Summary
Background/Objective
The participation of sensory neural regulation in bone metabolism has been widely studied. However, the physiological role of sensory neural regulation in the functional adaptation to weight bearing is not clear. This study was conducted to investigate the effect of capsaicin-induced sensory neuron lesions on cancellous architecture properties in a hindlimb suspension (HLS) model.
Methods
Thirty-two female rats were randomly assigned to four groups. Groups b and d underwent systemic capsaicin treatment, whereas Groups a and c were treated with vehicle. Then, Groups c and d were subjected to HLS, whereas Groups a and b were allowed hindlimbs full loading. The proximal trabecular and mid-shaft cortical bone structure were evaluated via microcomputed tomography, and the biomechanical properties of the tibial mid-shaft were assessed using the four-point bending test.
Results
The trabecular bone volume was reduced by 40% and 50% in Groups b and c, respectively, and was also reduced significantly in Group d. Trabecular thickness and trabecular separation in Group b were not significantly different from those of Group a. The cortical bone area fraction showed no significant difference among all groups. Compared with Group a, the ultimate strength in Group b decreased by 20.3%, whereas it did not change significantly in Group c.
Conclusion
The results suggest that capsaicin-sensitive sensory neurons play an important role in bone modelling. The effect of capsaicin is similar to HLS. However, HLS has no add-on effect to capsaicin in the reduction of bone density and mechanical properties.
Translational potential of this article: This study gives clues to the function of sensory neurons in bone modelling.
Keywords: bone, capsaicin, hindlimb suspension, sensory neuron
Introduction
It has been demonstrated that bone tissue is highly sensitive to mechanical stress and can change its shape, structure, and mineral density. The mechanism of bone loss due to immobilisation, paralysis, long-term bed rest, and spaceflight, and the mechanism of bone mass increase owing to regular resistance exercises indicate that bone turnover is sensitive to both external loads arising from gravitational loading and to internal loads generated by muscle activity [1], [2]. The ability of bone to sense the mechanical stimuli was considered to be a local interaction between the loading and the affected bone cells [3]. However, the participation of neural regulation has been demonstrated in both local and systemic bone metabolism based on the innervation of the sympathetic and peripheral sensory neurons in bone via osteoblastic and osteoclastic cell guidance [4], [5], [6]. Substance P (SP) and calcitonin gene-related peptide (CGRP), which are important neuropeptides, are synthesised in unmyelinated sensory neurons, which are the target of capsaicin, and released from their peripheral terminals [7]. It has been demonstrated that bone integrity was compromised by decreased levels of local neuropeptide in bone in some hereditary sensory neuropathies, such as familial dysautonomia [8], [9]. The innervation of the developing mouse femur was guided by nerve growth factor–neurotrophic tyrosine kinase receptor type 1 signalling, in turns, to promote vascular invasion of the ossification centres and osteoprogenitor cell lineage progression [6].
Capsaicin is the major pungent component of hot chili peppers. Transient receptor potential (TRP) vanilloid subfamily member 1 (TRPV1) is identified as the receptor of capsaicin [10]. TRPV1 is expressed in unmyelinated and small-diameter myelinated sensory neurons [11]. The activation of TRPV1 by capsaicin induces Ca2+ and Na+ influx into the sensory neuron, causing an excitotoxic effect. Large sensory neurons, motor neurons, and sympathetic neurons are affected by lack of vanilloid receptors [12], [13]. It has been demonstrated that only the unmyelinated and small-diameter myelinated sensory neurons are destroyed when capsaicin is administered in neonatal rats, whereas the large sensory afferent, motor, and sympathetic fibres are unaffected [14], [15]. Capsaicin treatment could deplete SP and CGRP in peripheral nerves, but not in the central nervous system [16], [17]. The depletion rate of the unmyelinated fibres in adult rat induced by capsaicin injection has been reported to reach 90–95% [18]. In addition, systemic capsaicin treatment of the adult rat results in the death of at least 50% of the vagal afferent neurons in dorsal root ganglia [19].
