Abstract
Background:
Morinda pubescens J.E.Sm. (Rubiaceae) is an important medicinal plant used in indigenous system of medicine i.e., Ayurveda, Siddha and Unani.
Objective:
The aim of this study is to evaluate the nephroprotective effect of M. pubescens in gentamicin induced acute renal failure in rats.
Methods:
Nephrotoxicity was induced in male Wistar rats by administration of gentamicin (100 mg/kg, i.p.) once daily for 10 days. Simultaneously, the treatment was conducted with water extract bark of M. pubescens (200 mg/kg, p.o.) and its ethyl acetate fractions (100 mg/kg, p.o.) once daily for ten days. Silymarin (50 mg/kg, p.o.) is used as standard drug. Using renal biochemical markers creatinine, urea, uric acid, BUN, albumin, protein, and other parameters are kidney weight, body weight, urine volume and histopathology of the kidney. Statistical analysis was performed by using one - way ANOVA followed by Dunnett's test.
Results:
It was observed that the water extract and its ethyl acetate fractions bark of M. pubescens has brought back the altered levels of biochemical markers and other parameters to the near normal levels. Histopathological study revealed treatment groups also shows the normal texture of kidney.
Conclusions:
The present study possessed nephroprotective activity but ethyl acetate fraction was found to exhibit greater nephroprotective activity than the water extract.
Keywords: Ethyl acetate fraction, Morinda pubescens, Nephroprotective, Water extract
Introduction
The term acute renal failure (ARF) is currently substituted by an acute kidney injury (AKI). AKI is a reversible condition in which there is a sudden decline in renal function, manifested by hourly/daily/weekly elevation in serum creatinine and blood urea nitrogen (BUN), etc.[1]
The incidence of AKI in the community is 2147 and 4085 per million populations per year respectively in developing and developed nations.[2,3] Recent reports in the developed world indicate that AKI is seen in 3.2 to 9.6% of hospital admissions with overall mortality of 20% to 50% in ICU patients.[4,5] AKI demanding renal replacement therapy is 5 to 6% with a high in hospital mortality rate of 60%. It is estimated that nearly 2 million people die of AKI every year globally.[6,7] Those who survive AKI are at a greater risk for later development of chronic kidney disease.
Since there is no effective pharmacotherapy for AKI, seeking alternative treatments becomes a necessity. Use of medicinal plants in renal failure goes back to ancient days.
Morinda pubescens J.E. Sm. [Figure 1: Aerial parts (a) and bark (b)] belonging to family Rubiaceae, commonly known as Brim stone tree, Wild ach root, Bartondi,[8,9] is used as folk medicine by the tribes for curing hypertension, diabetes, blood purification and treatment of renal toxicity symptoms etc., but the nephroprotective effects of these plants have not been proved scientifically.
Figure 1.

(a) Aerial parts and (b) bark of Morinda pubescens J.E.Sm.
In the present context, the in vivo nephroprotective activity of water extract and its ethyl acetate fraction of Morinda pubescens J.E. Sm. was evaluated in Wistar rats in the present study.
Materials and Methods
Phytochemical evaluation
The bark of Morinda pubescens J.E. Sm. was collected from Western ghat regions of (Satara - District) Maharashtra and (Belgaum - District) Karnataka state.
The plant material is identified and authenticated by the Botanist Dr. Harsha Hegde, Scientist ‘C’ Regional Medical Research Centre, Indian Council of Medical Research, Belgaum. The voucher specimen has been deposited at the same herbaria with accession no: RMRC - 990.
The water extract of dried coarse powder 1 kg. of the bark was prepared by using Maceration extraction for water extraction (Chloroform water; 2.5 ml of chloroform in 100 ml water).[10] The extracted solvent was evaporated in rota evaporator and this extract was concentrated on water bath. The water extract part was named WEMp.
