Abstract
We examined whether cognitive control moderates the effects of emotion on adolescent internalizing and externalizing symptomatology in a longitudinal study of 138 adolescents. Self-reported positive affect (PA) and negative affect (NA) and behavioral and neural indicators of cognitive control, indexed by performance and prefrontal hemodynamic response during a cognitive interference task, were collected at Time 1. Self-reported internalizing and externalizing symptomatology were collected at Time 1 and Time 2 (one year later). Results indicated that higher PA predicted decreases in externalizing symptomatology, but only for adolescents with poor neural cognitive control. No moderation effects were found for behavioral cognitive control. Findings imply the beneficial effects of PA on the development of externalizing problems among adolescents with poor prefrontal functioning.
Keywords: affect, cognitive control, psychopathology, prefrontal functioning
The social, biological, and intellectual changes associated with adolescence leave individuals at increased risk for maladjustment and psychopathology during this developmental period (Avenevoli, Swendsen, He, Burstein, & Merikangas, 2015; Cicchetti & Rogosch, 2002; Salk, Petersen, Abramson, & Hyde, 2016). In this way, adolescence is a sensitive period for emotional development characterized by heightened emotionality (Maciejewski, Lier, Branie, Meeus, & Koot, 2015) and the critical development of self-regulatory processes with the maturation of the prefrontal cortex (PFC) (Powers & Casey, 2015). These emotional changes likely contribute to adolescents’ adjustment outcomes. Specifically, it has been well established that negative affect (NA) is associated with internalizing and externalizing symptomatology in childhood and adolescence (Hanish et al., 2004; Kim, Walden, Harris, Karrass, & Catron, 2007; Silk, Steinberg, & Morris, 2003). However, considerably less attention has been brought to the role of positive affect (PA) in the development of internalizing and externalizing symptomatology (Gilbert, 2012). Preliminary evidence suggests that PA serves as a protective factor against maladaptation (Davis & Suveg, 2014), yet the role of PA (independent of NA) has not been systematically examined during the critical period of adolescence when emotional states are especially salient. Furthermore, continued growth and change in prefrontal functioning during adolescence (e.g., Ordaz, Velanova, & Luna, 2013) may influence how emotion affects psychopathology outcomes. Thus, it remains necessary to identify the nature of the relationship between affect and internalizing and externalizing symptomatology during adolescence and elucidate how neural and behavioral indicators of self-regulation may attenuate or enhance these effects.
PA and NA are distinct, orthogonal dimensions of affective structure (Watson, Clark, & Tellegen, 1988). For the purposes of the current study, affect and emotion are conceptually analogous and thus are used interchangeably. In our review of the literature we refer to the original terminology used by the authors. NA encompasses general feelings of distress and displeasure such as irritability, hostility, and nervousness. Low NA is characterized by the absence of these feelings. NA is well established in the literature as a risk factor for a host of psychological and adjustment problems, including externalizing symptomatology. In childhood, available findings indicate that children exhibiting higher NA, especially anger, are susceptible to higher levels of externalizing symptomatology than their peers (Kim et al., 2007). In addition, feelings of anger among children are related to the development of internalizing symptomatology (Eisenberg et al., 2009). Although this association has been less thoroughly examined in adolescence as compared to childhood, past research indicates that negative emotions, particularly anger, are associated with increases in adolescents’ aggressive behaviors (Neumann, van Lier, Frijns, Meeus, & Koot, 2011) and higher anger intensity is predictive of depressive symptoms as well as problem behaviors among adolescents (Silk et al., 2003). Similarly, higher hostility has been linked to delinquency among male college students (Langhinrichsen-Rohling, Arata, Bowers, O’Brien, & Morgan, 2004).
PA is best understood as liveliness and pleasurable engagement with one’s environment, with low PA reflecting lethargy or sadness (Watson et al., 1988). The effect of PA on child and adolescent outcomes (especially externalizing problems) is not as well understood as the effect of NA; however, there is reason to believe that high PA may serve as a protective factor against maladaptive outcomes, as higher PA has been linked to adaptation in social functioning and attachment (for a review, see Davis & Suveg, 2014). Indeed, early maternal report of PA has been shown to be inversely related to conduct problems in early childhood through adolescence (Lahey et al., 2008) and low self-reported positive emotion has been associated with more externalizing symptomatology among children (Kim et al., 2007). In contrast, low PA may exacerbate risk for internalizing symptomatology, such that children’s internalizing symptomatology is predicted by higher levels of sadness (Eisenberg et al., 2009; Jenkins & Oatley, 2000). Furthermore, a recent meta-analysis identified low levels of positive emotion as a specific risk factor for depression (Khazanov & Ruscio, 2016). We note that some prior research suggests that PA may function as a risk factor for later psychopathology in children and adolescents (Gilbert, 2012). However, measurement of PA in these studies often includes strong approach tendencies (e.g., temperamental surgency) as a facet of PA (Putnam, 2012), whereas the current study focuses on the nature of positive mood as representing pleasantness as opposed to unpleasantness (Watson et al., 1998). Previous research that has similarly operationalized PA as pleasantness has examined its beneficial effects on adjustment (e.g., Kim et al., 2007) and health outcomes (e.g.,Steptoe, Dockray, & Wardle, 2009). Further work has demonstrated that positive affective states can contribute to reduction in Stroop interference effects (Kuhl & Kazen, 1999), suggesting that PA may promote cognitive control abilities. However, current neuroscience literature emphasizes that cognitive control may modulate the effects of these affective states on adjustment outcomes.
