Abstract
Psychiatric comorbidities are common in people living with HIV (PLWH) and adversely affect life satisfaction, treatment adherence and disease progression. There are few data to inform the burden of psychiatric symptoms in older PLWH, a rapidly growing demographic in the U.S. We performed a cross-sectional analysis to understand the degree to which symptom burden was associated with cognitive disorders in PLWH over age 60. Participants completed a standardized neuropsychological battery and were assigned cognitive diagnoses using Frascati criteria. We captured psychiatric symptom burden using the Geriatric Depression Scale (GDS) and proxy-informed Neuropsychiatric Inventory-Questionnaire (NPI-Q). Those diagnosed with HIV-associated Neurocognitive Disorders (HAND, n = 39) were similar to those without HAND (n = 35) by age (median = 67 years for each group, p = 0.696), education (mean = 16 years vs. 17 years, p = 0.096), CD4+ T-lymphocyte counts (mean = 520 vs. 579, p = 0.240), duration of HIV (median = 21 years for each group, p = 0.911) and sex (92% male in HAND vs. 97% in non-HAND, p = 0.617). Our findings showed similarities in HAND and non-HAND groups on both NPI-Q (items and clusters) and GDS scores. However, there was a greater overall symptom burden in HIV compared to healthy elder controls (n = 236, p < 0.05), with more frequent agitation, depression, anxiety, apathy, irritability and nighttime behavior disturbances (p < 0.05). Our findings demonstrate no differences in psychiatric comorbidity by HAND status in older HIV participants; but confirm a substantial neurobehavioral burden in this older HIV-infected population.
Keywords: HIV, aging, mental health, behavioral disorders, cognition disorders
Introduction
Cognitive impairment is a frequent complication for people living with HIV (PLWH) in the U.S. Research completed at five academic centers among community dwelling patients with access to combination antiretroviral therapy (cART) identified a frequency of about 50%; although many individuals in this study had not achieved suppression of plasma HIV RNA (Heaton et al., 2010). Despite viro-immunological efficacy of cART, milder forms of HIV-associated neurocognitive disorders (HAND) still persist (Heaton et al., 2011; Clifford, 2008). The frequency of cognitive symptoms is substantially higher among older PLWH, a population that is rapidly growing due to widespread use of cART (Fazeli, 2015; Justice et al., 2010; Valcour et al., 2004).
Mental health comorbidities are common in HIV and mood symptoms such as depression and apathy have been associated with adverse functional outcomes including medication non-adherence, difficulties with everyday activities and poorer quality of life (Barclay et al., 2007; Kamat et al., 2013; Springer, 2012; Tate et al., 2003). Few studies have examined this psychiatric comorbidity associated with HAND in older PLWH, an emerging demographic at risk for age-associated comorbidity and social isolation (Gerst-Emerson & Jayawardhana, 2015). Based on a large nationally representative sample of HIV-infected persons receiving care in the U.S. (59% aged 45 or older), the prevalence of current major depression is over three times that seen in the general population. The increased frequency of depression remains present even when accounting for differences in demographic factors (Do et al., 2014).
Neuropsychological impairment in the setting of HIV is known to include cognitive, motor and behavioral attributes; thus, it is likely that individuals with HAND in the late cART era have the highest psychiatric symptom burden. This has not previously been evaluated and, it is possible that a high burden is noted in old age even among those without HAND. Some evidence suggests that depressive symptoms may be associated with neuropsychological testing in younger (20–39 years of age), but not in older (50 years of age or older) HIV-infected individuals (Shimizu et al., 2011). A more clear relationship between apathy and cognitive performance has reported on measures of learning efficiency and cognitive flexibility in a sample of 45 HIV-infected adults (mean age of 43 years) (Paul et al., 2005).
Recent work exploring the neuropsychiatric disturbances among older individuals without HIV infection notes a higher burden in those with cognitive impairment. Depression was the most common individual symptom in those with normal cognition (12%), cognitive impairment without dementia (30%) and mild dementia (25%), while apathy (42%), agitation (41%), and aberrant motor behaviors (31%) were the most common in those with severe dementia. A greater number of neuropsychiatric symptoms was associated with functional limitations in impaired and dementia groups (Okura et al., 2010). Our study was designed to capture neuropsychiatric symptom burden in older PLWH to test whether frequency was higher in participants with HAND. We also compare frequency of neuropsychiatric symptoms in HIV compared to historical controls.