Mechanical stimulation influences bone metabolism. However, will there be any difference in the response if the sensory nerve fibres in bone tissue are partially or completely destroyed? The aim of this study is to investigate the role of capsaicin-sensitive sensory neurons in bone modelling in a rat hindlimb suspension (HLS) model. In this study, the unmyelinated sensory fibres were depleted by systemic capsaicin treatment under a functional disuse HLS condition. The bone structure and biomechanical properties of the rat tibia were evaluated to assess the bone response to loading changes after capsaicin treatment.
Materials and methods
Animals
The in vivo experiment was approved by the Institutional Animal Care and Use Committee (IACUC), Stony Brook University (Stony Brook, NY, USA). Thirty-two 4-month-old female Sprague–Dawley rats weighing 245 ± 15 g were used in the study. The animals, which were randomly assigned in equal numbers (n = 8) to four groups, received different interventions as follows: Group a, control; Group b, capsaicin only; Group c, HLS only; Group d, combination (HLS after capsaicin treatment). All animals were raised in separate cages in a temperature-controlled room (22°C) with a 12:12-hour light–dark cycle. Standard rodent chow and water were provided ad libitum throughout the experiment. All experimental procedures were in accordance with the IACUC guidelines.
Capsaicin treatment
The rats were injected subcutaneously in the back with capsaicin (Sigma, St. Louis, MO, USA). To prevent reflux from the needle tract, the needle was left in the skin for 60 seconds after the injection. The capsaicin treatment protocol consisted of three injections. The initial one was 25 mg/kg, the second one was 50 mg/kg at 6 hours later, and the third one was 50 mg/kg at 24 hours after the first injection [20]. To prevent pulmonary oedema, all rats in the capsaicin treatment groups (Groups b and d) had no water supply 6 hours prior to the capsaicin injection [21]. The protocol was repeated every 2 weeks (Weeks 1, 3, 5, and 7), and all experiments were performed during Weeks 5 to 8. The groups without capsaicin treatment (Groups a and c) underwent the same injection protocol with vehicle (10% Tween 80, 10% ethanol and 80% saline).
Hindlimb suspension
The rats in Groups c and d were hindlimb suspended for 4 weeks during Weeks 5 to 8 following established procedures [22]. Briefly, the animal’s tail was cleaned with 70% ethanol and coated with tincture of benzoin. Once the tail is dry and sticky, a strip of adhesive tape (15 cm × 0.5 cm) was applied to the animal’s tail. The tape was then attached to a swivel apparatus that was suspended from the top of the cage. An approximately 30° head-down tilt was set to prevent contact of the animal's hindlimbs with the cage bottom. The animal’s forelimbs were allowed full access to the entire cage bottom.
Bone microarchitecture
All animals were sacrificed at the end of Week 8. The right tibia of each rat was removed and fixed in 70% ethanol. The proximal and midshaft tibial were scanned to evaluate trabecular and cortical microarchitecture using a microcomputed tomography system (μCT) (μCT 40; Scanco Medical AG, Basserdorf, Switzerland). The scanned region corresponded to a zone of 400 transverse slices below the growth plate of the proximal tibia and 200 transverse slices above the tibia–fibula junction. From the acquired data, the region of interest was delineated 1.5 mm below growth plate up to a height of 0.6 mm (50 slices) for trabecular bone and 1.0 mm above the tibia–fibula junction up to a height of 0.6 mm (50 slices) for cortical bone. For image smoothing purposes and to define the desired analysed objects, all images were evaluated using Gaussian filter, with specific sigma and support values of 0.1 and 1, respectively. To discriminate between bone and background, the threshold values for trabecular and cortical bone were 290 and 400, respectively. In trabecular bone, bone volume fraction (BV/TV), trabecular number (Tb.N; mm), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm) were evaluated. And cortical bone area fraction (Ct.A/TA) was evaluated to analyse the cortical bone structure.
