Abstract
Optical coherence tomography (OCT) and T2 mapping are emerging clinical imaging technologies with potential to detect subsurface changes in cartilage retaining a macroscopically intact articular surface. This study tests the hypothesis that OCT correlates with magnetic resonance imaging (MRI) T2 values, and that OCT signal is sensitive to cartilage matrix degeneration. Forty-five osteochondral cores were harvested from five human tibial plateau explants after MRI T2 mapping. Cores underwent OCT imaging and were graded as follows: A, obvious birefringence; B, no birefringence; C, subsurface voids and/or irregular surface. Extracellular matrix content was determined and cores underwent histologic and polarized light microscopy (PLM) evaluation. Grade B and C cores had 25% higher superficial T2 values (p = 0.047) and 50% higher deep T2 values (p = 0.012) than grade A cores. Grade B and C cores had 36% higher glycosaminoglycan (GAG) content compared to grade A cores (p = 0.009). Histology and PLM demonstrated increased surface irregularity and structural disorganization with increasing OCT grade. OCT grade and T2 value increased with increasing collagen disorganization, suggesting that MRI T2 mapping and OCT are sensitive to changes in collagen structure. Our results demonstrate the ability of OCT and T2 mapping to detect early cartilage degeneration in clinically normal appearing cartilage.
Keywords: cartilage, osteoarthritis, optical coherence tomography (OCT), magnetic resonance imaging (MRI), collagen
Osteoarthritis is reaching epidemic proportions in the United States.1 It is estimated that 67 million American adults will have arthritis by 2030,2 and nearly half of Americans will develop osteoarthritis of the knee.3 As the number of individuals with osteoarthritis continues to rise, improved methods for early detection and treatment are increasingly vital to reduce or delay the onset of osteoarthritis.
Progressive loss of articular cartilage is a key component in the pathology of osteoarthritis.4 Cartilage extracellular matrix has unique compressive and viscoelastic properties, and is composed primarily of type-II collagen and glycosaminoglycan (GAG).5 Proper cartilage function is maintained by a balance between anabolic and catabolic activity. In early cartilage degeneration, this balance is thought to be maintained by compensatory physiologic repair mechanisms including chondrocyte proliferation and matrix upregulation.6 When the body’s repair capacity is exceeded, pathologic features of osteoarthritis, including cartilage extracellular matrix destruction, are manifested clinically with joint pain and dysfunction. Current modalities used to evaluate cartilage health, including radiography, arthroscopy, and clinical MRI, lack the ability to reliably diagnose cartilage degeneration early in the disease process.7,8
Arthroscopy detects articular cartilage degeneration prior to radiographs and is the current clinical standard for grading preosteoarthritic lesions known as chondrosis.8 Although arthroscopy can detect gross cartilage softening, it is not sensitive to deep cartilage layer changes and biochemical alterations that may precede diminution of functional integrity.9,10 Histologic and compositional analyses can evaluate tissue cellularity, concentrations of GAG and type-II collagen, and extracellular matrix structure. Furthermore, polarized light microscopy (PLM) of histologic sections using picrosirius red staining provides an indirect assessment of collagen fiber architecture.11 These methods of cartilage assessment, however, are not practical tools for clinical use, as they require removal and destruction of the cartilage being evaluated.
Clinical MRI is a valuable noninvasive imaging tool. However, it is slow and typically lacks the standard resolution to detect microstructural changes within the cartilage. Unlike standard clinical MRI, T2 mapping can provide quantitative information about articular cartilage structure and biochemical integrity.12 T2 measurements are generally thought to be dependent on collagen concentration, collagen orientation, and tissue hydration.13,14 Similar to clinical MRI, however, T2 mapping takes time to acquire and process, and may lack adequate spatial resolution to detect microstructural changes.
