Summary
Maintenance of healthy bone quality and quantity requires a well-coordinated balance between bone formation by osteoblasts and bone resorption by osteoclasts. Chemerin is a novel adipokine with known functions such as regulating immunity and energy homeostasis through activation of chemokine-like receptor 1 (CMKLR1). G protein-coupled receptor 1 (GPR1) is the second mammalian chemerin receptor with similar binding affinity as CMKLR1. In male GPR1–/– mice, a phenotype with significantly low bone mineral density was observed. We hypothesise that GPR1 might participate the process of bone remodelling. In this study, we investigated the role of GPR1 in regulating bone mass maintenance in male mice, and for the first time, revealed that GPR1–/– male mice manifested seriously trabecular bone loss and lower serum testosterone levels compared to the wild type animals. Accordingly, the mRNA expression of biomarkers related to both osteoblast [collagen type I alpha 2 (Col1A2), osteocalcin (OCN)] and osteoclast [tartrate-resistant acid phosphatase (TRAP), Cathepsin K, NFATc1] were significantly decreased or increased in GPR1–/– mice relative to the wild type, respectively. However, other osteogenic markers, Osterix and ALP levels, were increased. Microcomputed tomography scanning and histological analyses proved that there was a myriad of trabecular bone loss in GPR1–/– mice. In the meantime, GPR1–/– mice presented a significant decrease in serum testosterone level. Taken together, these findings suggested that chemerin–GPR1 signalling might be directly or indirectly communicated with testosterone synthesis on bone turnover regulation. Further detailed studies are required to unveil how chemerin–GPR1 participates in bone metabolism.
The translational potential of this article: More studies and knowledge about GPR1 regulating function in bone turnover might supply a novel therapeutic target for osteoporosis in the future.
Keywords: bone loss, G protein-coupled receptor 1, inflammation, osteoporosis, testosterone
Introduction
Bone is a dynamic connective tissue consisting of different cell types, including osteoblasts, which are bone-forming cells involved in bone mineralisation; osteoclasts, which are multinucleated bone-resorbing cells in the body possessing the ability to degrade both the inorganic calcium matrix and the organic collagen matrix; and osteocytes, which build the basis of bone and appear to be able to regulate the activity of both osteoblasts and osteoclasts actively [1], [2], [3], [4]. Maintenance of the healthy bone quality and quantity requires a highly coordinated regulation among the cells [5]. If the balance between bone formation and bone resorption is disrupted, it will lead to metabolic bone diseases, such as osteoporosis [1], [3], [6]. Normally, the development and function of bone-forming and bone-resorbing cells are tightly regulated by signalling molecules such as sex hormones and other molecules.
Osteoporosis is a chronic disease characterised by low bone mineral density (BMD), deterioration of bone structure, ultimately leading to reduced bone strength, and increased risk of fractures at multiple skeletal sites, particularly at the spine, hip, or wrist in humans [7], [8], [9]. It has been estimated that more than 9 million osteoporotic fractures occurred worldwide in the year 2000 [10]. Osteoporosis stems from various causes, including gonadal dysfunction and ageing in both males and females [11]. Although osteoporosis is more prevalent in women, male osteoporotic fracture patients are subjected to a greater rate of mortality and second fracture compared to their female counterparts [6], [12]. It is well known that decreased serum testosterone levels are associated with reduced BMD [11], [13]. Testosterone exhibits a direct and indirect impact via conversion with oestrogen on those cells regulating bone remodelling [14]. Moreover, the evidence has demonstrated that testosterone deficiency affects calcium metabolism by increasing excretion of calcium from the skeletal compartment [15]. However, the pathogenesis of male osteoporosis remains to be elucidated, especially, the relationships among testosterone, oestrogen, sex hormone-binding globulin, age, and bone turnover.
Chemerin, previously called tazarotene-induced gene 2 (TIG2) or retinoic acid receptor responder protein 2 (RARRES2), was initially identified as a chemoattractant ligand for the G protein-coupled receptor (GPCR), called chemokine-like receptor 1 (CMKLR1) or chemerin receptor 23 (ChemR23) [16], [17]. It was also identified as a novel adipocytokine with functions for adipocyte differentiation [18], osteoblastogenesis [19], and energy homeostasis [20], [21]. It is secreted mainly from white adipocytes and is highly expressed in multiple tissues, except for in liver and white adipose tissue, also in placenta, skin, adrenal gland, lung, intestine, pancreas, and ovary [22]. After it is released as an 18-kDa inactive precursor protein called prochemerin, chemerin is quickly converted to its active form by proteolytic cleavage of its C terminus [16], [23].
To date, chemerin has been known to bind to three GPCR receptors: CMKLR1, CC-motif chemokine receptor-like 2 (CCRL2), and G protein-coupled receptor 1 (GPR1) [24]. CCRL2 does not stimulate calcium mobilisation or ligand internalisation after binding, but could increase the local concentration of chemerin and improve the interaction between chemerin and CMKLR1 [25], [26] to trigger functions such as intracellular calcium mobilisation, receptor and ligand internalization, and cell migration [16], [27]. GPR1 is the closest homologue of CMKLR1 with more than 40% amino acid sequence identity [27]. Unlike CMKLR1, there are only a few studies about chemerin–GPR1 and their physiological functions. It has been reported that GPR1 is expressed in the central nervous system, skin, adipose tissue, and skeletal muscle, as well as in few cell types including Leydig cells and granulosa cells [27], [28].