Biomechanical test
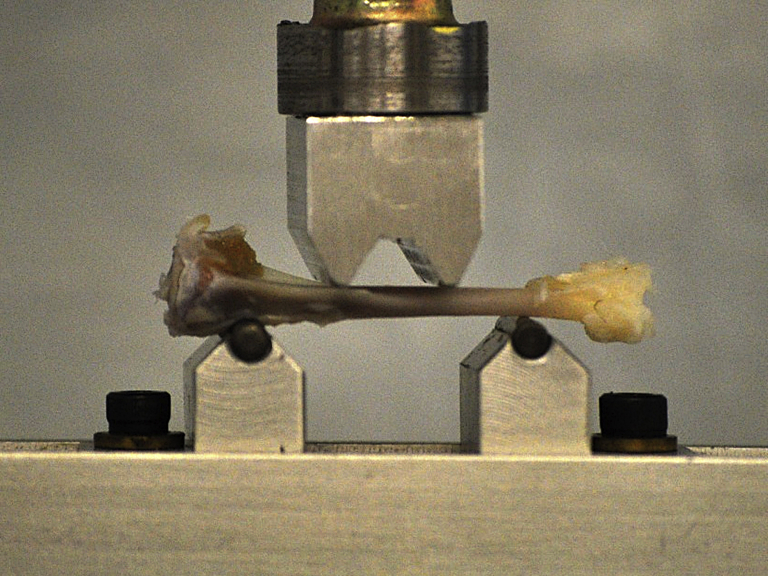
After μCT scanning, all tibiae were subjected to the four-point bending test to evaluate the biomechanical properties using the materials testing system (MTS Systems Corporation, Eden Prairie, MN, USA) following the established procedure [23]. The tibia was placed on its lateral surface on two rounded supporting bars that were 20 mm from each other. A preload of 1 N was applied by lowering the upper bar. A constant displacement rate of 6 mm/min was applied until bone break (Figure 1). The maximum load (ultimate load), displacement at ultimate load, the slope in the linear region of the ultimate load (extrinsic stiffness), and the area under the curve until ultimate load (energy to ultimate load) were determined.
Figure 1.
Tibia midshaft underwent the four-point bending test to evaluate the biomechanical properties of bone.
Statistical analysis
All data are presented as the means ± standard error of mean, and the statistical analysis of the data from the μCT and the four-point bending test was performed using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Statistical significance was considered when p < 0.05. SPSS 17.0 (SPSS Inc., Chicago, USA) was used to perform the data analysis.
Results
Bone microarchitecture
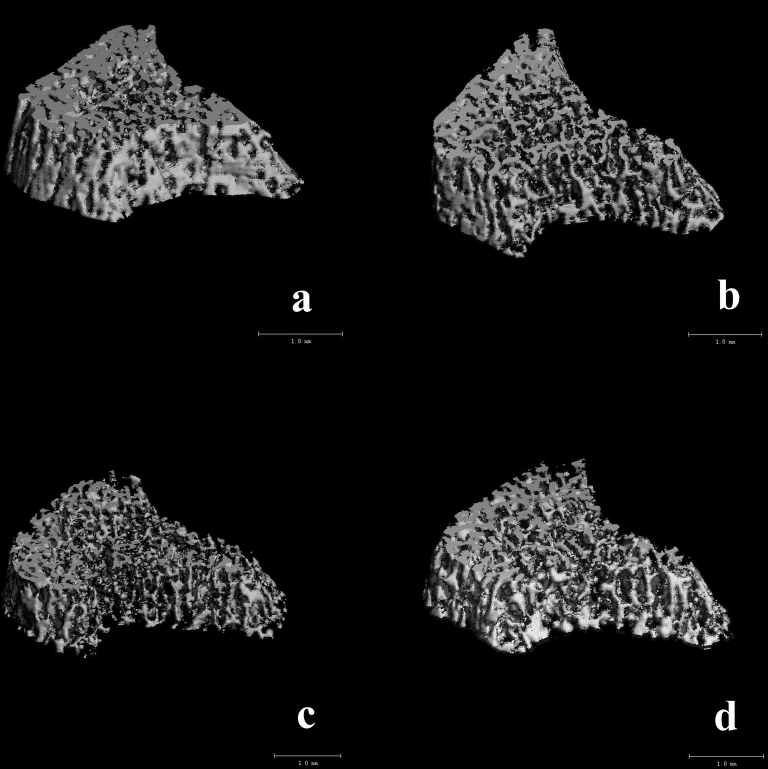
As shown in Table 1, one-way ANOVA showed that the effect of the intervention was significant in BV/TV (p = 0.003), Tb.N (p = 0.001), Tb.Th (p = 0.011), and Tb.Sp (p = 0.014), but not in Ct.A/TA (p = 0.325). Analyses using the Tukey’s post hoc test indicated that BV/TV reduced significantly in Group b (40%, p = 0.039), Group c (50%, p = 0.003), and Group d (45%, p = 0.008) compared with the normal control group (Group a). Tb.Th reduced significantly in Group c (p = 0.012), but not in Group b (p = 0.190) and Group d (p = 0.070). Tb.Sp increased significantly in Group d (p = 0.025), but not in Group b (p = 0.184) and Group c (p = 0.183). The 3D reconstruction μCT images (Figure 2) showed the different types of bone loss in Groups b and c; the trabecular bone loss in Group b was homogenous, whereas in Group c the bone loss was more severe in certain areas.
Table 1.
Bone microarchitecture of tibia.