Optical coherence tomography (OCT) is a novel imaging tool that captures cross-sectional echographs of infrared light and acquires microscopic high resolution images (<10 μm axial resolution) of articular cartilage.15 Light which is backscattered from the cartilage is processed by the OCT system to develop an image which is displayed on a computer monitor in near-real time. OCT has previously been incorporated into arthroscopes and can image human articular cartilage with resolution similar to low-powered histology.9,16 Clinically, arthroscopic OCT has been shown to detect subsurface changes, correlating with metabolic signs of early cartilage degeneration, in human articular cartilage appearing normal to conventional arthroscopic exam.17 In this study, loss of the characteristic dark bands, which create a multilaminar pattern known as OCT form birefringence, was associated with chondrocyte metabolic incompetence.17
As the rate of osteoarthritis increases, the ability to detect early cartilage changes at potentially reversible stages is of critical importance. We hypothesize that OCT signal is sensitive to early cartilage degeneration. To test this hypothesis, a spatially registered comparison was made between OCT, T2 MRI, and extracellular matrix composition and histology.
MATERIALS AND METHODS
Specimen Collection
Forty-five osteochondral specimens were harvested from five human tibial plateaus obtained from elective total knee replacement (n = 1) and human cadaver knees (n = 4). Tissue from the total knee replacement patient was obtained in accordance with protocols approved by the Institutional Review Board (IRB). Human cadaver knees were obtained in accordance with protocols approved by the Committee for the Oversight of Research for the Dead (CORID).
Magnetic Resonance Imaging
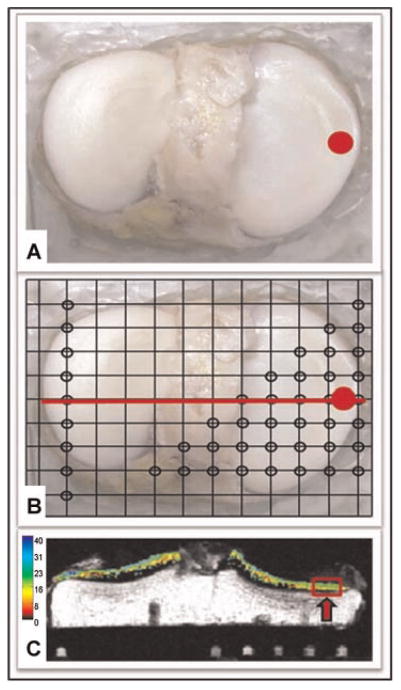
Tibial plateau explants were affixed to an acrylic plate with MRI fiducial markers to allow precise spatial registration of study locations for T2, OCT, and extracellular matrix composition measurements (Fig. 1). Precisely located wells within the fiduciary plate were filled with 4% agar doped with 2 mM Gd-DTPA2− (Magnevist, Berlex Imaging, Wayne, NJ). Quantitative T2 maps were acquired using a clinical 3T MRI scanner Siemens TIM Trio 3 Tesla MRI scanner (Siemens, Erlargen, Germany) with a standard extremity coil. A common multislice coronal 2-D FSE sequence was implemented with seven echo images ranging from 10 to 80 ms, repetition time 1800 ms, BW 326 Hz/pix, and four averages. Echo times were chosen to cover the range of physiologically likely T2 values in cartilage. The 20 2-D slices were collected with 417 × 417 μm in-plane resolution and 2-mm section thickness. Total T2 scan time was 12 min. Quantitative T2 maps were generated with a mono-exponential fitting routine using MRIMapper software (© Beth Israel Deaconess and MIT 2006). Mean T2 values within superficial and deep cartilage regions of interest (15–18 pixels wide) at each study location were quantified. T2 values less than 10 ms, or greater than 80 ms, were felt to reflect either signal average from the tidemark/calcified zone or factitious signal average from fluid, respectively, and thus were excluded from region-of-interest averages. The noise floor was not specifically measured for each fit.
Figure 1.
