Summary
Spinal pain and associated disability is a leading cause of morbidity worldwide that has a strong association with degenerative disc disease (DDD). DDD can begin in early–late adolescence and has a variable course. Biologically based therapies to treat DDD face significant challenges posed by the unique milieu of the environment within the intervertebral discs. Many potential promising therapies are still in the early stages of development with a hostile microenvironment within the disc presenting unique challenges.
The translational potential of this article: Patient selection, reasonable therapeutic goals, approach, and timing will need to be discerned in order to successfully translate potential therapeutics.
Keywords: back pain, disc injections, intervertebral disc disease, stem cell treatment
Introduction
Low back pain is a leading worldwide cause of disability with degenerative disc disease (DDD) being the most common source of low back pain [1]. In fact, evidence of DDD has been found in 40% of volunteers aged younger than 30 years, and this rises to more than 90% by age 55 years [2]. A recent systematic review of chronic back pain reported a prevalence of 4.2% between 24 years and 39 years of age, and amongst those between 20 years and 59 years old the prevalence increased to 19.6% [3]. Other studies involving persons aged older than 18 years reported chronic back pain to a similar to a similar degree at between 3.9% and 10.2%, with several others reporting between 13.1% and 20.3% [3]. A cross-sectional study of 876 family health clinic patients found that risk factors for chronic back pain included female sex, age 30 years or older, lower education status (4 years or less), anxiety, and an occupation requiring high exertion. Furthermore, quality of life and self-rated health scores were significantly worse among individuals with chronic spinal pain [4]. Furthermore, it has been demonstrated that sexual dimorphism exists with respect to DDD and that postmenopausal women are at an increased risk of disc degeneration perhaps because of the impact of the oestrogen receptor on collagen metabolism [5], [6]. Back pain has been reported to be the most common reason for healthcare visits among those with musculoskeletal disorders (more so than hypertension and arthritis) and has the greatest impact and resource use [7]. Back pain is also costly, with 5% of the American workforce missing at least 1 day of work per year, with direct and indirect costs estimated to range between $19.6 billion and $118.8 billion in the USA [1], [7], [8]. Like the lumbar spine, DDD affecting the cervical spine can be painful and disabling; moreover, it is also the main cause of cervical spondylotic myelopathy—the leading cause of spinal neurological impairment in persons aged older than 65 years [9]. DDD may overload segmental muscles, facet joints, and capsules, leading to pain arising from these spinal joints and soft tissues that might otherwise be classified as “muscular” or ill-defined soft tissue pain, perhaps underestimating the impact of DDD and spinal pain [10]. Treatment of spinal pain secondary to DDD is largely afforded by various modes of physical and cognitive behavioural therapy that achieve similar benefits as spinal fusion surgery, leaving the field with no effective disease-modifying therapy [11]. Therefore, new interventions including biologics and/or tissue engineering approaches are currently under intense investigation with a view to being able to influence the course of the disorder [12].
Intervertebral disc degeneration
Homeostatic regulation
The intervertebral disc (IVD) complex is composed of specialised cells and extracellular matrix (ECM) that are able to withstand high tensile strength as well as compressive and off-axis loading that affords the spinal column with strength, flexibility and protection of the spinal cord. In youth, the nucleus pulposus (NP) is gelatinous with a proteoglycan (PG) network rich in aggrecan and collagen type 2. PGs within the IVD NP ECM (principally aggrecan) are highly negatively charged; they bind water molecules and are responsible for the high net swelling pressure unique to the IVD NP. The healthy IVD NP is capable of resisting compression and deformation principally owing to the hydrophilic NP rich in highly negatively charged PGs that strongly bind water molecules. Homeostatic regulation of the healthy NP ECM involves a balance between anabolic and catabolic activity. However, in DDD this normally tightly regulated process becomes dysfunctional, such that ECM-degrading enzymes and proinflammatory molecules lead to progressive degeneration, loss of viable cells, and a fibrocartilaginous degenerative phenotype [13], [14], [15].
Apart from a limited vascular supply to the periphery of the annulus, the inner annulus and NP is hypoxic, ischaemic, aneural, and isolated from the immune system, and represents a unique tissue compartment. The cells within the NP have adapted to this harsh environment by relying upon glycolytic metabolism and diffusion of nutrients and waste products into and out of the NP via the vertebral endplates [16]. With maturity and DDD, the cellular and extracellular phenotype within the IVD NP changes from the youthful highly notochordal composition to one where small, chondrocyte-like cells predominate, where there is a gradual replacement of collagen type 1 and relative loss in collagen type 2 leading to the development of a fibrocartilaginous IVD NP [17]. The vertebral endplates form the superior and inferior boundaries of the IVD and act as diffusible barriers between the bone marrow of the vertebral body and the disc itself. With progressive DDD, the small pores within the endplates calcify, thus compromising their diffusion capacity and further compromising the already delicate molecular exchange within the IVD NP [18], [19].
Genetic and epigenetic influences
Some patients develop DDD to a greater degree than others, and it has been demonstrated that certain genes and/or small nucleotide polymorphisms such as collagen IX, the vitamin D receptor, collagen type 1, aggrecan, matrix metalloproteinase-3, and the interleukin (IL)-1 receptor can influence a patient’s predisposition to DDD [20], [21]. Data concerning the precise mechanisms whereby these genetic anomalies may influence the development and progression are not yet fully understood. However, many of the candidate genes involved with DDD (collagen 1, IX, XI, aggrecan, matrix metalloproteinase-3, and the vitamin D receptor) likely result in the deposition of flawed ECM proteins. In the case of IL-1 and its receptor, it is likely that impaired regulation of inflammation and/or even pain could be candidate targets [22]. All the factors listed above plus activities of daily living, trauma, and occupational demands lead to a net decrease in the main PG aggrecan, a decrease in type 2 collagen, an increase in the degradation of collagen type 2, and an increase in type 1 collagen within the NP [23]. The loss of functional aggrecan leads to a progressive inability of the NP ECM to bind water that, in turn, leads to a decrease in intradiscal pressure. The accumulated loss of ECM integrity such as enzymatic cleavage of PG core proteins (such as biglycan, decorin, and fibromodulin) further contribute to DDD, loss of disc height, and a reduced ability of the IVD to resist compressive/shear forces. With respect to epigenetic influences, Matsui et al [24] demonstrated a higher likelihood of DDD in patients with a relative who underwent herniated disc surgery. Furthermore, smokers and patients living with diabetes also have elevated risk of developing DDD [25]. It is therefore likely that certain mutated genes impair cell viability and the deposition and regulation of cellular–ECM interaction that along with multiple processes such as inflammation, leads to progressive DDD [26], [27].