Regulation of emotional reactivity is critical to healthy development (Derryberry & Rothbart, 1997; Ochsner & Gross, 2005). In the neuroscience literature, it has been theorized that successful self-regulation is dependent on interactions between the PFC and the subcortical regions involved in emotion and motivation (Casey, Getz, & Galvan, 2008; Ernst & Fudge, 2009; Heatherton & Wagner, 2011; Kim-Spoon et al., 2017). Neuroimaging research on emotion regulation has demonstrated that neural responses to emotional stimuli in the amygdala and associated limbic regions are regulated by the PFC (Davidson, Putnam, & Larson, 2000; Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003; Ochsner & Gross, 2005; see Guyer, Silk, & Nelson, 2016 for a review). Indeed, there is evidence that functional connectivity between the PFC and the amygdala is involved in successful self-regulation. Specifically, positive connectivity between these areas is observed in childhood, with a shift to negative connectivity during the transition to adolescence (Gee et al., 2013) with the inverse prefrontal-amygdala connectivity increasing from adolescence to young adulthood (Silvers, Shu, Hubbard, Weber, & Ochsner, 2015). This negative coupling between the amygdala and prefrontal areas is associated with better self-control and later substance use onset in adolescence (Lee & Telzer, 2016).
Unlike studies on amygdala-prefrontal connectivity, there remains a lack of clarity regarding developmental patterns of oxygenation in the PFC related to the increases in cognitive control abilities observed in adolescence. For example, a study examining age-related differences in neural responses in the left ventrolateral PFC during cognitive reappraisal (i.e., reframing emotional experience by interpreting in ways that change emotional responses) reported a linear increase across ages 10 to 22, in accordance with maturation of the PFC (McRae et al., 2012). In contrast, a study using a sample of participants aged 15 to 25 years reported significant age-related differences in neural responses during cognitive reappraisal, such that younger adolescents exhibited greater activation of temporal-parietal cortical regions, in combination with weaker suppression of amygdala reactivity during cognitive reappraisal of negative social images (Stephanou et al., 2016). We note that, in general, human neuroimaging work has demonstrated age-related decreases in PFC activation related to cognitive control in non-emotional contexts. For example, Durston and colleagues (2006) observed age-related decreases in lateral PFC activation during a go/no-go task and Ordaz and colleagues (2013) reported longitudinal decreases in PFC activation related to executive control during an antisaccade task. Taken together, these studies on PFC development related to cognitive control highlight how brain activation may differ across study tasks and samples (Crone & Dahl, 2012). Nevertheless, functional neuroimaging studies on PFC functioning during emotion regulation appear to suggest that the regulation of the PFC over reactivity of the subcortical regions, which indicates successful self-regulation, may improve throughout adolescence.
Following the work of Carver, Johnson, and Joormann (2008) whose two-mode model of self-regulation emphasizes the interaction between two simultaneous but distinct nervous systems—i.e., a reflexive lower order system that is reactive in nature (e.g., affect) and a reflective or regulatory higher order system that is more strategic or deliberate (e.g., cognitive control), we argue that the influence of affect on psychopathology in adolescents depends on the regulatory abilities of cognitive control. Indeed, emerging evidence within research on adolescent cognitive control highlights the regulating role of cognitive control, involving attentional and inhibitory control, in the associations between emotional reactivity and adjustment outcomes. In a longitudinal study testing whether attentional control modulated the effects of anger (based on reports by parents, teachers, and observers), the contribution of high anger to the development of externalizing behaviors was shown only among children with poor attentional control (Kim & Deater-Deckard, 2011). Another longitudinal study demonstrated that increased anger reactivity (i.e., approach motivation) between 9 and 11 years was related to increased risk-taking behaviors (including externalizing symptomatology) between 11 and 15 years only for adolescents with low attentional control (Kim-Spoon, Holmes, & Deater-Deckard, 2015). Similarly, Youssef and colleagues (2016) reported significant moderating effects of a cognitive control composite which combined both self-reported effortful control and behavioral inhibition performance, showing an attenuated link between self-reported temperamental frustration and risk-taking behaviors (including externalizing symptomatology) among adolescents with high cognitive control. However, there is also a study reporting no such moderating effect of self-reported emotional control in the link between positive and negative emotion and internalizing and externalizing symptomatology among children (Kim et al., 2007).
The current longitudinal study sought to further elucidate the joint contributions of affect and a specific aspect of self-regulation—cognitive control—on adolescents’ internalizing and externalizing symptomatology. In particular, we examined moderating effects of both behavioral and neural indicators of cognitive control, indexed by performance and prefrontal activation during a cognitive interference task. Available neuroimaging research on adolescent emotion regulation has primarily focused on neural responses during cognitive reappraisal and so there remains uncertainty about how PA and NA may interact with cognitive control to predict adjustment outcomes in adolescence. Since adolescents experience increases in negative emotion and decreases in positive emotion during this developmental period (Maciejewski, van Lier, Branje, Meeus, & Koot, in press), it is important to identify self-regulatory mechanisms that may play a role in modifying the effects that NA and PA may have on the development of psychopathology. Therefore, we first tested cross-sectionally whether both behavioral and neural measures of cognitive control moderated the associations between PA and NA at Time 1 and internalizing and externalizing symptomatology at Time 1, hypothesizing that the detrimental effects of high NA or low PA would be attenuated for adolescents with strong cognitive control. Next, we examined these associations longitudinally to detect whether cognitive control moderated the association between PA and NA at Time 1 and developmental changes in internalizing and externalizing symptomatology.
Method
Participants
The current sample included 157 adolescents (52% males, 48% females) who were 13 or 14 years of age at Time 1 (M = 14.13, SD = 0.54) and 14 or 15 years of age one year later at Time 2 (M = 15.05, SD = .55). Adolescents primarily identified as Caucasian (82%), 12% African-American, and 6% other. Median family income was $35,000 – $49,999 per year at Time 1. A total of 19 adolescents were excluded from final analyses because of unusable imaging data due to excessive movement during acquisition. Of the final 138 participants, 12 did not return at Time 2 (approximately one year later) for reasons including: declined participation (n = 7), and lost contact (n = 5). We performed a logistic regression analysis to predict attrition between Time 1 and Time 2 based on demographic and model variables. Attrition analyses indicated that the 12 adolescents who did not return for Time 2 were not significantly different on demographic or study variables from the 126 who did return (p = .94 for age, p = .62 for income, p = .60 for sex, p = .56 for race, p = .50 for PA, p = .46 for NA, p = .26 for neural cognitive control, p = .93 for behavioral cognitive control, p = .25 for externalizing symptomatology, and p = .95 for internalizing symptomatology).
