Summary
As the most common form of joint disorder, osteoarthritis (OA) imposes a tremendous burden on health care systems worldwide. Without effective cure, OA represents a unique opportunity for innovation in therapeutic development. In contrast to traditional treatments based on drugs, proteins, or antibodies, stem cells are poised to revolutionize medicine as they possess the capacity to replace and repair tissues and organs such as osteoarthritic joints. Among different types of stem cells, mesenchymal stem cells (MSCs) are of mesoderm origin and have been shown to generate cells for tissues of the mesoderm lineage, thus, raising the hope for them being used to treat diseases such as OA. However, given their ability to differentiate into other cell types, MSCs have also been tested in treating a myriad of conditions from diabetes to Parkinson's disease, apparently of the ectoderm and endoderm lineages. There are ongoing debates whether MSCs can differentiate into lineages outside of the mesoderm and consequently their effectiveness in treating conditions from the ectoderm and endoderm lineages. In this review, we discuss the developmental origin of MSCs, their differentiation potential and immunomodulatory effects, as well as their applications in treating OA. We suggest further investigations into new therapies or combination therapies that may provide more effective treatment for bone and joint diseases. Furthermore, cell-based therapy and its associated safety and effectiveness should be carefully evaluated before clinical translation. This review provides updated information on recent approval of clinical trials and related applications of MSCs, and discusses additional efforts on cell-based therapy for treating OA and other joint and bone diseases.
Keywords: Inflammation, Mesenchymal stem cells, Osteoarthritis
Introduction
Osteoarthritis (OA) is the most common form of joint disorder. It is characterized by degeneration of articular cartilage [1] and reactive new bone formation at the articular margins, causing pain and stiffness of the affected joints [2]. The macroscopic features of OA are revealed by radiography and magnetic resonance imaging (Figure 1), and the microscopic features by histology (Figure 2). OA can affect all synovial joints, with the hip and knee being the most common sites that often lead to physical disability [2]. Currently, OA is the leading cause of disability among the elderly population and this is often associated with depression and sleeping disorder [3]. It is estimated that 10–15% of adults over the age of 60 years suffer from OA worldwide [1]. As the global population ages, it has been forecasted that > 20% of the population will be suffering from OA and > 40 million people will be severely disabled by 2050 [1]. Unfortunately, without effective treatment [4], OA represents a significant economic burden for patients and society at large [1], [3], [5], [6], [7].
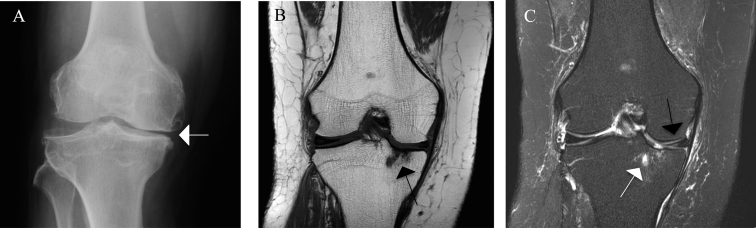
Fig. 1.
Radiograph and magnetic resonance images of a Kellgren–Lawrence grade 3 knee illustrating the features of osteoarthritis. (A) Anteroposterior radiograph: joint-space narrowing and definite marginal osteophytes at the distal femur and tibia plateau (white arrow). (B) Corresponding coronal T1-weighted image: diffuse cartilage loss at the tibia plateau and subchondral cysts (black arrow). (C) Corresponding coronal T2 weighted image: a subchondral cyst with bone marrow oedema (BML) of the medial tibial plateau (white arrow), which could not be visualized by X-ray. Partial maceration and oedema of the body of the medial meniscus and extrusion of the body of the medial meniscus (black arrow), both factors contributing to radiographic joint space narrowing.
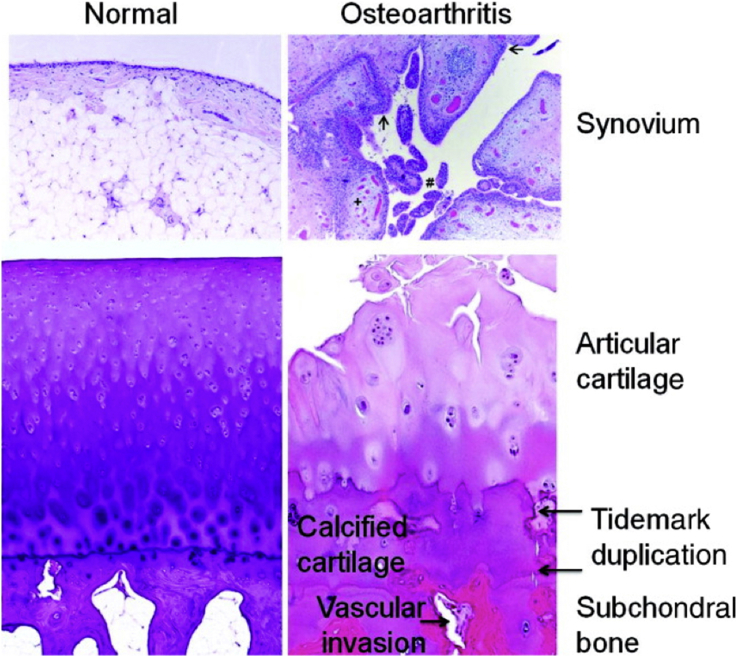
Fig. 2.
Histological features of OA. The normal synovium has a thin (1–2 cells thick) lining layer and a vascularized, loose connective tissue sublining layer. OA synovium demonstrates features of synovial villous hyperplasia (#), lining hyperplasia (arrows), increased vascularity (+), and perivascular mononuclear cell (inflammatory) infiltration. In OA articular cartilage, loss of cells and matrix is accompanied by areas of cell clusters. There is thickening of the calcified zone and duplication of the tidemark, which normally separates the articular cartilage from the underlying calcified cartilage. The subchondral bone is also thickened, and vascular invasion, which can extend through the tidemark and into the base of the articular cartilage, is seen. OA = osteoarthritis. Note. From “Osteoarthritis: a disease of the joint as an organ” by Loeser, et al., 2012. Arthritis Rheumatology, 64, p. 1697–1707. Copyright 2012, The American College of Rheumatology. Reprinted with permission.
Currently, there is no effective therapy that can reverse the progressive nature of OA. However, one promising therapy may rely on the therapeutic use of stem cells. The majority of the current stem cell therapies involve using mesenchymal stem cells (MSCs) due to their multilineage differentiation towards cell types in the joints and their immunoregulatory function. This could be efficacious to repair the damaged joints in OA, not only for cartilage repair but also for subchondral bone remodelling. However, the safety and effectiveness of the new cell-based therapies must be carefully evaluated before any clinical application. Thus, significant challenges remain and are addressed in this review.
Risk factors of OA
OA can be classified into primary and secondary forms [8]. Primary OA is a chronic degenerative disease related to aging [1] and heredity [2]. The exact aetiology of primary OA remains unknown [2], although genetic predisposition has been implicated [1]. Secondary OA can occur in any synovial joints and at any age due to articular injury [2], for instance, fracture, repetitive joint use, obesity [9], [10], [11], or metabolic disease such as diabetes [12], [13]. The aetiology of the primary and secondary of OA is different, however patients' symptoms and signs are similar [1]. Detailed risk factors for primary and secondary OA are summarized in Table 1.
Table 1.
| Forms of OA | Risk factors for OA | ||
|---|---|---|---|
| Primary OA | Constitutional risk factors | Hereditary and genetic factors | Age, sex, genetic inheritance |
| Idiopathic | |||
| Secondary OA | Congenital | Congenital and developmental disorders | |
| Acquired | Activity-related risk factors | Occupational/repetitive activities, sports activity, traumatic | |
| Local mechanical factors | High body mass index, obesity, muscle weakness, alignment, mechanical instability | ||
| Behavioural and hormonal factors | Smoking, oestrogen | ||
| Local osseous factor | Bone marrow lesion, bone mineral density | ||
| Inflammatory | |||
| Infection | |||
| Vascular | Avascular necrosis, haemarthrosis | ||
| Connective tissue disorder | Ehlers–Danlos syndrome, Marfan syndrome | ||
| Neuropathic | Diabetes mellitus, Charcot syndrome | ||
OA = osteoarthritis.