For more details with respect to the complexities and changes in cellularity and ECM, the reader is referred to several published reviews such as those by Feng et al [28] and Adams and Roughley [29].
Targeted IVD therapeutics
The “holy grail” of intradiscal therapy is to mediate the progressive degenerative cascade or even induce repair of the IVD. Necessarily, such putative therapy would need to inhibit inflammatory-driven catabolism of the IVD, stimulate anabolic repair/regeneration of the IVD, and deliver improved biomechanical status to the disc. To this end, there are a number of different therapeutic strategies currently under investigation.
Loss of disc height and swelling pressure within the IVD NP leads to degenerative disease and impaired biomechanical properties. Therefore, interventions that could ameliorate these pathological changes would capture the essence of a minimally invasive therapy. Interestingly, a study published by Klein et al [30] involved the percutaneous injection of a combination of glucosamine, chondroitin sulphate combined with hypertonic dextrose and dimethylsulphoxide. In this study, 30 patients with intractable low back pain of an average of 8.5 years’ duration that demonstrated concordant pain under provocative discography conditions received an injection of the intervention. One year after intervention, 57% of the patients experienced marked improvement in visual analogue pain and functional scores (Roland Morris). No patients experienced undue or serious side effects. Although this study did not propose a mechanism by which the treated patients experienced relief and the study did not have a control group, it remains one of the seminal publications with respect to early attempts to treat the confirmed painful DDD via percutaneous methods.
Biomolecules
Biomolecules may be able to augment the integrity of the IVD [NP and/or annulus fibrosus (AF)] ECM by directly implanting suitable anabolic/anticatabolic proteins such as bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs), and various members of the transforming growth factor (TGF)-β superfamily and/or catabolic enzyme inhibitors within the IVD NP [12]. Miyamoto et al [31] injected BMP-7, also known as OP-1, into the degenerative discs of rabbits that reportedly led to an increase in disc height with an improved elastic modulus. However, in a canine model of DDD, the injection of recombinant human BMP-7 failed to demonstrate any therapeutic effect on DDD, but did lead to undesired extensive formation of bone outside of the IVD [32]. With respect to therapeutic protein injection, it is vital to deliver the correct molecules in that akin to the experience with BMP-7 injection into canine IVD NPs, injection of BMP-2 into degenerative rabbit discs worsened degeneration with enhanced vascularisation and fibroblast proliferation [33]. An important question therefore with respect to biomolecule delivery is which molecule(s) is/are necessary and sufficient? A possible limitation to the use of therapeutic proteins may be only transient effects of the delivered biomolecules [12]. It may be possible to overcome this potential limitation by a slow release formulation or vector transmission in order to increase the duration of molecular delivery to the target tissue [12]. With respect to increasing the therapeutic effect of biomolecule delivery, Yan et al [34] investigated GDF-5 packaged in microspheres that reportedly enabled the slow release of GDF-5 for more than 42 days. This approach reportedly demonstrated some improvement in a rat-tail model of DDD in terms of increased disc height, sulphated glycosaminoglycan content and improved histological scores [34]. More recently, Matta et al [35] quantitatively demonstrated robust antidegenerative/regenerative effects in a rat-tail model of DDD through the use of a single injection of CTGF + TGFβ1.
Gene therapy
The delivery of therapeutic genes into cells within the IVD could potentially lead to the secretion of encoded proteins to enhance IVD homeostasis and possibly suppress degenerative disease. Several nonviral transmission vectors have been created including liposomes and “gene guns.” Although they eliminate the risk of viral vector infection, all of these methods are hampered by transient protein expression because these nonviral vectors do not efficiently incorporate with the host DNA. By contrast, a number of viral vector delivery systems have been evaluated, each with its own benefits and disadvantages. Some viruses can lead to infections, some integrate into the host DNA, and some remain separate. Amongst the most commonly used vectors, adenovirus and adeno-associated virus (AAV) have seen intense investigation. The adenoviral vector does not integrate into the host DNA, which eliminates the risk of mutation because the virus is not replicated with the host DNA. Therefore, the virus only expresses proteins transiently until it is degraded by the immune system that may attack the foreign material. AAV may be advantageous as compared to adenovirus as it requires a helper virus for expression, which limits immune system activation. However, AAV carries only a limited amount of genetic information [36]. Leckie et al [37] investigated AAV2-BMP2 and AAV2-TIMP1 for injection into the degenerated disc. They found there was a slowing of the degenerative cascade in the injected groups [37]. However, these vectors can be directly injected or transplanted in vivo. The ex vivo injection involves removal of targeted cells, culturing the cells, introducing genes, and replantation. The ex vivo introduction is reportedly safer but requires a two-step procedure, and there is difficulty in recreating the in vivo environment to expand the cells [36]. However, an element of caution must be introduced to the gene therapy approach, because in a safety study using rabbits, a higher dose of adeno-associated viral vectors expressing TGFβ1 and rhBMP-7 induced bilateral lower limb paralysis, clearly indicating the potential risk of this approach [38]. Therefore, although promising, it is clear that further investigation is necessary in order to identify the correct molecules, carriers, transfection method, procedures, and safety profile for gene therapy approaches to treat DDD.
