Abstract
Anterior cruciate ligament (ACL) injury is a known risk factor for future development of osteoarthritis (OA). This human clinical study seeks to determine if early changes to cartilage MRI T2 maps between baseline and 6 months following ACL reconstruction (ACLR) are associated with changes to cartilage T2 and cartilage thickness between baseline and 2 years after ACLR. Changes to T2 texture metrics and T2 mean values in medial knee cartilage of 17 human subjects 6 months after ACLR were compared to 2-year changes in T2 and in cartilage thickness of the same areas. T2 texture and mean assessments were also compared to that of 11 uninjured controls. In ACLR subjects, six-month changes in mean T2 correlated to 2-year changes in mean T2 (R = 0.80, p = 0.0001), and 6-month changes to T2 texture metrics, but not T2 mean, correlated with 2-year changes in medial femoral cartilage thickness in 9 of the 20 texture features assessed (R = 0.48–0.72, p ≤ 0.05). Both mean T2 and texture differed (p < 0.05) between ALCR subjects and uninjured controls.
Clinical Significance
These results show that short-term longitudinal evaluation of T2 map and textural changes may provide early warning of cartilage at risk for progressive degeneration after ACL injury and reconstruction.
Keywords: anterior cruciate ligament reconstruction (ACLR), post-traumatic osteoarthritis (PTOA), magnetic resonance imaging (MRI), cartilage T2, anterior cruciate ligament tear (ACLT)
Anterior cruciate ligament tear (ACLT) injury is a common injury that is known to increase risk for development of premature disabling osteoarthritis (OA).1–3 While acute cartilage damage is more readily observed by MRI to the lateral tibial cartilage,4,5 the ensuing OA appears to predominate to the medial compartment,6 with a 19-fold increase in risk for cartilage loss to the medial femoral condyle (MFC) observed just 7–11 years following ACL reconstruction (ACLR).4 A substantial portion of patients suffering acute ACL tear exhibit intact and arthroscopically firm MFC cartilage at the time of ligament reconstruction surgery without radiographic evidence of joint arthrosis.5,7 However, biochemical and histologic characteristics of idiopathic osteoarthritis (OA) have been observed in cartilage judged to be “normal” by arthros-copy.2,8,9 Consequently, potentially reversible cartilage matrix injury and early degeneration may be present prior to clinically detectable tissue softening and disruption of surface integrity. Clinical tools to detect biochemical and structural early warning signs are key to the timely introduction of targeted disease modifying therapies, which may ultimately permit more effective OA prevention and management strategies than is currently achievable.
The structural integrity of the cartilage collagen matrix and the changes that can occur in OA may be quantified by mapping T2 values obtained noninvasively with magnetic resonance imaging (MRI).10–13 Previous studies have reported increased cartilage T2 values with early stages of OA.14–16 In ACL-injured subjects, inconsistent cartilage T2 measurements relative to uninjured controls have been reported in the weeks after injury but prior to ligament reconstruction.5,17 An examination of cartilage T2 change over 2 years following ACLR revealed significant T2 elevations in superficial central medial femoral and deep posterior lateral tibial plateau cartilage.5 Other researchers have observed a “progressive prolongation” of T2 values in lateral femoral condylar and patellar cartilage of ACL-injured subjects followed annually for up to 11 years post-injury.4
With increasing cartilage degeneration, the collagen framework degrades resulting in a more disordered matrix. The degree of matrix disruption can be measured using image analysis techniques to evaluate the spatial distribution of cartilage T2 values. One such method, gray-level co-occurrence matrix (GLCM) image analysis,18 has demonstrated great potential as a tool to detect and quantify OA-induced changes to the spatial distributions of quantitative MRI (qMRI) T2 and T1rho values using second-order texture statistics.19–25 Previous GLCM texture analyses of T2 maps have shown that subjects with risk factors for OA, but without radiographic evidence of the disease, exhibit differences in several measurement variables. Elevated “contrast” and “variance” statistics compared to healthy controls in both medial femoral and tibial cartilage have been reported,24 and longitudinal decreases to “entropy” with concurrent increases to “contrast” and “variance” were demonstrated over 3 years in medial femoral cartilage.19 Patients with radiographically confirmed OA can be discriminated from healthy controls on the basis of relative disorder (“entropy”) and orderliness (“angular second moment”) in medial tibial cartilage,20 or “contrast” and “homogeneity” in weight-bearing MFC cartilage.22
Longitudinal GLCM cartilage measurements in subjects with moderate radiographic OA, Kellgren–Lawrence (KL) scores 2 or 3, demonstrated significant decreases to “contrast” and increases to “homogeneity” parallel to the articular surface over 2 years, as well as significant changes to several measures perpendicular to the surface.21 GLCM T2 texture analyses have also been examined for their ability to predict degenerative knee changes observed in subjects of the Osteoarthritis Initiative’s incidence cohort.26 Joseph et al. showed that elevated GLCM “entropy” in the medial tibial plateau was associated with meniscal degeneration over 3 years.23
Given that GLCM T2 textural analyses can detect structural changes to cartilage in both pre-radiographic and radiographic OA, this work seeks to identify associations between GLCM T2 texture and cartilage thickness changes in knee joints of human subjects over a 2-year period after ACLR. Specifically, this human clinical study focuses on individuals with arthroscopically intact articular cartilage to the medial compartment at the time of reconstructive surgery to test the hypotheses that changes to qMRI cartilage T2 maps (mean or textural) of medial femoral and tibial cartilage measured over the first 6 months following ACL-reconstruction correlates with 2-year T2 and cartilage thickness changes.