Measures
Positive and negative affect
Adolescents’ PA and NA were measured using the Positive and Negative Affect Schedule (PANAS; Laurent et al., 1999). The PA subscale consists of 10 words including “Excited”, “Interested”, and “Proud”. The NA subscale includes 10 words such as “Irritable”, “Afraid”, and “Upset”. Participants were asked to rate the extent they had felt that way in the past few weeks on a 5-point Likert scale from “1 = Very slightly or not at all” to “5 = Extremely.” The scale demonstrates reliability within the current sample at α = .82.
Externalizing and internalizing symptomatology
Adolescents’ levels of externalizing and internalizing symptomatology were assessed with the Youth Self-Report (YSR; Achenbach & Rescorla, 2001), a 102-item questionnaire that assesses behavior problems in children ages 11 to 17. Behaviors were rated on a 3-point scale ranging from “0 = Not true” to “2 = Very true”. T-scores from the externalizing (aggressive behavior and rule-breaking behavior) and internalizing (anxious-depressed, withdrawal-depressed, and somatic complaints) scales were used. The YSR has shown strong psychometric properties for internalizing and externalizing (α = .90; Achenbach & Rescorla, 2001) and demonstrates similar reliability in the current sample (α = .90 for internalizing symptomatology at both Time 1 and Time 2; and α = .86 and α = .84 for externalizing symptomatology at Time 1 and Time 2, respectively).
Cognitive control
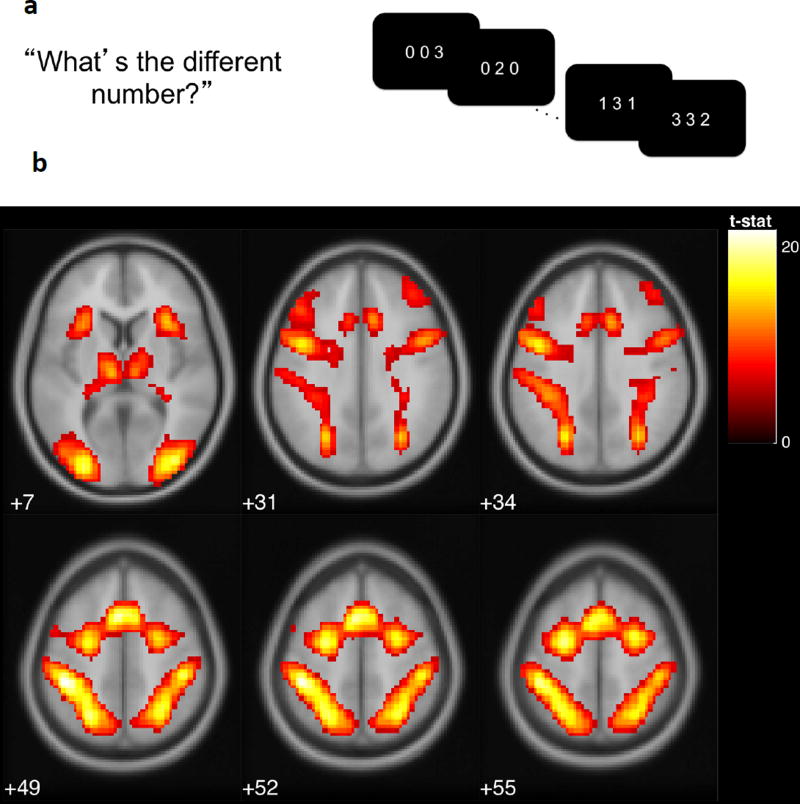
Cognitive control was measured at Time 1 with the Multi-Source Interference Task (MSIT; Figure 1a; Bush, Shin, Holmes, Rosen, & Vogt, 2003), a cognitive interference task shown to activate the anterior cingulate cortex, and the parietal, premotor and dorsolateral prefrontal cortices. In the MSIT, participants are presented with sequences of three digits, two of which are identical. Participants are instructed to indicate the identity (but not the position) of the unique, target digit. In the neutral condition, target digits are congruent with position (e.g., “2” is in the second position in the sequence “020”). In the interference condition, target digits are incongruent with position (e.g. “3” is in the second position in the sequence “131”). Four blocks of 24 interference trials and 4 blocks of 24 neutral trials were interleaved with an interstimulus interval of 1.75 seconds. To assess task performance, we used accuracy and intraindividual variability in reaction time, indexed as intraindividual standard deviations (ISDs; Macdonald, Karlsson, Rieckmann, Nyberg, & Bäckman, 2012) for correct responses in the interference condition. We also assessed hemodynamic response in prefrontal areas using functional magnetic resonance imaging (fMRI).
Figure 1.
a) In the multi-source interference task (MSIT), adolescents were asked to identify the digit that differed from two other concurrently presented digits, ignoring its position in the sequence. b) Adolescents exhibited greater activation for interference relative to neutral conditions in the regions of left posterior-medial frontal cortex, left and right inferior frontal gyrus, left and right inferior parietal lobules, right insula, right superior frontal gyrus, and left middle frontal gyrus, displayed at p(FWE) < .001 (see Table 1). Reprinted from [citation blinded].
