Summary
There are high expectations for the clinical application of regenerative medicine technologies to treat musculoskeletal disorders. However, there are still big hurdles in bringing cell-based products to the market, mainly due to strict regulatory frameworks to approve these. Recently, the Japanese Pharmaceuticals and Medical Devices Agency adopted new regulations under legislature. The translational potential of this article is to inform on the regulations to bring experimental phase regenerative concepts to market approval in the United States and Europe, and highlight the opportunities granted by Japanese regulatory framework. Furthermore, we discuss the perspectives on the quickly evolving regulatory environment.
Keywords: regenerative medicine, regulations, translational medicine
Introduction
Regenerative medicine has led the medical science scene in the past 20 years. However, only a handful of cell-based products have made their way to the market. Moreover, it has been almost a decade since the discovery of induced pluripotent stem cell (iPSC) technology [1]. While this discovery was sensationally reported globally, to date, only one patient was transplanted with autologous iPSC-derived retinal pigment epithelial cells for treatment of macular degeneration [2]. Up until now, clinical outcomes, including potential adverse effects, of the patient who received this treatment have not been reported. A second iPSC treatment for a macular degeneration patient was scheduled; however, this was temporarily suspended due to identification of a mutation found in a known oncogene. Recently, it has not been reported that the trial will be resumed with an allogeneic focus. The National Institutes of Health is estimated to fund US$898,000,000 on regenerative medicine and US$1,495,000,000 on stem cell-related research in 2016 [3]. Despite large research funding opportunities, very few regenerative medicine products are available to patients. The lack of cell-based products in clinics and market should be considered a major drawback of strict regulatory control by the competent authorities. This review aims to give an overview of the current global regulatory frameworks and highlight some current development that could potentially accelerate the translation of regenerative medicine to the market.
Regulatory framework
The fundamental regulatory concept of product manufacturing for regenerative medicine and cell therapy in Europe and the United States is based on the premise of public health protection. National regulatory authorities base their decisions on quality, safety, and efficacy, while financial aspects of achieving potential patient benefits are left out of the equation. A risk assessment-based approach is applied for each product. Logical and reasonable analyses of risk, specific for each product, are demanded for both developers and regulatory agencies. Risk profiles under scientific analysis for each product at an early phase of development should be obtained. However, regulatory agencies categorize products by the condition of “cell processing” and “non-homologous use” and not merely by a grading of potential risk. Spread of medical treatments with unproven safety and efficacy shadows breakdown of public health protection. Currently, there is no universal guideline for assessing biological products including cell-based therapies for regenerative medicine for human applications. In 1990, the idea of harmonizing regulatory requirements for the use of new pharmaceutical products was proposed by the European Union (EU), USA, and Japan, which gave rise to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Since then, more than 50 harmonized guidelines have been conducted in the areas of quality, safety, and efficacy. Good manufacturing practice (GMP) is a guideline designated to maintain quality and safety standards across a range of industries, including medical and food manufacturing. It is often mistaken that the GMP “grade” is most important for obtaining regulatory approval [4]. The level of manufacturing process may, however, vary between products if the product is meeting the quality standard for intended use. Therefore, equipment, supplies, materials, manufacturing processes, and management systems are usually evaluated independently for each cell-based product and manufacturer. All aspects of the product and the treatment will be tracked and followed, where possible, from the donor to the patient over time. Quality control of the manufacturing process and records will be maintained with the manufacturer.
United States
The regulatory authority in the USA is the Food and Drug Administration (FDA). The FDA is officially responsible for protecting public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, food supply, cosmetics, and products that emit radiation. Regulation of most regenerative medicine products is categorized under human cells, tissues, and cellular and tissue-based products (HCT/Ps) in the Code of Federal Regulations and the Food and Drug Safety Act, Title 42, Chapter 6A—Public health service, Public Health Service Act Section 361 and 351 (PHS Act361, Title 42 USC Section 264) and Code of Federal Regulations Title 21 Part 1271 (21CFR1271) [5]. The current FDA approval of HCT/Ps follows a sequential process starting from basic research and preclinical investigations under good laboratory practice guideline [4].