Methods
Participants
We recruited HIV-infected participants (n = 74) in a parent study focused on cognition in HIV-infected individuals aged 60 years and above (UCSF HIV Over 60 Cohort). Most were recruited through community fliers and physician referrals as previously described (Nir et al., 2013). Participants were required to provide contact information of a spouse, close friend or family member who could provide proxy information on the Neuropsychiatric Inventory-Questionnaire (NPI-Q). Participants with conditions that would significantly impact cognition aside from HIV were excluded; however, individuals were not selected or excluded solely due to cognitive symptoms (Chiao et al., 2013). All participants completed a 90-minute standardized, neuropsychological testing battery assessing multiple cognitive domains (see Supplemental Table 1). Proxy informant interviews were conducted using the Clinical Dementia Rating (CDR) scale as a guide to provide a global rating of functional capabilities (Morris, 1997).
HAND diagnoses were assigned in a group consensus conference with neurologists and neuropsychologists using clinical acumen informed by the Frascati criteria (Antinori et al., 2007). We measured CD4+ T-lymphocyte counts and plasma HIV viral load if current labs (+/− 3 months) were unavailable. For 13 cases that did not complete lab tests, we used the most recent self-reported results for CD4+ t-lymphocyte count and plasma HIV RNA.
Historical control data
As added value, we plotted the neuropsychiatric symptom burden against historical controls in our center with the goal of providing a sense of the symptom magnitude compared to healthy age-similar community dwelling individuals in the same catchment area of San Francisco. These healthy older (62–79 years old) uninfected participants had enrolled in a healthy aging study at the Memory and Aging Center, UCSF. All had normal performance on neuropsychological tests and were determined to have normal cognition in a consensus conference. They all reported normal everyday functioning, and denied cognitive symptoms. The study was approved by the Institutional Review Board of UCSF.
Measures
The NPI-Q is a brief and well-validated version of the Neuropsychiatric Inventory that assesses 12 psychiatric symptoms common to dementia: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, motor disturbance, changes in appetite/eating, and sleep/nighttime difficulties (Cummings JL, 1994; Kaufer et al., 2000). It is a structured interview of a caregiver, close relative, or friend of the patient who can provide detailed knowledge of the patient’s symptoms. Responses are coded as “yes” or “no” to indicate the presence of symptoms over the past month, and if present, symptom severity is rated from 1 to 3, with a total instrument score between 0 and 36. Higher scores indicate a higher symptom burden. In the current study the total NPI-Q score was defined as the sum of individual symptom scores, ranging therefore from 0 to 12. Adequate internal consistency was observed (Cronbach’s alpha = 0.70) in our sample.
The NPI-Q items were also explored as the following clusters: mood (euphoria, depression, apathy, and appetite); restlessness (aberrant motor activity, nighttime behavior, anxiety and disinhibition); agitation (agitation/aggression and irritability); and psychosis (delusions and hallucinations). These clusters were previously used among older patients with cognitive impairment (Azermai et al., 2013).
The 30-item Geriatric Depression Scale (GDS) is a widely used self-report screen for depression in older populations. It minimizes assessment of somatic symptoms common in depression and frequently noted by psychologically healthy elders suffering from acute or chronic illnesses (e.g. fatigue). The total score ranges from 0 to 30 with the following recommended cutoffs: normal = 0–9, mild depression = 10–19, and severe depression = 20–30 (Yesavage et al., 1982). Symptoms are endorsed as either “yes” or “no.” Good internal consistency was observed in our sample (Cronbach’s alpha = 0.89).
Statistical analyses
The sample was divided into two groups based on meeting criteria for HAND (n = 39) or not (non-HAND, n = 35). For descriptive statistics, we used independent samples t-test to assess differences in age, education, current CD4 cell count, CD4 nadir, and duration of HIV. Chi-square was used to examine differences in HIV viral load, route of HIV infection, and cART adherence, whereas Fisher’s Exact test was utilized to examine gender and ethnicity.