METHODS
Subject Demographics
This prospective cohort (level 2) study was performed according to Institutional Review Board approved procedures with signed informed consent obtained from all study participants. Seventeen subjects undergoing ACLR (mean age 31 ± 10 years, range 19–53 years; mean BMI 26 ± 6 kg/m2, range 20–39; 10/7 female/male; 12/5 left/right knees) completed pre-operative, 6-month (mean 6.0 ± 0.5 months) and 2-year (mean 25.3 ± 1.5 months) MRI and met inclusion criteria of having arthroscopically surface intact articular cartilage to the medial compartment at the time of ACLR (modified Outerbridge scores 0–1) with one subject noted to have intact “fibrocartilage.” Blinded review of preoperative MRI scans by a musculoskeletal radiologist (CSW) confirmed absence of cartilage lesions to study regions of interest by MRI criteria except for a lesion corresponding to the area previously described as “fibrocartilage” by the surgeon (CRC) at arthros-copy. Eight subjects had torn menisci and six subjects had MCL injuries. Health of the contralateral knee and other joints were not assessed. Anatomic ACLR was performed to the center of the ACL footprints on the femur and the tibia. Pre-operative, 6 months and 2 year 3.0 T MRI scans were obtained according to the investigational protocol. Eleven uninjured control subjects, with no known or suspected history of knee pathology (mean age 29 ± 4 years, range 25–35 years; mean BMI 25 ± 5 kg/m2, range 20–33; 6/5 female/male; 6/5 left/right knees) also received MRI T2 imaging according to the investigational protocol.
MR images from a separate set of control subjects, collected during pilot studies for the Osteoarthritis Initiative, were obtained from the National Institute of Arthritis and Musculoskeletal and Skin Disease and used for test-retest T2 texture assessments.27 T2 map data were obtained for five healthy controls (mean age 48 ± 2 years, range 45–50 years; mean BMI 26 ± 6 kg/m2, range 22–35; 4/1 female/male; 3/2 left/right knees).
MR Imaging
All ACLR subjects underwent MR examination of the injured knee on a three Tesla scanner (MAGNETOM Trio, Siemens, Erlangen, Germany) at three time-points: within 24 days prior to ACLR, and then 6 months and 2 years after surgery with an extremity coil or an eight-channel phased array coil. Coil type for each subject was kept the same over time, except for two subjects whose pre-operative and 6 months scans were collected with the extremity coil and whose 2 years scans were collected with the phased array coil. Uninjured control subjects were scanned once with an extremity coil. T2 images were acquired using an interleaved multi-slice, multi-echo spin-echo (MSME-SE) sequence with seven TEs ranging 11–88 ms, and repetition time of 2700 ms. The field of view (FOV) was 12–14 cm with a 384 × 384 matrix and 3 mm slice thickness in a scan time of approximately 12 min. 3-D double-echo steady-state (DESS) images with parameters identical to the NIH-sponsored Osteoarthritis Initiative sequence28 were acquired on ACLR subjects.
