Summary
Background
Hand flexor tendon injuries are compromised with tendon adhesion. Tendon adhesion forms between flexor tendon and tendon sheath, reduces the range of motion of fingers, and affects their function. Oxidative stress is increased in flexor tendon after injury and might play a role in tendon adhesion formation. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a water-soluble analog of vitamin E, is antioxidative. Trolox reduced oxidative stress and the expression of fibrotic cytokines in the bile gut ligation animal model. Vitamin C and Trolox are strong antioxidants, but they might also have prooxidant properties. The prooxidant properties of vitamin C and Trolox are different. In this study, our aim was to determine the effect of Trolox in reducing tendon adhesion formation.
Methods
Flexor digitorum profundus tendon injury was induced in 54 Kai-Mei Chicken according to a well-established protocol. After wound closure, an injection of 50 μL saline, 10mM Trolox, or 100mM Trolox was administered into the wound area. At 2 weeks or 6 weeks after the surgery, chicken feet were harvested for gliding test, high-resolution ultrasound measurement on a fibrotic area, and histology.
Results
At Week 2 after the surgery, Trolox has no effect on the flexion angle and gliding resistance, whereas a significant improvement was observed in the flexion angle and gliding resistance in the Trolox-treated groups at Week 6. However, no dose response was observed. In the ultrasound measurement, there was no significant difference in the fibrotic mass in the Trolox-treated group as compared to the saline group at Week 2. At Week 6, fibrotic mass was significantly reduced in both Trolox-treated groups. From the histological examination, the Trolox-treated groups presented a higher cellularity at Week 2 as compared to the saline group, and reduced fibrosis and adhesion at Week 6.
Conclusion
Our results suggest that local administration of Trolox can reduce tendon adhesion, and a higher dose of Trolox did not have negative effects.
Clinical Significance
Trolox solution might be feasible to reduce tendon adhesion via intraoperative injection at the wound area during tendon repair.
Keywords: oxidative stress, tendon adhesion, tendon injury, Trolox
Introduction
Flexor tendon adhesions are a common clinical problem presenting considerable challenge to orthopaedic surgeons. Repair of flexor tendon is complicated by the progression of fibrosis and a high rate of postoperative complication [1]. About 30–40% of cases resulted in a loss of digital range of motion and impaired hand function in the United States [2]. Tendon adhesion, found between flexor tendon and synovial sheath, is a result of fibrosis and immobilisation. The flexor tendon healing process involves both intrinsic and extrinsic mechanisms. The intrinsic mechanism involves tenocytes, whereas the extrinsic mechanism involves inflammatory cells and fibroblasts [3], [4] that promote tendon adhesion [5].
Different approaches have been taken to reduce tendon adhesion. For example, (1) an application of physical barriers, such as Interceed [6], expanded polytetrafluoroethylene, polyvinyl alcohol hydrogel [7], to prevent adhesion between the tendon and synovial sheath; (2) modified surgical techniques, such as multistrand repair and sheath repair [7]; (3) application of growth factors including VEGF, NF-κB, and bFGF [7], [8]; (4) using pharmacological adjuvants, such as anti-inflammatory drugs NSAIDs (nonsteroidal anti-inflammatory drugs) and TGFβ-1 (transforming growth factor beta 1), that suppress the extrinsic mechanism [8], [9]. Previously, we found that local administration of a low dose of vitamin C (5 mg/mL) can significantly reduce tendon adhesion by reducing oxidative stress and inflammation in a chicken model [10], [11]. However, a higher dose of vitamin C was ineffective, which suggests that vitamin C may elicit a different effect at higher doses. Vitamin C is a cofactor in hydroxyproline synthesis, which promotes collagen production and hence a fibrogenic response.
In contrast to vitamin C, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a water-soluble analogue of vitamin E, could significantly reduce oxidative stress and prevent the upregulation of fibrotic cytokines, such as TGFβ-1 and interleukin-10 [10]. It is possible that Trolox could be a better antioxidant as compared to vitamin C in reducing tendon adhesion. In the present study, we tested the hypothesis that intraoperative injection of Trolox following flexor digitorum profundus tendon injury will decrease tendon adhesion.