The 0.5 gm. water extract insoluble residue was removed by filtration and the solubles in the filtrate 100 ml. were fractionated into petroleum ether, chloroform, ethyl acetate, n – butanol and water.[11] The ethyl acetate fractions of WEMp were concentrated on water bath. Finally ethyl acetate fraction having faint brown solid was giving positive chemical tests for flavonoids, tannins and phenols, etc.[12,13]
The WEMp and its ethyl acetate fraction was separated and fraction compound was isolated by column chromatography with 100 gm. of aluminum oxide active - neutral and thin layer chromatography of isolated compound fraction was performed using the mobile phase Toluene:Ethyl acetate:Formic acid (5:5:1) for WEMp silica gel - G. The Rf value was 0.83 and isolated fraction part was named ISLTD mp – B.[14,15,16]
Pharmacological evaluation
Drugs and chemicals
Gentamicin and Silymarin was obtained from Abbott and Microlabs, India. The kits for all biochemical estimations were purchased from Transasia Biomedicals Ltd., India. The solvents and other chemicals used were of analytical grade.
Animals
Wistar rats (150-200 gm.) of male sex obtained from Sri Venkateshwara Enterprises, Bangalore were kept in standard environment conditions, fed with rodent diet (VRK Nutritional Solution, Sangali) and with water ad libitum. Approval from the institutional animal ethical committee for the usage of animals in the experiments was obtained. IAEC: 09/Mar-2014, KLE University's College of Pharmacy, Hubli, Karnataka).
Acute toxicity studies
The acute oral toxicity study was carried out as per the guideline 423 set by Organization for Economic Cooperation and Development received from Committee for the purpose of control and supervision of Experiments on Animals.[17]
In the acute toxicity assay it was found that no mortality was observed up to doses of 2000 mg/kg, orally and hence it was considered to be safe. Furthermore, there were no signs of any toxic reaction found until the end of the study period. One-tenth (200 mg/kg) of the median lethal dose 50 was taken as an effective dose.
Experimental design
Animals were randomly divided into five groups of six animals each. Group I normal with vehicle (distilled water, p. o.) was kept as normal. Group II toxicant group received gentamicin (100 mg/kg b. w., i. p.).[18] Group III standard group received silymarin (50 mg/kg b. w., p. o.) with toxicant.[19] Group IV treatment group received ethyl acetate fraction (from water extract) (100 mg/kg b. w., p. o.) with toxicant. Group V treatment group received water extract of Morinda pubescens J.E. Sm. (200 mg/kg b. w., p. o.) with toxicant. The extract, fraction and standard drug were administered for 10 days. On the 11th day of respective treatments, two hour fasted animals were sacrificed using diethyl ether anesthesia; blood samples were collected by puncturing the retro – orbit plexus. The blood so collected was centrifuged at 2500 rpm for 15 min. to get clear serum and analyzed for the estimation of biochemical marker enzymes, i.e., Creatinine, Urea, Uric acid, Blood Urea Nitrogen (BUN), Albumin, and Protein.[18,20,21] Other parameters, i.e., Kidney weight, Body weight, and Urine volume were also evaluated.
Histopathological study
After collection of blood for biochemical estimation, the rats were sacrificed and euthanized with an over-dosage of anesthetic diethyl ether and the kidney was carefully dissected, cleaned of extraneous tissue, and fixed in 10% formalin and dehydrated in alcohol. Then, the paraffin sections were prepared (automatic tissue processor Autotechnique) and cut into 5 μm. thick sections, using a rotary microtome. The sections were stained with hematoxylin-eosin dye and studied for histopathological changes.
Statistical analysis
Results were given as mean ± SEM, (N = 6). Data was analyzed using one – way ANOVA followed by Dunnett's test. The statistical significance of difference was taken as P < 0.05, P < 0.001, and P < 0.0001. The analysis was performed by using Prism software.
Results
Effect of bark of water extract Morinda pubescens J.E. Sm. (WEMp) and isolated ethyl acetate fraction (from water extracts) compound (ISLTD mp-B) with standard silymarin on biochemical parameters in gentamicin – induced kidney toxicity in rats is shown in [Table 1].
Table 1.