Test-Retest MR Imaging
Test-retest evaluation of the GLCM T2 texture assessment technique was performed on four sets of MSME-SE sequences, previously collected as part of pilot OAI studies,27 obtained for each of five control knees. Each knee underwent 3 T MR exams in each of two coils: a quadrature transmit/receive coil and an eight-channel phased array coil. Each knee was scanned twice per coil. MSME-SE images were collected with seven TEs ranging from 10 to 70 ms and other parameters as previously described.27
Cartilage T2 Mapping
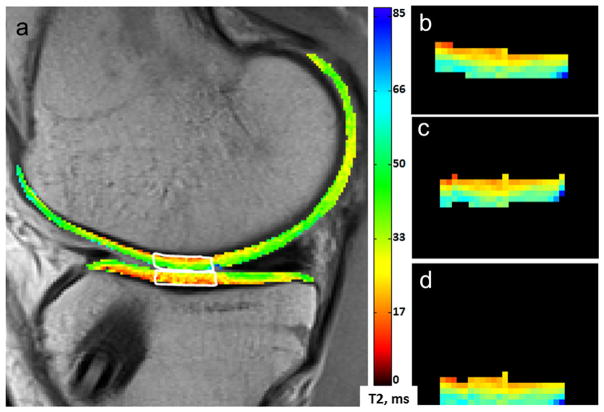
Prior to T2-curve fitting, the TE images were down-sampled using cubic interpolation in Matlab (TheMathWorks, Natick, MA), creating an effective resolution of 416 × 416 μm in-plane for all images. The down-sampling factor was optimized to increase signal-to-noise while maintaining <0.5 mm in-plane resolution. T2 maps were generated for a single section from the center of the medial femoral condyle using MRIMapper© software (Beth Israel Deaconess and MIT 2006) running on a Matlab platform. The shortest echo image was excluded from the T2 curve-fitting.29 Regions of interest (ROIs) were manually segmented, by one individual with 10 years prior segmenting experience (AW), from a single section in the cMFC and central medial tibial plateaus (cMTP), Figure 1a. To reduce the impact of noise and partial-voluming of bone or synovial fluid signals on cartilage T2 calculations, T2 maps were filtered to exclude pixels with non-physiologic T2 values. Pixels with T2 values <10 ms or >90 ms were excluded from all quantitative analyses. T2 thresholds were selected based on levels reported in the literature.8,13,30
Figure 1.
T2 map region of interest for textural analysis. MRI T2 mean value and texture features were calculated in in cMFC (a, top white outline) and cMTP (a, bottom white outline) cartilage from a mid-sagittal section. Prior to performing GLCM texture analysis, the extracted cMFC ROI was (b) rigidly rotated (c) and flattened (d).
T2 map Texture Analysis
Image texture analysis with GLCM statistics was performed on full-thickness cMFC and cMTP cartilage ROIs from calculated T2 maps using in-house developed Matlab based software utilizing the “graycomatrix” and “graycoprops” functions. Prior to performing GLCM analyses, ROIs were individually rigidly-rotated in-plane (≤5°) to minimize the angular offset between the bone-cartilage interface and rows of pixels in the T2 maps. After rotation, the segmented cartilage ROIs were aligned so that the bone-cartilage interface was perpendicular to columns of pixels and parallel to rows of pixels in the T2 maps (Fig. 1). Four statistical features (“contrast,” “homogeneity,” “correlation,” and “energy”) were investigated at three different spacings (1, 3, 5 pixel offsets) parallel (0°) to cartilage surface and at two different spacing (1, 3 pixel offset) perpendicular (90°) to cartilage surface for a total of 20 GLCM feature calculations per ROI. The maximum pixel spacing investigated in the perpendicular direction was limited to a 3-pixel offset due to the fact that cartilage in the cMFC and cMTP regions was typically 4–6 pixels thick. Briefly, “contrast” measures the amount of local T2 variation; “homogeneity” measures the T2 similarities between voxels and their neighbors; “correlation” measures linear dependencies of T2 values; and “energy” measures the orderliness of the T2 distribution.18 The equations below mathematically describe how each feature is calculated. In these equations, P(i,j) is the relative frequency with which two neighboring pixels, at a given offset and in a given direction, occur in the image with T2 values i,j, respectively; μ is the mean of the probability distribution, and σ is the variance.18
Morphometric MRI Assessment
Pre-operative and 2-year follow-up 3-D DESS images were sent to a company specializing in morphometric evaluation of knee MRI for cartilage thickness measurement according to validated protocols showing test-retest reproducibilities of 1.6% and 2.2% for the central medial tibial plateau and femoral cartilage, respectively (Chondrometrics GmbH, Ainring, Germany).31–33 Thickness measurements used in the analysis were obtained from the data set returned by the company for the central weight-bearing medial femoral condyle (ccMF.ThCtAB.aMe) and the central medial tibial plateau (cMT.ThCtAB.aMe).
Statistical Analyses
Statistical analyses were performed with SigmaPlot (Systat Software, San Jose, CA). All data were examined for normality with ShipiroWilk tests. T2 texture differences across test-retest scans in each of two coils were assessed with two-way repeated measures analysis of variance (RM-ANOVA) with post-hoc evaluation of simple main effects. Thickness changes over 2 years were evaluated with paired t-tests. Correlation of T2 texture changes over 6 months to thickness or mean T2 change over 2 years were assessed with Spearman’s rho correlations. Correlation of mean T2 change over 6 months with mean T2 change over 2 years was assessed with Pearson correlation. Correlations of age to change in T2 mean or T2 texture was assessed with Pearson correlation. Comparisons of T2 texture and mean differences between ACLR subjects and uninjured controls was assessed by two-tailed independent-samples t-tests (or Mann Whitney U-tests for non-normally distributed data).