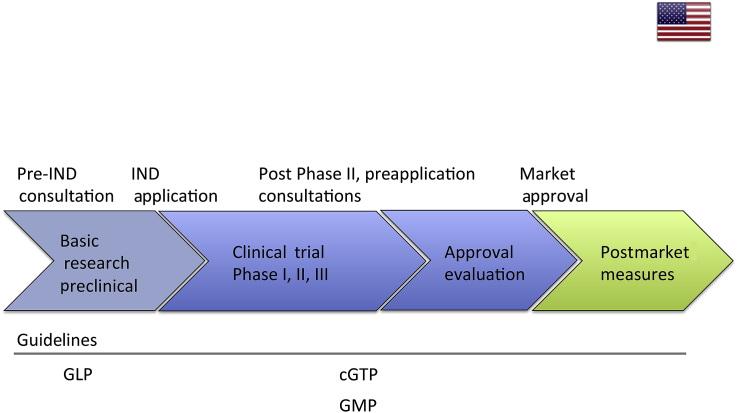
Researchers or manufacturers can consult the FDA for preinvestigational new drug application. Preinvestigational new drug is a request for authorization from the FDA to administer an investigational drug or biological product to humans. The FDA will then assess the risk of the product for potential human applications on a case-by-case basis. After preinvestigational new drug application, the product will go into phased clinical trials (Phase I: determining whether the product is safe to check for efficacy and to test dose range on healthy volunteers; Phase II: testing of safety and efficacy of the product; Phase III: testing of the product on patients to assess its efficacy, effectiveness, and safety) under current good tissue practice, a GMP for HCT/Ps [6]. FDA consultations can be held after Phases II and III before applying for the final approval. The whole process of bringing in new HCT/Ps to the market can take more than 12 years (Figure 1). In total, the FDA has, as of June 13, 2016, approved 12 cellular, and tissue- and gene therapy-based products [7] (Table 1). Among the 12 authorized therapies, six products are based on cord blood-derived hematopoietic progenitor cells for hematopoietic and immunological reconstitution. Furthermore, a cellular immunotherapy product Provenge by Dendreon Corporation (Seattle, WA, USA) has been approved for asymptomatic metastatic castrate-resistant prostate cancer. Another approved product is Imlygic, by Amgen Inc. (Thousand Oaks, CA, USA), that is targeted against recurrent melanoma. GINTUIT, by Organogenesis Incorporated (Canton, MA, USA), is an allogeneic cellularized scaffold for artificially creating a vascular wound bed for treatment of mucogingival conditions, and TheraCys, by Sanofi Pasteur Limited (Lyon, France), is used for treatment of a set of specific tumours. Additionally, the FDA permits usage of the autologous cultured chondrocyte product Carticel, by Genzyme Biosurgery (Cambridge, MA, USA), for symptomatic cartilage defects. Finally, Laviv (or Azficel-T), an autologous fibroblast product from Fibrocell Technologies Inc. (Exton, PA, USA), has been authorized to treat nasolabial fold wrinkles.
Figure 1.
Schematic overview of US regulation pathway. cGTP = current good tissue practice; GLP = good laboratory practice; GMP = good manufacturing practice; IND = investigational new drug.
Table 1.
Overview of authorized regenerative medicine products—a list of allowed cellular, tissue-based, and gene therapy products in the EU, USA, and Japan.