| Group | BV/TV | Tb.N (mm) | Tb.Th (mm) | Tb.Sp (mm) | Ct.A/TA |
|---|---|---|---|---|---|
| a: Control | 0.40 ± 0.04 | 8.46 ± 0.58 | 0.08 ± 0.005 | 0.12 ± 0.009 | 0.994 ± 0.001 |
| b: Capsaicin | 0.24 ± 0.02∗ | 6.28 ± 0.35∗ | 0.07 ± 0.003 | 0.16 ± 0.011 | 0.993 ± 0.003 |
| c: HLS | 0.20 ± 0.02∗∗ | 6.25 ± 0.37∗ | 0.06 ± 0.001∗ | 0.17 ± 0.009 | 0.995 ± 0.001 |
| d: HLS + C | 0.22 ± 0.03∗∗ | 5.88 ± 0.45∗∗ | 0.06 ± 0.004 | 0.18 ± 0.015∗ | 0.994 ± 0.001 |
BV/TV = trabecular bone, bone volume fraction; Ct.A/A = cortical bone area fraction; HLS = hindlimb suspension; Tb.N = trabecular number; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness.
p < 0.05 compared with the control group.
p < 0.01 compared with the control group.
Figure 2.
Three-dimensional reconstruction of microcomputed tomography (μCT) images of cancellous bone of proximal tibia in Groups a (control), b (Capsaicin), c (HLS) and d (HLS + Capsaicin).
Biomechanical test
Table 2 shows the bone mechanical property parameters. One-way ANOVA showed that the effects of interventions were significant in ultimate load (p = 0.010), displacement (p = 0.008), extrinsic stiffness (p = 0.014), and energy to ultimate load (p = 0.013). Tukey’s post hoc test indicated that compared with Group a, the ultimate load reduced significantly in Group b (20.3%, p = 0.023), whereas the ultimate load in Group c was not significantly changed (p = 0.830). The displacement increased significantly in Group d (48%, p = 0.012), whereas those in Groups b and c were not significantly changed. The stiffness of bones reduced significantly in Group b (20.9%, p = 0.030) compared with Group a. The energy to ultimate load in Group d was 68% (p = 0.006) higher than that in Group b.
Table 2.
Biomechanical properties of tibia according to the four-point bending test.
| Group | Load (N) | Disp. (mm) | Stiffness (N/mm) | Energy (mJ) |
|---|---|---|---|---|
| a: Control | 99.0 ± 6.6 | 0.68 ± 0.09 | 176.9 ± 13.7 | 67.7 ± 11.9 |
| b: Capsaicin | 78.9 ± 1.6∗ | 0.66 ± 0.08 | 140.0 ± 8.6∗ | 54.4 ± 7.8 |
| c: HLS | 101.5 ± 6.6 | 0.63 ± 0.03 | 179.1 ± 10.6 | 65.3 ± 7.5 |
| d: HLS+C | 89.5 ± 4.1 | 1.01 ± 0.14∗ | 159.7 ± 13.1 | 91.4 ± 15.5∗∗ |
Disp. = displacement.
p < 0.05 compared with the control group.
p < 0.01 compared with the capsaicin group.
