Summary
Background
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) induce inflammatory reactions, which can be described by changes in the neuroendocrine, cellular, protein, and cytokine systems. The aim of this study was to document the normal distribution pattern of the neutrophil-to-lymphocyte ratio (NLR) after THA and TKA and to compare it with postoperative C-reactive protein (CRP) patterns.
Methods
Changes in serum CRP levels, neutrophil count, and lymphocyte count were measured before and during the first 5 postoperative days in a prospective study performed on 387 patients undergoing total hip or knee arthroplasty.
Results
Mean CRP levels in patients undergoing THA were 7.7 mg/L, 184.8 mg/L, and 115.9 mg/L, respectively, at Days 0, 3 and 5. The mean NLR of patients undergoing THA was 2.9, 3.6, and 2.7, respectively, at Days 0, 3, and 5. Mean CRP levels in patients undergoing TKA were 7.8 mg/L, 192.6 mg/L, and 108.6 mg/L, respectively, at Days 0, 3 and 5. The mean NLR of patients undergoing TKA was 2.8, 3.4, and 2.6, respectively, at Days 0, 3, and 5. When comparing the preoperative value and the Day 3 value, CRP levels increased more than the NLR (almost a 24-fold increase in mean CRP values vs. a 1.2-fold increase in mean NLR values). In both groups, the NLR returned to preoperative values by the 5th postoperative day.
Conclusions
The present study demonstrated a significant elevation in CRP levels and the NLR following THA and TKA. In both groups, the NLR showed a faster kinetics pattern than CRP levels in response to surgical trauma.
The translational potential of this article: We describe results of the use of the NLR, as compared to a routinely used marker, CRP, as advantageous in clinical setting due to faster dynamics of change. Integrating the NLR in clinical practice seems easy and without extra cost.
Keywords: arthroplasty, C-reactive protein, immune response, lymphocytes, neutrophils
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) induce inflammatory reactions, which can be described by changes in the neuroendocrine, cellular, protein, and cytokine systems [1], [2]. C-reactive protein (CRP), an acute-phase protein produced in hepatocytes and macrophages, is the most commonly used marker in clinical practice [3]. Surgical trauma also triggers postoperative changes in white blood cell levels, leading to increased numbers of neutrophils and decreased lymphocyte counts [4], [5]. The neutrophil-to-lymphocyte ratio (NLR) is a simple and cost-effective biomarker of the inflammatory response that can be easily analysed at most hospital laboratories [6]. In elderly patients with hip fractures, high NLR values on admission are a significant risk factor of postoperative infection, myocardial injury, and in-hospital death [7].
The aim of this study was to document the normal distribution pattern of the NLR after THA and TKA and to compare it with postoperative CRP patterns. The clinical importance of this study would rely on testing the hypothesis that the NLR can be used as a surrogate marker for CRP to describe the inflammatory response to joint replacement in the immediate postoperative period.
Materials and methods
Patients
The study was conducted prospectively on 387 patients who underwent operations (235 THAs and 152 TKAs) at the tertiary care teaching hospital. The period of recruitment was from 2011 to 2014. There were two groups: the THA and TKA group. The mean age in the THA group was 63 ± 12 years (range, 21–89 years) with a median body mass index (BMI) of 29 kg/m2. The mean age in the TKA group was 68 ± 10 years (range, 35–85 years) with a median BMI of 31 kg/m2. Further demographics are given in Table 1.
Table 1.
Characteristics of the study group.
| THA | TKA | |
|---|---|---|
| Sex | ||
| Male | 90 | 32 |
| Female | 145 | 120 |
| Laterality (L/R) | 120/115 | 72/80 |
| ASA score | ||
| ASA 1 | 14 | 10 |
| ASA 2 | 154 | 94 |
| ASA 3 | 67 | 48 |
ASA = American Society of Anesthesiologists; L = left; R = right; THA = total hip arthroplasty; TKA = total knee arthroplasty.