We performed independent samples t-test to explore differences in total NPI-Q and GDS scores, and NPI-Q clusters, whereas NPI-Q symptom frequencies were analyzed by Chi-square. Analyses for the NPI-Q clusters were conducted using the total mean of symptom frequencies within their corresponding cluster between the groups. We used a similar approach to describe differences by serostatus compared to historical controls. The level of significance was set at p < 0.05 for all analyses. All analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Population characteristics
There were no statistically significant differences in clinical or demographic variables between the groups (Table 1). In the HAND group, 16 participants (41%) met criteria for ANI, 23 (59%) met criteria for MND and no cases met criteria for dementia. At the time of study entry, all HIV-infected participants reported being free of current substance abuse. The HAND vs. non-HAND groups did not differ in any of the clinical factors that might be associated with increased psychiatric burden such as psychoactive medications, ART regimen, body mass index, and past substance abuse (Table 1). Compared to our historical controls, the HIV participants were somewhat younger (mean = 67 vs. 72, p < 0.001), less educated (mean = 16 vs. 17, p = 0.008), and more likely male (% of male = 95% vs. 39%, p < 0.001). In our sample, 34% of informants for HIV-infected participants were spouse/partners compared to 66% for controls, while 51% of informants for the HIV subjects were friends versus 19% for controls.
Table 1.
Demographic and clinical characteristics by group
| HAND | non-HAND | Historical Controls |
P-value† HAND vs. non-HAND |
P-value† HIV vs. Controls |
|
|---|---|---|---|---|---|
| N | 39 | 35 | 236 | ||
| Age, median (range) | 67 (61–83) | 67 (60–80) | 72 (62–79) | 0.696 | <0.001 |
| Gender (% male) | 92% | 97% | 39% | 0.617 | <0.001 |
| Education, mean years (SD) | 15.7 (2.3) | 16.7 (2.2) | 17.1 (2.7) | 0.096 | 0.008 |
| Ethnicity, Caucasian (%) | 87% | 94% | 92% | 0.709 | 0.884 |
| MSM only (%) | 80% | 83% | - | 0.821 | - |
| BMI, mean (SD) | 25.8 (5.4) | 25.9 (5.1) | 0.925 | - | |
| CD4 count, mean (SD) | 520 (202) | 579 (227) | - | 0.240 | - |
| Nadir CD4, mean (SD) | 194 (160) | 224 (147) | - | 0.401 | - |
| HIV duration, median (range) | 21 (3–31) | 21 (6–28) | - | 0.911 | - |
| Duration of ART, mean years (SD) | 12.2 (6.4) | 13.7 (3.8) | - | 0.228 | - |
| Undetectable viral load* (%) | 64% | 86% | - | 0.060 | - |
| Past substance abuse (%) | 16% | 17% | - | 0.872 | - |
| On ART (%) | 92% | 100% | - | 0.242 | - |
| ART regimen | |||||
| i. NRTI (%) | 90% | 97% | - | 0.242 | - |
| ii. PI (%) | 64% | 56% | - | 0.475 | - |
| Other medications | - | ||||
| i. Antidepressant medications (%) | 44% | 34% | - | 0.479 | - |
| ii. Psychoactive medications (%) | 46% | 29% | - | 0.152 | - |
| iii. Anxiety medications (%) | 21% | 17% | - | 0.773 | - |
| HAND diagnosis | |||||
| • Cognitively normal (%) | - | 100% | - | - | - |
| • ANI (%) | 41% | - | - | - | - |
| • MND (%) | 59% | - | - | - | - |
Level of significance set at p < 0.05
less than 75 copies/ml
Abbreviations: HAND: HIV-Associated Neurocognitive Disorders; MSM: Men who have Sex with other Men; BMI: Body Mass Index; ART: Antiretroviral Therapy; NRTI: Nucleoside reverse Transcriptase Inhibitors; PI: Protease Inhibitor; ANI: Asymptomatic Neurocognitive Disorder; MND: Mild Neurocognitive Disorder
NPI-Q single items and clusters across groups
The HAND and non-HAND groups did not significantly differ on the total NPI-Q score (mean (SD) = 2.64 (± 2.38) vs. 2.14 (± 1.82), respectively; p = 0.319) or on any of the NPI-Q items (p > 0.05; Table 2). When compared to healthy controls, the NPI-Q symptoms’ frequencies were higher for both HIV groups regardless of the presence of HAND (p < 0.05). Specifically, among the NPI-Q items, agitation, depression, anxiety, apathy, irritability and nighttime behavior disturbances were the most frequent in both HIV groups compared to historical controls (p < 0.05; see Supplemental Table 2).