Imaging acquisition and analysis
We assessed hemodynamic correlates of cognitive conflict using the MSIT. Functional images were acquired using a 3.0T Siemens Tim Trio (Siemens, Erlangen, Germany) with the following parameters: echo-planar imaging, gradient recalled echo; repetition time (TR) = 2 s; echotime (TE) = 30 ms; flip angle = 90°; voxel size 3.4375 × 3.4375 × 4 mm; 34 axial slices, 4.0 mm slice thickness, 220 × 220 mm field of view (FOV), 64 × 64 grid, and hyperangulated slices acquired at 30 degrees from the anterior commissure posterior commissure line. During analysis the images were resliced so that voxels were 3 × 3 × 3 mm. The structural scan was acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence (TR = 1200 ms, TE = 3.02 ms, FoV = 245 × 245 mm, 1 mm slice thickness, 192 slices with spatial resolution of 1×1×1 mm). Data were processed and analyzed using SPM8 (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, London, UK). Functional images were corrected for head motion using a six-parameter rigid-body transformation, realigned, and normalized to template space before smoothing. Images were then realigned and normalized to the Montreal Neurological Institute (MNI) template using parameters derived from a segmented anatomical image coregistered to the mean EPI and were spatially smoothed using a 6 mm full-width at half-maximum Gaussian kernel. As described below, general linear models were specified for each participant and subsequent second level random effects analyses were conducted.
ROIs were selected based on a previous study using the same sample and task (CITATION BLINDED). For each participant, individual-level regions-of-interest (ROI) values were extracted at coordinates corresponding to peak activations in the interference minus neutral second-level contrast (see Table 1). Specifically, the first eigenvariate values of the contrast images were extracted using spherical masks of 5 mm surrounding MNI coordinates, thresholded at p < .001, family-wise error corrected. Among the extracted ROI values, variables representing (1) regions known to be engaged by cognitive control related to interference- and error-processing (Fitzgerald et al., 2010; Koechlin et al., 2003a, 2003b; Roberts & Hall, 2008) and (2) regions significantly correlated with behavioral performance (i.e., absolute magnitude of correlation > .20 with the behavioral performance factor score) were chosen as manifest indicators of the neural cognitive control factor. These ROIs included left posterior-medial frontal cortex, right inferior frontal gyrus, left and right inferior parietal lobules, right insula, right superior frontal gyrus, and left middle frontal gyrus (see Figure 1b).
Table 1.
Areas of significant activation for the contrast of Interference minus Neutral blocks of the Multi-Source Interference Task
| Cluster | Peak | MNI Coordinates |
Region | |||
|---|---|---|---|---|---|---|
| k | p(FWE) | t | x | y | z | |
| 759 | < .001 | 21.84 | −42 | −37 | 49 | L postcentral gyrus |
| 19.61 | −24 | −64 | 49 | L superior parietal lobule | ||
| 18.07 | −30 | −55 | 52 | L inferior parietal lobule | ||
| 265 | < .001 | 20.43 | −3 | 14 | 49 | L posterior-medial frontal |
| 506 | < .001 | 20.42 | −39 | −85 | −2 | L inferior occipital gyrus |
| 20.15 | −30 | −91 | −2 | L inferior occipital gyrus | ||
| 19.05 | −39 | −73 | −8 | L fusiform gyrus | ||
| 654 | < .001 | 19.21 | 42 | −64 | −8 | R fusiform gyrus |
| 19.17 | 42 | −82 | −2 | R inferior occipital gyrus | ||
| 18.46 | 33 | −91 | 1 | R inferior occipital gyrus | ||
| 15.78 | 39 | −67 | −23 | R cerebellum (crus 1) | ||
| 15.24 | 33 | −49 | −26 | R cerebellum (VI) | ||
| 245 | < .001 | 19.06 | −24 | −4 | 55 | L middle frontal gyrus |
| 431 | < .001 | 18.12 | 30 | −58 | 52 | R inferior parietal lobule |
| 18.11 | 45 | −31 | 49 | R postcentral gyrus | ||
| 16.52 | 30 | −64 | 40 | R superior occipital gyrus | ||
| 140 | < .001 | 17.38 | 27 | −4 | 55 | R superior frontal gyrus |
| 94 | < .001 | 16.89 | −45 | 2 | 34 | L inferior frontal gyrus (pars opercularis) |
| 46 | < .001 | 15.01 | −9 | −19 | 10 | L thalamus |
| 24 | < .001 | 14.50 | 33 | 20 | 7 | R insula lobe |
| 7 | < .001 | 13.47 | 6 | −73 | −20 | Cerebellar vermis (7) |
| 13 | < .001 | 13.32 | 48 | 8 | 31 | R inferior frontal gyrus (pars opercularis) |
| 12 | < .001 | 13.31 | −30 | 17 | 10 | L insula lobe |
| 5 | < .001 | 12.73 | 9 | −19 | 10 | R thalamus |
| 9 | < .001 | 12.66 | −27 | −55 | −23 | L cerebellum (VI) |
| 12.60 | −27 | −64 | −23 | L cerebellum (VI) | ||
Note. Voxel-wise thresholded at t = 12, equivalent to p = 2.00 × 10−23 uncorrected. k = the number of voxels in each significant cluster; FWE = family-wise error corrected; t = peak activation level in each cluster; x, y, z = MNI coordinates; L = left; R = right. Boldface indicates the regions included in the neural cognitive control factor scores. Reprinted from [citation blinded].
Plan of Analysis
For all study variables, descriptive statistics were examined to determine normality of distributions and outliers. Skewness and kurtosis were examined for all variable distributions and acceptable levels were less than 3 and 10, respectively (Kline, 2011). Outliers were identified as values ≥ 3 SD from the mean. In these cases (n = 7), values were winsorized to retain statistical power and attenuate bias resulting from elimination (Ghosh & Vogt, 2012). Multivariate GLM analyses indicated no significant effects of demographic variables at Time 1 on internalizing and externalizing at Time 1 (p = .41 for age, p = .13 for family income, p = .25 for race, p = .32 for sex). For residualized scores, sex reported at Time 1 was a significant predictor of internalizing symptomatology (p = .01) and thus was included as a covariate. Other demographic variables were not significantly associated with residualized internalizing and externalizing outcomes (p = .92 for age, p = .74 for family income, p = .11 for race). The hypothesized models were tested via Structural Equation Modeling (SEM) using AMOS statistical software for IBM SPSS (Arbuckle, 2014). Overall model fit indices were determined by χ2 value, degrees of freedom, corresponding p-value, Root Mean Square Error of Approximation (RMSEA), and Confirmatory Fit Index (CFI). RMSEA values of less than .05 were considered a close fit while values less than .08 were considered a reasonable fit (Browne & Cudeck, 1993), and CFI values of greater than .90 were considered an acceptable fit while values greater than .95 were considered an excellent fit (Bentler, 1990). Full information maximum likelihood estimation was used to handle missing data. We used two-group structural equation models, based on maximum likelihood estimation, to test our hypothesis regarding the moderating effects of cognitive control in the link between PA and NA and internalizing and externalizing symptomatology.