(A) Tibial plateau with selected core region of interest highlighted in red. (B) The plateau has been mounted on a registration plate with MRI fiducial markers located at the sites designated by the open black circles. (C) MRI T2 map. image of a coronal slice corresponding to the red line shown in (B). The selected region of interest is highlighted in red. The MRI fiducial markers are seen below the tibial plateau.
Osteochondral Cores
Following MRI, an 8.5-mm osteochondral coring device (Smith & Nephew) was used to take osteochondral cores from normal appearing regions of interest on the tibial plateau. Osteochondral core areas were determined, and the registration plate was used to locate the precise location of the core on the T2 map, so that a direct comparison could be made between T2 and OCT imaging. Four cores were taken from normal appearing areas on each tibial plateau compartment: two from the submeniscal region, and two from the central region. Additional cores were taken from regions of interest as determined by MRI and/or OCT evaluation.
Optical Coherence Tomography
Osteochondral cores were scanned using a custom polarized OCT scanning system (Bioptigen, Research Triangle Park, North Carolina) with a 1310-nm center wavelength, allowing a depth of penetration of approximately 2 mm. As OCT images can be affected by the signal polarization and orientation of the tissues being examined, a mark was made at the 12 o’clock position (aligned with the anterior/posterior axis of the plateau, where 12 o’clock pointed posteriorly) of each core at the time of harvest. Cartilage cores were then scanned at 0, 45, 90, and 135 degrees of orientation, relative to the mark. Images were saved and blinded for grading and analysis. OCT images were graded by a single reader according to the following scale: A, intact surface and obvious birefringence; B, intact surface and no birefringence; C, irregular articular surface and/or subsurface voids where a subsurface focal loss of OCT signal was interpreted as a void.
Glycosaminoglycan Assay
Osteochondral cores were bisected and one quarter of each core was processed for GAG content. The dry weight of the cartilage sections was determined, after which cartilage pieces were placed in 0.5 N NaOH, and GAG was extracted for 48 h at 4°C. GAG extracts were then diluted with saline to a concentration of 1:175 and assayed using dimethyl-methylene blue.18 GAG contents were normalized by their dry weights. In order to compare GAG contents across multiple tibial plateaus, individual core values were standardized to the mean GAG content per plateau.
Type-II Collagen ELISA
Osteochondral cores were bisected and the cartilage dry weight was determined for half of each core. A 2–5-mg sample was then processed for type-II collagen content analysis. Tissue was homogenized by freezing the samples in liquid nitrogen and then crushing them between metal plates to form a powder. The cartilage powder then underwent three incubations in pepsin (10 mg/ml dissolved in 0.05 M acetic acid) at 4°C for 48 h. This solution was incubated overnight in pancreatic elastase (1 mg/ml dissolved in 1 × TBS) at 4°C. Extracts were diluted between 1:25 and 1:200 and type-II collagen content was quantified using a type-II collagen ELISA assay (isotope 3D, MD Biosciences). Collagen content was normalized by the dry weight of each sample. In order to compare type-II collagen content across multiple tibial plateaus, individual core values were standardized to the mean type-II collagen content per plateau.
Histologic Analysis
One-half of each osteochondral core was fixed, processed, and paraffin-embedded for histologic analysis using standard techniques.9,19 Histologic sections were stained with hematoxylin/eosin, Safranin O, and picrosirius red. Histologic analysis was performed by a board certified pathologist who was blinded to the imaging and biochemical results. Sections stained with hematoxylin/eosin and Safranin O were used to evaluate cartilage structure and cellularity.20 Osteochondral sections stained with picrosirius red were evaluated using PLM to observe collagen structure and orientation. PLM images were obtained with two polarizers set perpendicular to each other, and with sections offset by 45 degrees to obtain optimal birefringence. PLM images were assessed by classifying the collagen architecture as either normal or disorganized.
Statistical Analysis
OCT grades were analyzed relative to spatially registered superficial and deep T2 values, as well as to extracellular matrix content and structure. Statistical significance was determined using one-way ANOVA and the Bonferroni t-test with p <0.05 being significant.