Table 2.
Frequency of Neuropsychiatric Inventory (NPI-Q) items across groups
| HAND | non-HAND | P-value† | |
|---|---|---|---|
| Delusions | 5% | 3% | 1.00 |
| Hallucinations | 0 | 0 | -- |
| Agitation | 36% | 51% | 0.241 |
| Depression | 39% | 26% | 0.321 |
| Anxiety | 33% | 17% | 0.182 |
| Elation | 10% | 3% | 0.361 |
| Apathy | 26% | 17% | 0.411 |
| Disinhibition | 15% | 6% | 0.267 |
| Irritability | 41% | 37% | 0.814 |
| Motor disturbance | 3% | 6% | 0.600 |
| Nighttime behaviour | 31% | 31% | 1.00 |
| Appetite | 26% | 17% | 0.411 |
Level of significance set at p<0.05
Abbreviation: HAND: HIV-Associated Neurocognitive Disorders
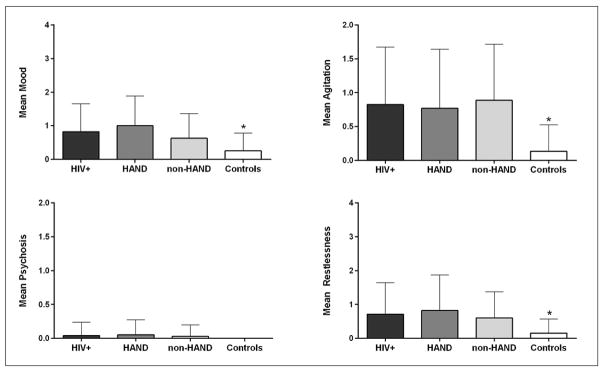
We did not find statistically significant differences between HAND vs. non-HAND in any of the NPI-Q clusters (Figure 1). Taken all together, the HIV-infected participants had higher mean scores compared to historical controls (p<0.001) on all of the NPI-Q domains.
Figure 1.
Neuropsychiatry Inventory (NPI-Q) clusters by group
Figure 1 shows means and standard deviations on each NPI-Q cluster by group. On the psychosis cluster, only few HIV-infected participants and no one historical control showed psychotic symptoms. Notes: NPI-Q clusters: mood (euphoria, depression, apathy, and appetite); agitation (agitation/aggression and irritability); psychosis (delusions and hallucinations); and restlessness (aberrant motor activity, nighttime behavior, anxiety and disinhibition). Abbreviation: HAND: HIV-Associated Neurocognitive Disorders * Significant difference between HIV-positive participants - regardless of the presence of cognitive impairment - and controls (p < 0.001)
GDS across groups
The mean GDS total score did not differ between HAND and non-HAND groups (mean (SD) = 9.72 (± 6.70) vs. 6.88 (± 6.12), respectively p = 0.064). The frequency of total scores at or above the standard GDS clinical cutoff of 10 (range = 10–30) varied by group, with 46% of the HAND group having mild-to-severe depressive symptoms compared to 30% for the non-HAND group (Figure 2). HIV-infected participants endorsed a higher number of depressive symptoms compared to historical controls (mean = 2.67 (± 2.85); p < 0.001), of which 3% endorsed mild-to severe depressive symptoms.
Figure 2.