Results
For cognitive control behavioral and neural variables, we created factor scores using confirmatory factor analysis (CFA). For the behavioral factor, CFA was used to calculate a factor score using standardized scores of accuracy difference scores (i.e., interference condition – neutral condition) and ISD of reaction time (reverse coded). The model was fully saturated and both loadings were significant (.72, p < .001). For the cognitive control neural factor, CFA testing a single factor model based on the 7 ROI variables indicated a good fit (χ2 = 12.05, df = 11, p = .36, CFI = 1.00, and RMSEA = .03). All factor loadings were significant (p < .001) ranging from .61 to .86. In this model, based on modification indices, three covariances between residuals were estimated: between left and right inferior parietal lobules, between right inferior frontal gyrus and left middle frontal gyrus, and between left posterior-medial frontal cortex and right insula. Activation for each selected ROI during the task was negatively correlated with the behavioral factor scores based on accuracy and ISD of reaction time (r = −.21 to −.34, ps < .05). Thus, the results indicated that adolescents who were more challenged by the interference condition exhibited greater differentiation in hemodynamic activity between the interference and neutral conditions. In contrast, those that had lower differentiation between the conditions were more efficient in interference conditions. To be consistent with behavioral scores, neural scores were reverse coded so that a higher score reflected better cognitive control.
Table 2 presents descriptive statistics and correlations for all model variables. We formed high cognitive control (above the median) vs. low cognitive control (below the median) groups using the MSIT factor scores (separately for the behavioral and neural scores). We then tested whether the relationship between positive and negative affect and internalizing and externalizing symptomatology was moderated by level of cognitive control (the grouping variable) by examining whether the affect and symptomatology associations were significantly stronger for the low cognitive control compared to the high cognitive control group. To test the statistical significance of the difference between high vs. low cognitive control groups, we used nested model comparisons starting with the configural invariance model in which all parameters were freely estimated across the two groups. In the following nested models, we imposed an equality constraint to test numeric invariance between the low and high cognitive control groups with respect to the effects of positive and negative affect on internalizing and externalizing symptomatology. If imposing an equality constraint on a particular path led to a significant model fit deterioration (as assessed by a significant chi-square difference test), this would indicate that that particular path significantly differed between the low and high cognitive control group, indicating a significant moderation effect. We tested model fit comparisons using the change in the Comparative Fit Index (CFI) which is not affected by sample size (i.e., ΔCFI > .01 reflects a meaningful difference in model fit; Cheung & Rensvold, 2002). Because we ran the hypothesized moderation models separately for the behavioral and neural MSIT scores, we used Bonferroni corrected p levels (.05/2 = .025) to evaluate statistical significance of the estimates in the SEM analyses.
Table 2.
Descriptive statistics and bivariate correlations among affect, cognitive control, and internalizing and externalizing symptomatology
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | M(SD) |
|---|---|---|---|---|---|---|---|---|
| 1. Positive affect | 3.53 (.67) | |||||||
| 2. Negative affect | .13 | 1.91 (.71) | ||||||
| 3. Internalizing symptomatology Time 1 | −.07 | .63** | 52.25 (9.98) | |||||
| 4. Internalizing symptomatology Time 2 | −.05 | .49** | .69** | 52.57 (10.87) | ||||
| 5. Externalizing symptomatology Time 1 | .06 | .46** | .50** | .30** | 49.01 (9.21) | |||
| 6. Externalizing symptomatology Time 2 | .02 | .29** | .27** | .34** | .71** | 49.21 (9.27) | ||
| 7. MSIT neural factor score | .01 | −.06 | −.07 | −.20* | −.03 | −.09 | 1.74 (.28) | |
| 8. MSIT behavioral factor score | −.07 | −.08 | .09 | .11 | .04 | .06 | .36** | 0.00 (.83) |
Note. MSIT = Multiple Source Interference Task. Neural MSIT scores were reverse coded such that higher scores indicate better cognitive control.
p < .05;
p < .01.
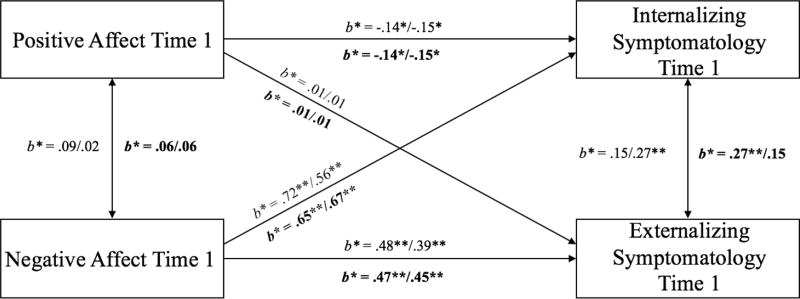
Model 1 was a cross-sectional analysis testing associations between affect and symptomatology at Time 1 and was a fully saturated model (Figure 2). We first fit the configural invariance model and the equality constraints were added on one path at a time beginning with the path from PA to internalizing symptomatology (see Table 3 for model comparison estimates). Moderating effects of neural and behavioral indicators of cognitive control were tested separately; results between the neural and behavioral models were consistent in that the best fitting model in which all paths were constrained to be equal indicated no significant differences between the groups. For behavioral cognitive control, higher levels of NA at Time 1 significantly predicted higher levels of externalizing (b = .62, SE = .12, p < .001) and internalizing (b = .99, SE = .10, p < .001) symptomatology, regardless of level of cognitive control. Higher levels of PA at Time 1 were significantly related to lower levels of internalizing (b = −.23, SE = .10, p = .02) symptomatology, but not related to externalizing (b = .01, SE = .11, p = .93) symptomatology. Similarly, for neural cognitive control, higher levels of NA at Time 1 significantly predicted higher levels of externalizing (b = .63, SE = .12, p < .001) and internalizing (b = .96, SE = .10, p < .001) symptomatology, regardless of level of cognitive control. Higher levels of PA at Time 1 were significantly associated with lower levels of internalizing (b = −.23, SE = .10, p = .02), but not externalizing (b = .02, SE = .11, p = .86), symptomatology. Standardized estimates are presented in Figure 2.