RESULTS
Optical Coherence Tomography and T2 Magnetic Resonance Imaging
Superficial T2 MRI
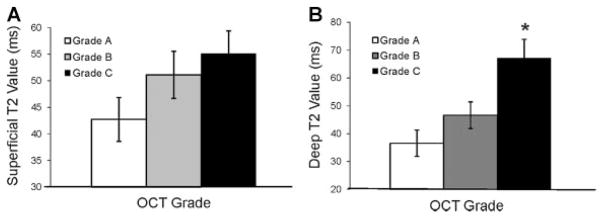
Osteochondral cores demonstrating clear OCT form birefringence (grade A) had a mean superficial T2 value of 43 ± 14 ms (n = 11). Cores with an OCT grade of B had a mean superficial T2 value of 51 ± 14 ms (n = 10), while grade C cores had a mean superficial T2 value of 55 ± 17 ms (n = 16). Although a trend was observed (Fig. 2A), the increase in superficial T2 relaxation time between OCT grades was not significant (p = 0.12). A significant difference was observed between superficial T2 values in birefringent cores (grade A), compared to cores without birefringence (grades B, C). Cores lacking birefringence demonstrated a 25% higher mean superficial T2 value than cores with birefringence (p = 0.047).
Figure 2.
(A) Superficial T2 relaxation time increased with increasing OCT grade (p = 0.12). Cores with OCT grades B, C had a 25% higher mean superficial T2 value compared to grade A cores (p = 0.047). (B) Deep T2 values increased with increasing OCT grade (p = 0.01). Within the groups, deep T2 values between grade A and C cores were significantly different (*p <0.05). Cores without birefringence (grades B and C) had an overall 50% higher mean deep T2 value compared to birefringent cores (p = 0.012). Error bars represent SEM.
Deep T2 MRI
The mean deep T2 value for cores assigned an OCT grade of A was 37 ± 14 ms, compared to a mean of 47 ± 16 ms for grade B and 67 ± 23 ms for grade C cores (Fig. 2B). ANOVA analysis revealed a significant difference between the groups (p = 0.01). Deep T2 values between grade A and C cores were significantly different (p <0.05), while the difference between A and B cores, as well as, between B and C cores was not significant. Cores without birefringence (grades B, C) had a 50% higher mean deep T2 value compared to birefringent cores (p = 0.012).
Optical Coherence Tomography and Cartilage Extracellular Matrix Content
ANOVA analysis revealed a significant difference in GAG content between OCT grades (p = 0.004, Fig. 3). OCT cores with a grade of A, exhibiting clear OCT form birefringence, had an average standardized GAG value of 0.76 (n = 12), while cores with grades B and C had mean values of 1.00 (n = 12) and 1.12 (n = 18), respectively. A significant difference was found between grade A and C GAG contents (p = 0.003), while a trend was seen between GAG contents for grade A and B (p = 0.081). Overall, cores without birefringence (grades B, C) had a 36% higher GAG content compared to cores with birefringence (p = 0.009).
Figure 3.