Platelet-rich plasma
Platelet-rich plasma (PRP) is a method of delivering a small volume of concentrated activated platelets to an area of injury in the hopes that growth factors secreted by the activated platelets might have a restorative effect upon the tissue. PRP has been shown to stimulate cell proliferation and induce PG and glycosaminoglycan synthesis in tendon and muscle tissue; however, its mechanism of action continues to be under investigation. For example, in tendon-derived fibroblasts, it has been hypothesised that PRP might stimulate reactive oxygen species-based oxidative stress pathways that, in turn, induce a transient proinflammatory event that subsequently triggers inflammation and finally tissue regeneration [39]. With respect to IVD treatment in a short-term, preclinical rat-tail model of injury-induced DDD, Gullung et al [40] suggest that PRP-injected discs prevented degenerative changes compared to a sham injection when injected acutely after injury. Overall, there have been several studies evaluating in vitro and in vivo injection of PRP as a potential DDD therapy; however, there is considerable inconsistency using in vivo preclinical animal models [41]. Tuakli-Wosornu et al [42] performed a double-blind study during discograms with either contrast and PRP or just contrast. The PRP group had significant improvement for at least 1 year in terms of the Functional Rating Index. However, in this study, the control group was limited to only 8 weeks, and although worst pain ratings were reported to be significantly different at 8 weeks after injection and the control group remained relatively constant, the PRP treatment group only dropped by 2 points. Nonetheless, there were reported statistically significant changes in some predictors such as the 36-item Short Form Health Survey and functional rating index, although these were also restricted to only 8 weeks after treatment [42]. Additionally, no imaging studies were performed on any study participants. Potential confounders/limitations to the use of PRP include the lack of standardisation of dose, large donor variability, and method of preparation, and the lack of understanding with respect to purported mechanism of action [41].
Cell-based therapeutics
Cells within the AF and NP have an intrinsic capacity to effect ongoing homeostasis and repair. To this end, cells expressing stemness genes have been isolated from various components of the IVD including vertebral endplates, NP, and AF tissues, indicating that as with other tissues, the machinery for cell-based repair exists within the IVD [43], [44], [45]. The degenerative disc includes senescent and apoptotic cells that do not respond to bioactive molecules such as growth factors. Therefore, biological therapy for more advanced DDD may benefit from the transplantation of healthy cells to repopulate the disc and/or to act as “nurse” cells to improve the NP degenerative milieu.
Stem cells
In addition to terminally differentiated cells such as donor chondrocytes, stem cells from a variety of sources are another potential choice of cellular replacement. Bone marrow-derived mesenchymal stem cells (BMSCs) possess significant proliferative and differentiation capacity. BMSCs have been transplanted in a rabbit model of DDD, and it has been reported that such cells differentiated into “NP-like” cells with increases in PG deposition within the IVD NP [46]. Elabd et al [47] injected autologous, hypoxic cultured, bone marrow-derived MSCs into the IVDs of five patients with back pain and reported improvement in a small number of patients 5 years after injection. Furthermore, Cao et al [48] cocultured bone marrow MSCs with NP cells of rabbit IVD origin and demonstrated an upregulation in aggrecan, collagen 2, and SOX-9 expression, suggesting an anabolic effect upon the NP ECM. It is noteworthy with respect to the work by Cao et al that NP cells obtained from the rabbit disc are primarily of notochordal origin and apart from ECM protein expression; there was no specific characterisation of the cellular phenotypic fate. These authors reported an increase in TGF-β signalling and decrease in nuclear factor-κB activity, suggesting that these two signalling pathways play a role in preventing degeneration [48]. BMSCs have been intensively studied as potential cellular replacements to treat DDD and in addition to their use in preclinical animal models; BMSCs have been the subject of a number of clinical trials. Despite some early evidence of improvement in some patients, studies have suffered from small sample size and lack of adequate controls [49]. Furthermore, beyond some limited data in preclinical animal studies, the fate of the transplanted cells remains largely unknown. One clinical trial by Red de Terapia Celular (https://clinicaltrials.gov/ct2/show/record/NCT01860417) involves the ex vivo expansion of bone marrow-derived stem cells and the injection of 25 × 106 stem cells/2 mL into the disc. Another trial by Mesoblast Ltd. involves either 6 × 106 or 18 × 106 cells/disc within a hyaluronic acid carrier (https://clinicaltrials.gov/ct2/show/NCT01290367?term=mesoblast&rank=3). The cellular concentration within the cervical IVD NP has been reported to be within the range of 4200/mm3. The human IVD NP disc contains cells that are approximately 0.25–1.0%/volume [50]. Therefore, it is important to consider that the transplantation of up to 25 × 106 cells is on a scale that is orders-of-magnitude greater than the normal cellular population. Given that DDD is associated with calcification of the endplates and impaired nutrient supply within the IVD NP, it does not appear feasible that such high numbers of transplanted cells could integrate and survive within such a harsh and compromised tissue compartment. Therefore, any beneficial effects conferred by such a massive cellular transplant is, at this point, a matter of conjecture but may be associated with transient growth factor and other secreted factors by the transplanted cells that may confer activity until the cells die. This is an area that requires further, quantitative study.
In addition to BMSCs, other sources of stem cells have been investigated including adipose stem cells, synovial MSCs, olfactory stem cells and neonatal dermal fibroblasts [44], [46], [51], [52], [53], [54], [55]. Colombier et al [51] used lipoaspirates from patients cultured within a TGF-β1 and GDF-containing medium and induced NP cell-like differentiation. In this study, the cells were suspended within a hydrogel and injected subcutaneously into nude mice where it is reported that the cells survived and differentiated into a cellular phenotype similar to NP cells in that they secreted aggrecan and collagen type 2 [51]. Synovial MSCs are other potential stem cell candidates and have been reported to have robust replication rates with in vitro cartilaginous ECM production [52], [55]. Miyamoto et al [52] implanted synovial MSCs into rabbit IVD NPs and demonstrated that these cells exhibited anticatabolic and anabolic activity after transplant. Other sources of potential stem cells to be used for IVD repair include olfactory and neonatal dermal fibroblasts; however, donor site morbidity and sufficient tissue sources confound their use [53], [54]. However, there have been negative reports with respect to the use of stem cells for IVD therapeutics. Vadalà et al [56] transplanted rabbits MSCs into rabbit IVDs and after transplant detected no signs of the transplanted cells but did detect a significant increase in osteophyte formation. Their conclusions were that cells possibly leaked from the injection site and differentiated into an unwanted osteogenic lineage [56].
Allogeneic and autologous disc and articular chondrocytes have both been explored as potential cells for transplant with Nomura et al [57] reporting that allogeneic rabbit IVD cells slowed degeneration in a needle puncture injury model of rabbit DDD [12], [58]. Interestingly, cells transplanted along with their ECM showed greater improvement than cells alone [57]. Another study by Zhang et al [58] used a rabbit degenerative disc model and following injection of articular chondrocytes into the disc. The authors demonstrated cell survival for at least 8 weeks with suppression of associated inflammation; however, the ECM did not resemble the normal NP cell phenotype, and the use of differentiated autologous cells carries with it donor site morbidity [58]. Finally, the choice of cells for transplant is vital in that rabbit IVDs are highly notochordal and contain stem cells. The cellular phenotype of the human IVD is, of course, entirely different; therefore, it is important to exercise caution when drawing conclusions from such animal studies.