Age is accepted as an independent risk factor for the development of OA [17], thus increasing the chance of total hip replacement [18]. Recently, more emphasis has been placed on genetic predisposition as another independent cause for OA. Genes such as those for the vitamin D receptor gene, insulin-like growth factor I, cartilage oligomeric proteins, and the human leukocyte antigen (HLA) region have all been associated with OA [19]. Post-traumatic OA can develop in joints after sustaining fractures or contusion [14]. These injuries invariably accelerate the nature of OA development and progression [2].
The development of OA also shows sex-specific prevalence and ethnic differences. Zhang et al. [20] have compared the prevalence of OA in Chinese patients from Beijing and Caucasians from Framingham, MA, USA. They reported that the prevalence of radiographic knee OA was higher in Chinese and Caucasian women than in men aged > 60 years. Ethnic variation also shows that Chinese women have a higher prevalence of knee OA than their Caucasian counterparts.
High body mass index is associated with a high risk of developing knee OA [21] and hip OA. The risk of knee OA increases ∼15% for each additional kg/m2 over 27 [16], [22]. However, development of OA in obesity is not confined to the lower limbs. Reports of upper limb involvement are just as common in OA of the hands and wrists [10]. Weight reduction and controlling obesity is the single patient control variable that can reduce the risk of OA development [18], [21].
Oestrogen deficiency might be a risk factor for developing OA as women have a high incidence of OA after menopause [14]. Lower prevalence of OA has been observed in women under oestrogen replacement therapy [2], [14]. However, oestrogen has complex effects on the degradation and repair of joint cartilage [2]. On the one hand, oestrogen preserves bone mass by preventing the activation of osteoclasts, therefore resulting in high bone density, which may increase the risk of OA [2], [14]. On the other hand, high bone mineral density may protect the progression of OA, therefore, oestrogen also has a protective effect on OA [14].
Diagnosis of OA
The pathophysiology of OA is multifactorial [2], involving mechanical, cellular and biochemical processes [15]. These lead to changes in the composition and loss of articular cartilage, remodelling of the subchondral bone, and changes to the homeostasis of the soft tissue and synovial joint environment [2], [14]. The diagnosis of OA mainly depends on the detailed history of patients, together with a complete physical examination. Ancillary diagnostic tests can be performed when the diagnosis remains uncertain (Table 2).
Table 2.
| Diagnostic tools | Details | Symptoms and signs |
|---|---|---|
| Clinical features | Patient's history | Pain, morning stiffness |
| Physical examination | Inflammation, swelling, deformities, joint enlargement, crepitus, limitation of motion | |
| Imaging | Radiography | Objective evidence on joint-space narrowing, osteophyte formation, pseudocysts, and sclerosis of subchondral bone |
| CT and CT arthrography | Visualization of cortical bone and soft-tissue calcification, quantifying amount of bone lost or size of subchondral cyst | |
| Magnetic resonance imaging | Assessment of cartilage morphology and soft-tissue associate with OA | |
| Ultrasonography | Evaluation of the quality of superficial articular cartilage and cartilage thickness | |
| Laboratory testing | Serum and molecular markers | Inflammation markers, bone markers, cartilage synthesis and degradation markers, transforming growth factor-β |
CT = computed tomography; OA = osteoarthritis.
Clinical features of OA
The principal symptom of OA is pain involved in one or a few joints [15]. Pain tends to progress with time and get worse with weight bearing, especially following a period of inactivity [23]. OA usually cause morning stiffness in the affected joints and resolves within 30 minutes [15]. This differs from rheumatoid arthritis in which stiffness can last for ≥ 45 minutes [24].
Physical examination reveals joint swelling caused by effusion and restricted joint motion associated with joint crepitus [25]. Joint enlargement, inflammation, and synovitis may also be observed [2]. In addition, Heberden's node or Bouchard's node may be present and cause joint deformity and functional limitations [2].
Radiographs
Radiography is a useful and cost-efficient tool in establishing OA [23]. It can reveal joint-space narrowing and osteophyte formation, as well as subchondral cysts and sclerosis of the subchondral bone [20], [23], [25]. Furthermore, radiography has been used in the assessment of joint-space width [26], [27] and there is radiographic classification to stage the severity of OA (Kellgren Lawrence grading). However, radiographic and symptomatic OA are not always consistent [20], [23]. For instance, some patients with radiographic changes do not show any symptoms of OA or any disability, and symptomatic OA patients may have no radiographic changes. Therefore, the presence or absence of radiographic changes cannot establish or exclude the diagnosis of OA [23].
Other valuable tools have also been applied in the diagnosis of OA due to their unique advantages. Specifically, computed tomography and computed tomography arthrography in the visualization of cortical bone and soft-tissue calcification; magnetic resonance imaging in assessment of cartilage morphology and soft-tissue associate with OA; and ultrasonography for evaluation of the quality of superficial articular cartilage and measurement of cartilage thickness [23], [27].
The clinical classification criteria developed by the American College of Rheumatology remain a popular method of classifying knee OA. This classification is based on clinical sign and symptoms, as well as radiographic or biochemical evidence (Table 3).
Table 3.
ACR clinical classification criteria for knee OA [28].
| Clinical and laboratory | Knee pain and at least 5 of 9 | Age > 50 y Stiffness < 30 min Crepitus on active motion Bony tenderness Bony enlargement No palpable warmth of synovium Erythrocyte sedimentation rate < 40 mm/h Rheumatoid factor < 1:40 Synovial fluid signs of OA |
| Clinical and radiographic | Knee pain and at least 1 of 3 | Age > 50 y Stiffness < 30 min Crepitus on active motion and osteophytes |
| Clinical | Knee pain and at least 3 of 5 | Age > 50 y Stiffness < 30 min Crepitus on active motion Bony tenderness Bony enlargement No palpable warmth of synovium |
ACR = American College of Rheumatology; OA = osteoarthritis.
Laboratory testing and biochemical markers
In general, laboratory testing is not required to make the diagnosis of OA. It is recommended when the diagnosis remains uncertain [2]. Bauer et al [29] proposed BIPED as a classification scheme for OA biomarkers, with B for burden of disease, I for investigative, P for prognostic, E for efficacy of intervention, and D for diagnostic. To assist the diagnosis and monitor the progression of OA, cartilage matrix components and molecular makers in synovial fluid, serum, and urine have been investigated for their critical role in cartilage and bone turnover and synovial inflammation in OA (Table 4).
Table 4.
| Categories | Biomarkers | Sources | Refs | |
|---|---|---|---|---|
| Inflammation markers | [23] | |||
| Bone markers | N-terminal cross-linked telopeptide of type I collagen | Urine | [30] | |
| CTX-I | Urine | |||
| Cartilage markers | Synthesis | C-propeptide of collagen type II | Serum, Synovial fluid | [30] |
| N-propeptide II of collagen type II | Serum | |||
| Degradation | CTX-II | Urine, synovial fluid | [14], [19], [30] | |
| Glc-Gal-Pyd | Urine | |||
| Cartilage oligomeric protein (COMP) | Serum | |||
| Transforming growth factor-β | [23] | |||
CTX = C-terminal cross-linked telopeptide; OA = osteoarthritis.
Subchondral bone in OA
Although cartilage degradation has been considered as an important feature of OA, subchondral bone also contributes to the pathogenesis of OA [31]. The subchondral bone with functional mechanical properties supports the integrity of the articular cartilage, and vice versa. Those patient with avascular necrosis with weaken subchondral bone support tend to have accelerated OA development. It is reported that bone anabolic drug dickkopf homolog 1 prevents OA and bone spurs [32] and anti-bone resorption drug alendronate can be used as a disease-modifying agent in the treatment of OA in rats [33].