| Product name | Manufacturer | Description | Indication of approval |  |
 |
 |
|---|---|---|---|---|---|---|
| Imlygic | Amgen Inc. | Gene therapy medicinal product | Unrespectable metastatic melanoma | × | × | |
| Glybera | UniQure Biopharma B.V. | Gene therapy medicinal product | Lipoprotein lipase deficiency with severe pancreatitis | × | ||
| TheraCys | Sanofi Pasteur Limited | Attenuated mycobacteria | Carcinoma in situ of the urinary bladder and Ta and/or T1 papillary tumours | × | ||
| GINTUIT | Organogenesis Incorporated | Allogeneic keratinocytes and fibroblasts in bovine collagen | Mucogingival conditions | × | ||
| TEMCELL | JCR Pharmaceuticals | Allogeneic mesenchymal stromal cells | Acute graft versus host disease | × | ||
| Holoclar | Chiesi Farmaceutici S.p.A. | Autologous corneal epithelial (including stem cells) | Limbal stem cell deficiency with burned corneal damage | × | ||
| PROVENGE | Dendreon Corporation | Activated autologous peripheral blood mononuclear cells | Prostate cancer | × | × | |
| HeartSheet | Terumo | Autologous skeletal myoblast sheet | Chronic ischaemic heart disease | × | ||
| JACE | Japan Tissue Engineering Co. Ltd | Autologous cultured keratinocytes sheet | Full-thickness skin burn wounds | × | ||
| Laviv | Fibrocell Technologies, Inc. | Autologous fibroblast | Nasolabial fold wrinkles | × | ||
| JACC | Japan Tissue Engineering Co. Ltd | Autologous cultured chondrocytes in atelocollagen gel | Traumatic cartilage defects and osteochondritis dissecans | × | ||
| Carticel | Genzyme Biosurgery | Autologous chondrocytes | Acute symptomatic femoral condyle cartilage defects | × | ||
| ChondroCelect | TiGenix N.V. | Autologous cartilage cells | Single-defect symptomatic cartilaginous femoral condyle defects | × | ||
| Maci | Aastrom Biosciences | Autologous chondrocytes | Full-thickness symptomatic cartilage defects | × | ||
| N/A | Bloodworks | CBdHPC | Hematopoietic system deficiency | × | ||
| N/A | LifeSouth Community Blood Centers Inc. | CBdHPC | Hematopoietic system deficiency | × | ||
| N/A | Clinimmune Labs, University of Colorado Cord Blood Bank | CBdHPC | Hematopoietic system deficiency | × | ||
| Ducord | Duke University School of Medicine | CBdHPC | Hematopoietic system deficiency | × | ||
| Hemacord | New York Blood Center, Inc | CBdHPC | Hematopoietic system deficiency | × | ||
| ALLOCORD | SSM Cardinal Glennon Children's Medical Center | CBdHPC | Hematopoietic system deficiency | × |
Marking under the flags indicate approval in corresponding territories.
CBdHPC = cord blood-derived hematopoietic progenitor cells; N/A = not-applicable.
European Union
Within the EU, the regulatory authority is the European Medicines Agency (EMA), and their official mission is to foster scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health. The Committee for Medicinal Products for Human Use under the EMA evaluates and approves all regenerative medicine products. All legislatures relating to medicinal products, including regenerative medicine products, are databased in the EudraLex, which consists of 10 volumes. Under this legislature, areas of advanced therapy medicinal products (ATMPs) were defined and categorized, which comprise gene therapy medicinal products, somatic cell therapy medicinal products, tissue-engineered products, and medical devices combined with cell or tissue parts [8].
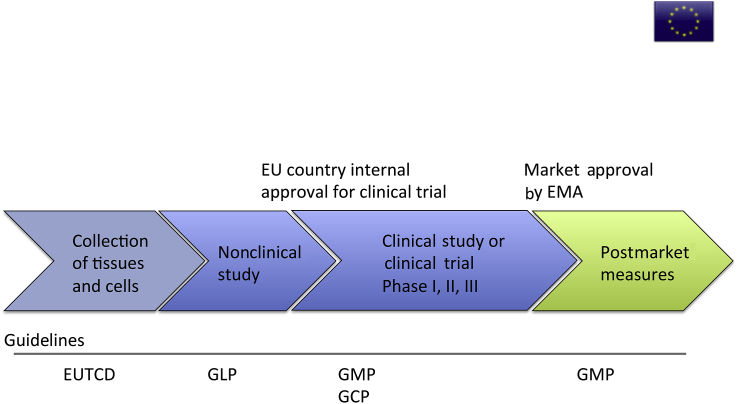
EMA also adopts an individual risk-based approach. The process of ATMP market approval starts with the collection of tissues and cells from donors and their evaluation under the European Union Tissue and Cells Directives, the EU version of good tissue practice. Preclinical testing for safety of the product will be performed under good laboratory practice, similar to the USA. If the product is qualified for a clinical trial, it will be submitted to the authorities of the originating EU country, where the trial will take place within the EU. Clinical studies (noncommercial) or clinical trials (commercial) will be performed under GMP and good clinical practice guidelines. When the product is adequate for market approval, it will lastly be evaluated by the EMA as a final step to market application. ATMPs will be required to obtain continuous postmarket evaluation on the traceability of the donors, products, and patients, as well as the development of risk management systems and pharmacovigilance, especially for follow-up on efficacy. Similar to the situation in the USA, the whole process may take multiple years (Figure 2).