The surgical technique, surgical team, and postoperative care were standardised and kept uniform within each group. Patients were mobilised and a standardised rehabilitation protocol was started on the 1st day after surgery in both groups. All patients with inflammatory conditions or receiving anti-inflammatory treatment before surgical intervention were excluded from this study. Patients with preoperative C-reactive protein levels > 10 mg/L were also excluded, as were patients who developed a prosthetic joint infection within 2 years of operation or any other inflammatory or medical complication during the hospital stay.
Surgical technique
Operations were performed under spinal anaesthesia. All patients received three doses of first generation cephalosporin intravenously as the perioperative prophylaxis (first dose within 30 min of skin incision and then 8 h and 16 h postoperatively). All THAs were cementless (BiContact stem and ScrewCup acetabular cup, Aesculap, Tuttlingen, Germany) and all TKAs were cemented (Triathlon knee and SimplexP cement, Stryker, Mahwah, NJ, USA). The postoperative analgesic protocol excluded the use of antiinflammatory drugs. The rehabilitation program was identical for all patients, with tolerated weight bearing with crutches along with active range of motion exercises conducted the day after the operation. Thrombosis prevention was performed with low molecular weight heparin for 30 days after surgery with enoxaparin. All patients initially included in this study were followed up for at least 2 years in order to fulfil the periprosthetic joint infection surveillance criteria of the Centers for Disease Control and Prevention [8].
Blood draws
Peripheral venous blood samples were collected 24 h before the day of operation and then on the 3rd and 5th postoperative days. To control for circadian rhythms, all samples were drawn at the same hour in the morning (7:00 am), and only the patients undergoing the first interventions each day were included. The samples were stored in 4 mL serum separation tubes with clot activator. The tubes were transported within an hour to the laboratory for biochemical processing. They were centrifuged at 1500g for 15 min at room temperature, after which the serum was collected and frozen at −80°C. The inflammatory parameter levels were determined after collection of all sera. As CRP and NLR are not quantified by the same units, the results of each are presented as a percentage, with the highest value in each patient normalised to 100%.
Ethics
This study has been carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. The Institutional Review Board approved this study before enrolment (no. 24/02/2010). All patients gave their written informed consent to participation in this study prior to enrolment.
Measurements of inflammatory parameters
All parameters were tested in the same laboratory.
The levels of CRP in the sera of all the participants were established by immunoturbidimetry, using the Turbitex CRP Ultra kit (Biocon Diagnostik, Vöhl/Marienhagen, Germany) on the Metrolab 2300 apparatus (UV-Vis Metrolab S.A., Buenos Aires, Argentina). The CRP content of each serum was tested three times and the results expressed as the mean in mg/L. The assays display a limit of detection of 0.14 mg/L with the normal program. Results were obtained at one decimal precision.
Leucocyte count was typically included in the routine preoperative and postoperative evaluation. All blood samples were anticoagulated by EDTA and processed in a Sysmex blood analyser (TOA Medical Electronics, Kobe, Japan) for the determination of the complete blood cell counts and differential counts of leucocytes. The neutrophil-to-lymphocyte ratio was calculated by dividing the recorded absolute total neutrophil and lymphocyte counts.
Statistical analysis
Patient demographics are presented as means and standard deviations for continuous variables. Frequencies and percentages are used for categorical and discrete variables. All laboratory values are expressed as mean ± standard deviation when the distribution was normal and median (with interquartile range or range) when the distribution was not normal. Pre- and postoperative marker levels were compared using Wilcoxon rank sum test; Student t test was also used when there was a normal distribution of means. All statistical analyses were performed using the R 3.0 software (R Foundation for Statistical Computing, Vienna, Austria). We considered probability values < 0.05 significant.
Results
The results for mean CRP levels and NLR are presented in Table 2, Table 3 for patients undergoing THA and TKA, respectively. The mean preoperative CRP level for patients undergoing THA was 7.79 mg/L, and it was 7.82 mg/L for patients undergoing TKA. The highest mean peak of CRP for patients undergoing both THA and TKA was at Day 3 (184.83 mg/L and 192.6 mg/L, respectively). At Day 5, CRP levels were lower than on Day 3 in 225 of 235 patients undergoing THA, and 147 of 152 patients undergoing TKA.
Table 2.