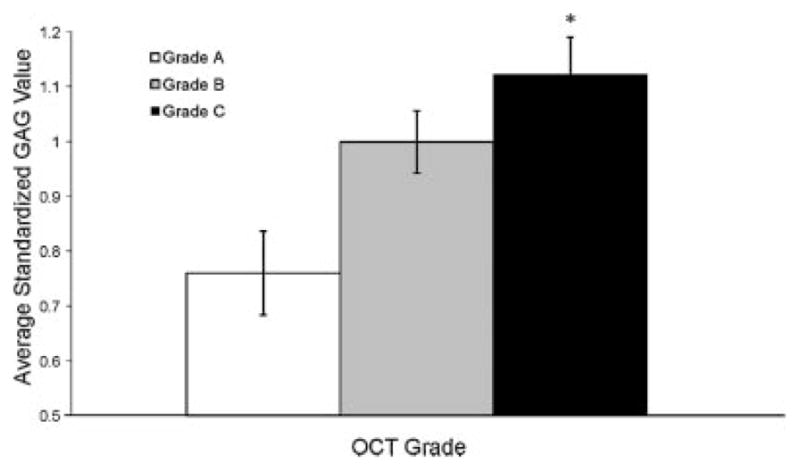
GAG content increased with increasing OCT grade (p = 0.004). Grade A osteochondral cores had significantly lower average GAG values than grade C cores (*p = 0.003), while differences between grade A and B GAG values approached significance (p = 0.08). Overall, cores without birefringence (grades B, C) had a 36% higher GAG content compared to cores with birefringence (p = 0.009). Error bars represent SEM
No significant relationship was found between OCT grade and type-II collagen content (p = 0.38). Grade A cores had a mean standardized type-II collagen content of 0.91 (n = 10), while grades B and C cores had average values of 0.85 (n = 8) and 1.13 (n = 16), respectively.
T2 Map and Cartilage Extracellular Matrix Content
No correlation was found between T2 Map and extra-cellular matrix content. GAG assays revealed no correlation with superficial (R2 = 0.028) or deep (R2 = 0.0002) T2 values. Similarly, no correlation was found between type-II collagen content and superficial (R2 = 0.066) or deep (R2 = 0.003) T2 values.
Histologic Analysis
Abnormalities in cartilage structure and cellularity were found to increase with increasing OCT grade (Table 1). All osteochondral cores with an OCT grade of A had a histologically intact surface, while 85% of grade B and only 33% of grade C cores had intact surfaces. Hematoxylin/eosin-stained cores showed no signs of disorganization in grade A cores, while 17% of grade B and 21% of grade C cores did show signs of disorganization. Cell clustering was lowest in grade A cores, with only 17% of cores exhibiting a moderate or high number of cell clusters. Half of grade B and C cores had moderate-to-high cell clustering.
Table 1.
Optical Coherence Tomagraphy Grades
| OCT Grade (%) | A | B | C |
|---|---|---|---|
| Cores with an intact surface | 100 | 85 | 33 |
| Cores with structural disorganization or absent layers | 0 | 17 | 21 |
| Cores with a moderate or high number of cell clusters | 17 | 50 | 50 |
| Cores with disorganization of collagen fibers | 10 | 50 | 88 |
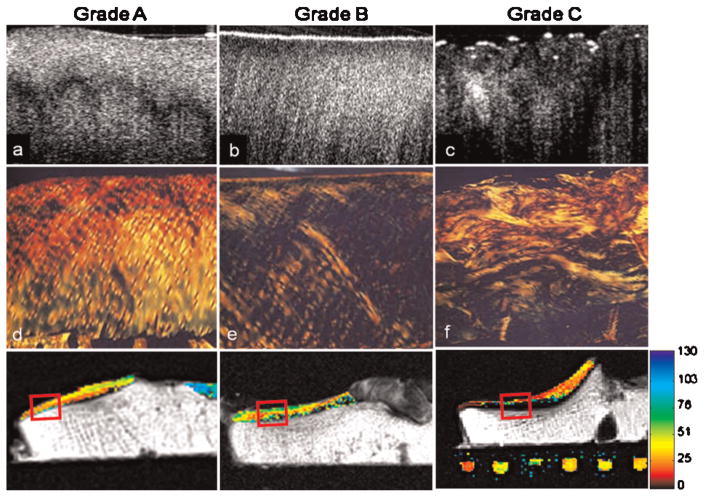
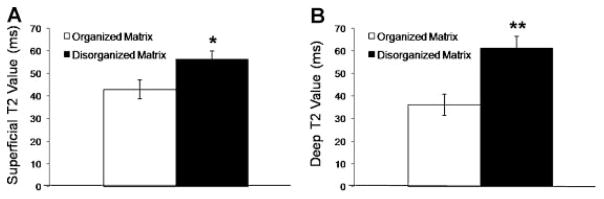
PLM imaging of osteochondral cores stained with picrosirius red also demonstrated an association with both OCT grade and MRI T2 maps (Fig. 4). Cores with an OCT grade of A consistently demonstrated organized collagen architecture by PLM, with only 10% of cores exhibiting signs of collagen fiber disorganization. This disorganization increased with increasing OCT grade, with 50% of grade B and 88% of grade C cores exhibiting signs of collagen fiber disorganization. Both superficial and deep T2 values correlated with collagen architecture as determined by PLM (Fig. 5). Superficial T2 values were found to be 31% higher in cores with evidence of collagen matrix disorganization, compared to cores with normal appearing collagen matrix (p = 0.04). Similarly, deep T2 values were 69% higher in cores with collagen disorganization as assessed by PLM, compared to normal appearing cores (p <0.001).