Figure 2.
Schematic overview of EU regulation pathway. EMA = European Medicines Agency; EU = European Union; EUTCD = European Union Tissue and Cells Directives; GCP = good clinical practice; GLP = good laboratory practice; GMP = good manufacturing practice.
As of March 16, 2016, the EMA has approved marketing authorization for a total of six ATMP-classified products (Table 1). Firstly, two gene therapy medicinal products, Glybera, from UniQure N.V., Amsterdam, Netherlands, and Imlygic, from Amgen Europe Bv. (Breda, Netherlands), have been granted authorization in 2012 and 2015, respectively. Glybera is aimed against lipoprotein lipase deficiency. Furthermore, the somatic cell medicinal product Provenge from Dendreon UK Ltd was approved as of 2013; however, marketing authorization has been withdrawn by Dendreon due to commercial reasons. Finally, a set of three tissue-engineered products was accepted for clinical use. Holoclar from Chiesi Farmaceutici S.p.A (Parma, Italy) received approval as of 2015 for use in patients with the indication of corneal burn damage associated with severe limbal stem cell deficiency. The first ATMP to be approved for clinic was ChondroCelect in 2009 from Tigenix N.V. (Leuven, Belgium) for the treatment of single cartilage defects within the femoral condyle. Lastly, Maci, a treatment option for symptomatic cartilage defects by Aastrom Bioscience (Ann Arbor, MI, USA), was granted marketing authorization in 2013; however, it has been suspended due to closure of the manufacturing facility within the EU territory. Notably, Provenge and Imlygic are the only two products approved for marketing in both the EU and the USA.
Japan
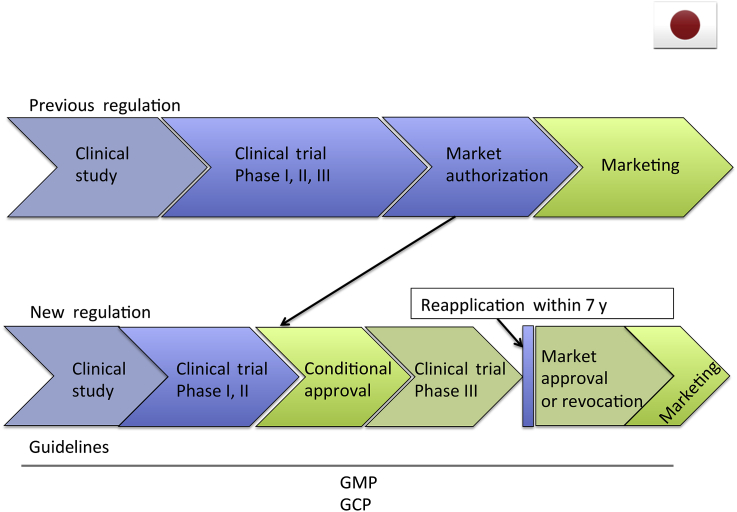
Until 2014, there were no statute-based regulations available for regenerative medicinal products including stem cell therapies. The regulatory pathways followed for cellular and tissue-based products were divided into three different categories accompanied by distinct governing rules, namely, “clinical research”, “clinical trials and marketing”, and “medical treatment at one's own expense”. “Clinical research” mainly points to a clinical research using stem cell treatments in an academic setting and following the academic guidelines that govern this, issued by the Japanese Ministry of Health, Labour and Welfare. “Clinical trials and marketing” refers to commercial trials following the traditional phased process, and this is governed by Pharmaceuticals Affairs Act, which is put in place by the Pharmaceuticals and Medical Devices Agency. “Medical treatment at one's own expense” refers to medical treatment not falling under the coverage of the Japanese National Health Insurance, which is governed by Medical Practitioners Act, issued by the Japanese Ministry of Health, Labour and Welfare. At present, two regenerative medicinal products have been approved under the “clinical trials and marketing” category, i.e., “autologous cultured epidermis” and “autologous cultured cartilage” manufactured by the Japan Tissue Engineering Co., Ltd. (Aichi, Japan) However, a need remained for an integrated regulatory system where medical professionals could safely and adequately provide regenerative medicine to improve the laboratory-to-market process. As a result, since November 25, 2014, “the Act on the Safety of Regenerative Medicine” has become effective [9]. This new law was issued to establish steps for the practice of regenerative medicine, in order to ensure the safe and ethical administration of regenerative medical technologies and the safe yet accelerated adoption of specific processed cellular products by establishing a manufacturing permit system. This new act also sets out regulations not only for regenerative medicine, but also for cell-based products. The act divides regenerative medicinal products into three classes depending on the potential risk to human health—Class I: identifying high-risk products including iPSC, embryonic stem cells, allogeneic, and xenogeneic cells; Class II: referring to medium-risk products covering all autologous somatic stem cells; and Class III: defining low-risk products including autologous somatic