Mean (± SD) CRP and NLR levels preoperatively and on the 3rd and 5th postoperative days after THA.
| CRP (mg/L) |
NLR |
|||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Pre-op | 7.7 ± 13.1 | 0–142.6 | 2.9 ± 1.7 | 0.85–16.88 |
| Day 3 | 184.8 ± 74.5 | 1.4–473.1 | 3.6 ± 1.7 | 1.04–11.89 |
| Day 5 | 115.9 ± 52.7 | 12–279.8 | 2.7 ± 1.2 | 0.64–9.33 |
CRP = C-reactive protein; NLR = neutrophil-to-lymphocyte ratio; Pre-op = preoperative; SD = standard deviation; THA = total hip arthroplasty.
Table 3.
Mean (± SD) CRP and NLR levels preoperatively and on the 3rd and 5th postoperative days after TKA.
| CRP (mg/L) |
NLR |
|||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Pre-op | 7.8 ± 8.5 | 0–43.3 | 2.8 ± 1.14 | 1.04–7.5 |
| Day 3 | 192.6 ± 71.9 | 47.9–555.9 | 3.4 ± 1.53 | 1.28–10.8 |
| Day 5 | 108.6 ± 45.8 | 7.4–302.4 | 2.6 ± 1.06 | 0.86–6.75 |
CRP = C-reactive protein; NLR = neutrophil-to-lymphocyte ratio; Pr-eop = preoperative; SD = standard deviation; TKA = total knee arthroplasty.
The mean preoperative NLR was 2.93 for patients undergoing THA and 2.75 for patients undergoing TKA. Its mean value reached a peak of 3.58 in patients undergoing THA and 3.38 in patients undergoing TKA on postoperative Day 3. At Day 5, 95.74% (225/235) of patients undergoing THA had a normal NLR (NLR < 5), and 96.05% (146/152) had a normal NLR in the TKA group.
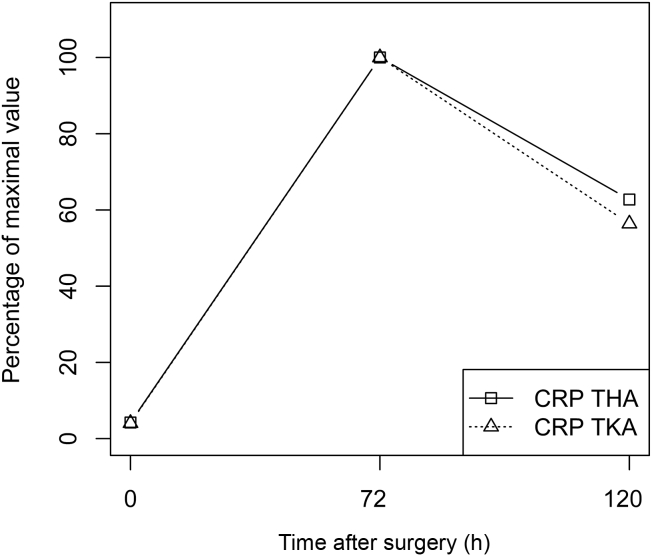
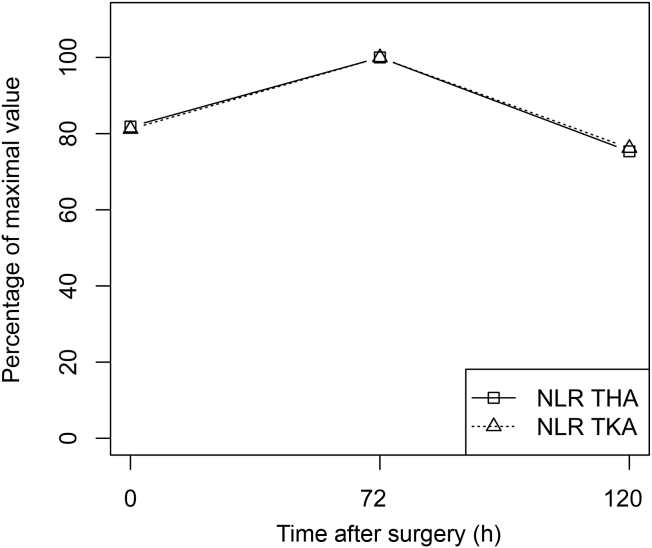
The CRP levels and the NLR expressed as percentages of their maximum values are presented in Figure 1, Figure 2, respectively. The logarithms of the mean CRP levels and NLR values are presented in Figure 3, Figure 4, respectively. The highest mean peak for both was at Day 3. When comparing the preoperative value and the Day 3 value, CRP levels increased more than the NLR (an almost 24-fold increase in mean CRP levels vs. a 1.2-fold increase in mean NLR).