RESULTS
Correlation of 6-Month and 2-Year Mean T2 Change
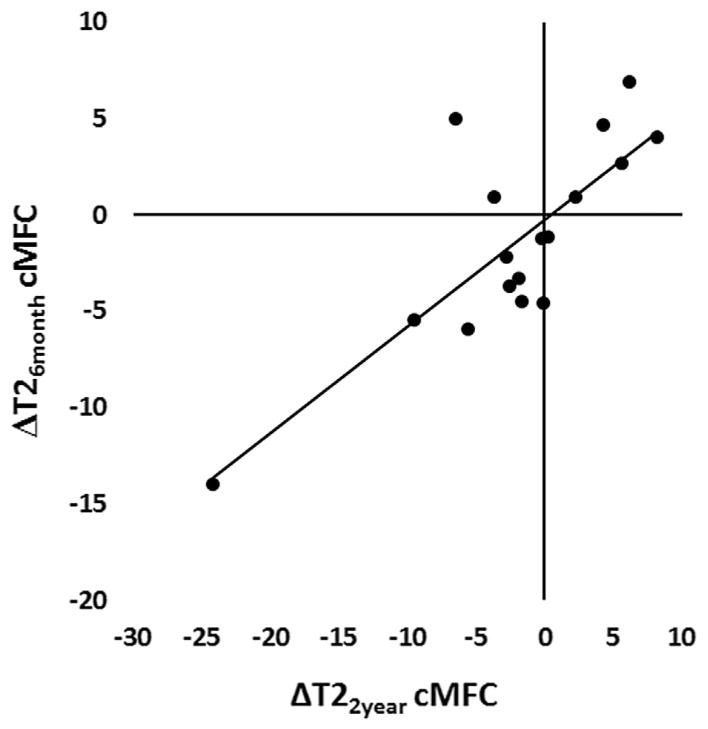
Change of mean T2 over 6 months in femoral cartilage (ΔT26-month cMFC) of ACLR subjects significantly correlated to change in mean cartilage T2 over 2 years (ΔT22-year cMFC) in the same region (n = 17, R = 0.80, p = 0.0001), Figure 2. The subject with MRI and arthroscopic evidence of a cMFC fibrocartilage lesion also demonstrated relatively large cMFC T2 changes (ΔT26-month = −14 ms, ΔT22-year = −24 ms). The correlation between mean ΔT26-month cMFC and mean ΔT22-year cMFC remains significant with exclusion of this outlier (n = 16, R = 0.62, p = 0.009). No correlation was detected for tibial cartilage between ΔT26-month cMTP and ΔT22-year cMTP mean in ACLR subjects (p > 0.06). Sample T2 maps at the three study time-points are shown in Figure 3.
Figure 2.
Short-term mean T2 change correlates to 2-year mean T2 change. Change of mean T2 over 6 months in cMFC cartilage demonstrated correlations with change in mean T2 over 2 years in the same region (n = 17; Pearson correlation R = 0.80; p = 0.0001). Solid line indicates linear fit. The correlation remains significant with exclusion of the outlier (n = 16, Pearson R = 0.62, p = 0.009).
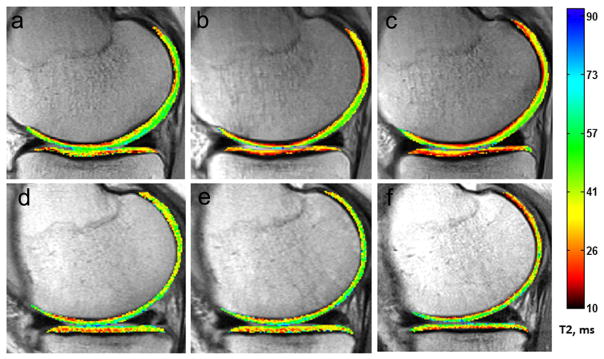
Figure 3.
Sample T2 maps acquired prior to (a,d), 6 months after (b,e), and 2 years after (c,f) ACL reconstruction surgery. Within a subject, change to mean T2 over 6 months correlates to change in mean T2 over 2 years. Top row: a subject demonstrating drops in mean central femoral and tibial T2 values between the pre-operative time-point and 6 months, and further decreases at 2 years post-ACL reconstruction. Bottom row: a subject demonstrating stable T2 values across all three time-points. Mean T2 ± std in cMFC cartilage at pre-SX, 6 months, and 2 years post-SX; (a–c) 38 ± 14 ms, 33 ± 20 ms, 29 ± 13 ms; (d–f) 46 ± 17 ms, 44 ± 17 ms, 46 ± 19 ms. Mean T2 ± std in cMTP cartilage at pre-SX, 6 months, and 2 years post-SX; (a–c) 39 ± 15 ms, 32 ± 16 ms, 30 ± 11 ms; (d–f) 36 ± 12 ms, 38 ± 15 ms, 37 ± 13 ms.