Nephroprotective activity of water extract and isolated ethyl acetate fraction compound in gentamicin induced toxicant
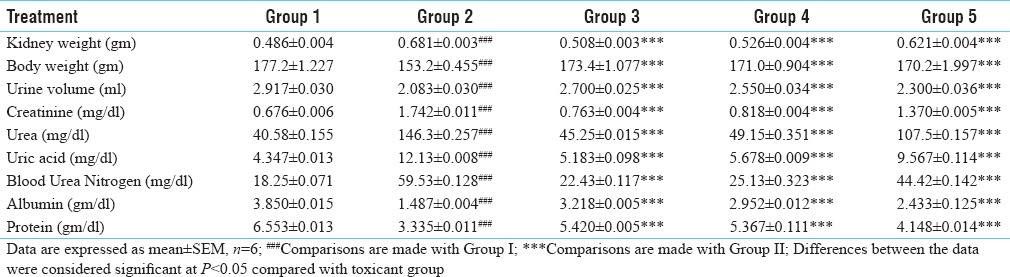
In our experiment it is observed that gentamicin induced group showed increased kidney weight, serum creatinine, urea, uric acid, blood urea nitrogen and decreased body weight, urine volume, albumin, and protein. All parameters in the toxicant group clearly indicates kidney damage due to gentamicin (P < 0.05) [Table 1].
Treatment with water extract Morinda pubescens J.E. Sm. (WEMp) and its isolated ethyl acetate fraction compound (ISLTD mp-B) showed decreased levels of various biochemical markers of the kidney i.e., serum creatinine, urea, uric acid, blood urea nitrogen, kidney weight and increased albumin, protein, body weight, and urine volume. (P < 0.0001) [Table 1].
Standard silymarin decreased the levels of various biochemical markers of the kidney i.e., serum creatinine, urea, uric acid, blood urea nitrogen, kidney weight and increased albumin, protein, body weight, and urine volume (P < 0.0001) [Table 1].
Toxicant effects of gentamicin on kidney histology caused a marked Glomerular and Peritubular congestion (Triangle), Loss of brush border (Right arrow), Tubular cast and inflammation (Left arrow), Interstitial hemorrhage (Star) [Figure 2].
Figure 2.
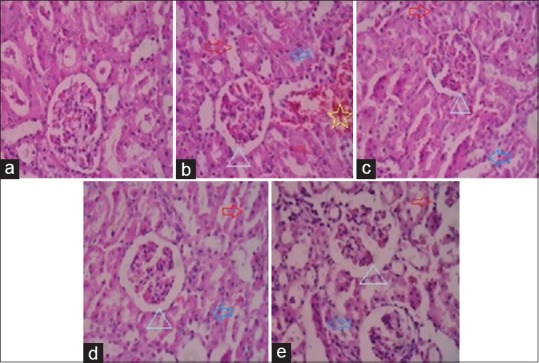
Histopathology of kidney rat. (a) Group I: Normal group, (b) Group II: Toxicant group, (c) Group III: Standard group, (d) Group IV: Treatment group, (e) Group V: Treatment group
Treatment with water extract, ethyl acetate fraction and silymarin in gentamicin induced kidney toxicity group shows decreased Glomerular and Peritubular congestion (Triangle), Loss of brush border (Right arrow), Tubular cast and inflammation (Left arrow), Interstitial hemorrhage etc., resembling to normal texture [Figure 2].
All parameters in serum and others observed to the near healthy levels or normal values of rat or reduce the toxicity of gentamicin.
Discussion
The aminoglycosides induce nephrotoxicity in 10-20% of therapeutic courses. The widespread therapeutic use of the gentamicin is limited because of its nephrotoxic side effect and oxidative damage which can lead to acute renal failure.[22,23,24]
Gentamicin is one of the effective antibiotic used in the treatment of gram-negative bacterial infection. A major complication of the use of these drugs is nephrotoxicity. The pathogenesis of aminoglycosides nephrotoxicity is a two–step process. The first step entails the transportation and accretion of antibiotics in high concentration by renal proximal tubular cells. The second step involves the adverse interaction between these polycationic drugs leading to cellular damage.[25,26,27] A direct interstitial hemorrhage is also observed during nephrotoxicity.