GPR1 signalling has been investigated on glucose homeostasis and reproduction [28], [29], but to date there has been no study related to GPR1 and bone metabolism. In this study, we will present our preliminary findings on the role GPR1 in bone metabolism in vivo.
Materials and methods
Reagents
Acid Phosphatase, Leukocyte (TRAP) Kit was purchased from Sigma-Aldrich (St. Louis, MO, USA). Anticollagen I mouse polyclonal antibody and anti-F4/80 mouse monoclonal antibody were purchased from Abcam (Cambridge, UK). Unless specified, all cell culture reagents were obtained from Thermo Fisher Scientific Inc. (Cleveland, OH, USA).
Animals
GPR1−/− mice (C57BL/6 genetic background, 12 months old) were bred in our certified animal facility with age-matched wild type (WT) C57BL/6 mice as controls (animal ethics approval number: SIAT-IRB-170305-YGS-LIJ-A0313). Mice were maintained under specific pathogen-free conditions at 25°C on a 12-hour light/12-hour darkness cycle with access to food and water ad libitum. Animal care was in compliance with the Guide for the Care and Use of Laboratory Animals of Guangdong province. All procedures were performed under the supervision and approval of the Ethics Committee for Animal Research, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China.
Microcomputed tomography scanning and analysis
The femora of 12-month-old WT (N = 15) and GPR1−/− male mice (N = 6) were microcomputed tomography (microCT) scanned (SkyScan 1176; Bruker, Kontich, Belgium) after dissection of the animals that were euthanised by CO2 inhalation. The scanning protocol used was as follows: 9 μm resolution, 0.5 mm aluminium filter, 60 kV voltage, and 425 μA current. All imaging data were acquired and reconstructed using the commercial software provided by the company. After three-dimensional reconstruction, the major parameters for bone quantity and quality evaluation—including BMD, the ratio of bone volume to tissue volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), degree anisotropy (DA), structure model index (SMI), and trabecular pattern factor (Tb.Pf)—were calculated.
Biochemical assays
Blood was obtained from the retro-orbital sinus of euthanised mice using unheparinised capillaries and collected into 1.5-mL centrifuge tubes after overnight fasting of animals. Serum was prepared by centrifugation for 30 minutes at 2000 rpm, aliquoted, and stored at −80°C until assay. The serum level of testosterone and estradiol was measured using commercial iodine [125I] radioimmunoassay (RIA) Kits by Beijing North Biotechnology Research Institute, Beijing, China. The serum concentration of interleukin (IL)-1β, IL-6, and tumour necrosis factor alpha (TNF-α) were measured using enzyme-linked immunosorbent assay kits (Dakewe, Beijing, China), respectively.
Cells
Isolation, culture, expansion, and differentiation of bone marrow-derived mesenchymal stem cells
The isolation and culture of bone marrow-derived mesenchymal stem cells were performed as previously described [30]. Briefly, bone marrow was collected from 14-week-old male mice that were euthanised by cervical dislocation after CO2 anaesthesia. After the femora and tibiae were dissected away from attached muscle and soft tissue, both ends were cut. The bone marrow cells were extruded by inserting a 21-gauge needle into the shaft of the bones and flushing with 2 mL of Dulbecco's modified Eagle's medium-low glucose (DMEM-LG) and filtered through 40 mesh nylon and plated overnight in DMEM-LG supplemented with 10% (v/v) foetal bovine serum, 2mM l-glutamine, and 100 units/mL penicillin–streptomycin at 37°C under 95% humidity with 5% CO2. After 48 hours, the medium containing nonadherent haematopoietic cells were removed by changing the medium, and the adherent cells were harvested by trypsinisation (0.25% trypsin–EDTA; Gibco, Carlsbad, CA, USA) when reaching about 80% confluence and then replated. The culture medium was replaced twice per week. The phenotype of cell population was identified by the presence of bMSC surface molecular markers (CD90, CD44, and CD105) and the absence of the haematopoietic lineage molecular markers, CD34 and CD45, by flow cytometric immunophenotyping (data not shown). Osteogenic and adipogenic differentiation capacity of MSCs were examined based on established methods [31]. For osteogenic differentiation, deposition of a calcium phosphate mineralised matrix was detected by staining with alizarin red S, and quantification was according to established protocols [32]. For adipogenic differentiation, cells were fixed in 4% paraformaldehyde and stained with a filtered 0.3% solution of oil red O in 60% isopropanol for 15 minutes (data not shown). The third or fourth passages of pure bMSCs were used for the following experiments.
Gene expression analysis
Total RNA was extracted from the whole long bones using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. cDNA was synthesised using the Reverse Transcription Kit (Takara, Tokyo, Japan). The mRNA levels for interested genes were measured by real-time quantitative polymerase chain reaction (RT-qPCR) using the SYBR Green Detection System with a LightCyclerV480 instrument (Roche, Basel, Switzerland). All experiments were done in triplicate, and the primers are listed in Table 1. RT-qPCR was performed for amplification according to the following cycling conditions: initial denaturation at 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 30 seconds. Post-PCR melting curves confirmed the specificity of single-target amplification, and the fold change of the gene of interest relative to β-actin was determined.