Figure 1.
Comparison of the kinetics of CRP distribution over 5 postoperative days after THA (squares) and TKA (triangles). Values are displayed as percentages of the maximum values. CRP = C-reactive protein; THA = total hip arthroplasty; TKA = total knee arthroplasty.
Figure 2.
Comparison of the kinetics of NLR distribution over 5 postoperative days after THA (squares) and TKA (triangles). Values are displayed as percentages of the maximum values. NLR = neutrophil-to-lymphocyte ratio; THA = total hip arthroplasty; TKA = total knee arthroplasty.
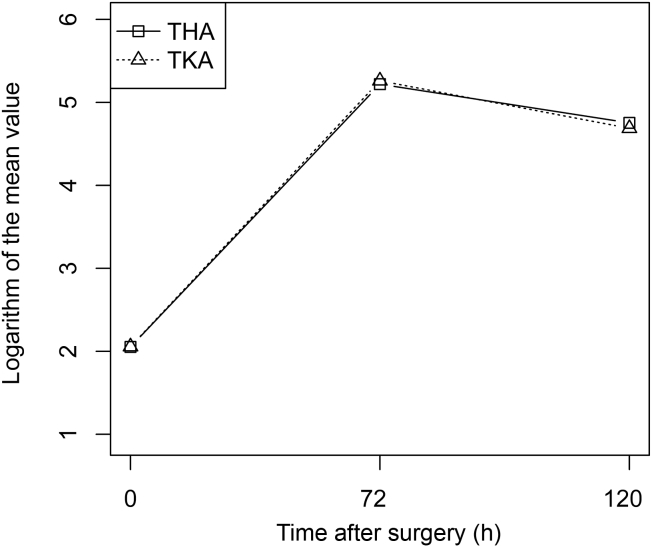
Figure 3.
Mean CRP changes for THA and TKA. A base-e log scale (ln) is used for the Y axis. Squares: THA; triangles: TKA. CRP = C-reactive protein; THA = total hip arthroplasty; TKA = total knee arthroplasty.
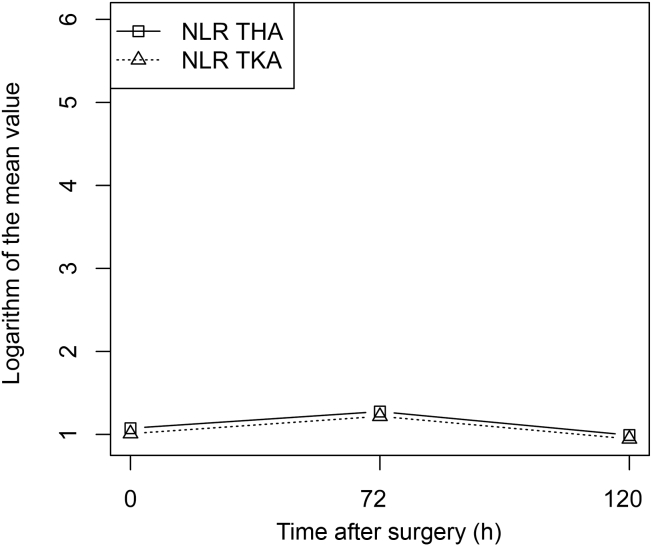
Figure 4.
Mean NLR changes for THA and TKA. A base-e log scale (ln) is used for the Y axis. Squares: THA; triangles: TKA. NLR = neutrophil-to-lymphocyte ratio; THA = total hip arthroplasty; TKA = total knee arthroplasty.