Data from recent studies showed that the cationic proteins and peptides, inhibit the uptake of nephrotoxic drug, gentamicin, which is highly accumulated in the kidneys. The mechanism underlying gentamicin–induced renal cellular damage by generation of superoxide anion, hydrogen peroxide (H2O2), hydroxyl radicals and reactive oxygen species (ROS) generation in kidneys and finally this has been attributed to its deleterious effect on the kidney.[28,29]
An association between nephrotoxicity and oxidative stress has been confirmed in many experimental models.[30,31,32]
In our experiment it is observed that gentamicin-induced group increased kidney weight, serum creatinine, urea, uric acid, blood urea nitrogen and decreased body weight, urine volume, albumin, protein. It is clearly indicates kidney damage due to gentamicin.
Treatment with silymarin, water extract Morinda pubescens J.E. Sm. (WEMp) and its isolated ethyl acetate fraction compound (ISLTD mp-B) has decreased the levels of various biochemical markers of the kidney i.e., serum creatinine, urea, uric acid, blood urea nitrogen, kidney weight and increased albumin, protein, body weight, and urine volume. All parameters in serum and others observed to the near healthy levels or normal values of rat or reduce the toxicity of gentamicin. The present study shows an increase in urine output of standard, (WEMp) and (ISLTD mp-B) groups of treatment animals which dilutes the concentration of kidney biochemical markers i.e., serum creatinine, urea, uric acid, and BUN. As a result, biochemical markers or waste products of the kidney are flushed out via the urine and there are lesser chances or prevention of renal toxicity. The kidney and body weights, albumin and protein also showed significant improvement compared to gentamicin toxicant group.
This prevention of renal toxicity by bark extracts may be due to the antioxidant activity which may be due to the presence of secondary metabolites tannins, flavonoids and phenolic compounds, etc.[21,27]
Histopathological profiles of rat kidney shown in [Figure 2] also reveals a major damage in the same groups. The observable fact was proved by kidney biopsy, [Figure 2a]; In group: I - all kidney cells are normal (vehicle). [Figure 2b]; In group: II – The kidney necrosis occured with Glomerular congestion and peritubular congestion (Triangle), (Loss of brush border (Right arrow), tubular cast and inflammation (Left arrow), interstitial hemorrhage (Star) has been observed gentamicin received group. [Figure 2c]; Group: III Standard silymarin treatment group shows reduced or cured kidney narcosis in Glomerular congestion and peritubular congestion (Triangle), (loss of brush border (Right arrow), tubular cast and inflammation (Left arrow), interstitial hemorrhage is similar to that normal group. [Figure 2d]; Group: IV Treatment with isolated ethyl acetate fraction compound (ISLTD mp-B) shows reduced Glomerular congestion and peritubular congestion (Triangle), (loss of brush border (Right arrow), tubular cast and inflammation (Left arrow), interstitial hemorrhage is similar like standard or normal group. [Figure 2e]; Group: V Treatment with water extract of bark Morinda pubescens J.E. Sm. (WEMp) shows reduced Glomerular congestion and peritubular congestion (Triangle), (loss of brush border (Right arrow), tubular cast and inflammation (Left arrow), interstitial hemorrhage refract minimal toxicity or better than toxicant group. All treatments groups repaired minimal injuries and better protection of kidney.
The graphical presentation (Prism 5 software) of nephroprotective activity of water extract (WEMp) and its isolated ethyl acetate fraction compound (ISLTD mp-B) is compared with standard silymarin in gentamicin-induced toxicant. Both extracts in treatment group exhibited nephroprotective action but in comparison, the ethylacetate fraction is more effective than the water extract [Graph 1].
Graph 1.