Table 1.
Primer sequences for real-time quantitative polymerase chain reaction.
| Gene | Primer (5′–3′) |
|
|---|---|---|
| Forward | Reverse | |
| Osterix | ATGGCTCGTGGTACAAGGC | GCAAAGTCAGATGGGTAAGTAGG |
| ALP | GCCCTCCA GATCCTGACCAA | GCAGAGCCTGCTGGTCCTTA |
| Col1A2 | CTGGAACAAATGGGCTCACTG | CAGGCTCACCAACAAGTCCTC |
| OCN | ACAAA'GCCTTCATGTCCAAG | TTTAGGGCAGCACAGGTC |
| TRAP | GCAACATCCCCTGGTATGTG | GCAAACGGTAGTAAGGGCTG |
| Cathepsin K | GAAGAAGACTCACCAGAAGCAG | TCCAGGTTATGGGCAGAGATT |
| NFATc1 | CCGTTGCTTCCAGAAAATAACA | TGTGGGATGTGAACTCGGAA |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| GPR1 | GTCTCCCAGCTTCCCCGCTG | CAAGCTGTCGTGGTGTTTGA |
| chemerin | TACAGGTGGCTCTGGAGGAGTTC | CTTCTCCCGTTTGGTTTGATTG |
| AR | CTGGGAAGGGTCTACCCAC | GGTGCTATGTTAGCGGCCTC |
| β-actin | GTATCCATGAAATAAGTGGTTAC AGG | GCAGTACATAATTTACACAGAAG CAAT |
Histological analysis
Dissected femora were fixed in 4% paraformaldehyde for 24 hours, decalcified with decalcifying solution (pH 7.2, 4°C) for 4 weeks, embedded in paraffin, and sliced into 7-μm sections (Leica RM2235; Leica Microsystems, Wetzlar, Germany). Haematoxylin–eosin (H&E, C0105; Beyotime, Beijing, China), TRAP, and immunohistochemistry (IHC) staining for collagen I and F4/80 were performed, respectively. Images were taken by using an Olympus IX71 microscope (Olympus, Tokyo, Japan).
Statistical analyses
Data are presented as means ± standard error of the mean, and statistical significance was assessed by either one-way analysis of variance followed by Bonferroni or two-tailed Student t test; a p value < 0.05 means statistically significant difference in this study.
Results
GPR1 deficiency results in bone loss and low sex hormones in male mice
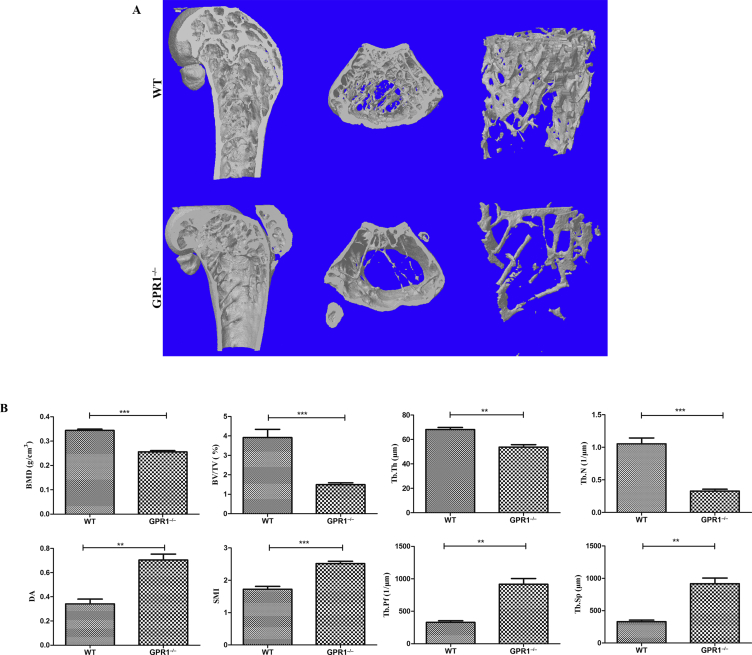
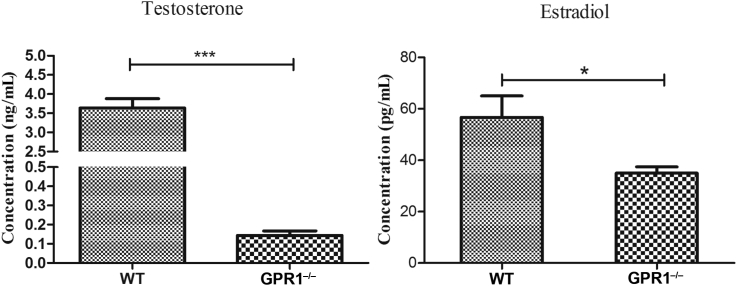
High-resolution microCT scanning was performed to compare the skeletal phenotype of 12-month-old WT and GPR1−/− male mice. GPR1−/− mice exhibited deterioration of trabecular bone at distal femora compared to WT mice, with obviously decreased bone mass (Figure 1A), as shown in BMD, BV/TV, Tb.N, and Tb.Th (Figure 1B). In addition, the parameters representing osteoporotic potential increased in GPR1−/− mice relative to the control, as shown by DA, Tb.Sp, SMI, and Tb.Pf (Figure 1B). Along with the fact that GPR1 deficiency leads to male bone loss in vivo, serum levels of testosterone and estradiol were obviously decreased in male GPR1−/− mice (Figure 2).