Less differentiated stem cells may be advantageous as they have the potential to propagate longer and might better differentiate into the desired lineages. These cells include induced pluripotent stem cells (iPSCs), human umbilical tissue-derived cells, and embryonic stem cells (ESCs) [46], [59], [60], [61], [62], [63]. iPSCs are somatic cells, such as dermal fibroblasts, that—through genetic reprogramming using a limited number of transcription factors (originally, Oct4, Sox2, c-myc, and Klf4)—assume an embryonic cell-like phenotype and because of their ease of harvest and minimal donor site morbidity offer a virtually limitless tissue source [64]. Since their discovery, it has been reported that considerable variability exists with respect to the transcription factors necessary to reprogramme cells. It has recently become clear that there is considerable tissue and cellular heterogeneity with respect to reprogramming and efficiency such that human papillary dermal fibroblasts and melanocytes can be reprogrammed following transfection with only Oct4 and Klf4 because of their high endogenous expression of Sox2 and c-myc; in these cases, however, there is a lower yield of iPSC clones [65]. Nonetheless, the use of iPS cells is accompanied by a number of risks including the integration of retroviral DNA into host cells, and the downstream interjection of coding regions that can affect subsequent transcription that heightens the risk of malignant transformation [66]. Takahashi et al [64] found that murine iPSC clones contained retroviral integrations for each transfection factor used, raising a concern that this technology could increase the risk of tumourigenesis [64]. This concern was validated by Okita et al [67], who reported that close to 20% of mice derived from an iPS cell line died or were killed because of weakness, wheezing or paralysis, and that approximately 14% of mice developed neck tumours with other tumours in a small number of other mice [67]. Therefore, although iPSCs offer promise in the future, their use must be restricted to research platforms for the present.
ESCs have been intensively investigated for use regenerative medicine. These are cells recovered from the inner cell mass of the developing embryo at the blastocyst stage and have totipotent characteristics. A search of clinicaltrials.gov using ESCs for DDD reveals no studies to date, and ESCs have had limited use as a potential therapy to treat disc disease with studies restricted to preclinical animal models. In a report by Sheikh et al [68], murine ESCs were expanded in a cocktail of growth factors in an attempt to prechondrodifferentiate the cells that were then transplanted in a rabbit model of DDD. This study demonstrated de novo notochordal cell-like growth within discs receiving transplants, suggesting that transplanted ESCs, after differentiation, are detectable at least 8 weeks after transplant and that they are capable of differentiation into a notochordal cell-like phenotype. Although ESCs, like iPSCs, offer considerable expansion and differentiation characteristics, ESCs, like iPSCs, have risks of oncogenesis as well as ethical concerns as they are harvested from developing embryos, raising challenges with translation into the clinic.
Another exciting area of cell-based therapeutics involves sourcing endogenous stem cells that reside within the IVD NP itself. Erwin et al [43] reported that stem cells obtained from canine IVD NPs display pluripotential differentiation characteristics and express stemness genes. However, it has been reported that stem cells obtained from degenerative human discs undergo “exhaustion,” possibly limiting their use in a cell-based therapy approach [69]. Finally, the process of tissue culture/cell expansion is labourious and costly, and the use of ESCs is fraught with ethical concerns including risks of malignancy/teratoma development.
Tissue engineering strategies
The goal of a tissue-engineered scaffold is to function as a load-bearing component. While under the proper conditions, transplanted cells suspended within the construct could repopulate the degenerative disc. The scaffold should be biocompatible, biodegradable and allow for sufficient cellular migration and ECM assembly. The scaffold also must have similar mechanical properties as the native NP or AF [70]. High molecular weight hyaluronic acid (hyaluronan) has potential advantages as a drug carrier, but also when cross-linked as a biomaterial. To this end, a study by Moss et al [71] reported that a thiol-modified hyaluronic acid and elastin-like polypeptide conferred an environment that was suitable for human NP cells and demonstrated desirable biomechanical properties both in vitro and in vivo in a preclinical rabbit model of DDD. This construct also provided a permissible environment for cell viability and ECM production, suggesting that such composite constructs might have a place in tissue engineering approaches to disc repair [71]. Composite constructs involving cross-linked hyaluronic acid with type II collagen have also been investigated that support cell viability and also offer a desirable high intradiscal swelling pressure [72].
Numerous reports have been published with respect to NP scaffolds though these strategies remain at the preclinical level. Gorapalli et al [73] used human NP cells obtained from discectomy patients and demonstrated that NP cells cultured within poly N-acetyl glucosamine resulted in significantly increased PGs, cell viability, metabolic activity, collagen type 2, and aggrecan production in vitro. Hyaluronic acid is a major component of the IVD NP ECM, and various forms of this macromolecule have been investigated for IVD tissue engineering including a thiol-modified hyaluronan and elastin-like polypeptide composite [71], [74].
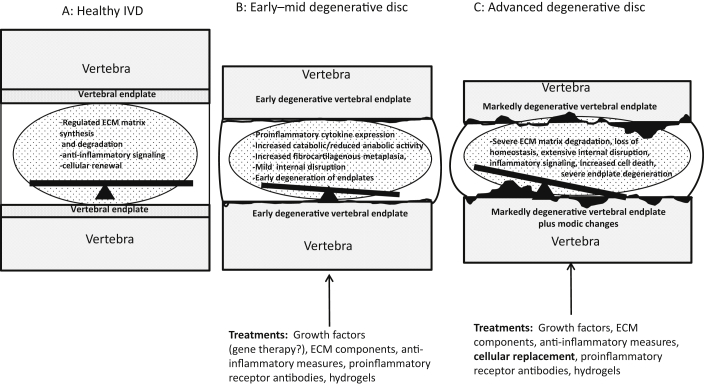
Each of the above treatment strategies—whether it is biomolecules, gene therapy, cell-based or tissue engineering—may have a role and possibly can be incorporated in treatment based on the extent of the degeneration of the disc. See Figure 1 for a summary of grades of DDD and possible intradiscal therapeutics.
Figure 1.