Figure 2.
Model 1: Cross-sectional model of the interaction effect between affect and cognitive control at Time 1 on internalizing and externalizing symptomatology at Time 1.
Note. Standardized parameter estimates are presented; estimates listed first are for the low cognitive control group; second for the high cognitive control group. Estimates above regression line are behavioral indicators of cognitive control, below (in bold) are neural. *p < .05; **p < .01
Table 3.
Model Fit Comparisons for Model 1: Structural Equation Modeling Analyses Testing the Cross-Sectional Associations among Affect and Internalizing/Externalizing Symptomatology for High vs. Low Cognitive Control Groups
| Model Label | χ2 | df | p(exact) | CFI | RMSEA | p(close) | Comparison | Δχ2 | Δdf | p(d) | ΔCFI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High vs. low groups by MSIT behavioral scores | |||||||||||
| 1a. Configural invariance model | 0 | 0 | - | 1.00 | - | - | |||||
| 1b. Equal PA effect on internalizing symptomatology | .01 | 1 | .95 | 1.00 | .00 | .95 | a vs. b | .01 | 1 | .94 | .00 |
| 1c. Equal PA effect on externalizing symptomatology | .65 | 2 | .72 | 1.00 | .00 | .79 | b vs. c | .64 | 1 | .42 | .00 |
| 1d. Equal NA effect on internalizing symptomatology | 1.87 | 3 | .60 | 1.00 | .00 | .72 | c vs. d | 1.22 | 1 | .27 | .00 |
| 1e. Equal NA effect on externalizing symptomatology | 2.52 | 4 | .64 | 1.00 | .00 | .77 | d vs. e | .65 | 1 | .42 | .00 |
| High vs. low groups by MSIT neural scores | |||||||||||
| 1a. Configural invariance model | 0 | 0 | - | 1.00 | - | - | |||||
| 1b. Equal PA effect on internalizing symptomatology | .74 | 1 | .39 | 1.00 | .00 | .47 | a vs. b | .74 | 1 | .39 | .00 |
| 1c. Equal PA effect on externalizing symptomatology | .77 | 2 | .68 | 1.00 | .00 | .76 | b vs. c | .03 | 1 | .86 | .00 |
| 1d. Equal NA effect on internalizing symptomatology | 1.42 | 3 | .70 | 1.00 | .00 | .80 | c vs. d | .65 | 1 | .42 | .00 |
| 1e. Equal NA effect on externalizing symptomatology | 1.43 | 4 | .84 | 1.00 | .00 | .91 | d vs. e | .01 | 1 | .91 | .00 |
Note. PA = Positive Affect; NA = Negative Affect; p(exact) = probability of an exact fit to the data; CFI = comparative fit index; RMSEA = root mean square error of approximation; p(close) = probability of a close fit to the data; Δχ2 = difference in likelihood ratio tests; Δdf = difference in df; p(d) = probability of the difference tests; ΔCFI = difference in CFI. Best-fitting model is in bold face.
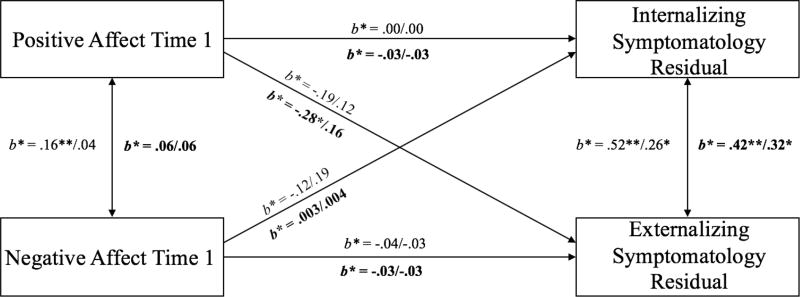
Model 2 longitudinally tested change in internalizing and externalizing symptomatology between Time 1 and Time 2 by using standardized residual scores as the outcome variables. Sex was entered as a covariate due to its significant association with change in internalizing symptomatology. As with Model 1, equality constraints were added on one path at a time. For behavioral cognitive control (see Table 4), the chi-square difference test indicated that adding an equality constraint on the effect of PA on internalizing symptomatology between the two groups (“Equal PA effect on internalizing” model) did not significantly degrade the overall model fit. Next, adding the “Equal PA effect on externalizing” model yielded a marginally significantly worse fit (p = .06). Keeping this constraint yielded a poor fitting model, so this particular path was freely estimated between the two groups in subsequent models. Adding equality constraints on the effects of NA on internalizing (“Equal NA effect on internalizing” model) also yielded significantly worse fit (compared to “Equal PA effect on internalizing”) and so this path was freely estimated. Equality constraints on the path between NA and externalizing (“Equal NA effect on externalizing” model) symptomatology did not degrade the model fits significantly. Therefore, the best-fitting model (“Equal NA effect on externalizing” model) freely estimated paths for the effect of PA on externalizing symptomatology and the effect of NA on internalizing symptomatology while imposing equality constraints on all other regression paths. However, a closer inspection of the best-fitting model indicated no significant effects of PA and NA on changes in adjustment outcomes. PA did not contribute to change in externalizing symptomatology for the low (b = −0.29, SE = .18, p = .10) or high group (b = 0.18, SE = .18, p = .33). NA did not predict change in internalizing symptomatology for either the low (b = −0.15, SE = .15, p = .33) or high (b = 0.32, SE = 0.21, p = .12) cognitive control groups. PA at Time 1 did not predict change in internalizing symptomatology and the effects were comparable between the low and high (b = 0.00, SE = .13, p = .71) cognitive control groups. NA at Time 1 did not predict change in externalizing symptomatology and the effects were comparable between the low and high (b = −0.05, SE = .13, p = .99) cognitive control groups. Standardized estimates are shown in Figure 3.