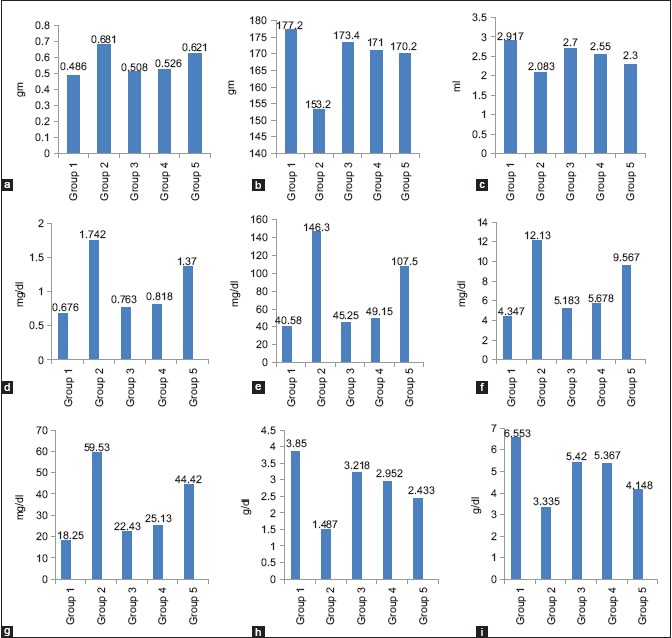
The graphs presents nephroprotective activity of water extract and its isolated ethyl acetate fraction compared with standard silymarin in gentamicin induced toxicant. (a) Kidney weight, (b) body weight, (c) urine volume, (d) creatinine, (e) urea, (f) uric acid, (g) blood urea nitrogen, (h) albumin, (i) protein
Conclusion
The results demonstrate the nephroprotective activity but water extract is less effective than its ethyl acetate fraction bark of M. pubescens J.E. Sm. The nephroprotective mechanism for its protection against cellular damage may be due to presence of flavonoids, tannins (gallic acid) and phenolic compounds etc., having good antioxidant activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are thankful for K.L.E. University Belgaum and Principal, K.L.E. University's College of Pharmacy Hubli - 31 (Karnataka) India, for providing the necessary facilities to carry out the work.
References
- 1.Schrier R, Wang W, Poole B, Mitra A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114(1):5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–98. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 3.Hsu C, McCulloch C, Fan D, Ordonez J, Chertow G, Go A. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–12. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y, Ding X, Zhong Y, Zou J, Teng J, Tang Y, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30(2):120–26. doi: 10.1159/000319972. [DOI] [PubMed] [Google Scholar]
- 5.Lafrance J, Miller D. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21(2):345–52. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Continuous renal replacement therapy: A worldwide practice survey. Intensive Care Med. 2007;33(9):1563–70. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 7.Murugan R, Kellum J. Acute kidney injury: What's the prognosis? Nat Rev Nephrol. 2011;7(4):209–17. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magadi RG. Botanical and Vernacular Names of South India Plants. 1st ed. Bangalore: Smt B M Komala for Divyachandra Prakashan; 2001. p. 282. [Google Scholar]
- 9.Ghorband DP, Biradar SD. Folk medicine used by the tribes of kinwat forest of Nanded district Maharashtra India. Indian J Nat Prod & Resources. 2012;3(1):118–22. [Google Scholar]
- 10.Anonymous: Phytochemical Reference Standards of Selected Indian Medicinal Plants. Vol. 1. New Delhi: Indian Plants Unit, Indian Council of Medical Research; 2003. Appendix – II- 343. [Google Scholar]
- 11.Houghton PJ, Raman A. Laboratory Handbook for the Fractionation of Natural Extracts. 1st ed. London: Thomson publication; 1998. pp. 54–65. [Google Scholar]
- 12.Khandelwal KR. In: Practical Pharmacognosy and Experimental Techniques. 22nd ed. Sethi V, editor. Pune: Nirali Prakashan; 2012. pp. 251–9. [Google Scholar]
- 13.Kokate CK, Purohit AP, Gokhale SB. Text Book of Pharmacognosy. 41st ed. Pune: Nirali Prakashan; 2008. pp. A5–A6. [Google Scholar]
- 14.Harborne JB. Phytochemical methods. A Guide to Modern Techniques of Plant Analysis. 3rd ed. London: Chapman and Hall; 2004. pp. 40–96. [Google Scholar]
- 15.Stahl E. Kurt E. Hydrophilic plant constituents and their Derivatives. New York: Springer - Verlag Berlin Heidelberg; 1969. Thin Layer Chromatography, A laboratory handbook; pp. 687–705. [Google Scholar]