Therapeutic strategies for OA
The current therapeutic strategies for OA include nonpharmacological, pharmacological, complementary alternative, and surgical, with the primary goal to reduce pain and improve function and quality of life (Table 5). Nonpharmacological and pharmacological treatments are usually considered in the early stage of OA: complementary alternative treatment such as supplements with glucosamine and chondroitin for moderate and severe OA; and surgical intervention should be reserved for patients with OA symptoms that cannot be controlled by other treatments [4], [24]. However, currently, no therapy has been shown to protect articular cartilage [4], nor approved to prevent the progression of OA [3]. Due to the complex pathology and its slow progression, and the lack of biomarkers for early diagnosis of OA [3], a challenge remains for researchers to develop efficient therapies to treat and modify OA.
Table 5.
| Stages of OA | Strategies | Measures |
|---|---|---|
| Early stage | Nonpharmacological | Lifestyle adjustment, weight loss if necessary, elimination of damaging influences on the joint, physical and occupational therapy |
| Pharmacological | NSAIDs, glucocorticoids, opioids, symptomatic slow-acting drugs for OA, anticytokines | |
| Moderate and severe OA | Complementary alternative | Acupuncture, supplements with glucosamine and chondroitin, therapeutic touch, antioxidants such as vitamins C, D and E |
| Severe OA | Surgical treatment | Joint-preserving surgical treatment: bone-stimulating, joint surface restoration including ACT and OCT; total joint replacement: THR or TKR |
ACT = autologous chondrocyte transplantation; NSAIDs: nonsteroidal anti-inflammatory drugs; OA = osteoarthritis; OCT = autologous osteochondral transplantation; THR = total hip knee replacement; TKR = total knee replacement.
MSCs are a good candidate to meet the challenge in treating OA. They can repair the damaged tissues or provide immunomodulatory function to reduce inflammation in OA. Since OA is a degenerative joint disease likely involving the depletion of endogenous MSCs [3], and adult MSCs have the potential to differentiate into cells of chondrogenic lineage, investigation into MSC-based therapy should be supported for potential articular cartilage repair and regeneration [3], [4].
MSCs
MSCs can be isolated from various sources but bone marrow harvesting remains the primary source of most MSCs [34]. MSCs are widely distributed in other tissues, including periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle, tendon, lung, and deciduous teeth [35], [36], [37]. Due to their similar behaviour and potential with perivascular cells both in vivo and in vitro, the relationship between MSCs and pericytes has been investigated [38], [39], [40]. Furthermore, the perivascular niche for MSCs has been suggested [39], [40] and an in vitro study has confirmed that pericytes in human tissues are positive for MSC markers [39]. However, Kurth et al [41] have reported that MSCs isolated from the synovium in vivo are distinct from pericytes phenotypically and functionally.
Isolation and characterization of MSCs
The first attempt to isolate MSCs was reported by Friedenstein and co-workers [42], [43], [44]. They were the first to isolate fibroblastic cells from the stromal compartment of bone marrow, which could differentiate into bone tissue and bone marrow stroma in vivo. These fibroblastic cells were named stromal progenitor cells then stromal stem cells [42], [44]. MSCs were then isolated from many other tissues [3], including adipose tissue [45], skeletal muscle [39], umbilical cord blood [46], and Wharton's jelly [47]. As progenitors for the mesoderm lineage, MSCs have shown the multilineage differentiation potential that enables MSCs to differentiate into bone, fat, cartilage, and muscle cells in vitro [3]. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has proposed the criteria to classify MSCs, which includes: fibroblast-like morphology; plastic-adherent property under standard culture conditions; differentiation potential into osteoblasts, adipocytes, and chondroblasts in vitro; expression of surface markers including CD105, CD73 and CD90; and lack of expression of CD45, CD34, CD14, or CD11b, CD79α or CD19, and HLA-DR [48].
Differentiation potential of MSCs
As progenitors for the mesoderm lineage, MSCs can be differentiated into lineages of mesodermal tissues, including bone, cartilage, fat, muscle, tendon/ligament, and bone marrow stroma, dermis, and other connective tissues as diagrammed in Figure 3.
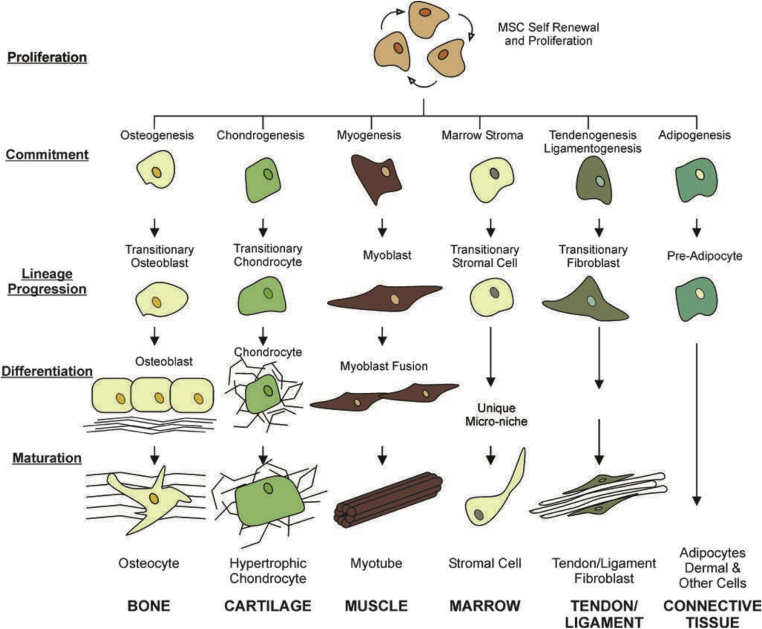
Fig. 3.
The mesengenic process. MSC self-renewal, proliferation, and potential lineage specific differentiation pathways are depicted in this diagram. MSCs differentiate by committing, differentiating, and maturing in a lineage-specific fashion. MSC = mesenchymal stem cell. Note. From “Identification of functional progenitor cells in the pulmonary vasculature” by Firth and Yuan, 2012. Pulmonary Circulation, 2, p. 84–100. Copyright 2012, Pulmonary Circulation. Reprinted with permission.
Osteogenic differentiation of MSCs requires β-glycerol-phosphate, ascorbic acid-2-phosphate, dexamethasone, and fetal bovine serum. Successful differentiation can be assessed by the upregulation of alkaline phosphatase activity and deposition of calcium-rich mineralized extracellular matrix readily detected by Alizarin Red or other stain [34], [35]. Adipogenic differentiation of MSCs involves several factors including nuclear receptor, transcription factor, peroxisome proliferator-activated receptor-γ, and fatty acid synthetase. The resulting adipocytes can be identified by their morphology of large lipid-filled vacuoles and staining with Oil Red [34], [35]. The differentiation potential of MSCs into other cell types such as myocytes and neurons has also been reported. These cells can be identified through immunocytochemistry with antibodies specific for antigens [34], [35].
Chondrogenic differentiation occurs when MSCs grow in serum-free nutrient medium and three-dimensional culture, with the addition of a member of the transforming growth factor (TGF)-β super family [35]. These cells have characteristic cartilage-specific markers such as collagen type II, aggrecan, and sulphated proteoglycans. Previous studies on MSC chondrogenesis were usually performed in high-density micromass cultures [49] or pellet culture [50] to promote cellular condensation. Caplan and co-workers [51], [52], [53] have developed an in vitro method of chondrogenic differentiation for animal and human MSCs using pellet or aggregate culture. A later study revealed that human bone-marrow-derived MSCs can form chondrocytes with TGF-β in the growth medium, while human adipose-tissue-derived MSCs require TGF-β and bone morphogenetic protein 6 [51], [54].