Schematic of (A) healthy versus (B) early degenerative disc and (C) more severe DDD. (A) Balanced homeostatic regulation of ECM synthesis, cell renewal, and apoptosis. (B) For early/mid degenerative disease, there is a loss of ECM regulation, accelerated cell death, increased proinflammatory enzymatic activity, mild loss of disc height, bulging of the annulus, and early degeneration of the vertebral endplate. (C) For more advanced DDD, the changes seen in (B) are more severe with extensive internal disruption of the IVD NP, more severe endplate changes, reactive bone marrow oedema (modic changes), extensive cell death, and marked loss of ECM regulation. Possible therapeutic interventions are highlighted for (B) and (C). DDD = degenerative disc disease; ECM = extracellular matrix; IVD = intervertebral disc; NP = nucleus pulposus.
AF repair or augmentation
Coexistent with degeneration of the IVD NP are similar changes affecting the AF. Such changes involve progressive delamination of the concentric lamellar rings composed primarily of type 1 collagen, fibroblasts, and interposed ECM [75]. AF repair or augmentation could theoretically assist with the mediation of DDD and prevent leakage of injectable or implantable treatment modalities. Currently, two main therapeutic strategies exist for AF repair: (1) suture or implant repair; and (2) augmentation. Bateman et al [76] have reported the feasibility of a suture application device that was able to close the AF in a porcine model that was maintained for at least 4 weeks. Other approaches include implantable hydrogels designed to mimic the material properties of native AF tissues or the use of cell-based therapy or tissue-engineered scaffolds [77], [78]. Additionally, various other strategies have been used in attempts to address AF insufficiency including biodegradable and synthetic materials as well as composites, different forms of bioactive glass, electrospun collagens, and nanofibers [79]. However, vexing problems remain with these tissue engineering strategies including immunogenicity of construct/materials, sufficient biomaterial strength, cellular/tissue milieu, and incorporation and degradation of constructs.
Modelling IVD degeneration
In order to test potential intradiscal therapies, a number of different animal models of DDD have been investigated. However, differences between species are another potential confounder when one considers the interpretation of scientific literature. O’Connell et al [80] compared the geometry of the human lumbar disc to lumbar discs of baboons, sheep, rabbits, rats, and mice along with bovine, mouse, and rat-tail disc geometry, and concluded that with respect to geometry, the lumbar disc of the mouse, rat, and mouse-tail were most similar to the human lumbar disc. With respect to animal models, the cellular phenotype is of vital importance when attempting to model human DDD. The composition of the human NP at birth and in youth is largely notochordal in nature, but this declines by adolescence/early adulthood into a mostly chondrocyte-like IVD NP. Bovine, ovine, and chondrodystrophic canine IVD NPs resemble the human IVD NP that is characterised by chondrocyte-like cells and a fibrocartilaginous NP. By contrast, the IVD NP in mice, rats, cats, minks, pigs, and rabbits remain rich in notochordal cells largely throughout life—a very different situation than the human condition.
In most animal models, it is necessary to induce DDD through either mechanical or chemical methods. Commonly, DDD is induced by stab injuries using a needle or scalpel that, in turn, leads to degeneration of the tissue compartment to assume the more acellular, fibrocartilaginous morphology typical of DDD [12], [37], [81], [82], [83], [84], [85]. In addition to acute mechanical injury, biomechanically induced DDD has also performed in the form of compression and distraction through the use of affixed clamps to the rodent tail disc [85]. Furthermore, the introduction of digestive enzymes such as chymopapain and/or chondroitinase ABC also results in degenerative change; however, it must be noted that these methods by definition utilise both needle puncture injury (injection) as well as enzymatic activity. Also, such enzymatically induced DDD occurs very rapidly, unlike DDD that occurs over time or secondary to needle puncture that requires many weeks to achieve a mature DDD phenotype [35], [85]. Pathologies induced by these methods include the loss of disc height as well as collagen type 2 and notochordal cells and an increase in the expression of ECM remodelling and proinflammatory enzymes such as MMPs, aggrecanases, and IL-1β and tumour necrosis factor (TNF)-α.
Any experimentally-induced animal model of DDD has its own inherent limitations that must be considered when interpreting experimental results. For example, the de novo IVD cellular phenotype, age of the animal, weight bearing (relevant in rodent models), and genetic variations figure prominently in the response of the animal’s IVD to experimental manipulations [12]. With respect to genetic variations, recently, the chondrodystrophic dog and the ovine models have been proposed to be the best animal models of disc degeneration because of their similarity to the human condition in terms of cellular and ECM phenotypes [83], [86]. Notwithstanding the inherent limitations in animal models of DDD, understanding the key pathologies and mechanisms involved with the progression of DDD provide vital experimental platforms for the controlled investigation of novel therapeutics.
Clinical challenges
There are significant challenges involved with the development and implementation of biological therapy for the disc that include diagnostic methods, the best choice of therapeutic agent, method of delivery, outcome measures and, of course, patient selection. With respect to patient selection, the ability to specifically isolate and determine pain of discogenic origin remains a vexing challenge. Cheung et al [2] found that 60% of symptomatic and nonsymptomatic people older than 50 years had more than three degenerative intervertebral levels on magnetic resonance imaging (MRI) examination. Another study involving MRI imaging of 98 asymptomatic people reported that only 36% of individuals had nondegenerative discs at all levels [87]. It is widely accepted that many people display imaging changes on MRI but do not experience spinal pain, indicating that contemporary imaging modalities are not capable of specifically detecting symptomatic disc disease. With respect to determining the presence of a painful disc, the gold standard for many years has been provocative discography. Xi et al [88] performed discograms for patients with presumed discogenic back pain for more than 6 months and performed arthrodesis in patients with positive discograms. Fourteen of 18 patients had significant improvement after arthrodesis in terms of the visual analogue scale and Oswestry Disability Index scores over a year postoperatively [88]. However, provocative discography is interventional, painful and is associated with a number of potential morbidities including accelerated disc degeneration [89]. In order to circumvent some of the limitations of discography, new technologies are under development including single-voxel MRI spectroscopy, whereby noninterventional imaging may be able to differentiate painful discs from nonpainful ones [90]. Such technology would substantially enhance the clinician’s ability to detect the painful disc without invasive means and develop an appropriate therapeutic strategy thereafter.