Materials and methods
Animal model and injection
The Animal Research Ethics Committee of the Chinese University of Hong Kong approved all animal experiments conducted in this study. A total of 54 3-month-old female Kamei chickens [Hong Kong Poultry (Kamei Chicken) Development Limited, Hong Kong] were used in this study; a well-established flexor digitorum profundus tendon injury animal model was used [11], [12]. In brief, 1–1.5 mL of ketamine/xylazine (1:1) was intravenously injected to anesthetise the chicken. A 10-mm medial incision was made at the proximal interphalangeal joint area of the third toe to approach the flexor digitorum profundus tendon. Flexor sheath was exposed via a blunt dissection to mobilise the skin. A2 pulley, which is an annular pulley equivalent to the A2 pulley in the human finger, was located just distal to the proximal interphalangeal joint area, on the side of the flexor sheath. After flexing the toe, the flexor digitorum profundus was withdrawn through the opening. The superficial tendon and vinculum longus were left untouched. At the medial aspect of the tendon, three partial cuts at 4-mm intervals were made. Each cut was 50% of the diameter of the tendon. To repair the cuts, a single strand stitch using 5–0 Ethilon nonabsorbable nylon suture (Johnson & Johnson, New Brunswick, NJ, USA) was used to pass through all three cuts simultaneously, and returned to the sheath in neutral position. The 5–0 Ethilon nonabsorbable nylon suture was used to close the opening of the sheath and skin wound. The injured foot was immobilised in neutral position for 2 weeks with a fiberglass cast (3M, St. Paul, MN, USA), whereas the contralateral side served as an uninjured and free-moving control.
After animal surgery, chickens were randomly assigned to three different groups to receive a local injection of 50 μL saline, 10mM Trolox solution, or 100mM Trolox solution after the tendon sheath closure with suture. Leakage of solutions was not observed after the injection. To determine the effective dose for reducing tendon adhesion, two doses of Trolox were used. The chickens were sacrificed at 2 weeks or 6 weeks after the surgery (n = 9 in each group at each time point) for ultrasonography evaluation, gliding test, and histological examination. Both the injured and contralateral feet were harvested. Feet were kept in saline-solution-moistened gauze at −20°C until the gliding test was performed (within 1 month after sample harvest).
Ultrasound imaging and video-assisted gliding test
The chicken feet were thawed to room temperature prior to taking ultrasound images and performing the gliding test. Ultrasound and video-assisted gliding test were performed as previously described [13]. In brief, to expose the flexor sheath over the A2 pulley, the skin and subcutaneous adipose tissue over the second phalanx of the long toe were removed. Coupling gel was applied on top for ultrasound imaging. A Vevo 770 high-resolution ultrasound imaging system (Visualsonic, Toronto, Ontario, Canada) with an RMV 711 scan head (6-mm focal length and 30-μm axial resolution) was used to capture three-dimensional (3-D) ultrasound images for a total length of 12 mm with a step size of 32 μm. The A2 pulley was identified from the acquired images by the built-in software. The cross-sectional area of the flexor digitorum superficialis and the flexor digitorum profundus were measured at 4 mm proximal to the A2 pulley by two independent sonographers according to the methods described in our previous study [13]. 3-D ultrasound images were used to examine the fibrotic response, tendon adhesion, and peritendinous reactions near the injured sites in all groups. The chicken feet were then mounted onto a custom-made testing jig for the gliding test using a mechanical testing machine (H25KM; Tinius Olsen, Salfords, UK) as described previously [13]. In brief, the toe joints of the long toe were labeled and the flexor digitorum profundus tendon was clamped to the clamp head of a 50-N load with the long toe fully extended in a naturally relaxed position. Flexor digitorum profundus tendon was preloaded with 0.1 N. Displacement of the gliding test was 15 mm, and the speed was 30 mm/min. The gliding test was videorecorded. By matching the video clip, force–displacement curve, mechanical data, and maximum flexion angle, the gliding resistance could be calculated. The joint flexion angle was measured from the force–displacement curve using ImagePro (Media Cybernetics, Bethesda, MN, USA). The extent of tendon adhesion in the injured flexor digitorum profundus tendon was presented as a percentage of the maximum flexion angle and the gliding resistance in the contralateral control. The tendon was kept moist during the gliding test, and the chicken feet underwent histological processing after the gliding test.