Table 4.
Model Fit Comparisons for Model 2: Structural Equation Modeling Analyses Testing the Longitudinal Associations among Affect and Change in Internalizing/Externalizing Symptomatology for High vs. Low Cognitive Control Groups
| Model Label | χ2 | df | p(exact) | CFI | RMSEA | p(close) | Comparison | Δχ2 | Δdf | p(d) | ΔCFI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High vs. low groups by MSIT behavioral scores | |||||||||||
| 1a. Configural invariance model | 1.79 | 2 | .41 | 1.00 | .00 | .52 | |||||
| 1b. Equal PA effect on internalizing symptomatology | 2.86 | 3 | .41 | 1.00 | .00 | .55 | a vs. b | 1.07 | 1 | .30 | .00 |
| 1c. Equal PA effect on externalizing symptomatology | 6.46 | 4 | .17 | .87 | .07 | .31 | b vs. c | 3.59 | 1 | .06 | .13 |
| 1d. Equal NA effect on internalizing symptomatology | 6.68 | 4 | .15 | .86 | .07 | .29 | b vs. d | 3.81 | 1 | .05 | .14 |
| 1e. Equal NA effect on externalizing symptomatology | 3.22 | 4 | .52 | 1.00 | .00 | .67 | b vs. e | .35 | 1 | .55 | .00 |
| High vs. low groups by MSIT neural scores | |||||||||||
| 1a. Configural invariance model | .83 | 2 | .66 | 1.00 | .00 | .74 | |||||
| 1b. Equal PA effect on internalizing symptomatology | 2.96 | 3 | .40 | 1.00 | .00 | .54 | a vs. b | 2.13 | 1 | .14 | .00 |
| 1c. Equal PA effect on externalizing symptomatology | 10.54 | 4 | .03 | .65 | .11 | .09 | b vs. c | 7.58 | 1 | .01 | .35 |
| 1d. Equal NA effect on internalizing symptomatology | 3.05 | 4 | .55 | 1.00 | .00 | .70 | b vs. d | .08 | 1 | .77 | .00 |
| 1e. Equal NA effect on externalizing symptomatology | 3.08 | 5 | .69 | 1.00 | .00 | .82 | d vs. e | .04 | 1 | .85 | .00 |
Note. PA = Positive Affect; NA = Negative Affect; p(exact) = probability of an exact fit to the data; CFI = comparative fit index; RMSEA = root mean square error of approximation; p(close) = probability of a close fit to the data; Δχ2 = difference in likelihood ratio tests; Δdf = difference in df; p(d) = probability of the difference tests. ΔCFI = difference in CFI. Best-fitting model is in bold face.
Figure 3.
Longitudinal model of the interaction effect between affect and cognitive control on change in internalizing and externalizing symptomatology between Time 1 and Time 2.
Note. Standardized parameter estimates are presented; estimates listed first are for the low cognitive control group; second for the high cognitive control group. Estimates above regression line are behavioral indicators of cognitive control, below (in bold) are neural. Covariances between predictors and control variable were estimated, but are not included in figure for clarity of presentation (PA ↔ sex = .03/−.05; NA ↔ sex = .08/.07). *p < .05; **p < .01
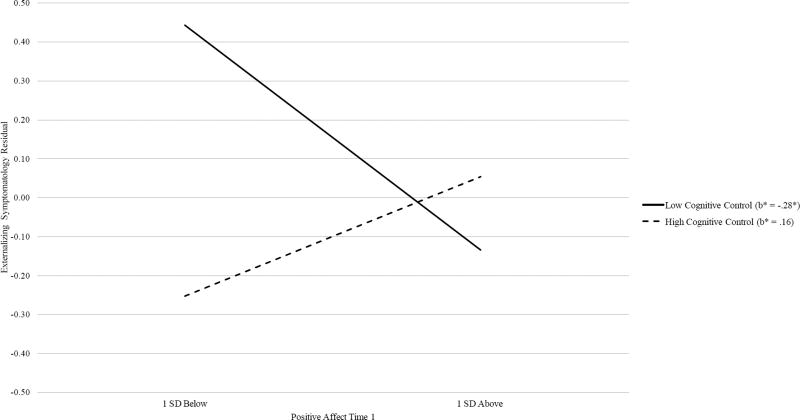
As shown in Table 4, for neural cognitive control, the chi-square difference test indicated that adding an equality constraint on the effect of PA on internalizing symptomatology between the two groups (“Equal PA effect on internalizing” model) did not significantly degrade the overall model fit, indicating no significant moderation effect of cognitive control in the link between PA and internalizing symptomatology. Next, adding the “Equal PA effect on externalizing” model yielded a significantly worse fit as compared to the configural invariance model. This finding suggested that the low and high cognitive control groups showed significantly different magnitudes for the effect of PA on change in externalizing symptomatology. Therefore, this particular path was freely estimated between the two groups in subsequent models. Adding equality constraints on the effects of NA on internalizing (“Equal NA effect on internalizing” model) and externalizing (“Equal NA effect on externalizing” model) symptomatology did not degrade the model fits significantly. Therefore, the best-fitting model (“Equal NA effect on externalizing” models) included the path for the effect of PA on externalizing symptomatology freely estimated while imposing equality constraints on all other regression paths. Specifically, higher PA was predictive of decreases in externalizing symptomatology for the low cognitive control group (b = −0.43, SE = .18, p = .02), but not for the high cognitive control group (b = 0.23, SE = .18, p = .21). Simple slope analyses for the moderation effect of cognitive control are depicted in Figure 4. PA did not predict change in internalizing symptomatology for either the low or high cognitive control group (b = −0.04, SE = .13, p = .77). NA did not predict change in internalizing (b = 0.01, SE = .13, p = .97) or externalizing (b = −0.05, SE = .13, p = .70) symptomatology for either group. Standardized estimates are shown in Figure 3.