Figure 1.
GPR1−/− deficiency male mice have much lower bone mineral density. (A) MicroCT images of secondary spongy bone of the metaphysis of distal femora from GPR1−/− and WT control mice. (B) The quantitative microCT bone parameters BMD, BV/TV, Tb.Th, Tb.N, DA, SMI, Tb.Pf, and Tb.Sp. Statistical significance was assessed with two-tailed Student t test; **p < 0.01 versus WT, ***p < 0.001 versus WT, NWT = 15, NGPR1−/− = 6. BMD = bone mineral density; BV/TV = ratio of bone volume to tissue volume; DA = degree anisotropy; GPR1 = G protein-coupled receptor 1; microCT = microcomputed tomography; SMI = structure model index; Tb.N = trabecular number; Tb.Pf = trabecular pattern factor; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness; WT = wild type.
Figure 2.
Serumal levels of testosterone and estradiol in male GPR1−/− and WT male mice (14 weeks old) measured by RIA. Statistical significance was assessed using two-tailed Student t test; *p < 0.05 versus WT, ***p < 0.001 versus WT, NWT = 12, NGPR1−/− = 3. GPR1 = G protein-coupled receptor 1; RIA = radioimmunoassay; WT = wild type.
GPR1, chemerin, and androgen receptor expression exists in bMSC osteogenesis
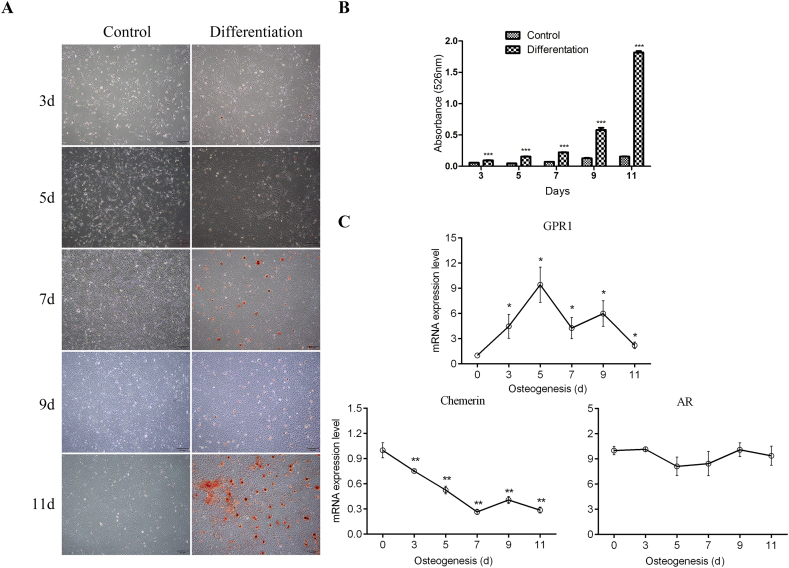
To understand the roles of GPR1–chemerin in bone metabolism, we investigated the expression of chemerin, GPR1, and androgen receptor (AR) in bMSCs. We compared their expression at different differentiating stage of bMSCs, from Day 0 to Day 11. Prior to this, bMSCs' osteogenic differentiation was verified by alizarin red S staining, and extracellular matrix calcium nodules were quantified. In Figures 3A and 3B, the bMSCs successfully differentiated into osteoblast-like cells, which started to deposit mineral in the extracellular matrix at Day 11. Then mRNA expression of the related genes was investigated in these osteoblastic differentiated bMSCs. GPR1 mRNA expression increased continuously with cell osteoblastic differentiation and summited on Day 5 with about a 9-fold increase compared to Day 0 (Figure 3C), whereas the mRNA level of chemerin decreased all along during osteoblastic differentiation of bMSCs. As for mRNA expression of AR, there was almost no change during the process (Figure 3C).
Figure 3.
GPR1, chemerin, and AR expression in osteogenic differentiated bMSCs isolated from WT mice. (A) Alizarin Red S staining of ECM of bMSCs, photomicrographs (×4). (B) Quantification of ECM mineralisation by measuring Alizarin Red S extracted in 10% CPC solution. Statistical significance was assessed by the two-tailed Student t test; ***p < 0.001 versus control in each day; N = 3. (C) RT-qPCR analysis of mRNA levels for GPR1, chemerin and AR during WT MSC osteogenesis. All values are expressed relative to the mRNA levels measured on Day 0 and represent the mean ± standard error of the mean. Statistical significance was assessed by one-way ANOVA followed by Bonferroni; *p < 0.05 versus Day 0, N = 3. ANOVA = analysis of variance; AR = androgen receptor; bMSCs = bone marrow-derived mesenchymal stem cells; ECM = extracellular matrix; GPR1 = G protein-coupled receptor 1; RT-qPCR = real-time quantitative polymerase chain reaction; WT = wild type.