Histological analysis
After the gliding test, a joint segment from the third toe (including the A2 pulley) was dissected and fixed in 10% buffered formalin overnight. After fixation, samples were decalcified in 9% formic acid for 2 weeks, and embedded in paraffin. Samples were sectioned at 7 μm sagittal section for hematoxylin and eosin staining. The severity of tendon adhesion was evaluated by examining the cellularity and fibrotic response of the healing tendon in the peritendinous region.
Statistical methods
The maximum flexion angle and the gliding resistance are shown in box plots. The nonparametric Kruskal–Wallis test was used to perform multiple group comparisons, and p values were reported. Two-group comparisons were performed using the Mann–Whitney U test with Bonferroni correction when necessary (α = 0.05/2 = 0.025). All data analyses were done using the Statistical Package for the Social Sciences (SPSS) 16.0 (IBM, Armonk, NY, USA). A p value ≤0.050 was regarded as statistically significant.
Result
Gliding test
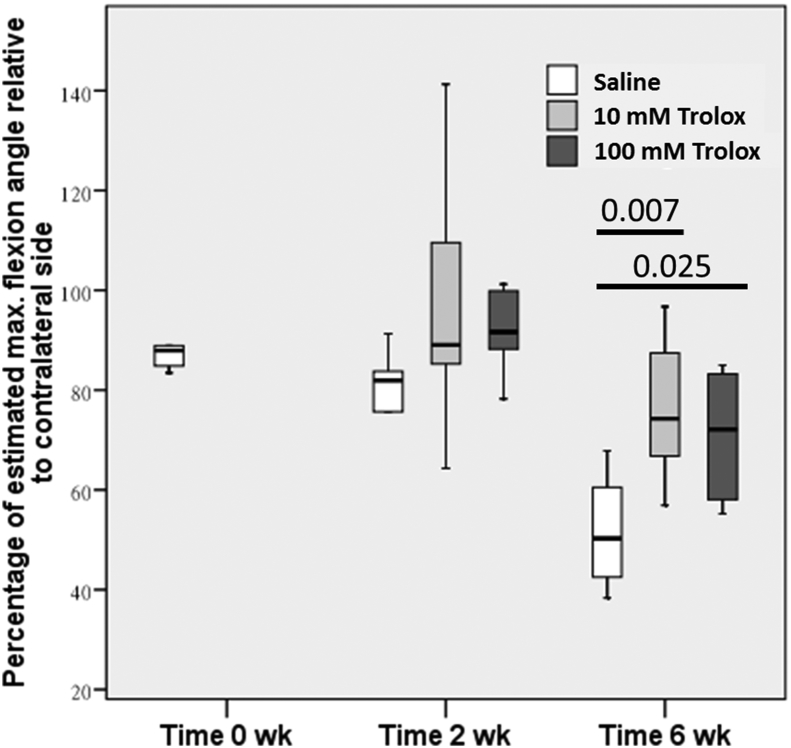
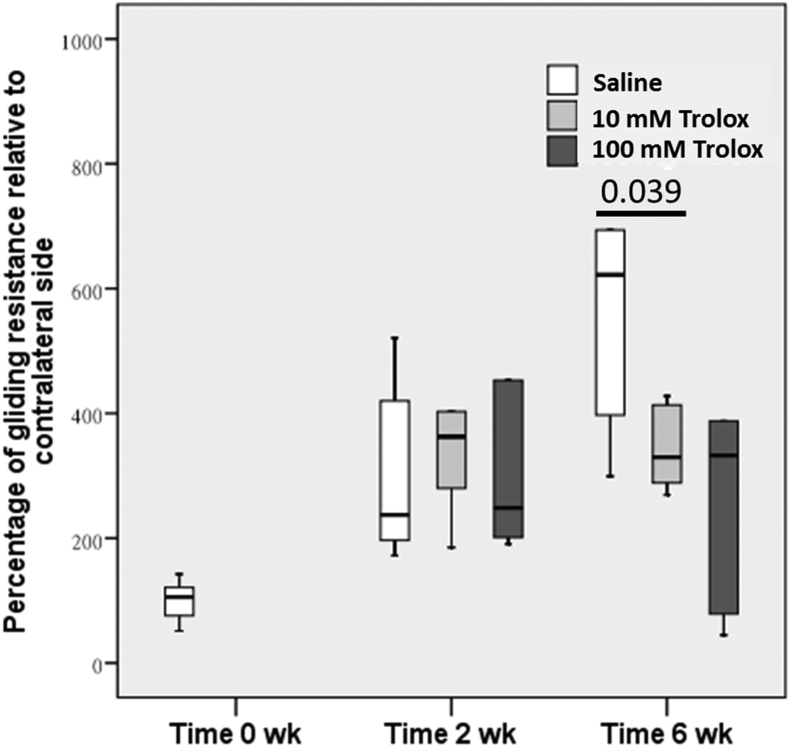
At 2 weeks after the surgery, no significant difference was found in the maximum flexion angle (Figure 1) and gliding resistance (Figure 2) at the proximal interphalangeal joint between the Trolox groups and the saline group. At 6 weeks after the surgery, significant improvements in the restoration of the maximum flexion angle were found in the 10mM (p = 0.007) and 100mM (p = 0.025) Trolox groups as compared to the saline group. However, only 10mM Trolox significantly reduced the gliding resistance (p = 0.039), whereas 100mM Trolox did not (p = 0.078).
Figure 1.