cells. Regenerative medicine using iPSCs will thus be categorized as high-risk medicine, and all aspects of product development to market will be handled at the national level. The act was issued to promote safe clinical studies. After this preclinical study stage, clinical trials will be performed under the “Pharmaceuticals, Medical Devices and Other Therapeutic Products Act”. This new law, issued on August 10, 1960, has been revised to provide an accelerated route to the clinic for regenerative medicine. It also aims to establish regulations for regenerative medicine that are independent of regular ethical drugs, medical devices, and nonmedical and cosmetic products. Although this act updates regulation for many of the medical products, most momentous is the incorporation of the novel “conditional approval” system. If the regenerative medical product satisfies the following conditions, then entity can obtain input from a subcommittee of the Pharmaceutical Affairs and Food Sanitation Council and receive conditional approval. Regenerative medicinal products are oftentimes processed during production. This “processing” can introduce certain risks, including “the manifestation of additional properties that differ from the cells that were originally processed” and “an inconsistency of quality”. To help deal with these inherent risks adequately, regenerative medicinal products that are provided with conditional approval must stay within the following boundaries: they must not be carcinogenic; conditional approval will not last longer than 7 years, and during this period, measures must be taken to ascertain the proper use of the regenerative medical products; and upon reapplication they must demonstrate adequate efficacy and safety. To summarize this novel system, by treating regenerative medicine products in a similar manner to orphan drugs, the approved product will typically skip Phase III trial and obtain marketing authorization after demonstration of safety and minimal signs of efficacy after a solid Phase I and II trial. The Ministry of Health, Labour and Welfare aimed to accelerate the process of testing the efficacy in humans, which previously took many years, similar to other countries. This system will also benefit the manufacturer by potentially lowering the overall cost of the final product. Regenerative medicinal products that are approved under the Pharmaceuticals, Medical Devices and Other Therapeutic Products Act will not be subject to the Act on the Safety of Regenerative Medicine (except for off-label use) insofar as they are being used within the conditions of their approval (Figure 3).
Figure 3.
Schematic overview of Japan regulation pathway. GCP = good clinical practice; GMP = good manufacturing practice.
By September 18, 2015, two cell products TEMCELL (JCR Pharmaceuticals (Hyogo, Japan); licensee of Mesoblast Ltd in Japan) and HeartSheet (Terumo, Tokyo, Japan) were approved for the Japanese market (Table 1). TEMCELL product consists of allogeneic mesenchymal stromal cells for the treatment of graft versus host disease. This product was approved with the review of two clinical trials: a US-based clinical trial containing 51 placebo patients and a Japan-based clinical trial consisting of a single cohort of 25 patients with their statistical comparison to determine whether it possessed adequate efficacy for approval. On November 27, 2015, TEMCELL obtained authorization of Japanese ¥868,680 (approximately US$7079) per bag of 72 million cells for reimbursement from the national health insurance [10], [11].
HeartSheet is a cell sheet product processed with autologous skeletal muscle cells. Terumo applied for approval with a Japan-based clinical trial consisting of only seven patients and found it difficult to evaluate efficacy due to patient scarcity. However, Terumo was allowed to combine data from a clinical research consisting of 19 patients, which was eventually regarded sufficient to provide “adequate safety” and “probable efficacy” data, and to be granted conditional approval. HeartSheet treatment obtained an unpredicted reimbursement cost of Japanese ¥147,600,000 (approximately US$122,000) [10].