GPR1 affects osteoblast and osteoclast development in vivo
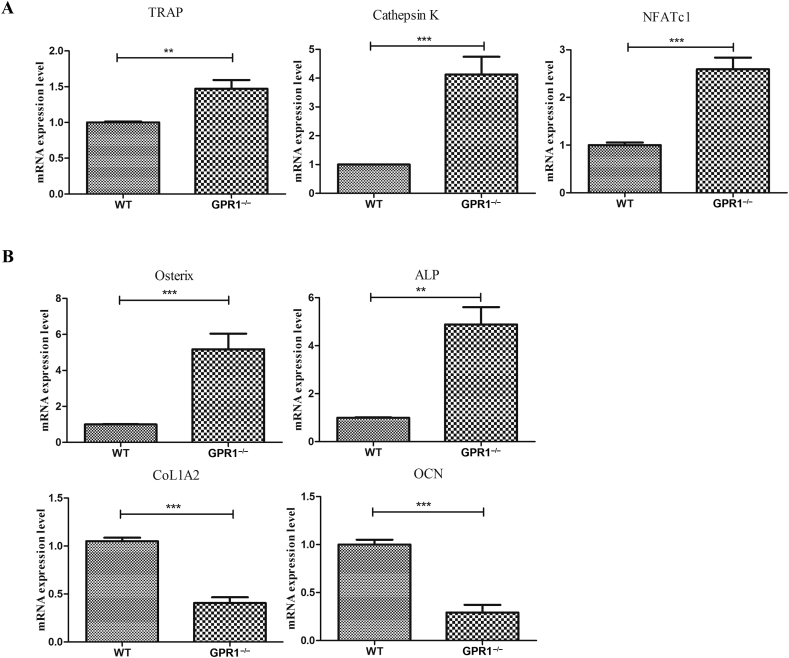
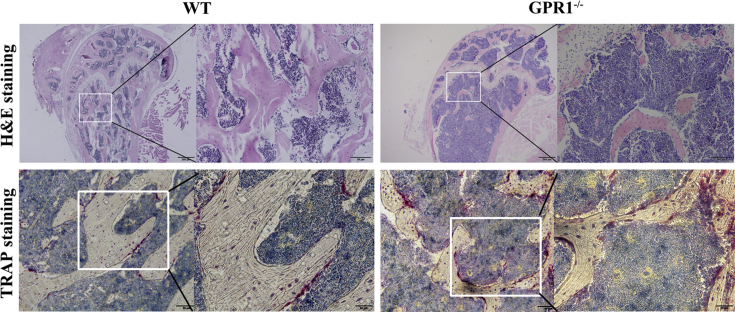
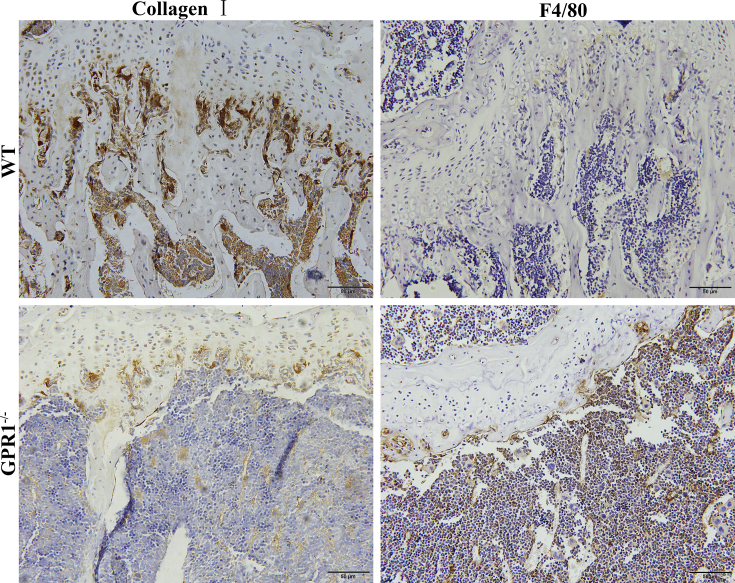
In order to evaluate how GPR1 affects osteoblast and osteoclast function, we examined the related genes of osteoblast and osteoclast biomarkers in GPR1-deficient and WT male mouse bone tissue. The osteoclast markers TRAP, Cathepsin K, and NFATc1 significantly increased in GPR1−/− mice bones (Figure 4A), whereas the osteoblast markers Col1A2 and OCN significantly decreased (Figure 4B). Surprisingly, in GPR1−/− mice, the other osteoblast markers including ALP and Osterix's mRNA expression significantly enhanced (Figure 4B). Histomorphologic analysis was performed to obtain more information about GPR1 and bone metabolism. Femoral bone H&E staining showed obvious trabeculae lost in GPR1−/− mice when compared to WT mice (Figure 5). In Figure 5, TRAP staining indicates that there were more osteoclasts in the femora of GPR1−/− mice than in the femora of WT mice. Corresponding with osteogenic biomarkers' mRNA expression, IHC staining revealed lower collagen I level in GPR1−/− mice than in WT mice (Figure 6).
Figure 4.
RT-qPCR analysis of the mRNA expression of biomarkers related to both (A) osteoclast including TRAP, Cathepsin K, NFATc1; and (B) osteoblast including CoL1A2, ALP, Osterix, and OCN. Statistical significance was assessed using two-tailed Student t test; **p < 0.01 versus WT, ***p < 0.001 versus WT, NWT = 15, NGPR1−/− = 6. CoL1A2 = collagen type I alpha 2; OCN = osteocalcin; RT-qPCR = real-time quantitative polymerase chain reaction; TRAP = tartrate-resistant acid phosphatase; WT = wild type.