Discussion
The results have shown that both capsaicin and HLS can result in dramatic bone loss according to the BV/TV data in the proximal tibia trabecular bone. Capsaicin treatment causes bone loss that was close to the magnitude of bone loss caused by HLS. However, interestingly, HLS after capsaicin treatment does not lead to a further decrease in bone density.
HLS is a model that causes mechanical unloading of the hindlimbs [24]. It has been reported that HLS results in large reduction in bone formation and cancellous bone loss in both sexes, and the mechanisms are similar in both sexes [25]. Mechanical strain [26] and fluid shear stress [27] are the two most commonly accepted mechanisms for coupling the loading with bone formation changes. The current theory about the mechanisms of bone reaction to mechanical stimulus considers that mechanical strain to the cell membrane activates stretch-sensitive ion channels and other membrane-associated proteins such as integrins, which are involved in various cellular processes and structures [28]. According to the mechanostat theory, when the loading of bone is insufficient to produce bone strains that are above a minimum level of effective strain, bone mass and architecture are adjusted until bone strains are within the minimum effective strain range; as a result, bone resorption prevails over bone formation [29]. The fluid flow through cancellous bone and the lacuna canalicular network of cortical bone caused by mechanical loading exacerbates the flow-induced shear stress applied on surface bone cells [30]. Regardless of the mechanical strain or the fluid shear stress, bone cells respond through autocrine or paracrine signals that regulate remodelling process [31], [32], [33]. The cells detect the changes in fluid flow and physical deformation. Osteocytes and osteoblasts on the bone surface are the mechanosensors of bone. After a mechanical stimulus, these cells release prostaglandins and nitric oxide that promote bone formation and inhibit bone resorption, respectively [34]. Thus, when under an unloading situation, bone resorption is uncoupled from bone formation, contributing to the resultant bone loss.
Capsaicin, a neurotoxic agent, induces sensory denervation by inducing the death of most small dorsal root ganglion cells and leads to a loss of unmyelinated sensory axons [35]. Unmyelinated sensory neurons contain a variety of transmitter peptides, such as CGRP and SP. CGRP has been reported to inhibit osteoclastogenesis [36] and bone resorption [37] and to stimulate osteoblasts proliferation and bone formation [38], [39]. SP could stimulate osteoclast resorption and is involved in the pathogenesis of bone changes [40]. SP can both inhibit and stimulate bone formation in vitro [41], [42], and the absence of SP reduces the bone formation rate in an adult model of endochondral ossification [43]. Some studies suggest that neuronal SP during bone regeneration has a role in bone formation, whereas during remodelling, increased SP fibre density in unloaded areas may be related to bone resorption [44]. However, because there are less SP-containing nerve fibres than CGRP-containing nerve fibres [45], the capsaicin-sensitive sensory nerve fibres could be considered to be CGRP-positive nerve fibres. The depletion of CGRP-positive nerve fibres after regular capsaicin treatment inhibits bone formation and enhances bone resorption [46]. In the current study, the bone mass of tibia cancellous bone also decreased significantly after capsaicin treatment, even though the hindlimbs were fully loaded. This finding suggests that normal mechanical loading does not necessarily lead to normal balance of bone remodelling. HLS does not lead to additional bone loss after depletion of CGRP-positive nerve fibres by capsaicin treatment. A previous study [47] has demonstrated that sciactomy decreased the density of CGRP-positive nerve fibres in tibia fracture callus and resulted in low response of callus to ultrasound stimulation, which is well known in accelerating fracture healing in bone with normal innervations [48].
