in a canine disc degeneration model. Eur Cells Mater. 2016;31:95–106. doi: 10.22203/ecm.v031a07. [DOI] [PubMed] [Google Scholar]