Cartilage Thickness Changes Over 2 Years
The mean cMFC cartilage thickness was 2.28 ± 0.40 ms at the pre-surgery time-point and was 2.36 ± 0.38 ms at 2 years following reconstruction (mean difference =+0.08 mm, range = −0.24 to 0.44 mm, 95%CI [−0.165 to 0.00793], p = 0.07). cMTP thickness did not change significantly over the same time period (p = 0.14).
Correlation of Cartilage qMRI Change Over 6 Months to Thickness Change Over 2 Years
Nine of 20 T2 texture features assessed in femoral cartilage of ACLR subjects demonstrated 6 month changes (Δ texture over 6 months) that correlated (p < 0.05) with 2-year cartilage thickness changes within the same region (Δ thickness over 2 years), Table 1. There was a negative correlation between 2-year thickness change and 6-month T2 texture feature “contrast” in cMFC cartilage in the direction parallel (at 1- and 5-pixel offsets, R = −0.56, −0.54; p = 0.020, 0.024) and perpendicular to the bone-cartilage interface (at a 3-pixel offset, R = −0.51, p = 0.036). “Energy” and “homogeneity” changes positively correlated to 2-year thickness change in the direction parallel to the bone-cartilage interface at all pixel offsets tested: “energy” 1-, 3-, 5-pixel offsets, R = 0.62, 0.49, 0.54; p < 0.008, 0.047, 0.026; “homogeneity” 1-, 3-, 5-pixel offsets, R = 0.72, 0.67, 0.48; p = 0.001, 0.003, 0.05). By contrast, no correlation was found between 6-month mean T2 value change and 2-year thickness change in the cMFC cartilage (p = 0.48). In tibial cartilage, changes to the assessed texture features and mean T2 value over 6 months did not correlate to 2-year cMTP thickness changes (p > 0.33).
Table 1.
Correlations Between 6 Month Change to T2 Texture and 2 Year Change to Thickness in ACLR Subjects’ Central Medial Femoral Cartilage
| Texture Feature | Direction | Pixel Offset | R | p-Value |
|---|---|---|---|---|
| Contrast | ↕ | 1 | −0.20 | 0.439 |
| ↕ | 3 | −0.51 | 0.036 | |
| ↔ | 1 | −0.56 | 0.020 | |
| ↔ | 3 | −0.47 | 0.055 | |
| ↔ | 5 | −0.54 | 0.024 | |
| Homogeneity | ↕ | 1 | 0.41 | 0.10 |
| ↕ | 3 | 0.31 | 0.224 | |
| ↔ | 1 | 0.72 | 0.001 | |
| ↔ | 3 | 0.67 | 0.003 | |
| ↔ | 5 | 0.48 | 0.050 | |
| Energy | ↕ | 1 | 0.27 | 0.295 |
| ↕ | 3 | −0.07 | 0.794 | |
| ↔ | 1 | 0.62 | 0.008 | |
| ↔ | 3 | 0.49 | 0.047 | |
| ↔ | 5 | 0.54 | 0.026 | |
| Correlation | ↕ | 1 | −0.08 | 0.751 |
| ↕ | 3 | −0.01 | 0.963 | |
| ↔ | 1 | 0.13 | 0.619 | |
| ↔ | 3 | −0.03 | 0.911 | |
| ↔ | 5 | 0.26 | 0.324 |
↕, ↔ Feature measured perpendicular, parallel to the bone cartilage interface. Bold indicates statistical significance.
Test-Retest T2 Texture Assessment
Cartilage T2 texture values were stable across test-retest examinations and across coils for all 20 features assessed in cMTP cartilage (p > 0.07) and 19/20 features assessed in cMFC cartilage (p > 0.05). In cMFC cartilage, the T2 texture feature “energy,” assessed in the direction parallel to the bone interface with 5-pixel offset, differed between test and retest scans (n = 5, F = 10.41, p = 0.03). However, the differences appeared specific to the quadrature coil since post-hoc pairwise examination of simple main effects showed a 28% difference between test-retest values of this feature when imaged by the quadrature coil (mean diff −0.019, 95%CI[0.002, 0.036], unadjusted p = 0.04), and no difference by the phased-array coil (p = 0.34).