Chondrogenic differentiation potential of MSCs and OA treatment
Given the capacity of MSCs to differentiate towards the chondrogenic lineage, OA has been proposed as one of the primary areas for MSC-based therapy. OA may be the result of dysfunction in the MSC population, giving rise to degenerative changes in the absence of repair [3]. Thus, MSCs could be effective in treating OA by repairing the worn out tissues and lost cells. However, conflicting findings have been reported on the chondrogenic differentiation of MSCs and OA. Barry and Murphy [35] have reported that chondrogenic and adipogenic activity of bone-marrow-derived MSCs is reduced in patients with advanced OA; and they have argued that these changes in differentiation profile of MSCs may explain the loss of cartilage in OA patients [55]. In contrast, Scharstuhl et al [56] have revealed that chondrogenic differentiation potential of bone-marrow-derived MSCs from patients with OA is independent of age and OA [56]. MSCs isolated from all the three types of OA-aetiology groups including age-related, joint trauma, and joint dysplasia show adequate chondrogenic differentiation potential, and therefore, can be applied to cartilage regeneration. Furthermore, synovium-derived MSCs are reported to be larger from patients with rheumatoid arthritis and OA than healthy joints [57]. Caplan and Correa [58] have proposed MSCs as drug stores during injury. MSCs were released and activated from perivascular location, Further studies are needed to verify and compare those results, especially in the context of OA aetiology and therapy.
Recent investigations have advanced our understanding on the paracrine signalling by MSCs, with the secretion of biologically active molecules that might be more important than differentiated cells in stimulating repair responses, therefore, effectively widening the range of MSC therapeutic applications [3], [59]. Thus, more attention should be shifted from cell-surface markers and differentiation to paracrine factors by MSCs for assessment of MSC therapeutic potency [3], [59]. MSC paracrine effects can be categorized into trophic (nurturing) in terms of antiapoptosis, angiogenesis, and support of growth and differentiation of stem and progenitor cells, immunomodulation, antiscarring, and chemoattraction (Figure 4). Insights from these paracrine mechanisms may lead to revolutionary solutions to OA treatment.
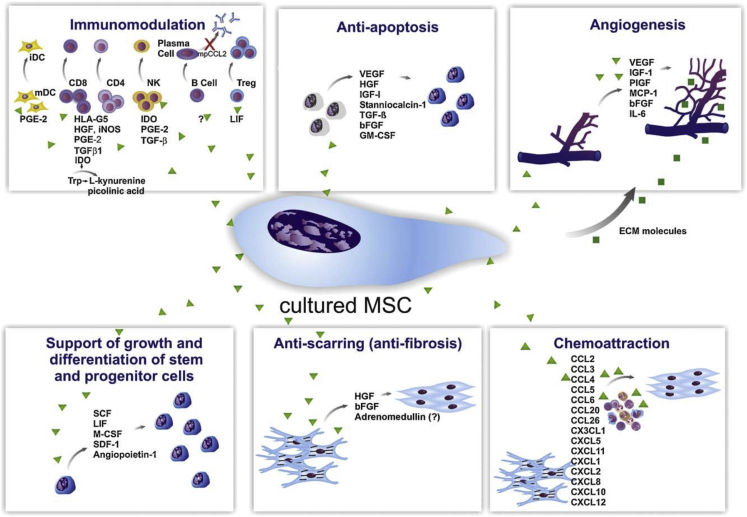
Fig. 4.
Paracrine effects of cultured MSCs. The secretion of a broad range of bioactive molecules is now believed to be the main mechanism by which MSCs achieve their therapeutic effect and it can be divided into six main categories: (1) immunomodulation; (2) antiapoptosis; (3) angiogenesis; (4) support of the growth and differentiation of local stem and progenitor cells; (5) antiscarring; and (6) chemoattraction. bFGF = basic fibroblast growth factor; CCL = CC chemokine ligand; CXCL = chemokine (C-X-C motif) ligand; ECM = extracellular matrix; GM-CSF = granulocyte–macrophage colony-stimulating factor; HGF = hepatocyte growth factor; iDC = invasive ductal carcinoma; IGF-1 = insulin growth factor-1; LIF = leukaemia-inhibitory factor; M-CSF = macrophage colony-stimulating factor; mDC = macrophage-derived chemokine; NK = natural killer; PGE2 = prostaglandin E2; SCF = stem cell factor; SDF-1 = stromal cell-derived factor 1; TGF-β = transforming growth factor-β; VEGF = vascular endothelial growth factor. Note. From “Mechanisms involved in the therapeutic properties of mesenchymal stem cells” by da Silva Meirelles, et al., 2009. Cytokine Growth Factor Reviews, 20, p. 419–427. Copyright 2009, Elsevier Ltd. Reprinted with permission.
Biomaterials in cell therapy
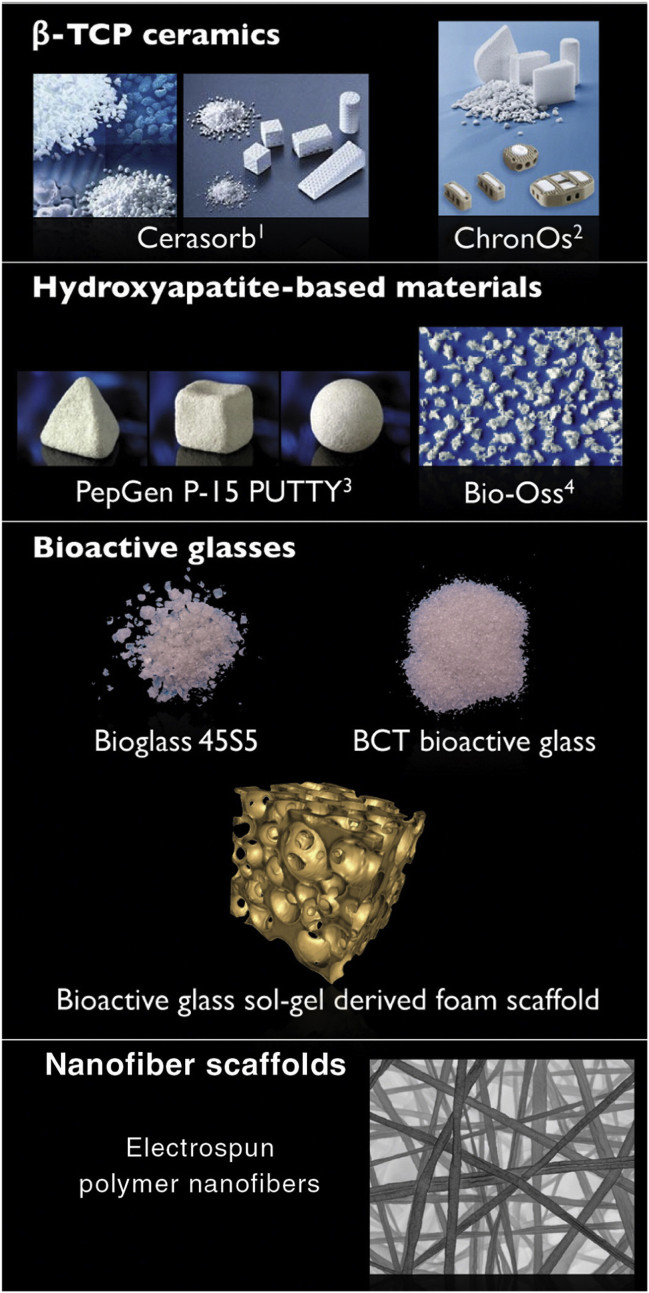
It is of great interest to combine biomaterials and cell therapy in the treatment of OA. Both primary adult osteoblasts and bone marrow MSCs have been used with biomaterials for bone tissue engineering [60]. Generally, autologous or allogeneic bone biomaterials can be divided into three groups: (1) bioactive inorganic materials, including bioactive ceramics such as tricalcium phosphate, hyaluronic acid (HA) and bioactive glasses; (2) biological and synthetic polymers; biological polymers include collagen and HA, while synthetic polymers include polylactic acid, polyglycolic acid, copolymers of polylactic and polyglycolic acids, and polycaprolactone; and (3) inorganic–organic composites, such as tissue-engineered HA–collagen nanocomposite systems (Figure 5). Several types of scaffolds have been used in tissue engineering in the study of MSC differentiation. For instance, silk-based scaffolds [61], agarose [62], [63], hydrogels [64], [65], [66], nanocomposite [67], and affinity-bound TGF-β scaffolds [68].