The μCT result showed that Tb.Th in the HLS group was significantly lower than that in the control group, whereas Tb.Th in the capsaicin group was not significantly different from that of the control group. In addition, the 3D reconstruction μCT image revealed the different types of bone loss in these two groups. The difference between the capsaicin group and the HLS group suggests that CGRP-positive nerve fibres may not be the only mechanosensors in bone. In addition, results from the biomechanical tests showing that the ultimate load of the capsaicin group was lower compared with that of the control group, whereas the ultimate load of the HLS group was not significantly changed could be an evidence of the existence of different mechanisms.
The effect of capsaicin on sensory neurons varies with age, strain, and species. Ding et al [21] used a single dosage of 150 mg/kg to treat rats and achieved a 16.9% decrease in BV/TV in the proximal trabecular bone. Offley et al [20] used a dosage of 125 mg/kg to treat rats once every 2 weeks for 4 weeks, and the BV/TV was reduced by 28%. In addition, the present study used the same dosage as that used by Offley et al [20], but the animals were treated four times; this resulted in a 40% reduction in BV/TV. These findings imply that the effect of capsaicin on bone might be dose- and duration-dependent. The dosage and duration should also be taken into consideration while evaluating the effect of capsaicin treatment.
In conclusion, capsaicin-sensitive sensory neurons play an essential role in bone remodelling. While the reduction of bone density is similar between capsaicin treatment and HLS groups, the different bone microarchitecture and biomechanical properties results in capsaicin and disuse groups imply that capsaicin-sensitive sensory neurons are not the sole mechanism of induced bone loss.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study has been kindly supported by the National Institute of Health (AR49286, AR52379, and AR61821), the National Space Biomedical Research Institute through NASA Cooperative Agreement NCC 9-58 (SMST01603), NYSTAR, and Hong Kong Polytechnic University (G-YBBL) Study Abroad Program. The authors also thank the kind assistance from Fred Serra-Hsu, Liangjun Lin, Minyi Hu, Alyssa Tuthill and Jiqi Cheng.
References
- 1.Qin Y.X., Hu M. Mechanotransduction in musculoskeletal tissue regeneration: effects of fluid flow, loading, and cellular–molecular pathways. Biomed Res Int. 2014 doi: 10.1155/2014/863421. http://dx.doi.org/10.1155/2014/863421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.L., Tseng S.Y., Chen C.N., Liao W.C., Wang C.H., Lee M.C. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging. 2013;8:1603–1609. doi: 10.2147/CIA.S53591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein-Nulend J., Bacabac R.G., Bakker A.D. Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur Cells Mater. 2012;24:278–291. doi: 10.22203/ecm.v024a20. [DOI] [PubMed] [Google Scholar]
- 4.Takeda S., Elefteriou F., Lavasseur R., Liu X., Zhao L., Parker K.L. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 5.Sample S.J., Collin R.J., Wilson A.P., Racette M.A., Behan M., Markel M.D. Systemic effects of ulna loading in male rats during functional adaptation. J Bone Miner Res. 2010;25:2016–2028. doi: 10.1002/jbmr.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomlinson R.E., Li Z., Zhang Q., Goh B.C., Thorek D.L., Rajbhandari L. NGF–TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 2016;16:2723–2735. doi: 10.1016/j.celrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardini N., Neuhuber W., Reeh P.W., Sauer S.K. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. doi: 10.1016/j.neuroscience.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich P., Dragatsis I. Familial dysautonomia: mechanisms and models. Genet Mol Biol. 2016;39:497–514. doi: 10.1590/1678-4685-GMB-2015-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maayan C., Bar-On E., Foldes A.J., Gesundheit B., Pollak R.D. Bone mineral density and metabolism in familial dysautonomia. Osteoporos Int. 2002;13:429–433. doi: 10.1007/s001980200050. [DOI] [PubMed] [Google Scholar]