- 16.Wagner H, Bladt S. Rickl V. Colored photograph. 2nd ed. New York: Springer - Verlag Berlin Heidelberg; 1996. Plant Drug Analysis, A Thin Layer Chromatography Atlas; pp. 195–244. [Google Scholar]
- 17.OECD [Organization for Economic Co-operation and Development]. Guideline 423 acute oral toxicity - Fixed dose procedure. Paris: OECD; 2001. [Google Scholar]
- 18.Gaurav VH, Chandragauda RP, Mahesh RP. Protective effect of Kalanchoe pinnata pers.(Crassulaceae) on gentamicin - induced nephrotoxicity in rats. Indian J Pharmacol. 2007;39(4):201–05. [Google Scholar]
- 19.Ingale KG, Thakurdesai PA, Vyawahare NS. Protective effect of Hygrophila spinosa against cisplatin induced nephrotoxicity in rats. Indian J Pharmacol. 2013;45(3):232–06. doi: 10.4103/0253-7613.111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pani SR, Mishra S, Sahoo S, Panda PK. Nephroprotective effect of Bauhinia variegata (linn.) whole stem extract against cisplatin – Induced nephropathy in rats. Indian J Pharmacol. 2011;43(2):200–02. doi: 10.4103/0253-7613.77370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anupam KB, Mumtaz SMF. Hepatoprotective and nephroprotective activity of hydroalcoholic extract of Ipomoea staphylina leaves. Bangladesh J Pharmacol. 2013;8(3):263–68. [Google Scholar]
- 22.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62(3):179–86. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223(1):86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Stojiljkovic N, Mihailovic D, Veljkovic S, Stoiljkovic M, Jovanovic I. Glomerular basement membrane alterations induced by gentamicin administration in rats. Exp Toxicol Pathol. 2008;60(1):69–75. doi: 10.1016/j.etp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed TB, Sadeghnia HR. Protective effect of safranal against gentamicin – Induced nephrotoxicity in rat. Iranian J Med Sci. 2009;34(4):285–88. [Google Scholar]
- 26.Kaloyanides GJ. Aminoglycosides induced functional and biochemical defects in the renal cortex. Fundam Appl Toxicol. 1984;4(6):930–43. doi: 10.1016/0272-0590(84)90231-8. [DOI] [PubMed] [Google Scholar]
- 27.Sonkar N, Ganeshpurkar A, Yadav P, Dubey S, Bansal D, Dubey N. An experimetal evaluation of nephroprotective potential of Butea monosperma extract in albino rats. Indian J Pharmacol. 2014;46(1):109–12. doi: 10.4103/0253-7613.125190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis. 1997;29(3):465–77. doi: 10.1016/s0272-6386(97)90212-2. [DOI] [PubMed] [Google Scholar]
- 29.Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999;21(3-4):433–42. doi: 10.3109/08860229909085109. [DOI] [PubMed] [Google Scholar]
- 30.Ghaznavi R, Faghihi M, Kadkhodaee M, Shams S, Khastar H. Effects of nitric oxide on gentamicin toxicity in isolated perfused rat kidneys. J Nephrol. 2005;18(5):548–52. [PubMed] [Google Scholar]
- 31.Ozbek E, Turkoz Y, Sahna E, Ozugurlu F, Mizrak B, Ozbek M. Melatonin administration prevents the nephrotoxicity induced by gentamicin. BJU Int. 2000;85(6):742–46. doi: 10.1046/j.1464-410x.2000.00531.x. [DOI] [PubMed] [Google Scholar]
- 32.Bashan I, Bashan P, Secilmis MA, Singirik E. Protective effect of L-arginine on gentamicin-induced nephrotoxicity in rats. Indian J Pharmacol. 2014;46(6):608–12. doi: 10.4103/0253-7613.144915. [DOI] [PMC free article] [PubMed] [Google Scholar]