Fig. 5.
Macromorphology of some examples of different bone graft materials. BCT = bioceramic therapeutics; TCP = tricalcium phosphate. Note. From “Biomaterials for bone tissue engineering” by Stevens, 2008, Materials Today, 11, p.18–25, Copyright 2008, Elsevier Ltd. Reprinted with permission.
Commercial tissue engineering products for cartilage repair for clinical use is growing fast (Table 6). On December 13, 2016, The US Food and Drug Administration approved MACI (autologous cultured chondrocytes on porcine collagen membrane) for the repair of symptomatic, full-thickness cartilage defects of the knee in adult patients [70]. MACI is the first US Food and Drug Administration-approved autologous cell therapy product that is composed of autologous cultured chondrocytes from healthy cartilage tissue of a patient, and expanded on scaffolds with bioresorbable, porcine-derived collagen membrane and implanted over the defective or damaged area of the knee. MACI is produced by Vericel Corporation, Cambridge, MA, USA, which markets autologous cell-based therapies for patients with serious diseases.
Table 6.
Commercial cartilage tissue engineering products and biomaterials [69].
| Product | Regulatory status | Description | Use | Source | Cell | Form |
|---|---|---|---|---|---|---|
| Synvisc, Genzyme | 1997 | HA (Hylan GF-20 and Hylan B) | Synovial fluid replacement | Animal derived Resorbable | Gel | |
| CaReS, Arthro Kinetics | 2007 Germany | Rat-tail type I collagen matrix | Articular cartilage injury | Animal derived Resorbable | Autologous chondrocytes | 3D disc |
| Bioseed-C, Biotissue Technologies | Clinic trial 2001 | PLA/PGA and PDS scaffold | Articular cartilage injury | Synthetic Resorbable | Autologous chondrocytes | 3D disc |
| Menaflex, Regenbiologics | 2008 | Bovine type I collagen with HA and GAG, hydrated | Meniscus cartilage injury | Animal derived Resorbable | Mesh | |
| Hyalograft C autograft, Fidia advanced biopolymers | 2008 EU | HYAFF (esterified derivative of HA) scaffold | Articular cartilage injury | Resorbable Plant or bacteria derived | Autologous chondrocytes | 3D disc |
| MACI, Vericel Corporation | Clinic trial 2016 FDA | porcine-derived collagen membrane | Articular cartilage injury | Human derived Resorbable | Autologous chondrocytes | Sheet |
HA = hyaluronic acid; PLA = polylactic acid; PGA = polyglycolic acid; GAG = glycosaminoglycan; PDS = polydioxane; 3D = three-dimensional; EU = European Union; FDA = US Food and Drug Administration.
Clinical roadmap of MSCs as OA treatment
Choice of stem cells
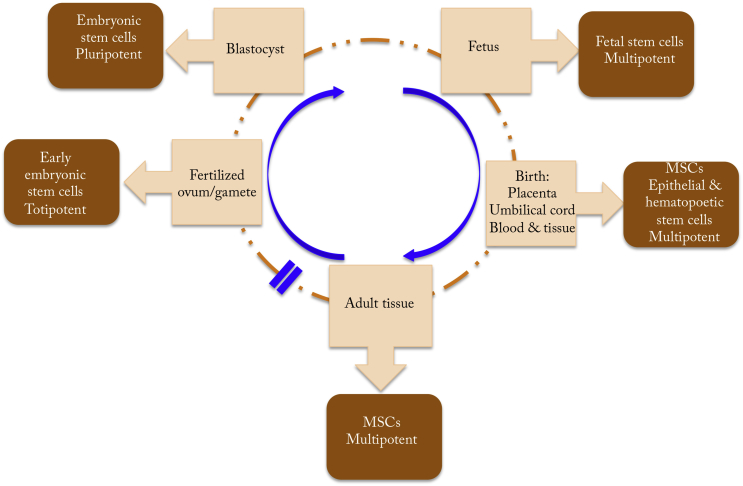
Stem cells can be of embryonic, fetal, or adult origin (Figure 6) [71]. Embryonic stem cells (ESCs) originate from the early embryo [71]. However, the use of fetal stem cells or ESCs has been a complex ethical issue. Currently, human ESCs from antenatal origin are not allowed in many countries [72]. Therefore, placenta, umbilical cord blood, and tissue obtained postnatally offer another source of MSCs (Figure 6). The MSCs isolated from these sources share similar properties in both their cell surface markers and morphology with MSCs from other sources. Different from ESCs with pluripotent differentiation capacity, fetal MSCs show multipotent differentiation capacity, with the differentiation pathways in terms of adipogenic, osteogenic and chondrogenic, myogenic, neurogenic, and endothelial potential [72]. Adult MSCs can be isolated from bone marrow or marrow aspirates. Besides bone-marrow-derived MSCs, muscle- and adipose-tissue-derived MSCs have been investigated and serve as important sources of MSCs [73]. It is critical to evaluate MSCs from all these sources and determine which is more efficacious for OA therapy.
Fig. 6.
Stem cells obtained at different stages of development can have varying levels of potential for differentiation. MSC = mesenchymal stem cell. Note. From “Prospects of stem cell therapy in osteoarthritis” by Roberts S, Genever P, McCaskie A, De Bari C, 2011, Regenerative Medicine, 6, p. 351–66. Copyright, 2011, Future Medicine Ltd. Modified with permission.
Preclinical models
Given the success in generating cartilage from MSCs, preclinical models have been applied to investigate MSCs in treating OA. The effect of MSCs on cartilage has been observed on many animal models including cartilage protection in mice [74], complete repair of full-thickness defects of joint cartilage [75] and cartilage improvement in rabbits [76], regeneration of the meniscus in goats [77], enhanced cartilage quality in horses [78], and partial cartilage repair in guinea pigs [79]. MSCs have also shown efficacy in preventing OA in a mouse model [80], meniscal repair in rats [81], and meniscal regeneration in sheep [82] (Table 7).
Table 7.
Preclinical models of MSC therapy for treating OA.
| Animal models | Delivery systems | MSC sources | Outcome | Refs |
|---|---|---|---|---|
| Mouse | Intra-articular injection of MSCs | Adipose-derived stem cells | Cartilage protection | [74] |
| Intra-articular injection of MSCs | Bone marrow | Efficacy in preventing OA | [80] | |
| Rat | Fibrin glue embedded with MSCs | Bone marrow | Meniscal repair | [83] |
| Intra-articular injection of human MSCs | Synovial stem cells | Meniscal repair | [84] | |
| Hyaluronan-based scaffold of MSCs | Bone marrow | Reduced the development of early/mild OA lesions | [85] | |
| Rabbit | Type-I collagen gel with adherent cells from bone marrow or periosteum | Bone marrow or periosteum | Complete repair of full-thickness defects of joint | [75] |
| Implantation of MSCs | Synovial SCs | Meniscal regeneration | [81] | |
| Direct intra-articular injection of MSCs | Subcutaneous adipose tissue | Cartilage improvement | [76] | |
| Goat | Direct intra-articular injection of MSCs | Bone marrow | Regeneration of the meniscus | [77] |
| Sheep | Intra-articular Injection of MSCs | Chondrogenic-induced bone marrow SCs | Meniscal regeneration | [82] |
| Horse | Intra-articular injection of MSCs | Adipose-derived or bone marrow-derived MSCs | Improved synovial fluid effusion PGE2 levels | [78] |
| Guinea pig | Intra-articular injection of MSCs with HA | Bone marrow | Cartilage repair | [86] |
| Intra-articular injection of MSCs with HA | Commercially available human MSCs | Partial cartilage repair | [79] | |
| Donkey | Intra-articular injection of MSCs | Bone marrow | Homing evidence and the reparative effect | [87] |
MSC = mesenchymal stem cell; OA = osteoarthritis; PGE2 = prostaglandin E2.