- 10.Pingle S.C., Matta J.A., Ahern G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 11.de la Roche J., Walther I., Leonow W., Hage A., Eberhardt M., Fischer M. Lactate is a potent inhibitor of the capsaicin receptor TRPV1. Sci Rep. 2016;6:36740. doi: 10.1038/srep36740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frias B., Merighi A. Capsaicin, nociception and pain. Molecules. 2016:21. doi: 10.3390/molecules21060797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helliwell R.J.A., McLatchie L.M., Clarke M., Winter J., Bevan S., McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 14.Nakao A., Takahashi Y., Nagase M., Ikeda R., Kato F. Role of capsaicin-sensitive C-fiber afferents in neuropathic pain-induced synaptic potentiation in the nociceptive amygdala. Mol Pain. 2012;8:51. doi: 10.1186/1744-8069-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy J.I., Iversen L.L., Goedert M., Chapman D., Hunt S.P. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci. 1983;3:399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foss J.D., Wainford R.D., Engeland W.C., Fink G.D., Osborn J.W. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–R122. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madasu M.K., Roche M., Finn D.P. Supraspinal transient receptor potential subfamily V member 1 (TRPV1) in pain and psychiatric disorders. Mod Trends Pharmacopsychiatry. 2015;30:80–93. doi: 10.1159/000435934. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez-Andrade J.M., Bloom A.P., Mantyh W.G., Koewler N.J., Freeman K.T., Delong D. Capsaicin-sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience. 2009;162:1244–1254. doi: 10.1016/j.neuroscience.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czaja K., Burns G.A., Ritter R.C. Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats. Neuroscience. 2008;154:621–630. doi: 10.1016/j.neuroscience.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Offley S.C., Guo T.Z., Wei T., Clark J.D., Vogel H., Lindsey D.P. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005;20:257–267. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y., Arai M., Kondo H., Togari A. Effects of capsaicin-induced sensory denervation on bone metabolism in adult rats. Bone. 2010;46:1591–1596. doi: 10.1016/j.bone.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z.K., Li J., Liu J., Guo B., Leung A., Zhang G. Icaritin requires phosphatidylinositol 3 kinase (PI3K)/Akt signaling to counteract skeletal muscle atrophy following mechanical unloading. Sci Rep. 2016;6:20300. doi: 10.1038/srep20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwers J.E., van Reitbergen B., Ito K., Huiskies R. Effects of vibration treatment on tibial bone of ovariectomized rats analyzed by in vivo micro-CT. J Orthop Res. 2010;28:62–69. doi: 10.1002/jor.20951. [DOI] [PubMed] [Google Scholar]
- 24.Morey-Holton E.R., Globus R.K. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 25.Hefferan T.E., Evans G.L., Lotinun S., Zhang M., Morey-Holton E., Turner R.T. Effect of gender on bone turnover in adult rats during simulated weightlessness. J Appl Physiol. 1985;2003(95):1775–1780. doi: 10.1152/japplphysiol.00455.2002. [DOI] [PubMed] [Google Scholar]
- 26.Brown G.N., Sattler R.L., Guo X.E. Experimental studies of bone mechanoadaptation: bridging in vitro and in vivo studies with multiscale systems. Interface Focus. 2016;6:20150071. doi: 10.1098/rsfs.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittkowske C., Reilly G.C., Lacroix D., Perrault C.M. In vitro bone cell models: impact of fluid shear stress on bone formation. Front Bioeng Biotechnol. 2016;4:87. doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosa N., Simoes R., Magalhães F.D., Marques A.T. From mechanical stimulus to bone formation: a review. Med Eng Phys. 2015;37:719–728. doi: 10.1016/j.medengphy.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Lerebours C., Buenzli P.R. Towards a cell-based mechanostat theory of bone: the need to account for osteocyte desensitisation and osteocyte replacement. J Biomech. 2016;49:2600–2606. doi: 10.1016/j.jbiomech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Kwon R.Y., Meays D.R., Tang W.J., Frangos J.A. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res. 2010;25:1798–1807. doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J., Zhang T., Mi L., Zhang B., Wang B., Yang L. Stepwise increasing and decreasing fluid shear stresses differentially regulate the functions of osteoblasts. Cell Mol Bioeng. 2010;3:376–386. doi: 10.1007/s12195-010-0132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moustafa A., Sugiyama T., Prasad J., Prasad G., Zaman G., Gross T.S. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporosis Int. 2012;23:1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam H.Y., Qin Y.X. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–1100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marenzana M., Chenu C. Sympathetic nervous system and bone adaptive response to its mechanical environment. J Musculoskelet Neuronal Interact. 2008;8:111–120. [PubMed] [Google Scholar]
- 35.Dux M., Santha P., Jancso G. The role of chemosensitive afferent nerves and TRP ion channels in the pathomechanism of headaches. Pflugers Arch. 2012;464:239–248. doi: 10.1007/s00424-012-1142-7. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N., Matsuda Y., Sato K., de Jong P.R., Bertin S., Tabeta K. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci Rep. 2016;6:29294. doi: 10.1038/srep29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granholm S., Henning P., Lerner U.H. Comparisons between the effects of calcitonin receptor-stimulating peptide and intermedin and other peptides in the calcitonin family on bone resorption and osteoclastogenesis. J Cell Biochem. 2011;112:3300–3312. doi: 10.1002/jcb.23256. [DOI] [PubMed] [Google Scholar]
- 38.Tian G., Zhang G., Tan Y.H. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin. 2013;34:1467–1474. doi: 10.1038/aps.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J., Ma G., Liu W., Liu Y., Ding Y. The influence of the sensory neurotransmitter calcitonin gene-related peptide on bone marrow mesenchymal stem cells from ovariectomized rats. J Bone Miner Metab. 2016 doi: 10.1007/s00774-016-0780-9. [DOI] [PubMed] [Google Scholar]
- 40.Gaus S., Moriwaki K., Suyama H., Kawamoto M., Yuge O. Capsaicin treatment inhibits osteopenia and heat hyperalgesia induced by chronic constriction injury to the sciatic nerve in rats. Hiroshima J Med Sci. 2003;52:43–51. [PubMed] [Google Scholar]
- 41.Liu H.J., Yan H., Yan J., Li H., Chen L., Han L.R. Substance P promotes the proliferation, but inhibits differentiation and mineralization of osteoblasts from rats with spinal cord injury via RANKL/OPG system. PLoS One. 2016;11:0165063. doi: 10.1371/journal.pone.0165063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda A., Goto T., Kuroishi N., Gunjigake K.K., Kataoka S., Kobayashi S. Hemokinin-1 competitively inhibits substance P-induced stimulation of osteoclast formation and function. Neuropeptides. 2013;47:251–259. doi: 10.1016/j.npep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Niedermair T., Kuhn V., Doranehgard F., Stange R., Wieskotter B., Beckmann J. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014;38:22–35. doi: 10.1016/j.matbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Ahmed M., Bergstom J., Ackermann P., Stark A., Kreicsberg A. Occurrence of substance P in bone repair under different load comparison of straight and angulated fracture in rat tibia. J Orthop Res. 2010;28:1643–1650. doi: 10.1002/jor.21169. [DOI] [PubMed] [Google Scholar]
- 45.Imai S., Matsusue Y. Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc Res Technol. 2002;58:61–69. doi: 10.1002/jemt.10119. [DOI] [PubMed] [Google Scholar]
- 46.Adam C., Llorens A., Baroukh B., Cherruau M., Saffar J.L. Effects of capsaicin-induced sensory denervation on osteoclastic resorption in adult rats. Exp Physiol. 2000;85:62–66. doi: 10.1017/s0958067000019308. [DOI] [PubMed] [Google Scholar]
- 47.Lam W., Guo X., Kwong K., Leung K. Sciatic nerve section decreases callus response to low intensity pulsed ultrasound. Calcified Tissue Int. 2007;80:S80–S81. [Google Scholar]
- 48.Carlson E.J., Save A.V., Slade J.F., 3rd, Dodds S.D. Low-intensity pulsed ultrasound treatment for scaphoid fracture nonunions in adolescents. J Wrist Surg. 2015;4:115–120. doi: 10.1055/s-0035-1549276. [DOI] [PMC free article] [PubMed] [Google Scholar]