The maximum flexion angle of the proximal interphalangeal joint and the gliding resistance were calculated to evaluate the extent of restrictive tendon adhesion. Significant differences were observed at Week 6 between Trolox-treated groups and the saline group. No difference was observed at Week 2 between all groups. Trolox = 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid.
Figure 2.
The gliding resistance was calculated to evaluate the extent of restrictive tendon adhesion. A significant difference was observed between Trolox (10 mg)-treated group and saline group at Week 6. Similar to the results of maximum flexion angle, no differences were observed between all groups at Week 2. Trolox = 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid.
Ultrasound measurement fibrotic size around injured tendon
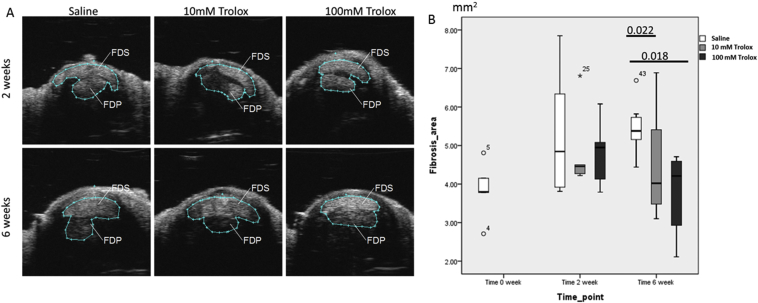
The fibrotic size of the injured flexor tendon was measured using 3-D ultrasound imaging as described previously [13]. Injections of Trolox did not significantly reduce the fibrotic size at Week 2 after the surgery (Figure 3); however, both the low and high doses of Trolox reduced fibrotic size at Week 6 after the surgery (p = 0.022 and 0.018, respectively) (Figure 3).
Figure 3.
(A) The cross-sectional area of flexor tendons (flexor digitorum superficialis and flexor digitorum profundus) at the injured site was measured with 3-D ultrasound imaging to evaluate the magnitude of fibrosis. (B) Significant difference in reduction of the fibrotic area was observed in the Trolox (100 mg)-treated group at Week 6, whereas there was no difference across all groups at Week 2. FDP = flexor digitorum profundus tendon; FDS = flexor digitorum sperficialis tendon; Trolox = 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid.