Figure 4.
Comparison of OCT grade to polarized light microscopy (PLM). Representative OCT images of osteochondral cores with grades A through C (a–c). Corresponding PLM images of sections stained with picrosirius red demonstrating increased collagen fiber disorganization with increasing OCT grade (d–f). Corresponding T2 MRI maps demonstrating a correlation with OCT and increasing collagen disorganization (g–i).
Figure 5.
(A) Superficial T2 values were 31% higher in cores with evidence of collagen matrix disorganization as assessed by PLM, compared to cores with normal appearing collagen matrix (*p = 0.04). (B) Deep T2 values were 69% higher in cores with collagen disorganization compared to normal appearing cores (** p <0.001). Error bars represent standard error of the mean.
DISCUSSION
The results show that OCT birefringence patterns correlate with T2 MRI relaxation time and with histological signs of early articular cartilage degeneration. Osteochondral cores with clear birefringence had lower T2 values compared to cores without clear birefringence. Using PLM, T2 relaxation time has been shown to correspond to collagen fiber orientation.21–23 Prolongation of the T2 relaxation time has also been shown to correlate with the development of osteoarthritis and disruption of normal collagen structure.13,23–25 These findings are consistent with our data, which demonstrate prolonged T2 relaxation times in osteochondral cores exhibiting signs of collagen disorganization as assessed by PLM. Our results further demonstrate that cores without birefringence show signs of early cartilage degeneration despite having a grossly intact surface, as demonstrated by a significant increase in T2 relaxation time, increased histologically evident cell clustering, and greater collagen disorganization by polarized microscopy. These data indicate that OCT birefringence patterns reflect the structural integrity and organization of the cartilage matrix.
Consistent with previous studies,11,12 T2 relaxation time did not correlate with type-II collagen content. Loss of OCT detectable birefringence also did not correlate with type-II collagen content. These findings suggest that both MRI T2 maps and OCT differences reflect alterations in collagen structure rather than changes in type-II collagen content. This is further supported by histological findings using PLM which permits assessment of collagen fiber architecture.11 Using PLM, a strong association was found between OCT grade and collagen structure, with cores showing OCT detectable birefringence having the greatest degree of collagen organization, while progressively greater disorganization of collagen fibers was observed in cores without OCT form birefringence. A similar association was also found between T2 MRI and collagen structure, as T2 values were significantly higher in cores with signs of collagen organization as identified by PLM. This finding is consistent with past studies which have demonstrated increased T2 values in less organized cartilage.21,23
Previous studies have shown that type-II collagen is evenly distributed between the superficial and deep layers of healthy cartilage.26 Similar to other studies showing that overall content of collagen remains unchanged in early osteoarthritis,27,28 we observed no change in collagen content with increasing OCT grade. Kempson et al., however, showed that type-II collagen content is decreased in superficial cartilage with early degeneration.29 Further research has also demonstrated a simultaneous upregulation of collagen in deep cartilage layers.30 This means that if a decrease in superficial collagen content is matched by a comparable increase in deep collagen content, this would result in no net change in collagen content. It is therefore possible that we did not observe a change in collagen content, because we did not separate superficial and deep layers in our analysis of collagen content.