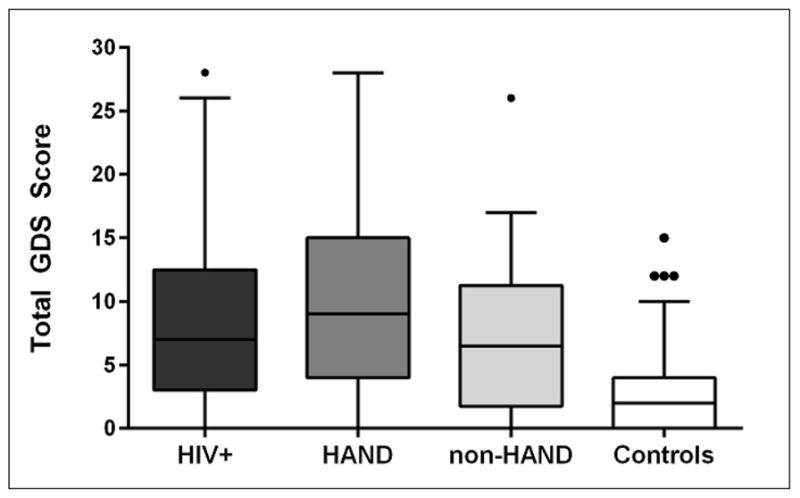
Total score on the Geriatric Depression Scale (GDS) by group
Figure 2 shows the median value of the GDS total score and the 25th percentile and 75th percentile for each group, with the median represented by a horizontal line and percentiles by the bars. A significant difference was found between the overall HIV group and controls (model adjusted for age, gender and education; p < 0.001), but not between HAND vs. non-HAND (p > 0.05). Abbreviations: GDS: Geriatric Depression Scale; HAND: HIV-Associated Neurocognitive Disorders
Discussion
The primary aim of this study was to determine if the neuropsychiatric symptom burden was greater in PLWH who had cognitive impairment compared to those without. Our findings demonstrate similarity in HAND and non-HAND groups on either NPI-Q items or clusters; yet, we find a greater overall symptom burden associated with HIV compared to historical healthy elder control participants from our center. Proxy reports on the NPI-Q from both HIV groups identified greater frequency of agitation, anxiety, apathy, irritability, nighttime behavior disturbances, and depression in PLWH. This unique finding may be driven by a higher baseline high frequency of neuropsychiatric symptoms in older PLWH, thus limiting our ability to identify an augmentation of these symptoms in the setting of HAND. Similarly, depressive symptoms differed by HIV status; yet, the burden was similar across the HIV groups.
The relationship between psychiatric symptoms and cognitive impairment in geriatric HIV-infected patients in the cART era remains controversial. While some studies suggest that depressive symptoms do not affect neuropsychological testing performance, others found an association between subtypes of depressive symptoms and cognitive deficits (Bornstein et al., 1993; Castellon et al., 2006; Cole et al., 2007; Cysique et al., 2007; Rourke, Halman, & Bassel, 1999). Our findings provided further evidence that neuropsychiatric symptoms were not associated with cognitive functioning in older PLWH. As elsewhere proposed, patients with cognitive impairment may lack sufficient insight into their symptoms, impacting their ability to report symptoms accurately (Chiao et al., 2013). In the current study we used a self-completed and a proxy-report scale to assess neuropsychiatric burden in our sample, lessening the impact of potential lack of insight of cognitively impaired subjects. However, although psychiatric symptom screening may be facilitated by access to proxy informants, our data suggest that this may be less feasible in typical HIV clinical settings since proxy informants were required for participation, but often became unavailable at the time of testing (Murray et al., 2016).
The frequency and severity of neuropsychiatric symptoms were significantly higher in HIV compared to healthy elders: a result that is even more worrisome given that elders with HIV are less likely to receive needed mental health care compared to their younger counterparts (28% and 40%, respectively) (Zanjani, Saboe, & Oslin, 2007). Regular psychiatric symptom screening is pertinent. This may be most effective if a caregiver or proxy is engaged.
Among all the etiological hypotheses for late-life depression, the vascular depression hypothesis postulated that white matter (WM) abnormalities might determine depressive symptoms due to the disruption of frontostriatal circuits (Herrmann, Le Masurier, & Ebmeier, 2008; Vu & Aizenstein, 2013). In the context of aging with HIV, comprehending the etiological mechanisms underlying depressive symptomatology is even more challenging due to the greater risk for cardiovascular disease and medical comorbidities associated with longer life expectancy in HIV-infected individuals (Greene, Justice, Lampiris, & Valcour, 2013). WM changes have been associated with medical and demographic variables such as hepatitis C, cART treatment, race, cardiovascular factors and advancing age in HIV-infected patients (Haddow et al., 2014; Morgello, Murray, Van Der Elst, & Byrd, 2014; Soontornniyomkij et al., 2014). Further investigation of the multifactorial nature of neuropsychiatric burden in older PLWH might be of outstanding value to optimize the care of these patients.