Cartilage qMRI Cross-Sectional Comparison to Uninjured Controls
T2 texture and mean values of ACLR subjects measured at the pre-operative time-point were compared to texture and mean values of uninjured control subjects. A total of eight T2 texture features differed between ACLR subjects and uninjured controls: two features in femoral cartilage and six features in tibial cartilage (p < 0.044), Table 2. In femoral cartilage, the mean T2 value was 7% higher in ACLR subjects (39.4 ± 3.2 ms, n = 17) compared to controls (36.9 ± 5.2 ms, n = 11) at the preoperative timepoint (p = 0.01). In tibial cartilage, the preoperative mean T2 values appeared 7% higher in ACLR subjects (35.9 ± 3.7 ms, n = 17) compared to controls (33.4 ± 2.7 ms, n = 11), but the difference was not significant (p = 0.11).
Table 2.
T2 Map GLCM Texture Differences Between ACLR Subjects and Uninjured Controls
| ROI | Pre ACL-Reconstruction | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Texture Feature | Direction | Pixel Offset | ACLR Versus Controls | % Difference | p-Value | |
| cMFC | Contrast | ↔ | 3 | > | 36 | 0.036* |
| Correlation | ↕ | 3 | > | 28 | 0.042 | |
| cMTP | Correlation | ↕ | 1 | < | 59 | 0.011* |
| Correlation | ↔ | 3 | < | 35 | 0.009 | |
| Correlation | ↔ | 5 | < | 45 | 0.006 | |
| Homogeneity | ↕ | 3 | > | 17 | 0.034 | |
| Energy | ↕ | 1 | > | 81 | 0.030* | |
| Energy | ↔ | 1 | > | 38 | 0.043* | |
↕, ↔ Feature measured perpendicular, parallel to the bone cartilage interface.
Statistical significance assessed with non-parametric Mann Whitney U Tests. All other p values assessed with two-tailed t-tests.
T2 Metrics in ACLR Subjects Age 40-and-Under
Two of the 17 subjects enrolled in this study were older than 40 years (ages 49, 53 years). The remaining 15 subjects were 40 years or younger (mean age 28± 6 years) and similar in age to the uninjured controls (mean age 29± 4 years). Repeat examinations, excluding these two older individuals, reveals T2 mean and textural findings consistent with those of the full cohort. Specifically, in those 40 years of age or younger, mean ΔT26month cMFC significantly correlated to mean ΔT22year cMFC (n = 15, R = 0.78, p = 0.0006), and seven of 20 T2 texture features assessed in femoral cartilage demonstrated 6-month changes that correlated (p < 0.042) with 2-year cartilage thickness changes within the same region. Further, 9 of 20 texture metrics differed between uninjured controls and ACLR subjects 40-and-under: three in the cMFC and six in the cMTP, p < 0.048. Analyses of subjects aged 40 and under revealed that age did not correlate to ΔT26month in any texture metric or T2 mean in medial femoral or tibial cartilage (p > 0.05).
DISCUSSION
This study shows that early changes to medial compartment cartilage MRI T2 after ACLR in subjects with arthroscopically intact cartilage at the time of surgery correlate with later changes to both compositional and structural MRI. These findings support the hypothesis that compositional MRI may provide early warning on subclinical cartilage matrix changes reflective of longer-term trends in cartilage health. Of note, short-term (6-month) change to cartilage mean T2 correlated with longer-term (2-year) changes to mean T2. In addition, short-term (6-month) change to cartilage T2 texture variables were associated with longer-term (2-year) changes to cartilage thickness. Furthermore, textural and mean cartilage T2 differences were observed between baseline MRI of ACLR patients and uninjured control subjects suggesting that these changes reflect altered matrix structure in the ACLR subjects.
Quantitative evaluations of cartilage T2 texture changes may provide additional insight into cartilage matrix properties reflective of injury and degeneration. Given the previously observed complex and non-monotonic relationship between cartilage T2 time and disease severity assessed both histologically and arthroscopically,8,10,34 recent evidence shows that mean T2 value alone may not be adequate in determining cartilage health over time. The results of this study demonstrate both differences in textural metrics between ACLR and uninjured controls and an association between early changes to T2 texture metrics and later changes to cartilage thickness after ACLR.
Elevation of cartilage T2 “contrast,” like that observed here, has previously been noted in OA populations. Prior reports found medial femoral cartilage T2 “contrast” to be higher in patients with symptomatic or mild OA (KL 1,2) compared to controls,22,25 and to decrease over 2 years in subjects with moderate OA (KL 2,3).21 Previous longitudinal study of cartilage T2 “energy” and “homogeneity” in subjects with moderate radiographic OA (KL 2,3) also showed increases over 2 years of follow-up.21 In the current work, 6-month changes to femoral cartilage T2 “energy” and “homogeneity” positively correlated to 2-year change in cartilage thickness where subjects who demonstrated early increases to these texture features later exhibited an increase in cartilage thickness. Given that these ACLR subjects had arthroscopically intact articular cartilage at the time of reconstructive surgery, these intriguing findings potentially point to elevated cartilage T2 “energy” and “homogeneity” as markers of reduced matrix health leading to subsequent cartilage swelling consistent with early degeneration at 2 years after ACLR.