The translation of a biologic therapy that could influence the course of the disease would revolutionise the treatment of DDD. Such a revolution in therapy has been seen in the treatment of inflammatory arthropathies such as rheumatoid arthritis, ankyloses spondylitis and, lately, for inflammatory bowel disease, all because of a commonality in the inflammatory pathway modulating the response to TNFα activity. In previous years, joint replacement because of profound destruction was common in rheumatoid arthritis; however, the emergence of anti-TNF therapy has markedly reduced the burden of illness such that surgical intervention for joint replacement occurs with far less frequency. The convergence of TNFα signalling pathways has a commonality in all of these diseases such that suppressing the proinflammatory effects of this inflammatory enzyme can significantly slow down/arrest disease progression and even induce remission. The development of a biologic therapy that could improve cellular viability, mitigate ECM degeneration and maintain the biomechanical properties of the IVD would have a profound effect on the treatment of DDD.
Measuring success
Treatment success can be measured using a number of available metrics. It is important to consider both image-based as well as patient-derived functional outcome measure success. Therapeutic enhancement of disc structure as determined by conventional medical imaging may not necessarily translate into reduced patient pain and reported functional outcome. In considering the use of patient-reported measures, it remains important to record both generic (e.g., 36-item Short Form) and disease-specific measures of outcome (e.g., Oswestry Disability Index). As previously described, the emergence of new noninvasive imaging techniques to diagnose painful discs also holds promise as another potential way to objectively monitor a patient’s response to therapy.
Conclusion/future perspectives
The development of a biologic therapy that can influence the course of DDD and its associated morbidity will yield a transformative solution in the management of DDD and lead to tremendous cost savings, avoidance of morbid surgical procedures and untoward effects (such as adjacent segment degeneration and further surgery) and drastically reduce pain medication dependence such as is the case with the current opioid crisis.
Conflict of interest
The authors wish to confirm that there is no conflict of interest with any contents of this manuscript.
Funding
Nothing to disclose.
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung K.M., Karppinen J., Chan D., Ho D.W., Song Y.Q., Sham O. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 3.Dalke M.R., Gastal F.A., Xavier F.N.M. Prevalence of chronic low back pain: systematic review. Rev Saude Publ. 2015;49:49–53. doi: 10.1590/S0034-8910.2015049005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depintor J.D., Bracher E.S., Cabral D.M., Eluf-Neto J. Prevalence of chronic spinal pain and identification of associated factors in a sample of the population of São Paulo, Brazil: cross-sectional study. Sao Paulo Med J. 2016;134:375–384. doi: 10.1590/1516-3180.2016.0091310516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song X.X., Yu Y.J., Li X.F., Liu Z.D., Yu B.W., Guo Z. Estrogen receptor expression in lumbar intervertebral disc of the elderly: gender and degeneration degree-related variations. Joint Bone Spine. 2014;81:250–253. doi: 10.1016/j.jbspin.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Calleja-Agius J., Muscat-Baron Y., Brincat M.P. Estrogens and the intervertebral disc. Menopause Int. 2009;15:127–130. doi: 10.1258/mi.2009.009016. [DOI] [PubMed] [Google Scholar]
- 7.Hoy D., March L., Brooks P., Blyth P., Woolf A., Bain C. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968–974. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 8.Katz J.N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 9.Karadimas S., Erwin W.M., Ely C.G., Dettori J.R., Fehlings M.G. The pathophysiology and natural history of cervical spondylotic myelopathy. Spine. 2013;S38:S21–S36. doi: 10.1097/BRS.0b013e3182a7f2c3. [DOI] [PubMed] [Google Scholar]
- 10.Panjabi Manohar M. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–676. doi: 10.1007/s00586-005-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannion A.F., Brox J.I., Fairbank J.C. Comparison of spinal fusion and nonoperative treatment in patients with chronic low back pain: long-term follow-up of three randomized controlled trials. Spine J. 2013;13:1438–1448. doi: 10.1016/j.spinee.2013.06.101. [DOI] [PubMed] [Google Scholar]
- 12.Moriguchi Y., Alimi M., Khair T., Manolarakis G., Berlin C., Bonassar L.J. Biological treatment approaches for degenerative disk disease: a literature review of in vivo animal and clinical data. Global Spine J. 2016;6:497–518. doi: 10.1055/s-0036-1571955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips K., Cullen K., Chiverton N., Michael A.L., Cole A.A., Breakwell L.M. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. 2015;23:1165–1177. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Feng C., Liu H., Yang M., Zhang Y., Huang B., Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15:1674–1684. doi: 10.1080/15384101.2016.1152433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freemont A. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 17.Kadow T., Sowa G., Vo N., Kang J.D. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Rel Res. 2015;473:1903–1912. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajasekaran S., Venkatadass K., Naresh B.J. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs. Eur Spine J. 2008;17:626–643. doi: 10.1007/s00586-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosikova Y., Santerre J.P., Grynpas M., Gibson G., Kandel R.A. Characterization of the annulus fibrosus–vertebral body interface: identification of new structural features. J Anat. 2012;221:577–589. doi: 10.1111/j.1469-7580.2012.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kepler C.K., Ponnappan R.K., Tannourr C.A., Risbud M.V., Anderson D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Kalb S., Martirosyan N.L., Kalani M.Y., Broc C.G., Theodore N. Genetics of the degenerated intervertebral disc. World Neurosurg. 2012;77:491–501. doi: 10.1016/j.wneu.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y., Egan B., Wang J. Genetic factors in intervertebral disc degeneration. Genes Dis. 2016;3:178–185. doi: 10.1016/j.gendis.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoniou J., Steffen T., Nelson F., Winterbottom N., Holander A.P., Poole R.A. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui H., Kanamori M., Ishihara H., Yudoh K., Naruse Y., Tsuji H. Familial predisposition for lumbar degenerative disc disease: a case-control study. Spine. 1998;23:1029–1034. doi: 10.1097/00007632-199805010-00013. [DOI] [PubMed] [Google Scholar]