Figure 5.
Histomorphologic analysis. H&E and tartrate-resistant acid phosphatase (TRAP) staining of distal femora sections from male GPR1−/− and control WT mice (14 weeks old). GPR1 = G protein-coupled receptor 1; H&E = haematoxylin–eosin; WT = wild type.
Figure 6.
Immunohistochemical staining with collagen I and F4/80-specific antibody of the bone tissue from male GPR1−/− and control WT mice (14 weeks old). GPR1 = G protein-coupled receptor 1; WT = wild type.
Enhanced inflammation correlates with GPR1 deficiency
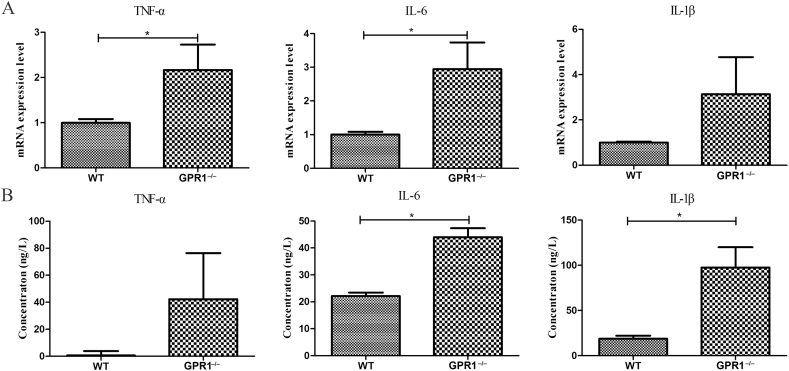
We performed macrophage/monocyte marker F4/80 IHC staining, and we found more positive staining in the GPR1−/− group (Figure 6). Then several major inflammatory cytokines' existence was analysed in bone. The RT-qPCR results showed that the mRNA expression of TNF-α, IL-6, and IL-1β increased to some extent in GPR1−/− bone as compared to WT bone (Figure 7A). The enzyme-linked immunosorbent assay results supported the mRNA expression data (Figure 7B).
Figure 7.
Analyses of the mRNA and protein levels of IL-1β, IL-6, and TNF-α in bone tissues and serum from male GPR1−/− and WT mice (12 months old), respectively. (A) RT-qPCR analysis and (B) enzyme-linked immunosorbent assay (ELISA) analysis. Statistical significance was assessed using two-tailed Student t test; *p < 0.05 versus WT, NWT = 15, NGPR1−/− = 6. GPR1 = G protein-coupled receptor 1; IL = interleukin; RT-qPCR = real-time quantitative polymerase chain reaction; TNF-α = tumour necrosis factor alpha; WT = wild type.
Discussion
Bone remodelling is a dynamic process requiring the well-coordinated action of bone formation and bone resorption [4]. If the bone cells respond wrongly to the signalling carried by hormones, cytokines or minerals, and so on, dysregulation of the remodelling process could happen and a net bone loss could be detected [33], and chronic bone loss disorders such as osteoporosis occurred [7], [34]. Chemerin is a novel adipocyte-derived signalling protein already known to be involved in adipogenic differentiation of MSCs [19] and other biofunctions such as glucose homeostasis through activation of CMKLR1 [35]. GPR1 binds chemerin with a similar affinity as CMKLR1; however, there are only a few studies about chemerin and GPR1 physiological functions [23], [36]. In this study, we investigated the function of GPR1 in regulating bone metabolism in vivo and in vitro in male mice.
The apparent bone loss in GPR1−/− mice was detected by microCT scanning, presented by histological staining, and also reflected by the fragility of the bones during processing. For example, we opted for 7-μm sections instead of the usually used thinner ones owing to the fragility of the GPR1 null mice bone samples. Because of the insufficiency of the null mice and their tissues, unfortunately, in this study we had no chance to study female mice and to do mechanical analysis on the bones. According to the data presented, the significant deterioration of trabeculae in bone of GPR1−/− mice indicates that GPR1 should be important in bone metabolism, and could be related to osteoporosis.
A paralleled notable phenotype with osteoporosis in male GPR1−/− mice is the dramatically low testosterone and relatively low estradiol level. Steroid sex hormones play a key role in bone growth and development in childhood and adolescence [37], and are also essential for the maintenance of bone health in an adult male or female [38]. Evidence indicated that reduced BMD is correlated with decreased serum testosterone levels [13]. In this study, it is possible that the reduced estradiol level could be a consequence of the extremely lowered testosterone level as mentioned by others [11], [13]. It has been reported that GPR1 could activate Gαi/o, ERK1/2, p38, and RHOA/ROCK-dependent signalling [16]. However, the molecular mechanisms underlying the effects of chemerin–GPR1 in inhibiting testosterone secretion are not yet clear. How chemerin–GPR1 plays its role in the regulation of testosterone and estradiol is an interesting topic to be elucidated in our future studies. We investigated the expression of GPR1, chemerin, and AR in differentiated WT bMSCs, but we were unable to do so in GPR1−/− mice bMSCs. For unknown reasons, bMSCs isolated from GPR1−/− mice could not be successfully cultured in vitro for further investigation. We are interested in the unculturable characteristic of the GPR1 null mice bMSCs, and we believe this is important for revealing the mechanism behind the osteoporosis phenotype of the null mice.