Several animal models have been used for evaluating the effect of intra-articular injection of MSCs for treating OA, such as mice [74], [80], rats [84], rabbits [76], goats [77], sheep [82], guinea pigs [79], [86], and donkeys [87] (Table 5). Mouse and rat models used for OA research include transgenic mouse models, aging models, and obesity models [88]. Specifically, Wakitani et al [75] observed complete repair of full-thickness defects of articular cartilage after they placed rabbit MSCs from bone marrow, or cells from the periosteum into a full-thickness chondral defect of an adult rabbit. Murphy et al [77] evaluated the effects of autologous injection of bone-marrow-derived MSCs in tissue repair and joint regeneration in goats with knee OA [77]. They observed marked regeneration of meniscal tissue and a decrease in degeneration of the articular cartilage, osteophytic remodelling, and subchondral sclerosis in cell-treated joints. In addition, Lee et al [86] used direct intra-articular injection of bone-marrow-derived MSCs suspended in HA for treating cartilage defects in pigs. Cartilage repair was improved both histologically and morphologically at 6 weeks and 12 weeks after injection. Mokbel et al [87] showed significant reparative effect of MSCs both clinically and radiologically in animals injected with green-fluorescent-protein-transduced MSCs. These preclinical studies have indicated that MSCs can serve as a viable and practical option for treating different degrees of OA. Taken together, insights from these animal models provide the rationale for human clinical trials detailed below.
It is accepted that animal studies have improved our understanding of disease mechanisms, while it has been controversial to use animal experiments to predict the effectiveness of treatment strategies in clinical trials. There are three good reasons for continuing animal studies: (1) the recurrent failure of interventions; (2) methodological flaws in animal studies; and (3) critical disparities between the animal models and the clinical trials [89]. Therefore, clinical trials should only be performed after a systematic analysis of all available animal studies [89], [90].
Clinical trials
Clinical trials with MSCs for treating human joint cartilage defects have been performed in the past several decades. Brittberg et al [91] reported that cultured autologous chondrocytes could be used to repair human deep cartilage defects of the knee joint [91]. The clinical benefits from this study led to the development of MSC-based strategies for cartilage repair. Wakitani et al [92], [93], [94] applied human autologous culture expanded bone-marrow-derived MSCs for repair of cartilage defects in osteoarthritic knees. They found that patients treated with bone-marrow-derived MSCs had higher arthroscopic and histological grades than the control group, while with no significant clinical improvement. This study revealed the potential to use MSC therapy for cartilage repair and regeneration, and reducing arthritis signs and symptoms through the immune regulatory effect of MSCs.
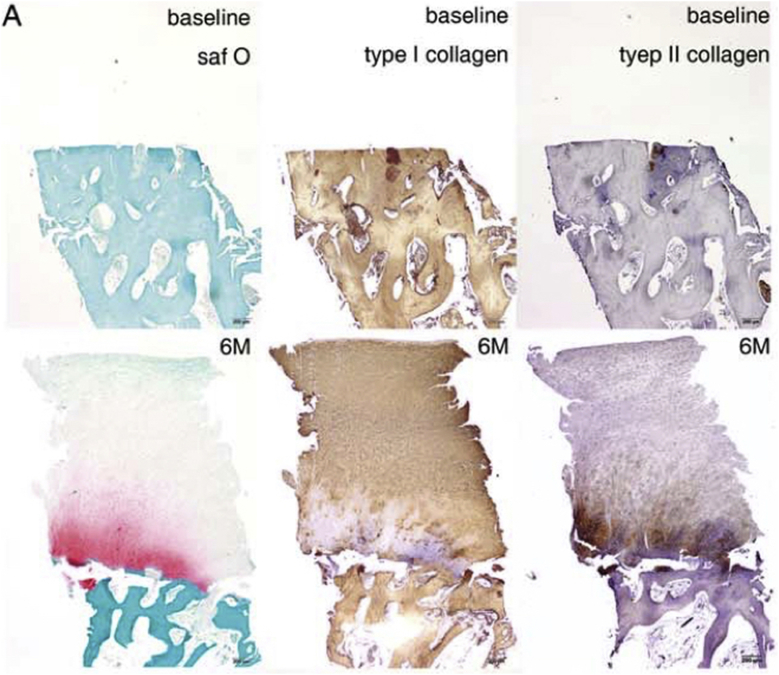
Bone-marrow- or adipose-tissue-derived MSCs have been used in most of the clinical studies, either with the approach of intra-articular injection or delivering the cells to the synovial fluid compartment [3]. Several studies have reported the effect of intra-articular injection of autologous MSCs for the treatment of knee OA [95], [96], [97], [98]. Centeno et al [95] reported significant cartilage and meniscus growth, and reduced pain and increased joint mobility in patients with degenerative joint disease at 24 weeks after autologous bone-marrow-derived MSC injection [95]. Emadedin et al [97] also observed satisfactory effects of intra-articular injection of bone-marrow-derived MSCs in patients with knee OA. In their study, after 6 months injection of MSCs, patients showed improvements in terms of pain, functional status of the knee, and walking ability. Furthermore, an increase in cartilage thickness and extension of the repair tissue over the subchondral bone was found in three out of six patients after MSC injection [97]. In addition, Jo et al [98] evaluated the potential of intra-articular injection of adipose-tissue-derived MSCs for the treatment of knee OA at low, medium and high doses. After 6 months of intra-articular injection at high dose (108) of MSCs into the osteoarthritic knee, they observed improvement in pain and function of the knee joint without causing adverse events, and cartilage defects were also reduced by regeneration of hyaline-like articular cartilage (Figure 7).
Fig. 7.
Histological evaluation of regenerated articular cartilage of biopsy from the medial femoral condyle after intra-articular injection of adipose-tissue-derived MSCs (AD MSCs). (A) Typical biopsy sample from the medial femoral condyle of a patient with International Cartilage Repair Society Grade 3C in the high-dose group at baseline and 6 months after AD MSC injection, stained with safranin O and anti-type I and II collagen antibodies. Although no articular cartilage is seen at baseline, a thick, hyaline-like cartilage with a smooth surface is regenerated and integrated with the subchondral bone 6 months after injection. In the superficial and the upper half of the middle zones, regenerated cartilage is composed of type I collagen and contains minimal type II collagen. Collagen fibrils in the superficial zone run parallel to the articular surface, while those in the middle zone are aligned obliquely. Safranin O and type II collagen is stained mostly in the lower half of the middle and deep zones. Collagen fibrils in these zones run vertically. Typical columnar chondrocytes or tide mark is not definite. However, chondrocytes are flattened in the superficial zone, and round in the middle and deep zones similar to those in the deep zone of hyaline cartilage. Small chondrocytes are also present in the in the calcified cartilage zone. saf O = safranin O. Note. From “Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial” by Jo et al., 2014, Stem Cells, 32, p.1254–66. Copyright 2014, Alpha Med Press. Reprinted with permission.