Our study is strengthened by use of validated instruments commonly employed in studies of elders with cognitive impairment. The GDS is intended to maximize sensitivity and thus, over-estimates symptom burden depression compared to a standard structured diagnostic interview. Our work is also strengthened by use of expert consensus panel at a specialty memory disorders clinic for the diagnosis of HAND. We dichotomized outcomes to the presence or absence of symptoms to calculate the NPI-Q total score, in an attempt to minimize the risk of over- or under-reporting symptoms. This issue was recently explored using the full NPI in dementia; although both scoring methods are comparable and adequately sensitive to treatment-related changes over time, this has not been examined in HIV (Cummings, Ihl, Herrschaft, Hoerr, & Tribanek, 2013).
The HIV-infected groups had fewer cases where informants were available to complete the NPI-Q, which may speak to the unique social network of this population, but also limits our study and potentially provides a bias in reporting. Although our historical controls were more commonly women, our results remained similar in sensitivity analyses including only male controls. Future studies on sex-matched samples might be valuable in confirming our findings. External validity of our findings is a limitation, since our sample was well educated, mostly Caucasian, and from one geographic region. Psychiatric diagnoses of participants were unavailable, thus the study relied on self- and proxy report of symptoms. Lastly, we acknowledge that our study might have some limitations due to the cross-sectional design and the small sample size.
In sum, our study evidenced a substantial neurobehavioral burden in a sample of HIV-infected participants aged 60 and older, regardless of cognitive status, compared to historical healthy controls. Elucidating the impact of neuropsychiatric symptoms on everyday functioning may help inform interventions aimed at reducing the burden for patients, caregivers and healthcare systems.
Supplementary Material
Acknowledgments
We wish to thank the study participants. This study was supported by the following grants from the National Institutes of Health: K24-MH-098759 (VV), P50-AG-023501 (ADRC, PI: Bruce Miller); P30-AI-027763 (UCSF/GIVI Center for AIDS Research), and the UL1-RR-024131 (UCSF GCRC), with additional support from the Larry L. Hillblom Foundation and the UCSF AIDS Research Institute.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, … Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azermai M, Petrovic M, Engelborghs S, Elseviers MM, Van der Mussele S, Debruyne H, … Vander Stichele RH. The effects of abrupt antipsychotic discontinuation in cognitively impaired older persons: a pilot study. Aging & Mental Health. 2013;17(1):125–132. doi: 10.1080/13607863.2012.717255. [DOI] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, … Durvasula RS. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26(1):40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein RA, Pace P, Rosenberger P, Nasrallah HA, Para MF, Whitacre CC, Fass RJ. Depression and neuropsychological performance in asymptomatic HIV infection. The American Journal of Psychiatry. 1993;150(6):922–927. doi: 10.1176/ajp.150.6.922. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, … Moore L. Components of depression in HIV-1 infection: their differential relationship to neurocognitive performance. Journal of the Clinical and Experimental Neuropsychology. 2006;28(3):420–437. doi: 10.1080/13803390590935444. VP21110U5681W123 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, … Valcour V. Deficits in Self-Awareness Impact the Diagnosis of Asymptomatic Neurocognitive Impairment in HIV. AIDS Research Human Retroviruses. 2013;29(6):949–956. doi: 10.1089/AID.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Topics in HIV medicine. 2008;16(2):94–98. [PubMed] [Google Scholar]
- Cole MA, Castellon SA, Perkins AC, Ureno OS, Robinet MB, Reinhard MJ, … Hinkin CH. Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. Journal of the International Neuropsychological Society. 2007;13(3):549–554. doi: 10.1017/S135561770707066X. S135561770707066X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Ihl R, Herrschaft H, Hoerr R, Tribanek M. Sensitivity to change of composite and frequency scores of the Neuropsychiatric Inventory in mild to moderate dementia. International Psychogeriatrics. 2013;25(3):431–438. doi: 10.1017/S1041610212001974S1041610212001974. [pii] [DOI] [PubMed] [Google Scholar]