Discussion
In concert with the advancement of regenerative medicine technologies, regulatory authorities confront emerging new obstacles in regulating safety of these products for human applications. Timely revisions of policies are needed to adequately control the use of regenerative medicinal products. With the explosive increase of industry within the regenerative medicine market, it is becoming increasingly important to selectively identify and invest in products with apparent efficacy limited to strict indications in order to smoothly enter the market.
Moreover, costs for clinical trials are rising, which have several plausible explanations. Firstly, the increasing number of medications makes it more difficult for pharmaceutical companies to prove product superiority compared with existing products [12]. Secondly, it may be argued that the increasing costs of conducting large clinical trials have be covered by equally high reimbursements, while, e.g., patents are securing market position. Furthermore, when the precise working mechanisms of complex regenerative treatments have not been explained by experimental studies, testing of efficacy in clinical studies is used to verify causality—especially when compared with placebo in randomized clinical trials. In such cases, cohort studies without control groups, which serve as validation of safety and efficacy, may require additional large placebo-controlled trials to justify their use.
Simplifying and streamlining the regulatory processes from the laboratory to the patient by stratification and characterization of regenerative treatments based on their expected safety profiles have several advantages in terms of making better and more advanced treatments available with the potential benefit of reduced cost. Evidence-based medicine is supposed to rely on high-level evidence such as randomized placebo-controlled trials. To avoid situations that may, to some extent, discredit the use of regenerative treatments that have been approved without the use of randomized clinical trials, companies should ensure prospective follow-up of the patients treated with their products. The companies should be encouraged to enrol all their patients in independent national or international registers that can be used by the scientific community. This may be one of the crucial steps for enhancing and accelerating future collaborations of the researchers, clinicians, industry, and authorities to deliver safe and effective regenerative medicine for the benefits of the patients.
Conflicts of interest
One coauthor receives personal financing from Regience K.K., Japan, and DiscGenics Inc., United States.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jot.2017.02.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotech. 2015;33:890–891. doi: 10.1038/nbt0915-890. [DOI] [PubMed] [Google Scholar]
- 3.WHO. WHO good manufacturing practices for pharmaceutical products: main principles—2014. Available at: http://www.who.int/medicines/areas/quality_safety/quality_assurance/TRS986annex2.pdf?ua=1. Accessed June 25, 2016.
- 4.WHO. Handbook for good laboratory practise (GLP)—2009. Available at: http://www.who.int/tdr/publications/documents/glp-handbook.pdf. Accessed June 25, 2016.
- 5.FDA. Guidance for industry: regulation of human cells, tissues, and cellular and tissue-based products (HCT/Ps)— small entity compliance guide—2007. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/ucm073366.htm. Accessed June 25, 2016.
- 6.FDA. Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps)—2011. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Accessed June 25, 2016.
- 7.FDA. Marketed products from the office of cellular, tissue and gene therapies (OCTGT)—2016. Available at: http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/. Accessed June 25, 2016.
- 8.EMA. Advanced therapy medicinal products—2016. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000294.jsp&mid=WC0b01ac05800241e0. Accessed June 25, 2016.
- 9.MHLW. Translated English overview of 'the Act on the Safety of Regenerative Medicine’—2014. Available at: http://www.mhlw.go.jp/english/policy/health-medical/medical-care/dl/150407-01.p. Accessed June 25, 2016.
- 10.Buckler L., Novick C.L. Japan's regulatory gamble and what it means for the industry. Bioinsights. Cell Gene Therapy Insights. 2015 (Proceedings) [Google Scholar]
- 11.JCR Pharmaceuticals Co. L. TEMELL HS Inj. receives NHI reimbursement price listing—2015. Available at: http://www.jcrpharm.co.jp/wp2/wp-content/uploads/2016/01/ir_news_20151126.pdf. Accessed June 25, 2016.
- 12.Roger C. Rapidly rising clinical trial costs worry researchers. CMAJ. 2009;180:277–278. doi: 10.1503/cmaj.082041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.