Concerns about MSC-based therapy
Direct intra-articular injection of MSCs has shown improvement in the condition of patients with OA and other degenerative joint diseases. Besides intra-articular injection, there are also other MSC delivery systems, such as scaffolds, aligned nanofibrous scaffolds, fibrin glue/gel/clot, tissue-engineered construct, and hydrogel systems [99]. However, the number and type of MSCs and the timing for injection, and the stage of the disease for MSC therapy and the delivery strategy need further investigation to achieve optimal response [96], [100]. More importantly, simplicity and ease of the injection for MSCs in clinical practice could avoid surgery and the associated adverse effects, and provide better treatment opportunities for OA. Each patient should be assessed individually for the severity of OA and a stepwise treatment with combination therapy could be applied for best performance. MSC treatment effects could be optimized together with patient education and counselling as well as conservative treatment or other options.
It is also worth noting that there are other concerns regarding therapy with MSCs and other stem cells. First, autologous cells should not be encouraged for genetic disorders due to their genetic influence, such as in OA. Therefore, allogeneic MSCs could be considered. Second, the quality of the cells might be too low for older patients [57]. Lastly, the safety of stem cell therapy is always a concern. The first safety study reported that human bone-marrow-derived mesenchymal progenitor cells obtained from cancer patients could be collected, expanded in vitro, and infused intravenously without toxicity. Five years later it was reported that autologous human bone-marrow-derived MSC infusion at the time of peripheral blood progenitor cell transplantation was feasible and safe. However, the risk of tumourigenicity with uncontrolled cell division and disease transmission remained a concern [72]. It takes time and a great amount of careful, quantitative research and safety assessment for us to answer these questions.
Conclusion and future directions
Without an effective cure, OA remains a significant clinical burden on our elderly population. The advancement of regenerative medicine and innovative stem cell technology offers a unique opportunity to treat this disease. In this review, we examine OA and the likely resolution with MSCs. MSCs have been one of the highlights in stem cell research in recent years. Although the application of MSCs in joint repair is well established, it is particularly exciting about MSCs being used for OA treatment. Indeed, animal testing has provided much needed encouragement and the rationale to move forward with human trials. Future challenges may include the efficient isolation and culture of MSCs from defined and reliable sources. MSCs made through good manufacturing practices must be carefully evaluated through a combination of means including biochemical, genetic, and epigenetic markers, as well as bioactive assays to establish the efficacy of cells, their proliferative activity, and reparative potentials before they can be used in humans. Furthermore, delivery systems for MSCs and evaluation of their safety and effectiveness also need to be investigated. It is hopeful that these studies can be accomplished in the near future and OA patients may receive much needed help soon. In this review, we provide some updated information, including most recent approvals of the clinical trials and related applications of MSCs. Future aspiration of MSCs resides in the realization of clinical translation and the treatment of other musculoskeletal disorders.
Conflicts of interest
All authors have no conflicts of interest.
Acknowledgements/Funding
This work was supported by Hong Kong RGC Collaborative Research Fund (C4028-14GF) and General Research Fund (GRF 473013).
References
- 1.Wittenauer R., Smith L., Aden K. World Health Organization; Geneva: 2013. Background paper 6.12 osteoarthritis. [Google Scholar]
- 2.Birchfield P.C. Osteoarthritis overview. Geriatr Nurs. 2001;223:124–130. doi: 10.1067/mgn.2001.116375. [DOI] [PubMed] [Google Scholar]
- 3.Barry F., Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;910:584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 4.Noth U., Steinert A.F., Tuan R.S. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;47:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 5.Maetzel A., Li L.C., Pencharz J., Tomlinson G., Bombardier C., Pro C.H.A. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Anna Rheum Dis. 2004;634:395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinert A.F., Rackwitz L., Gilbert F., Noth U., Tuan R.S. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;13:237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen C., Lu W.W., Chiu K.Y. Importance of subchondral bone in the pathogenesis and management of osteoarthritis from bench to bed. J Orthop Transl. 2014;21:16–25. [Google Scholar]
- 8.Arden N., Cooper C. Informa Healthcare; London: 2005. Osteoarthritis handbook. Osteoarthritis: epidemiology. [Google Scholar]
- 9.Felson D.T., Anderson J.J., Naimark A., Walker A.M., Meenan R.F. Obesity and knee osteoarthritis. The Framingham study. Ann Intern Med. 1988;1091:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 10.Carman W.J., Sowers M., Hawthorne V.M., Weissfeld L.A. Obesity as a risk factor for osteoarthritis of the hand and wrist—a prospective study. Am J Epidemiol. 1994;1392:119–129. doi: 10.1093/oxfordjournals.aje.a116974. [DOI] [PubMed] [Google Scholar]
- 11.Cicuttini F.M., Baker J.R., Spector T.D. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996;237:1221–1226. [PubMed] [Google Scholar]
- 12.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Postgrad Med J. 2012;881038:240–242. doi: 10.1136/pgmj.2010.146399rep. [DOI] [PubMed] [Google Scholar]
- 13.Schett G., Kleyer A., Perricone C., Sahinbegovic E., Iagnocco A., Zwerina J. Diabetes is an independent predictor for severe osteoarthritis results from a longitudinal cohort study. Diabetes Care. 2013;362:403–409. doi: 10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson D.T., Lawrence R.C., Dieppe P.A., Hirsch R., Helmick C.G., Jordan J.M. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;1338:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 15.Hinton R., Moody R.L., Davis A.W., Thomas S.F. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician. 2002;655:841–848. [PubMed] [Google Scholar]
- 16.Sharma L., Berenbaum F. Elsevier Health Sciences; Amsterdam: 2007. Osteoarthritis: a companion to rheumatology. [Google Scholar]
- 17.Loeser R.F. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;251:108–113. doi: 10.1097/BOR.0b013e32835a9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlson E.W., Mandl L.A., Aweh G.N., Sangha O., Liang M.H., Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;1142:93–98. doi: 10.1016/s0002-9343(02)01447-x. [DOI] [PubMed] [Google Scholar]
- 19.Sarzi-Puttini P., Cimmino M.A., Scarpa R., Caporali R., Parazzini F., Zaninelli A. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;351:1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Xu L., Nevitt M.C., Aliabadi P., Yu W., Qin M. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2001;449:2065–2071. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Coggon D., Reading I., Croft P., McLaren M., Barrett D., Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;255:622–627. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J.J., Felson D.T. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;1281:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 23.Roemer F.W., Mohr A., Genant H.K. 2005. Diagnosis of osteoarthritis. Osteoarthritis handbook; p. 49. [Google Scholar]
- 24.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;851:49–56. [PubMed] [Google Scholar]
- 25.Salter R.B. Lippincott Williams & Wilkins; Philadelphia: 1999. Textbook of disorders and injuries of the musculoskeletal system: an introduction to orthopaedics, fractures, and joint injuries, rheumatology, metabolic bone disease, and rehabilitation. [Google Scholar]
- 26.Neumann G., Hunter D., Nevitt M., Chibnik L.B., Kwoh K., Chen H. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthr Cartil. 2009;176:761–765. doi: 10.1016/j.joca.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roemer F.W., Crema M.D., Trattnig S., Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;2602:332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 28.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K. Development of criteria for the classification and reporting of osteoarthritis—classification of osteoarthritis of the knee. Arthritis Rheum. 1986;298:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 29.Bauer D.C., Hunter D.J., Abramson S.B., Attur M., Corr M., Felson D. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthr Cartil. 2006;148:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Rousseau J.C., Delmas P.D. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;36:346–356. doi: 10.1038/ncprheum0508. [DOI] [PubMed] [Google Scholar]
- 31.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;196:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M.S., Dwyer D. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;132:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 33.Hayami T., Pickarski M., Wesolowski G.A., McLane J., Bone A., Destefano J. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;504:1193–1206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 34.Nardi N.B., da Silva Meirelles L. Springer; Berlin: 2008. Mesenchymal stem cells: isolation, in vitro expansion and characterization, in stem cells; pp. 249–282. [PubMed] [Google Scholar]