Unculturable bMSCs of GPR1−/− mice might be able to result in lower BMD, but confusingly, not all the osteoblast biomarkers that have been investigated were reduced in our study. Besides the ubiquitously expressed bone formation marker ALP, Osterix is known as a master driver in the early stages of differentiation of bMSCs into preosteoblasts [39]. The upregulated Osterix in this study might give us a new clue of their new functions under GPR1 deficiency and extremely low testosterone conditions. As expected, three investigated osteoclast biomarkers increased at different levels, in agreement with histological information. At present, we cannot attribute the imbalance of bone turnover to osteoblasts or osteoclasts, either number- or function-wise [3], [40]. However, what is certain is that in GPR1−/− mice, the bone mass is dramatically decreased.
In addition to osteoblasts, bone marrow bMSCs can also give rise to adipocytes. Generally, factors that promote bMSC adipogenesis inhibit osteoblastogenesis and thereby, reduce bone formation. Chemerin was reported as a regulator that stimulates adipogenesis of bMSCs [18]. Maybe this explains why chemerin expression kept going down during the differentiation of bMSCs to osteoblast. Osteoporosis is a chronic disease usually accompanied by inflammation, so we detected the inflammatory cell, macrophage/monocyte biomarker. We also found that several inflammatory factors were upregulated significantly in GPR1−/− mice. These inflammatory cytokines might help to enhance bone resorption through stimulation of osteoclastogenesis, leading to bone loss in GPR1−/− mice, too [41].
In conclusion, in this study, we reported that GPR1 deficiency in mice would result in severe osteoporosis associated with lower sex hormones in serum. To the best of our knowledge, this is the first time that chemerin–GPR1 signalling is being connected with bone metabolism. Another bone-regulating factor, sex hormones' involvement, makes the story more interesting and complicated. To elucidate the mechanisms of chemerin–GPR1 and sex hormone on bone metabolism, more detailed studies are necessary before we can unveil them.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
We gratefully thank the following grants to support our study. National Natural Science Foundation of China (No. 31671562 and No. 81500684), the Guangdong Public Research and Capacity Building Special Program (No. 2015A020212030), the Shenzhen Peacock Program, China (No. 110811003586331), the Shenzhen Basic Research Program (No. JCYJ20150401150223631, No. JCYJ20160531185738467, No. JCYJ20150401145529020, No. JCYJ20160331190714896 and No. JCYJ20140417113430710), and the SIAT Innovation Program for Excellent Young Researchers (Y5G010).
Contributor Information
Jian V. Zhang, Email: jian.zhang@siat.ac.cn.
Pei-Gen Ren, Email: pg.ren@siat.ac.cn.
References
- 1.Chung H.J., Kim W.K., Oh J., Kim M.R., Shin J.S., Lee J. Anti-osteoporotic activity of harpagoside by upregulation of the BMP2 and Wnt signaling pathways in osteoblasts and auppression of differentiation in osteoclasts. J Nat Prod. 2017;80:434–442. doi: 10.1021/acs.jnatprod.6b00964. [DOI] [PubMed] [Google Scholar]
- 2.Papachroni K.K., Karatzas D.N., Papavassiliou K.A., Basdra E.K., Papavassiliou A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Henriksen K., Bollerslev J., Everts V., Karsdal M.A. Osteoclast activity and subtypes as a function of physiology and pathology—implications for future treatments of osteoporosis. Endocr Rev. 2011;32:31–63. doi: 10.1210/er.2010-0006. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen K., Neutzsky-Wulff A.V., Bonewald L.F., Karsdal M.A. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- 5.Seeman E., Delmas P.D. Bone quality—the material and structural basis of bone strength and fragility. New Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 6.Bord S., Ireland D.C., Beavan S.R., Compston J.E. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 7.Seo B.K., Ryu H.K., Park Y.C., Huh J.E., Baek Y.H. Dual effect of WIN-34B on osteogenesis and osteoclastogenesis in cytokine-induced mesenchymal stem cells and bone marrow cells. J Ethnopharmacol. 2016;193:227–236. doi: 10.1016/j.jep.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Xu X., Zhang Y., Hao H., Chen L., Su T. A model of health education and management for osteoporosis prevention. Exp Ther Med. 2016;12:3797–3805. doi: 10.3892/etm.2016.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin K.Y., Gengatharan D., Mohd Nasru F.S., Khairussam R.A., Ern S.L., Aminuddin S.A. The effects of annatto tocotrienol on bone biomechanical strength and bone calcium content in an animal model of osteoporosis due to testosterone deficiency. Nutrients. 2016;8:808. doi: 10.3390/nu8120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y., Tando T., Morita M., Miyamoto K., Kobayashi T., Watanabe R. Selective estrogen receptor modulators and the vitamin D analogue eldecalcitol block bone loss in male osteoporosis. Biochem Biophys Res Commun. 2017;482:1430–1436. doi: 10.1016/j.bbrc.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 12.Bliuc D., Nguyen N.D., Milch V.E., Nguyen T.V., Eisman J.A., Center J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 13.Walsh J.S., Eastell R. Osteoporosis in men. Nat Rev Endocrinol. 2013;9:637–645. doi: 10.1038/nrendo.2013.171. [DOI] [PubMed] [Google Scholar]