- 25.Sakellaridis N., Androulis A. Influence of diabetes mellitus on cervical intervertebral disc herniation. Clin Neurol Neurosurg. 2008;110:810–812. doi: 10.1016/j.clineuro.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Brown S., Melrose J., Caterson B., Roughley P., Eisenstein S.M., Robert S. A comparative evaluation of the small leucine-rich proteoglycans of pathological human intervertebral discs. Eur Spine J. 2012;21:S154–S159. doi: 10.1007/s00586-012-2179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vergroesen P.-P., Kingma I., Emanuel K.S., Hoogendoorn R.J., Weltin T.J., van Royen B.J. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Feng H., Danfelter M., Stromqvist B., Heinegard D. Extracellular matrix in disc degeneration. J Bone Joint Surg. 2006;88-A:25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 29.Adams M.A., Roughley P.J. What is intervertebral disc degeneration and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 30.Klein R.G., Eek B.C., O'Neill C.W., Elin C., Mooney V., Derby R.R. Biochemical injection treatment for discogenic low back pain: a pilot study. Spine J. 2003;3:220–226. doi: 10.1016/s1529-9430(02)00669-1. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto K., Masuda K., Kim J.G., Inoue N., Akeda K., Andersson G.B. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692–703. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Willems N., Bach F.C., Plomp S.G., van Rijen M.H., Wolfswinkel J., Grinwis G.C. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res Ther. 2015;17:137. doi: 10.1186/s13075-015-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang K.-Y., Yan J.J., Hsieh C.C., Chang M.S., Lin R.M. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007;32:1174–1180. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 34.Yan J., Yang S., Sun H., Guo D., Wu B., Ji F. Effects of releasing recombinant human growth and differentiation factor-5 from poly(lactic-co-glycolic acid) microspheres for repair of the rat degenerated intervertebral disc. J Biomater Appl. 2014;29:72–80. doi: 10.1177/0885328213515034. [DOI] [PubMed] [Google Scholar]
- 35.Matta A., Karim M.Z., Isenman D., Erwin W.M. Notochordal cell-based therapeutics can regenerate the degenerative disc. Proceedings of the 31st Annual Meeting of the North American Spine Society. Spine J. 2016;16:S169. [Google Scholar]
- 36.Woods B.I., Vo N., Sowa G., Kang J.D. Gene therapy for intervertebral disk degeneration. Orthop Clin N Am. 2011;42:563–574. doi: 10.1016/j.ocl.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Leckie S.K., Bechara B.P., Hartman R.A., Sowa G.A., Woods B.I., Coelho J.P. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12:7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallach C.J., Kim J.S., Sobajima S., Lattermann C., Oxner W.M., McFadden K. Safety assessment of intradiscal gene transfer: a pilot study. Spine J. 2006;6:107–112. doi: 10.1016/j.spinee.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Hudgens J.L., Sugg K.B., Grekin J.A., Gumucio J.P., Bedi A., Mendias C.L. Platelet-rich plasma activates proinflammatory signlaing pathways and induces oxidtive stress in tendon fibroblasts. Am J Sports Med. 2016;44:1931–1940. doi: 10.1177/0363546516637176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gullung G.B., Woodall J.W., Tucci M.A., James J., Black D.A., McGuire R.A. Platelet-rich plasma effects on degenerative disc disease: analysis of histology and imaging in an animal model. Evidence Based Spine Care J. 2011;2:13–18. doi: 10.1055/s-0031-1274752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monfett M., Harrisson J., Boachie-Adjei K., Lutz G. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: an update. Int Orthop. 2016;40:1321–1328. doi: 10.1007/s00264-016-3178-3. [DOI] [PubMed] [Google Scholar]
- 42.Tuakli-Wosornu Y.A., Terry A., Boachie-Adjei K., Harrisson J., Gribbin C.K., LaSalle E.E. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double blind, randomized controlled study. PM R. 2016;8:1–10. doi: 10.1016/j.pmrj.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Erwin W.M., Islam D., Eftekarpour E., Inman R.D., Karim M.Z., Fehling M.G. Intervertebral disc-derived stem cells: Implications for regenerative medicine and repair. Spine. 2013;38:211–216. doi: 10.1097/BRS.0b013e318266a80d. [DOI] [PubMed] [Google Scholar]
- 44.Blanco J.F., Graciani I.F., Sanchez-Guijo F.M., Muntion S., Hernandez-Campo D., Santamaria C. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine. 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 45.Feng G., Yang X., Shang H., Marks I.W., Shen F.H., Katz A. Multipotential differentiation of human anulus fibrosus cells. J Bone Joint Surg Am. 2010;92:675–685. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai D., Mochida J., Iwashina T., Watanabe T., Nakai T., Ando K. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 47.Elabd C., Centeno C.J., Schultz J.R., Lutz G., Ichim T., Silva F.J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016;14:253. doi: 10.1186/s12967-016-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao C., Zou J., Liu X., Shapira A., Moral M., Luo Z. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF-κB pathway. Spine J. 2015;15:530–538. doi: 10.1016/j.spinee.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Pennicooke B., Moriguchi Y., Hussain I., Bonssar L., Hartl R. Biological treatment approaches for degenerative disc disease: a review of clinical trials and future directions. Cureus. 2016;8:11 e892. doi: 10.7759/cureus.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urban J.P.G., Roberts S., Ralphs J.R. The nucleus of the intervertebral disc from development to degeneration. Ame Zool. 2000;40:53–61. [Google Scholar]
- 51.Colombier P., Clouet J., Boyer C., Ryel M., Bonin G., Lesoeur J. TGF-β1 and GDF5 act synergistically to drive the differentiation of human adipose stromal cells toward nucleus pulposus-like cells. Stem Cells. 2016;34:553–567. doi: 10.1002/stem.2249. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto T., Muneta T., Tabuchi T., Matsumoto K., Saito H., Tsuji K. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murrell W., Sanford E., Anderberg L., Cavanagh B., Mackay-Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009;9:585–594. doi: 10.1016/j.spinee.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Chee A., Shi P., Cha T., Kao T.H., Yang S.H., Zhu J. Cell therapy with human dermal fibroblasts enhances intervertebral disk repair and decreases inflammation in the rabbit model. Global Spine J. 2016;6:771–779. doi: 10.1055/s-0036-1582391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nimura A., Muneta T., Koga H., Mochizuki T., Suzuki K., Makino H. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 56.Vadalà G., Sowa G., Hubert M., Gilbertson L.G., Denaro V., Kang J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–355. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 57.Nomura T., Mochida J., Okuma M., Nishimura K., Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Rel Res. 2001;389:94–101. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Chee A., Shi P., Wang R., Moss I., Chen E.Y. Allogeneic articular chondrocyte transplantation downregulates interleukin 8 gene expression in the degenerating rabbit intervertebral disk in vivo. Am J Phys Med Rehabil. 2015;94:530–538. doi: 10.1097/PHM.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Fu S., Rahaman M.N., Mao J.J., Bal B.S. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res Part A. 2015;103:1053–1059. doi: 10.1002/jbm.a.35243. [DOI] [PubMed] [Google Scholar]