- 35.Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;364:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Ni M., Rui Y.F., Tan Q., Liu Y., Xu L.L., Chan K.M. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials. 2013;348:2024–2037. doi: 10.1016/j.biomaterials.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 37.He Q., Wan C., Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;251:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 38.Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;33:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;33:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Bianco P., Cossu G. Uno, nessuno e centomila: searching for the identity of mesodermal progenitors. Exp Cell Res. 1999;2512:257–263. doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- 41.Kurth T.B., Dell'Accio F., Crouch V., Augello A., Sharpe P.T., De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;635:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 42.Friedenstein A.J., Piatetzky-Shapiro, Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 43.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. Development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;34:393403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 44.Owen M., Friedenstein A.J. Stromal stem-cells—marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 45.Bunnell B.A., Estes B.T., Guilak F., Gimble J.M. Differentiation of adipose stem cells. Adipose Tissue Protoc. 2008:155–171. doi: 10.1007/978-1-59745-245-8_12. [DOI] [PubMed] [Google Scholar]
- 46.Weiss M.L., Troyer D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006;22:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troyer D.L., Weiss M.L. Concise review: Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;263:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;84:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 49.Denker A.E., Haas A.R., Nicoll S.B., Tuan R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation. 1999;642:67–76. doi: 10.1046/j.1432-0436.1999.6420067.x. [DOI] [PubMed] [Google Scholar]
- 50.Bosnakovski D., Mizuno M., Kim G., Ishiguro T., Okumura M., Iwanaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;325:502–509. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Caplan A.I. Mesenchymal stem cells: the past, the present, the future. Cartilage. 2010;11:6–9. doi: 10.1177/1947603509354992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;2381:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 53.Yoo J.U., Barthel T.S., Nishimura K., Solchaga L., Caplan A.I., Goldberg V.M. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Jt Surg Am. 1998;80A12:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Estes B.T., Wu A.W., Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;544:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 55.Murphy J.M., Dixon K., Beck S., Fabian D., Feldman A., Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;463:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 56.Scharstuhl A., Schewe B., Benz K., Gaissmaier C., Buhring H.J., Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;2512:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 57.Jones E.A., English A., Henshaw K., Kinsey S.E., Markham A.F., Emery P. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;503:817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 58.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;91:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meirelles L.D., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;205–6:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Stevens M.M. Biomaterials for bone tissue engineering. Mater Today. 2008;115:18–25. [Google Scholar]
- 61.Bhardwaj N., Kundu S.C. Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials. 2012;3310:2848–2857. doi: 10.1016/j.biomaterials.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 62.Awad H.A., Wickham M.Q., Leddy H.A., Gimble J.M., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;2516:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 63.Huang C.Y.C., Reuben P.M., D'Ippolito G., Schiller P.C., Cheung H.S. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol. 2004;278A1:428–436. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 64.Kundu B., Kundu S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials. 2012;3330:7456–7467. doi: 10.1016/j.biomaterials.2012.06.091. [DOI] [PubMed] [Google Scholar]
- 65.Teixeira L.S.M., Feijen J., van Blitterswijk C.A., Dijkstra P.J., Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;335:1281–1290. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 66.Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;94:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 67.Niamsa N., Srisuwan Y., Baimark Y., Phinyocheep P., Kittipoom S. Preparation of nanocomposite chitosan/silk fibroin blend films containing nanopore structures. Carbohydr Polym. 2009;781:60–65. [Google Scholar]
- 68.Re'em T., Kaminer-Israeli Y., Ruvinov E., Cohen S. Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials. 2012;333:751–761. doi: 10.1016/j.biomaterials.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Place E.S., Evans N.D., Stevens M.M. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;86:457–470. doi: 10.1038/nmat2441. [last accessed in April 2017] [DOI] [PubMed] [Google Scholar]
- 70.FDA News Release. FDA approves first autologous cellularized scaffold for the repair of cartilage defects of the knee. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm533153.htm.
- 71.Roberts S., Genever P., McCaskie A., De Bari C. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011;63:351–366. doi: 10.2217/rme.11.21. [DOI] [PubMed] [Google Scholar]
- 72.Gucciardo L., Lories R., Ochsenbein-Kolble N., Done E., Zwijsen A., Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;1162:166–172. doi: 10.1111/j.1471-0528.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 73.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;2132:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 74.ter Huurne M., Schelbergen R., Blattes R., Blom A., de Munter W., Grevers L.C. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;6411:3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 75.Wakitani S., Goto T., Pineda S.J., Young R.G., Mansour J.M., Caplan A.I. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Jt Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Toghraie F., Razmkhah M., Gholipour M.A., Faghih Z., Chenari N., Nezhad S.T. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;158:495–499. [PubMed] [Google Scholar]
- 77.Murphy J.M., Fink D.J., Hunziker E.B., Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;4812:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 78.Frisbie D.D., Kisiday J.D., Kawcak C.E., Werpy N.M., Mcllwraith C.W. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;2712:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 79.Sato M., Uchida K., Nakajima H., Miyazaki T., Guerrero A.R., Watanabe S. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diekman B.O., Wu C.L., Louer C.R., Furman B.D., Huebner J.L., Kraus V.B. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transpl. 2013;228:1395–1408. doi: 10.3727/096368912X653264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horie M., Driscoll M.D., Sampson H.W., Sekiya I., Caroom C.T., Prockop D.J. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Jt Surg Am. 2012;948:701–712. doi: 10.2106/JBJS.K.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al Faqeh H., Nor Hamdan B.M., Chen H.C., Aminuddin B.S., Ruszymah B.H. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;476:458–464. doi: 10.1016/j.exger.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 83.Izuta Y., Ochi M., Adachi N., Deie M., Yamasaki T., Shinomiya R. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;123:217–223. doi: 10.1016/j.knee.2001.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Horie M., Sekiya I., Muneta T., Ichinose S., Matsumoto K., Saito H. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;274:878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 85.Grigolo B., Lisignoli G., Desando G., Cavallo C., Marconi E., Tschon M. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;154:647–658. doi: 10.1089/ten.TEC.2008.0569. [DOI] [PubMed] [Google Scholar]
- 86.Lee K.B.L., Hui J.H.P., Song I.C., Ardany L., Lee E.H. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells. 2007;2511:2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 87.Mokbel A.N., El Tookhy O.S., Shamaa A.A., Rashed L.A., Sabry D., El Sayed A.M. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. 2011;12:259. doi: 10.1186/1471-2474-12-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen D., Shen J., Zhao W., Wang T., Han L., Hamilton J.L. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sena E., van der Worp H.B., Howells D., Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;309:433–439. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 90.van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O'Collins V. Can animal models of disease reliably inform human studies? Plos Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;33114:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 92.Wakitani S., Yamamoto T. Response of the donor and recipient cells in mesenchymal cell transplantation to cartilage defect. Microsc Res Tech. 2002;581:14–18. doi: 10.1002/jemt.10111. [DOI] [PubMed] [Google Scholar]
- 93.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;103:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 94.Wakitani S., Nawata M., Tensho K., Okabe T., Machida H., Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;11:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 95.Centeno C.J., Busse D., Kisiday J., Keohan C., Freeman M., Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;113:343–353. [PubMed] [Google Scholar]
- 96.Davatchi F., Abdollahi B.S., Mohyeddin M., Shahram F., Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;142:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 97.Emadedin M., Aghdami N., Taghiyar L., Fazeli R., Moghadasali R., Jahangir S. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;157:422–428. [PubMed] [Google Scholar]
- 98.Jo C.H., Lee Y.G., Shin W.H., Kim H., Chai J.W., Jeong E.C. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;325:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 99.Yu H., Adesida A.B., Jomha N.M. Meniscus repair using mesenchymal stem cells—a comprehensive review. Stem Cell Res Ther. 2015;61:86. doi: 10.1186/s13287-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu L., Li G. Circulating mesenchymal stem cells and their clinical implications. J Orthop Transl. 2014;21:1–7. [Google Scholar]