Full-thickness GAG content was significantly higher in cores without clear OCT form birefringence. While it is possible that separating superficial and deep cartilage layers in the GAG content analysis may have led to a different result, our findings are consistent with a known increase in GAG during the repair phase of early cartilage degeneration and with numerous studies which have shown an increase in GAG synthesis in early and mild osteoarthritis.31,32 Differences in GAG content did not correlate with T2 values, consistent with other studies which have demonstrated no change in T2 relaxation time after GAG depletion.33
Histologic evaluation with Safranin O and hematoxylin/eosin staining revealed progressive structural and cellular changes with increasing OCT grade. All of the cores which had clear OCT birefringence had a normal surface and structural organization. Furthermore, surface irregularity and structural disorganization increased with increasing OCT grade. The number of cell clusters, a histologically evident sign of degeneration,34 increased between grade A and grade B cores, and this number remained elevated in grade C cores. It is important to note that despite histologic findings showing a progressive increase in cartilage degenerative changes, 85% of specimens appearing intact by OCT, but lacking OCT form birefringence, had microscopically intact articular surfaces, confirming the normal appearance on visual surface examination. This observation, along with our OCT findings, further supports the ability of OCT to detect early degenerative changes in grossly normal appearing cartilage.
In this spatially registered comparison study, OCT was also found to correlate with MRI T2 mapping. This finding is additionally notable given the large disparity in resolution between OCT (axial resolution <10 μm) and MRI. For a given region of interest, each OCT scan had thousands of pixels, while the same region on MRI had less than 50 pixels. The limited MRI data in these small regions of interest likely account for the relatively large standard deviations in T2 values observed in this study. The wide standard deviations may also reflect the variability of relaxation times within this degenerative osteoarthritic environment.
This study demonstrates that in macroscopically normal appearing articular cartilage, loss of OCT form birefringence is a marker of early degeneration, as determined by histology and MRI T2 mapping. This study supports the utility of OCT to detect early subsurface degenerative changes in articular cartilage appearing normal upon surface inspection, such as what would be observed during conventional arthroscopy and structural MRI. Because OCT technology can be incorporated into arthroscopes and used clinically during arthroscopic surgery,16,17 this ability to detect subclinical evidence of microstructural and biochemical degeneration could be useful in the development of new options for early treatment to prevent or delay the onset of osteoarthritis.
Acknowledgments
The authors thank Elise Pringle for preparation of histology. This study was funded by National Institutes of Health (RO1 AR052784-CRC and P60 AR054731-CRC), the Orthopaedic Research and Education Foundation (PAG00 19989-DMB), and the Albert B. Ferguson Endowed Chair-CRC.