Histology
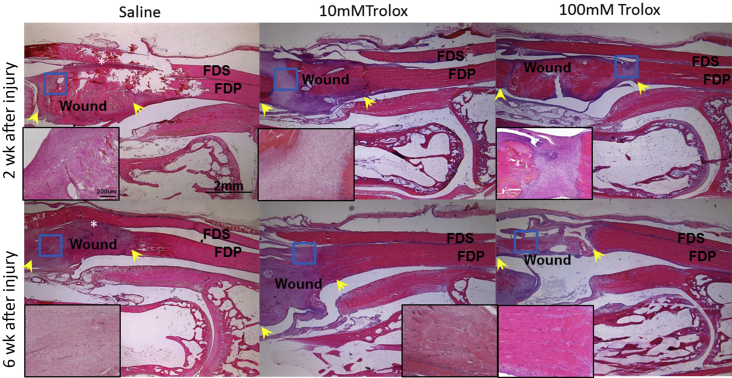
At Week 2 after the surgery, swelling of tendinous tissues was observed at the wound site in all three groups, with more peritendinous adhesion on the vinculum side. However, the cellularity in the gap of the wound was higher in the Trolox-treated groups. At 6 weeks after the surgery, fibrosis and tendon adhesion were still evident between the flexor digitorum superficialis and flexor digitorum profundus in the saline group. The fibrotic response was reduced in both Trolox-treated groups, but the edematous swelling was observed in the high dose Trolox group. Peritendinous adhesion was further resolved in the Trolox-treated groups at 6 weeks after the surgery, and the cellularity in the gap of the wound was decreased as compared to 2 weeks after the surgery (Figure 4).
Figure 4.
Histological examination of injured flexor digitorum profundus tendon at Week 2 and Week 6 after the surgery (H&E, 12.5× optical magnifications). Higher magnification (200×) view of the wound is shown in the corner. Wound: the wound resulted from 50% tenotomy. FDP = flexor digitorum profundus tendon; FDS = flexor digitorum sperficialis tendon; H&E = hematoxylin and eosin; Trolox = 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. Yellow arrow indicates the boundary of wound; white asterisk indicates fibrosis and adhesion; red rectangle indicates the higher magnification area.
Discussion
Effects of Trolox on tendon adhesion
We found that Trolox significantly reduced tendon adhesion formation, resulting in a better restoration of the range of motion and gliding properties at 6 weeks after the surgery. Although its effect on tendon adhesion was not evident at 2 weeks after the surgery, Trolox promoted cell recruitment to the wound site as shown in histology, which may imply its potential in promoting healing at the proliferation phase. As Trolox can decrease the expression of inflammatory cytokines [11], its inhibitory effect on the formation of tendon adhesion may be mediated through reducing fibrosis associated with inflammatory processes [12]. Moreover, its antioxidative effect may also contribute to the suppression of fibrogenesis related to oxidative stress [14]. As compared to vitamin C supplementation in our previous study [13], the effect of Trolox to reduce tendon adhesion is less dose-dependent. Because Trolox does not promote collagen synthesis as vitamin C does, this may account for the difference in dose response. Moreover, the differences in antioxidative effects between Trolox and vitamin C may also contribute to the different outcomes in reducing tendon adhesion. Trolox can suppress lipid peroxidation, whereas vitamin C can become prooxidative in the presence of ferrous ions [15]. Therefore, our preliminary data support the concept that Trolox is probably a better choice for local administration of antioxidants in reducing tendon adhesion.
Limitation
Although we observed treatment effects of Trolox in reducing tendon adhesion, the mechanism of the treatment effect remains speculative. Further investigation on the effects of Trolox on the recruitment of fibroblasts and tenoblasts may help explain the present observations. As compared to the saline group, large intragroup variations in treatment effects have been observed in Trolox-treated groups, which may imply some uncontrolled variables, such as the location of and retention of injected Trolox. As the intrasynovial space is large, a specific drug carrier may be necessary to localise the drug in the wound site where tendon adhesion is more likely to develop. As Trolox is relatively safe and can be metabolised [16], its future clinical use as a pharmacological adjuvants is envisaged.
Conclusion
The local administration of Trolox can reduce tendon adhesion.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
The work described in this paper was supported by the Research Grants Council of the Hong Kong Special Administrative Region (Project number: CUHK4500/06M). The high-resolution ultrasound imaging system was kindly supported by the Hong Kong Jockey Club Sports Medicine and Health Sciences Centre. The authors also acknowledge the Department of Orthopaedics & Traumatology and the Lee Hysan Laboratory, Faculty of Medicine, Chinese University of Hong Kong, for providing facilities for this study to be carried out. Funding from the General Research Fund under the University Grants Committee in Hong Kong was used to support the expenses of this study.