Only a few patients (10 patients undergoing THA and five patients undergoing TKA) displayed abnormal (defined as nondecreasing concentration between Days 3 and 5 postoperatively) CRP kinetics. Abnormal NLR kinetics were noted in 55 patients undergoing THA and 32 patients undergoing TKA. A complete data set is provided in Table 4, Table 5.
Table 4.
Total hip arthroplasty patients divided by expected vs. abnormal kinetics of CRP and NLR postoperatively.
| NLR 5 < NLR 3 | NLR 5 ≥ NLR 3 | |
|---|---|---|
| CRP 5 < CRP 3 | 173 | 52 |
| CRP 5 ≥ CRP 3 | 7 | 3 |
Data are presented as number of patients in each group.
Abnormal is defined as a failure to decrease in concentration between Days 3 and 5 postoperatively.
CRP = C-reactive protein; CRP 3 = C-reactive protein on postoperative Day 3; CRP 5 = C-reactive protein on postoperative Day 5; NLR = neutrophil-to-lymphocyte ratio; NLR 3 = neutrophil-to-lymphocyte ratio on postoperative Day 3; NLR 5 = neutrophil-to-lymphocyte ratio on postoperative Day 5.
Table 5.
Total knee arthroplasty patients divided by expected vs. abnormal kinetics of CRP and NLR postoperatively.
| NLR 5 < NLR 3 | NLR 5 ≥ NLR 3 | |
|---|---|---|
| CRP 5 < CRP 3 | 116 | 31 |
| CRP 5 ≥ CRP 3 | 4 | 1 |
Data are presented as number of patients in each group.
Abnormal is defined as a failure to decrease in concentration between Days 3 and 5 postoperatively.
CRP = C-reactive protein; CRP 3 = C-reactive protein on postoperative Day 3; CRP 5 = C-reactive protein on postoperative Day 5; NLR = neutrophil-to-lymphocyte ratio; NLR 3 = neutrophil-to-lymphocyte ratio on postoperative Day 3; NLR 5 = neutrophil-to-lymphocyte ratio on postoperative Day 5.
When calculated as area under the curve, CRP values were similar in the THA and TKA groups (230 vs. 242, Wilcoxon rank sum test p = 0.49). The same relationship existed for the NLR values (5.73 for THA and 5.65 for TKA, Wilcoxon rank sum test p = 0.44).
Discussion
The most important finding of this study was that the NLR showed a return to preoperative values within 5 days after surgery, while CRP was still elevated, after both THA and TKA. This would be especially important in patients with dubious in-hospital postoperative courses, in whom clinicians frequently suspect infection, as CRP may be used as a factor in determining the need for early revision surgery [9]. In those patients, the lack of, or only slow normalisation of CRP levels will not help clinicians in their diagnostic algorithms. For physicians unfamiliar with normal CRP distribution patterns, it could trigger unnecessary, expensive, and potentially dangerous investigations for the patient, or even lead to unnecessary surgery [10]. In cases when CRP levels do not decrease after surgery, the NLR could provide additional information at no cost, as it is calculated based on complete blood count, which is performed routinely with CRP. However, those values should be interpreted with caution for two reasons. First, the percentage of patients in our study displaying abnormal NLR kinetics was higher than those displaying abnormal CRP kinetics. Second, since this study excluded patients with complications, the predictive value of the NLR as a diagnostic test for infection is not known. Therefore, as with all additional studies, their results should be interpreted along with other clinical characteristics.
Our findings of a postoperative increase in CRP serum concentrations are consistent with previous reports [11], [12]. The peak median values were observed at 72 h postoperatively. Peak CRP levels vary between 100 mg/L and 260 mg/L for both procedures in the literature [1], [13].
The NLR has been used in multiple clinical settings, including sepsis, infective endocarditis, bacteraemia, and Crohn's disease [14], [15], [16]. It has also been used to predict outcomes not only in infection, but also in cardiovascular and oncological patients [17], [18], [19]. An NLR > 10 was shown to have higher prognostic accuracy than CRP for predicting bacteraemia [6].