- 14.Mohamad N.V., Soelaiman I.N., Chin K.Y. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317–1324. doi: 10.2147/CIA.S115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauras N., Hayes V.Y., Vieira N.E., Yergey A.L., O'Brien K.O. Profound hypogonadism has significant negative effects on calcium balance in males: a calcium kinetic study. J Bone Miner Res. 1999;14:577–582. doi: 10.1359/jbmr.1999.14.4.577. [DOI] [PubMed] [Google Scholar]
- 16.Rourke J.L., Dranse H.J., Sinal C.J. CMKLR1 and GPR1 mediate chemerin signaling through the RhoA/ROCK pathway. Mol Cell Endocrinol. 2015;417:36–51. doi: 10.1016/j.mce.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Zabel B.A., Zuniga L., Ohyama T., Allen S.J., Cichy J., Handel T.M. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol. 2006;34:1021–1032. doi: 10.1016/j.exphem.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Goralski K.B., McCarthy T.C., Hanniman E.A., Zabel B.A., Butcher E.C., Parlee S.D. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 19.Muruganandan S., Parlee S.D., Rourke J.L., Ernst M.C., Goralski K.B., Sinal C.J. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem. 2011;286:23982–23995. doi: 10.1074/jbc.M111.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi M., Okimura Y., Iguchi G., Nishizawa H., Yamamoto M., Suda K. Chemerin regulates beta-cell function in mice. Sci Rep. 2011;1:123. doi: 10.1038/srep00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banas M., Zegar A., Kwitniewski M., Zabieglo K., Marczynska J., Kapinska-Mrowiecka M. The expression and regulation of chemerin in the epidermis. PloS One. 2015;10:e0117830. doi: 10.1371/journal.pone.0117830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittamer V., Franssen J.D., Vulcano M., Mirjolet J.F., Le Poul E., Migeotte I. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy A.J., Yang P., Read C., Kuc R.E., Yang L., Taylor E.J. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bondue B., Wittamer V., Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Zabel B.A., Allen S.J., Kulig P., Allen J.A., Cichy J., Handel T.M. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 26.Monnier J., Lewen S., O'Hara E., Huang K., Tu H., Butcher E.C. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol. 2012;189:956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Henau O., Degroot G.N., Imbault V., Robert V., De Poorter C., McHeik S. Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PloS One. 2016;11:e0164179. doi: 10.1371/journal.pone.0164179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rourke J.L., Muruganandan S., Dranse H.J., McMullen N.M., Sinal C.J. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J Endocrinol. 2014;222:201–215. doi: 10.1530/JOE-14-0069. [DOI] [PubMed] [Google Scholar]
- 29.Nazarko V.Y., Thevelein J.M., Sibirny A.A. G-protein-coupled receptor Gpr1 and G-protein Gpa2 of cAMP-dependent signaling pathway are involved in glucose-induced pexophagy in the yeast Saccharomyces cerevisiae. Cell Biol Int. 2008;32:502–504. doi: 10.1016/j.cellbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Meirelles Lda S., Nardi N.B. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q., Shou P., Zhang L., Xu C., Zheng C., Han Y. An osteopontin–integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells. 2014;32:327–337. doi: 10.1002/stem.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory C.A., Gunn W.G., Peister A., Prockop D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Kim H., Lee K., Ko C.Y., Kim H.S., Shin H.I., Kim T. The role of nacreous factors in preventing osteoporotic bone loss through both osteoblast activation and osteoclast inactivation. Biomaterials. 2012;33:7489–7496. doi: 10.1016/j.biomaterials.2012.06.098. [DOI] [PubMed] [Google Scholar]
- 34.Lane N.E., Yao W. Developments in the scientific understanding of osteoporosis. Arthritis Res Ther. 2009;11:228. doi: 10.1186/ar2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimura T., Oppenheim J.J. Chemokine-like receptor 1 (CMKLR1) and chemokine (C–C motif) receptor-like 2 (CCRL2): two multifunctional receptors with unusual properties. Exp Cell Res. 2011;317:674–684. doi: 10.1016/j.yexcr.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L., Mao C., Wang X., Liu R., Li L., Mou X. Association of chemerin levels and bone mineral density in Chinese obese postmenopausal women. Medicine. 2016;95:e4583. doi: 10.1097/MD.0000000000004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachrach L.K. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab TEM. 2001;12:22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 38.Martin T.J., Seeman E. New mechanisms and targets in the treatment of bone fragility. Clin Sci. 2007;112:77–91. doi: 10.1042/CS20060046. [DOI] [PubMed] [Google Scholar]
- 39.Lian J.B., Gordon J.A., Stein G.S. Redefining the activity of a bone-specific transcription factor: novel insights for understanding bone formation. J Bone Miner Res. 2013;28:2060–2063. doi: 10.1002/jbmr.2076. [DOI] [PubMed] [Google Scholar]
- 40.Dougall W.C., Chaisson M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev. 2006;25:541–549. doi: 10.1007/s10555-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Wang L., Kikuiri T., Akiyama K., Chen C., Xu X. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]