- Cummings JLMM, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L, … Heaton RK. Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychology Society. 2007;13(1):1–11. doi: 10.1017/S1355617707070026. S1355617707070026 [pii] [DOI] [PubMed] [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, … Skarbinski J. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One. 2014;9(3):e92842. doi: 10.1371/journal.pone.0092842PONE-D-13-31444. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Crowe M, Ross LA, Wadley V, Ball K, Vance DE. Cognitive Functioning in Adults Aging with HIV: A Cross-Sectional Analysis of Cognitive Subtypes and Influential Factors. Journal of Clinical Research in HIV AIDS and Prevention. 2015;18:155–169. doi: 10.14302/issn.2324-7339.jcrhap-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst-Emerson K, Jayawardhana J. Loneliness as a public health issue: the impact of loneliness on health care utilization among older adults. American Journal of Public Health. 2015;105(5):1013–1019. doi: 10.2105/AJPH.2014.302427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. [Case Reports Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Journal of the American Medical Association. 2013;309(13):1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow LJ, Dudau C, Chandrashekar H, Cartledge JD, Hyare H, Miller RF, Jager HR. Cross-sectional study of unexplained white matter lesions in HIV positive individuals undergoing brain magnetic resonance imaging. AIDS Patient Care STDS. 2014;28(7):341–349. doi: 10.1089/apc.2013.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, … Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S … HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(6):619–624. doi: 10.1136/jnnp.2007.124651. jnnp.2007.124651 [pii] [DOI] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, … Bryant KJ. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Medicine. 2010;11(2):143–151. doi: 10.1111/j.1468-1293.2009.00757.x. HIV757 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Morgan E, Marcotte TD, Badiee J, Maich I, Cherner M, … Ellis R. Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. Journal of Affective Disorders. 2013;150(3):1069–1075. doi: 10.1016/j.jad.2012.11.040S0165-0327(12)00807-5. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, … DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry & Clinical Neurosciences. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Morgello S, Murray J, Van Der Elst S, Byrd D. HCV, but not HIV, is a risk factor for cerebral small vessel disease. Neuroimmunology and Neuroinflammation. 2014;1(3):e27. doi: 10.1212/NXI.0000000000000027NEURIMMINFL2014002113. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177–178. [DOI] [PubMed] [Google Scholar]
- Murray KJ, Cummins D, Batterham M, Trotter G, Healey L, O’Connor CC. Does the informal caregiver notice HIV associated mild cognitive impairment in people living with HIV? AIDS Care. 2016;28(2):221–227. doi: 10.1080/09540121.2015.1084989. [DOI] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, Valcour VG. Mapping white matter integrity in elderly people with HIV. Human Brain Mapping. 2013 doi: 10.1002/hbm.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. Journal of the American Geriatrics Society. 2010;58(2):330–337. doi: 10.1111/j.1532-5415.2009.02680.xJGS2680. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Navia B, Hinkin C, Malloy PF, Jefferson AL, … Flanigan TP. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. Journal of Neuropsychiatry & Clinical Neurosciences. 2005;17(2):167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Halman MH, Bassel C. Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology. 1999;21(6):737–756. doi: 10.1076/jcen.21.6.737.863. [DOI] [PubMed] [Google Scholar]
- Shimizu SM, Chow DC, Valcour V, Masaki K, Nakamoto B, Kallianpur KJ, Shikuma C. The Impact of Depressive Symptoms on Neuropsychological Performance Tests in HIV-Infected Individuals: A Study of the Hawaii Aging with HIV Cohort. World Journal of AIDS. 2011;1(4):139–145. doi: 10.4236/wja.2011.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, … Achim CL. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014 doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS and Behavior. 2012;16:2119–2143. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate D, Paul RH, Flanigan TP, Tashima K, Nash J, Adair C, … Cohen RA. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care and STDS. 2003;17(3):115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, … Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu NQ, Aizenstein HJ. Depression in the elderly: brain correlates, neuropsychological findings, and role of vascular lesion load. Current Opinion in Neurology. 2013;26(6):656–661. doi: 10.1097/WCO.0000000000000028. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zanjani F, Saboe K, Oslin D. Age difference in rates of mental health/substance abuse and behavioral care in HIV-positive adults. AIDS Patient Care and STDS. 2007;21(5):347–355. doi: 10.1089/apc.2006.0043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.