There is little data on the usefulness of the NLR in total joint replacement. Katoh et al. [20] demonstrated that the neutrophil level after total joint arthroplasty significantly rose from Day 1, peaked at Day 3, and decreased significantly following Day 5. In our study, similar observations were made. The mean lymphocyte levels displayed a reverse pattern; they decreased at Day 3 and increased on Day 5. Therefore, the NLR, which is based on neutrophil and lymphocyte levels, peaked on postoperative Day 3 and returned to the preoperative values on Day 5.
In a study of 255 patients with a nonvertebral bone fracture, including 167 with a hip fracture, Fisher et al. [7] demonstrated that high NLR on admission is an independent indicator of fracture presence, and a significant risk factor and moderate predictor of postoperative myocardial injury, exaggerated inflammatory response or infection, and in-hospital death. However, the strongest relationships were noted for high NLR values (> 8.5), which were rarely seen in our patients. They calculated a positive predictive value of the NLR for any of the complications to be only 23.8%, 58.2%, and 40.0%, respectively, for each of the NLR tertiles at admission. This indicated that a higher NLR does not necessarily predict postoperative complications. Furthermore, they studied only admission values and not NLR kinetics. Although this approach might be useful as a screening tool due to its simplicity, it does not take into account interpersonal variability [9]. Of importance, they point out that a higher NLR might simply represent age-related changes in the immune system and chronic inflammation [21]. Therefore, the authors concluded that the “NLR's prognostic value in relation to individual patients is modest (but superior to the majority of other recommended predictors) and it does not indicate the type of possible adverse outcome” [7].
Yombi et al. [22] confirmed a faster normalisation of the NLR, compared to CRP levels, in the early postoperative period after 587 TKA cases. They examined CRP and NLR values preoperatively and at postoperative Days 2, 4, 21, and 42. One-fifth of their patients had not reached normal CRP levels within 3 weeks after operation. However, only 4.5% had elevated NLR values (> 5) at the same time point. They concluded that, of the two tests, the NLR was potentially a better biomarker to follow postoperative inflammation or early infection after TKA.
This study has some limitations. One of the limitations lies in the fact that we performed the blood tests only within the 5-day hospital stay. Therefore, we cannot comment on the value of the NLR in the postoperative outpatient setting.
An important limitation of this study is that we did not compare two completely matched groups, which rendered it impossible to perform in-depth analysis of the influence of comorbidities and clinical events, such as hypovolemia and blood transfusion, on inflammatory response [2]. However, we feel that our study setup, with the recruitment of consecutive, nonselected patients, resembles one similar to the everyday clinical practice and therefore, with enough judiciousness, our findings could be extrapolated to the general population.
The strength of this study is that results were available for both the NLR and CRP levels, allowing comparison of both markers.
Conclusion
The present study demonstrated a significant elevation in CRP levels and the NLR following THA and TKA. We describe results of the use of the NLR, as compared to a routinely used marker, CRP, as advantageous in the clinical setting due to faster dynamics of change. Integrating the NLR into clinical practice seems easy and without extra cost.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Funding/Support statement
The study was supported by grants from the National Science Centre (N N403 1064 40) and the Medical Centre of Postgraduate Education in Warsaw, (2012/KOCZNR) Poland.
Acknowledgements
The authors wish to thank all participants of the study for their cooperation and the staff of the anaesthesia and orthopaedic units for collection of samples. The authors also wish to thank the SPSK laboratory staff, who ran or helped run the analyses.