References
- 1.Momeni A., Grauel E., Chang J. Complications after flexor tendon injuries. Hand Clin. 2010;26:179–189. doi: 10.1016/j.hcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Aydin A., Topalan M., Mezdegi A., Sezer I., Ozkan T., Erer M. Single stage flexor tendoplasty in the treatment of flexor tendon injuries. Acta Orthop Traumatol Turc. 2004;38:6. [PubMed] [Google Scholar]
- 3.Wang E.D. Tendon repair. J Hand Ther. 1998;11:105–110. doi: 10.1016/s0894-1130(98)80006-9. [DOI] [PubMed] [Google Scholar]
- 4.Docheva D., Muller S.A., Majewski M., Evans C.H. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [Epub 2014/12/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan U., Occleston N.L., Khaw P.T., McGrouther D.A. Differences in proliferative and collagen lattice contraction between endotenon and synovial fibroblasts. J Hand Surg. 1998;23A:8. doi: 10.1016/S0363-5023(98)80125-1. [DOI] [PubMed] [Google Scholar]
- 6.Temiz A., Ozturk C., Bakunov A., Kara K., Kaleli T. A new material for prevention of peritendinous fibrotic adhesions after tendon repair: oxidised regenerated cellulose (Interceed), an absorbable adhesion barrier. Int Orthop. 2008;32:389–394. doi: 10.1007/s00264-007-0335-8. [Epub 2007/03/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna A., Friel M., Gougoulias N., Longo U.G., Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [Epub 2009/04/28] [DOI] [PubMed] [Google Scholar]
- 8.Rouhani A., Tabrizi A., Ghavidel E. Effects of non steroidal anti inflammatory drugs on flexor tendon rehabilitation after repair. Arch Bone Joint Surg. 2013;1:3. [PMC free article] [PubMed] [Google Scholar]
- 9.Wong J.K., Metcalfe A.D., Wong R., Bush J., Platt C., Garcon A. Reduction of tendon adhesions following administration of Adaprev, a hypertonic solution of mannose-6-phosphate: mechanism of action studies. PLoS One. 2014;9:e112672. doi: 10.1371/journal.pone.0112672. [Epub 2014/11/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galicia-Moreno M., Favari L., Muriel P. Trolox mitigates fibrosis in a bile duct ligation model. Fundam Clin Pharmacol. 2013;27:308–318. doi: 10.1111/j.1472-8206.2011.01020.x. [Epub 2012/01/04] [DOI] [PubMed] [Google Scholar]
- 11.Eum H.A., Lee S.M. Effects of Trolox on the activity and gene expression of cytochrome P450 in hepatic ischemia/reperfusion. Br J Pharmacol. 2004;142:35–42. doi: 10.1038/sj.bjp.0705758. [Epub 2004/03/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas R., Bunderson-Schelvan M., Holian A. Potential role of the inflammasome-derived inflammatory cytokines in pulmonary fibrosis. Pulm Med. 2011;2011:105707. doi: 10.1155/2011/105707. [Epub 2011/06/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung L.K., Fu S.C., Lee Y.W., Mok T.Y., Chan K.M. Local vitamin-C injection reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J Bone Joint Surg Am Vol. 2013;95:e41. doi: 10.2106/JBJS.K.00988. [Epub 2013/04/05] [DOI] [PubMed] [Google Scholar]
- 14.Toyama T., Nakamura H., Harano Y., Yamauchi N., Morita A., Kirishima T. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem Biophys Res Commun. 2004;324:697–704. doi: 10.1016/j.bbrc.2004.09.110. [Epub 2004/10/12] [DOI] [PubMed] [Google Scholar]
- 15.Carr A.F. Balz. Does vitamin C act as a pro oxidant under physiological conditions. FASEB J. 1999;13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 16.Messier E.M., Bahmed K., Tuder R.M., Chu H.W., Bowler R.P., Kosmider B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013;4:e573. doi: 10.1038/cddis.2013.96. [Epub 2013/04/06] [DOI] [PMC free article] [PubMed] [Google Scholar]