- 60.Erwin W.M., Inman R.D. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine. 2006;31:1094–1099. doi: 10.1097/01.brs.0000216593.97157.dd. [DOI] [PubMed] [Google Scholar]
- 61.Erwin W.M., Islam D., Inman R.D., Fehlings M.G., Tsui F.W. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leckie S.K., Sowa G., Bechara B.P., Hartman R.A., Coelho J.P., Witt W.T. Injection of human umbilical tissue–derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13:263–272. doi: 10.1016/j.spinee.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheikh H., Zakharian K., De La Torre R.P., Facek C., Vasquez A., Chaudhry G.R. In vivo intervertebral disc regeneration using stem cell–derived chondroprogenitors: laboratory investigation. J Neurosurg Spine. 2009;10:265–272. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda M. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Medvedev S.P., Shevchkenko A.I., Zakian S.M. Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine. Acta Nat. 2010;2:18–27. [PMC free article] [PubMed] [Google Scholar]
- 66.Vadalà G., Russo F., Ambrosio L., Loppini M., Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8:185. doi: 10.4252/wjsc.v8.i5.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 68.Sheikh H., Zakharian K., De la Torre R.P., Facek C., Vasquez A., Chaudhry C.G.R. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009;10:265–272. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 69.Sakai D., Nakamura Y., Nakai T., Mishima T., Kato S., Grad S. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Rev. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Q., Xu H.W., Hurday S., Xu B.S. Construction strategy and progress of whole intervertebral disc tissue engineering. Orthop Surg. 2016;8:11–18. doi: 10.1111/os.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moss I.L., Gordon L., Woodhouse K.A., Whyne C.M., Yee A.J. A novel thiol-modified hyaluronan and elastin like polypeptide composite material for tissue engineering of the nucleus pulposus of the intervertebral disc. Spine. 2011;36:1022–1029. doi: 10.1097/BRS.0b013e3181e7b705. [DOI] [PubMed] [Google Scholar]
- 72.Calderon L., Collin E., Velasco-Bayon D., Murphy M., O’Halloran D., Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cells Mater. 2010;20:134–148. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 73.Gorapalli D., Seth A., Vournakis J., Whyne C., Akens M., Zhang A. Evaluation of a novel poly N-acetyl glucosamine (pGlcNAc) hydrogel for treatment of the degenerating intervertebral disc. Life Sci. 2012;91:1328–1335. doi: 10.1016/j.lfs.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 74.Pfeiffer M., Boudriot U., Pfeiffer D., Ishaque N., Goetz W., Wilke A. Intradiscal application of hyaluronic acid in the non-human primate lumbar spine: radiological results. Eur Spine J. 2003;12:76–83. doi: 10.1007/s00586-002-0478-7. [DOI] [PubMed] [Google Scholar]
- 75.Gregory D.E., Bae W.C., Sah R.L., Masuda K. Disc degeneration reduces the delamination strength of the anulus fibrosis in the rabbit anular disc puncture model. Spine J. 2014;1:1265–1271. doi: 10.1016/j.spinee.2013.07.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bateman A.H., Balkovec C., Akens M.K., Chan A.H., Harrisson R.D., Oakden W. Closure of the annulus fibrosus of the intervertebral disc using a novel suture application device—in vivo porcine and ex vivo biomechanical evaluation. Spine J. 2016;16:889–895. doi: 10.1016/j.spinee.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Long R.G., Torre O.M., Hom W.W., Assael D.J., Iatridis J.C. Design requirements for annulus fibrosus repair: review of forces, displacements, and material properties of the intervertebral disk and a summary of candidate rydrogels for repair. J Biomech Eng. 2016;138:021007. doi: 10.1115/1.4032353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharifi S., Bulstra S.K., Grijpma D.W., Kuijer R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J Tissue Eng Regen Med. 2015;9:1120–1132. doi: 10.1002/term.1866. [DOI] [PubMed] [Google Scholar]
- 79.Li X., Dou Q., Kong Q. Repair and regenerative therapies of the annulus fibrosus of the intervertebral disc. J Coll Physicians Surg Pak. 2016;26:138–144. [PubMed] [Google Scholar]
- 80.O’Connell G.D., Vresilovic E.J., Elliott D.M. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 81.Issay A.C., Castania V., Castania M., Salmon C.E., Nogueira-Barbosa M.H., Bel E.D. Experimental model of intervertebral disc degeneration by needle puncture in Wistar rats. Braz J Med Biol Res. 2013;46:235–244. doi: 10.1590/1414-431X20122429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sobajima S. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine. 2004;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 83.Bergknut N., Rutges J.P., Kranenberg H.J., Smolders L.A., Hagman R., Schmidt H.J. The dog as an animal model for intervertebral disc degeneration? Spine. 2012;37:351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 84.Han B., Zhu K., Li F.C., Xiao Y.X., Feng J., Shi Z.L. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33:1925–1934. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 85.Singh K., Masuda K., An H.S. Animal models for human disc degeneration. Spine J. 2005;5:267S–279S. doi: 10.1016/j.spinee.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 86.Alini M., Eisenstein S.M., Ito K., Little C., Kettler A.A., Masuda K. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., Modic M.T., Malkasia D., Ross J.S. Magnetic resonance imaging of the lumbar spine in people without back pain. New Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 88.Xi M.A., Tong H.C., Fahim D.K., Perez-Cruet M. Using provocative discography and computed tomography to select patients with refractory discogenic low back pain for lumbar fusion sintervertebral disc Cureus. 2016;8:e514. doi: 10.7759/cureus.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker J., III, El Abd O., Isaac Z., Muzin S. Discography in practice: a clinical and historical review. Curr Rev Musculoskelet Med. 2008;1:69–83. doi: 10.1007/s12178-007-9009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lotz J.C., Gornet M.F., Peacock J.C., III, Hu S.S., Schranck F.W., Stewart D. Single voxel MR spectroscopy distinguishes nonherniated painful from herniated painful and non-painful lumbar discs. Spine J. 2013;13(9):101S. [Google Scholar]