References
- 1.White J., Kelly M., Dunsmuir R. C-reactive protein level after total hip and total knee replacement. J Bone Joint Surg Br. 1998;80:909–911. doi: 10.1302/0301-620x.80b5.8708. [DOI] [PubMed] [Google Scholar]
- 2.Hall G., Peerbhoy D., Shenkin A., Parker C., Salmon P. Hip and knee arthroplasty: a comparison and the endocrine, metabolic and inflammatory responses. Clin Sci (Lond) 2000;98:71–79. [PubMed] [Google Scholar]
- 3.Niskanen R., Korkala O., Pammo H. Serum C-reactive protein levels after total hip and knee arthroplasty. J Bone Joint Surg Br. 1996;78:431–433. [PubMed] [Google Scholar]
- 4.Menger M., Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–484. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 5.Mimasaka S., Funayama M., Hashiyada M., Nata M., Tsunenari S. Significance of levels of IL-6 and IL-8 after trauma: a study of 11 cytokines post-mortem using multiplex immunoassay. Injury. 2007;38:1047–1051. doi: 10.1016/j.injury.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 6.de Jager C., van Wijk P., Mathoera R., de Jongh-Leuvenink J., van der Poll T., Wever P. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2009;14 doi: 10.1186/cc9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher A., Srikusalanukul W., Fisher L., Smith P. The neutrophil to lymphocyte ratio on admission and short-term outcomes in orthogeriatric patients. Int J Med Sci. 2015;13:588–602. doi: 10.7150/ijms.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangram A. A brief overview of the 1999 CDC Guideline for the Prevention of Surgical Site Infection. Centers for Disease Control and Prevention. J Chemother. 2001;13:35–39. doi: 10.1179/joc.2001.13.Supplement-2.35. [DOI] [PubMed] [Google Scholar]
- 9.Wasko M., Bobecka-Wesołowska K., Tomasiuk R., Kowalczewski J. Measurement of the inflammatory response in the early postoperative period after hip and knee arthroplasty. Clin Chem Lab Med. 2015;53:1785–1792. doi: 10.1515/cclm-2014-1055. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A., Zywiel M., Stroh A., Marker D., Mont M. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35:1621–1626. doi: 10.1007/s00264-010-1175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall G., Peerbhoy D., Shenkin A., Parker C., Salmon P. Relationship of the functional recovery after hip arthroplasty to the neuroendocrine and inflammatory responses. Br J Anaesth. 2001;87:537–542. doi: 10.1093/bja/87.4.537. [DOI] [PubMed] [Google Scholar]
- 12.Larsson S., Thelander U., Friberg S. C-reactive protein (CRP) levels after elective orthopedic surgery. Clin Orthop Relat Res. 1992;275:237–242. [PubMed] [Google Scholar]
- 13.Bilgen O., Atici T., Durak K., Bilgen M. C-reactive protein values and erythrocyte sedimentation rates after total hip and total knee arthroplasty. J Int Med Res. 2000;29:7–12. doi: 10.1177/147323000102900102. [DOI] [PubMed] [Google Scholar]
- 14.Bozbay M., Ugur M., Uyarel H., Cicek G., Koroglu B., Tusun E. Neutrophil-to-lymphocyte ratio as a prognostic marker in infective endocarditis: in-hospital and long-term clinical results. J Heart Valve Dis. 2014;23:617–623. [PubMed] [Google Scholar]
- 15.Loonen A., de Jager C., Tosserams J., Kusters R., Hilbink M., Wever P. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One. 2013;9 doi: 10.1371/journal.pone.0087315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao S., Huang L., Dai R., Chen D., Hu W., Shan Y. Neutrophil-lymphocyte ratio: a controversial marker in predicting Crohn's disease severity. Int J Clin Exp Pathol. 2014;8:14779–14785. [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat T., Teli S., Rijal J., Bhat H., Raza M., Khoueiry G. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 18.Lowsby R., Gomes C., Jarman I., Lisboa P., Nee P., Vardhan M. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J. 2015;32:531–534. doi: 10.1136/emermed-2014-204071. [DOI] [PubMed] [Google Scholar]
- 19.Templeton A., McNamara M., Šeruga B., Vera-Badillo F., Aneja P., Ocaña A. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:1–11. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 20.Katoh N., Nishino J., Nishimura K., Kawabata C., Hotta Y., Matsui T. Normal sequential changes in neutrophil CD64 expression after total joint arthroplasty. J Orthop Sci. 2013;18:949–954. doi: 10.1007/s00776-013-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panda A., Arjona A., Sapey E., Bai F., Fikrig E., Montgomery R. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yombi J., Schwab P., Thienpont E. Neutrophil-to-lymphocyte ratio (NLR) distribution shows a better kinetic pattern than C-reactive protein distribution for the follow-up of early inflammation after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24:3287–3292. doi: 10.1007/s00167-015-3921-0. [DOI] [PubMed] [Google Scholar]