References
- 1.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 3.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004;50:341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 5.Wollheim FA. Serum markers of articular cartilage damage and repair. Rheum Dis Clin North Am. 1999;25:417–432. viii. doi: 10.1016/s0889-857x(05)70076-4. [DOI] [PubMed] [Google Scholar]
- 6.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recht MP, Resnick D. MR imaging of articular cartilage: current status and future directions. AJR Am J Roentgenol. 1994;163:283–290. doi: 10.2214/ajr.163.2.8037016. [DOI] [PubMed] [Google Scholar]
- 8.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CR, Lin D, Geisler JL, et al. Arthroscopic microscopy of articular cartilage using optical coherence tomography. Am J Sports Med. 2004;32:699–709. doi: 10.1177/0363546503261736. [DOI] [PubMed] [Google Scholar]
- 10.Bradley MP, Provencher MT, Nelson FR. Bilateral subchondral cysts without arthroscopic evidence of articular cartilage degradation. Arthroscopy. 2002;18:E41. doi: 10.1053/jars.2002.29940. [DOI] [PubMed] [Google Scholar]
- 11.Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picrosirius red FBA. Beitr Pathol. 1973;150:174–187. doi: 10.1016/s0005-8165(73)80016-2. [DOI] [PubMed] [Google Scholar]
- 12.Potter HG, Black BR, Chong le R. New techniques in articular cartilage imaging. Clin Sports Med. 2009;28:77–94. doi: 10.1016/j.csm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.David-Vaudey E, Ghosh S, Ries M, et al. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 14.Menezes NM, Gray ML, Hartke JR, et al. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Y, Li Z, Xie T, et al. Hand-held arthroscopic optical coherence tomography for in vivo high-resolution imaging of articular cartilage. J Biomed Opt. 2003;8:648–654. doi: 10.1117/1.1609201. [DOI] [PubMed] [Google Scholar]
- 17.Chu CR, Izzo NJ, Irrgang JJ, et al. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. J Biomed Opt. 2007;12:051703. doi: 10.1117/1.2789674. [DOI] [PubMed] [Google Scholar]
- 18.Mort JS, Roughley PJ. Measurement of glycosaminoglycan release from cartilage explants. Methods Mol Med. 2007;135:201–209. doi: 10.1007/978-1-59745-401-8_12. [DOI] [PubMed] [Google Scholar]
- 19.Rohde RS, Studer RK, Chu CR. Mini-pig fresh osteochondral allografts deteriorate after 1 week of cold storage. Clin Orthop Relat Res. 2004;427:226–233. doi: 10.1097/01.blo.0000138955.27186.8e. [DOI] [PubMed] [Google Scholar]
- 20.Mankin HJ, Dorfman H, Lippiello L, et al. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg [Am] 1971;53:523–537. [PubMed] [Google Scholar]
- 21.Nieminen MT, Rieppo J, Toyras J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Moody JB, Burton-Wurster N, et al. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 23.Kelly BT, Potter HG, Deng XH, et al. Meniscal allograft transplantation in the sheep knee: evaluation of chondroprotective effects. Am J Sports Med. 2006;34:1464–1477. doi: 10.1177/0363546506287365. [DOI] [PubMed] [Google Scholar]
- 24.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayami T, Funaki H, Yaoeda K, et al. Expression of the cartilage derived anti-angiogenic factor chondromodulin-I decreases in the early stage of experimental osteoarthritis. J Rheumatol. 2003;30:2207–2217. [PubMed] [Google Scholar]
- 27.Appleyard RC, Burkhardt D, Ghosh P, et al. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthritis Cartilage. 2003;11:65–77. doi: 10.1053/joca.2002.0867. [DOI] [PubMed] [Google Scholar]
- 28.Lippiello L, Hall D, Mankin HJ. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977;59:593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempson GE, Muir H, Pollard C, et al. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosamoglycans. Biochim Biophys Acta. 1973;297:456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 30.Pfander D, Rahmanzadeh R, Scheller EE. Presence and distribution of collagen II, collagen I, fibronectin, and tenascin in rabbit normal and osteoarthritic cartilage. J Rheumatol. 1999;26:386–394. [PubMed] [Google Scholar]
- 31.Carney SL, Billingham ME, Caterson B, et al. Changes in proteoglycan turnover in experimental canine osteoarthritic cartilage. Matrix. 1992;12:137–147. doi: 10.1016/s0934-8832(11)80055-7. [DOI] [PubMed] [Google Scholar]
- 32.Saris DB, Dhert WJ, Verbout AJ. Joint homeostasis. The discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg [Br] 2003;85:1067–1076. doi: 10.1302/0301-620x.85b7.13745. [DOI] [PubMed] [Google Scholar]
- 33.Borthakur A, Shapiro EM, Beers J, et al. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthritis Cartilage. 2000;8:288–293. doi: 10.1053/joca.1999.0303. [DOI] [PubMed] [Google Scholar]
- 34.van der Sluijs JA, Geesink RG, van der Linden AJ, et al. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10:58–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]