The elevated tibial cartilage T2 “energy” and “homogeneity” observed in ACLR subjects compared to uninjured controls at the pre-operative time-point further support the hypothesis that these metrics are sensitive to cartilage matrix changes following ACL injury. While T2 elevations to articular cartilage occur with age, Mosher et al. demonstrated that T2 values and profiles in subjects aged 31–45 years were not found to differ from those of younger subjects aged 18–30 years.13,35 The findings of textural differences between ACL-injured subjects 40-and-under (mean age 28 years) and comparably aged uninjured controls (mean age 29 years) further supports the interpretation that T2 texture is sensitive to subtle changes in cartilage matrix organization in ACL-injured knees.
The relatively small cohort with MRI at the three evaluation time points renders an analysis of etiological factors for the observed changes in T2 metrics such as surgical technique, concomitant injuries, or the health of the contralateral knee or other lower extremity joints outside the scope of this study. As these factors potentially affect the health of the index knee by altering mechanical loading, they likely contributed to variability between individuals in short and long-term changes to T2 metrics. The between person variability did, however, serve to facilitate the primary goal of evaluating whether within person short term changes correlated to longer term changes in these metrics.
This study has several limitations. Both T2 texture and thickness measures in this study were determined in the central weight-bearing medial femoral and tibial cartilage compartments. However, the analyses and segmentations for T2 map and for thickness metrics were performed using different techniques. Consequently, the amount and location of cartilage tissue assessed for the texture and thickness measurements in this study is not exactly the same. Cartilage thicknesses reported here reflect the averages over a strip-like region (approximately 1 cm wide) stretching across the central third of the subchondral bone area in the weight-bearing femur or the central 20% of the tibal region.32 T2 texture, by contrast, was measured from a single 3 mm section within the broader region assessed for thickness. While T2 texture and cartilage thickness measurements represent different amounts of cartilage tissue, they nevertheless reflect tissue properties in their overlapped regions.
As well, while previous studies of GLCM T2 texture measurements in knee cartilage show good reproduc-ibility,24,36 we did not perform test-retest examinations of T2 texture assessments in this ACLR cohort. However, test-retest evaluations of our T2 texture methods performed on T2 data from healthy volunteers obtained from the OAI showed that the measurement technique used is stable. Since T2 texture test-retest precision is likely dependent on SNR, imaging protocol, and magnet system, future larger studies of cartilage T2 texture should include test-retest as part of the imaging protocol development.
While limitations to this study additionally include ethical and feasibility barriers to obtaining confirmatory tissue biopsies purely for research purposes, the findings are consistent with expected early changes to articular cartilage following ACLR. Validation of short-term compositional MRI techniques, like cartilage T2 textural changes as indicators of cartilage health, is difficult considering that patients, especially those with surface intact articular cartilage at the time of ACLR, are unlikely to exhibit post-ACLR clinical OA for many years. However, that these metrics differed between ACLR subjects and uninjured controls and that increases to cartilage thickness, elevations of T2 mean, and changes to T2 texture features, have previously been associated with OA,14,21,37 lend credence to the potential utility of these metrics in assessment of OA risk.
CONCLUSION
The ability to identify signs of potentially reversible cartilage injury and degeneration prior to breakdown of the articular surface are key to development of OA prevention strategies through efficacious application of early treatment strategies to those with cartilage “at risk.” While articular cartilage has not shown a capacity to heal wounds compromising the articular surface, it may have ability to heal and restore subsurface matrix changes due to injury or degeneration. Of the compositional imaging techniques, cartilage T2 map is most widely available. The results of this study suggest that measurement of early longitudinal changes to cartilage T2 maps, including cartilage T2 texture metrics, may reflect cartilage matrix changes that are potentially useful for assessing OA risk in ACL-reconstructed knees. Longer follow-up of larger cohorts are needed to evaluate the predictive value of early and persistent T2 and T2 textural changes in the development of clinical OA.
Acknowledgments
Grant sponsor: R01 AR; Grant number: 052784; Grant sponsor: NIAMS; Grant numbers: NO1-AR-2-2261, N01-AR-2-2262, N01-AR-2-2258.
This study was funded by R01 AR 052784 (CR Chu). The authors contracted with Chondrometrics for cartilage morphometry data.
Footnotes
Conflicts of interest: None.
AUTHORS’ CONTRIBUTIONS
CRC conceived of the study and contributed human subjects. AW designed and performed quantitative image analysis. CRC and AW contributed to data collection, interpretation of study results, and drafted the manuscript. CSW reviewed study MRI and participated in interpretation of data and critical revision of the manuscript. CRC is responsible for the overall study.
References
- 1.Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33:621–636. doi: 10.1016/s0030-5898(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 2.Nelson F, Billinghurst RC, Pidoux I, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2006;14:114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potter HG, Jain SK, Ma Y, et al. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40:276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 5.Su F, Hilton JF, Nardo L, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2013;21:1058–1067. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barenius B, Ponzer S, Shalabi A, et al. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42:1049–1057. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 7.Williams A, Coyle C, Bruno S, et al. predicts T2 change over 6 and 12 months in subjects with early articular cartilage degeneration. 57th Annual Meeting of the Orthopaedic Research Society; Long Beach, CA. 2011. Oct, p. 96. [Google Scholar]
- 8.Chu CR, Williams A, Tolliver D, et al. Clinical optical coherence tomography of early articular cartilage degeneration in patients with degenerative meniscal tears. Arthritis Rheum. 2010;62:1412–1420. doi: 10.1002/art.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price JS, Till SH, Bickerstaff DR, et al. Degradation of cartilage type II collagen precedes the onset of osteoarthritis following anterior cruciate ligament rupture. Arthritis Rheum. 1999;42:2390–2398. doi: 10.1002/1529-0131(199911)42:11<2390::AID-ANR18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.David-Vaudey E, Ghosh S, Ries M, et al. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin DW, Wadghiri YZ, Zhu H, et al. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182:311–318. doi: 10.2214/ajr.182.2.1820311. [DOI] [PubMed] [Google Scholar]
- 12.Liess C, Lusse S, Karger N, et al. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 13.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2-preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 14.Dunn TC, Lu Y, Jin H, et al. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2007;15:1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Stahl R, Luke A, Li X, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients-a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2-initial experience with 1-year follow-up. Radiology. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE SMC. 1973;3:610–621. [Google Scholar]
- 19.Baum T, Joseph GB, Nardo L, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res. 2013;65:23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenkrantz G, Stahl R, Carballido-Gamio J, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2008;16:584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carballido-Gamio J, Joseph GB, Lynch JA, et al. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65:1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 22.Carballido-Gamio J, Stahl R, Blumenkrantz G, et al. Spatial analysis of magnetic resonance T[sub 1ρ] and T[sub 2] relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36:4059. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph GB, Baum T, Alizai H, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years-data from the Osteoarthritis Initiative. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2012;20:727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph GB, Baum T, Carballido-Gamio J, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls-data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Pai A, Blumenkrantz G, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osteoarthritis Initiative. National Institutes of Health; www.oai.ucsf.edu. [Google Scholar]
- 27.Dardzinski BJ, Schneider E. Radiofrequency (RF) coil impacts the value and reproducibility of cartilage spin-spin (T2) relaxation time measurements. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2013;21:710–720. doi: 10.1016/j.joca.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier CF, Tan SG, Hariharan H, et al. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17:358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Tao H, Hua Y, et al. Quantitative magnetic resonance imaging assessment of cartilage status: a comparison between young men with and without anterior cruciate ligament reconstruction. Arthroscopy. 2013;29:2012–2019. doi: 10.1016/j.arthro.2013.09.075. [DOI] [PubMed] [Google Scholar]
- 31.Eckstein F, Hudelmaier M, Wirth W, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirth W, Hellio Le Graverand MP, Wyman BT, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckstein F, Kunz M, Hudelmaier M, et al. Impact of coil design on the contrast-to-noise ratio, precision, and consistency of quantitative cartilage morphometry at 3 Tesla: a pilot study for the osteoarthritis initiative. Magn Reson Med. 2007;57:448–454. doi: 10.1002/mrm.21146. [DOI] [PubMed] [Google Scholar]
- 34.Jungmann PM, Kraus M, Nardo L, et al. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: longitudinal data from the osteoarthritis initiative. J Magn Reson Med. 2013;38:1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosher TJ, Liu Y, Yang QX, et al. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004;50:2820–2828. doi: 10.1002/art.20473. [DOI] [PubMed] [Google Scholar]
- 36.Carballido-Gamio J, Link TM, Majumdar S. New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intra- and inter-subject comparisons. Magn Reson Med. 2008;59:1472–1477. doi: 10.1002/mrm.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstein F, Mc Culloch CE, Lynch JA, et al. How do short-term rates of femorotibial cartilage change compare to long-term changes? Four year follow-up data from the osteoarthritis initiative. Osteoarthritis Cartilage/OARS, Osteoarthritis Research Society. 2012;20:1250–1257. doi: 10.1016/j.joca.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]