Figure 4.
Regression lines for relations between positive affect and change in externalizing symptomatology, moderated by neural cognitive control.
b* = standardized regression coefficient (simple slope).
* p < .05.
In order to evaluate the nature of the link between PA and change in externalizing symptomatology among adolescents with poor neural cognitive control, we probed changes in simple slopes at various cut-points below the mean of neural cognitive control. Effect sizes of PA on externalizing symptomatology decreased as neural cognitive control decreased: (1) the mean (b* = −.39, p = .03, one-tailed), (2) 1 SD below the mean (b* = −.25, p = .03, one-tailed) and (3) 2 SD below the mean (b* = −.10, p = .33, one-tailed).
Discussion
Current neuroscience theories of self-regulation suggest that the interaction between neural systems is key to understanding the etiology of individual differences in affective behavior, as the regulatory system (associated with prefrontal regions) modulates the operation of the reactive system (associated with subcortical regions) in the service of goal directed behavior (Casey et al., 2008; Ernst & Fudge, 2009; Heatherton & Wagner, 2011). Therefore, the present longitudinal study sought to elucidate the joint contributions of affect and cognition to adolescents’ internalizing and externalizing symptomatology using behavioral, neural, and self-report measures. Specifically, we examined relative, longitudinal contributions of PA and NA to internalizing and externalizing symptomatology outcomes, with particular interest in testing whether neural and behavioral cognitive control may strengthen or attenuate these associations. Our findings indicated that higher PA predicted decreases in externalizing symptomatology, independent of NA, but such effects were present only for adolescents with poor neural cognitive control. Thus, our findings suggest that PA is particularly important for adolescents with poor neurobiological cognitive control, as it influences their development of externalizing symptomatology. This pattern is specific to developmental change in externalizing symptomatology and may be particularly informative for adolescent trajectories of maladaptation. The moderation results may also be understood as lower (or lack of) PA exacerbates risk for externalizing symptomatology in the context of poor prefrontal functioning. However, given the prominent literature emphasizing the protective effects of positive affect on mental and physical health outcomes (e.g., Davis & Suveg, 2014; Steptoe et al., 2009), we have chosen to focus on the potential benefits of high PA in our interpretation.
To date, statistical interactions between emotion and cognitive control have not been extensively studied regarding adolescent psychopathology, but available longitudinal (e.g., Kim & Deater-Deckard, 2011; Kim-Spoon et al., 2015) and cross-sectional (e.g., Youssef et al., 2016) work suggests that the risk-promoting effects of NA (anger and frustration) on externalizing symptomatology can be attenuated by strong cognitive control among children and adolescents. Our results extend this work and offer the first evidence that the beneficial effects of PA on externalizing symptomatology are particularly beneficial for adolescents with poor cognitive control reflected by neural functioning. These findings clearly support the current theorization of the interaction between prefrontal functioning and emotional reactivity in neuroscience research on adolescence (Casey, Galván, & Somerville, 2016).
The joint contribution of affect and cognitive control on the development of externalizing psychopathology also aligns with existing theoretical models of risk and resilience. Specifically, Luthar, Cicchetti, and Becker’s (2000) model of protective-enhancive effects illustrates how PA may be protective particularly for adolescents with considerable risk. This model of risk and resilience posits that individuals who are at increased risk demonstrate better outcomes in the presence of a protective attribute. The protective factor allows individuals to engage with adversity in a way that facilitates adaptation. Within this framework, our findings suggest that PA may serve as a protective-enhancing attribute in that PA is related to positively altered trajectories of externalizing symptomatology among those who may be at risk due to poor neural cognitive control. Though affect is more often examined in relation to internalizing symptomatology, existing research demonstrates a link between PA and externalizing symptomatology. For example, Lengua, West, and Sandler (1998) found that PA was negatively associated with self-reported and parent-reported conduct problems among children (aged 9 – 12 years). Our findings further highlight that in adolescence, this association between affect and psychopathology is contingent on adolescents’ prefrontal functioning. The PFC is critical for self-regulation related to inhibitory and interference control (Bush et al., 2000) and underdevelopment or impairment in this region may leave adolescents at increased risk for externalizing symptomatology (Young et al., 2009). Our data suggest that these adolescents may reap the protective benefits PA may offer.
The mechanism by which PA fosters adaptation is not yet entirely clear; PA may be protective against acting out among adolescents with poor cognitive control by broadening their thought-action repertoires and thus building the compensatory resources (physical, intellectual, and social resources) they need to effectively handle challenges (Fredrickson, 1998). In particular, given the difficulties in social relationships that adolescents with poor self-regulation experience (Holmes, Kim-Spoon, & Deater-Deckard, 2016; see Farley & Kim-Spoon, 2014 for a review), PA may promote protective psychosocial factors such as social connectedness, perceived social support, optimism, and preference for adaptive coping responses (Steptoe et al., 2009).
Probing simple slopes at various levels below the mean of neural cognitive control provided a nuanced understanding of the beneficial effects of PA. The results revealed an inflection point such that the effects of PA on changes in externalizing symptomatology were strong among adolescents with relatively poor cognitive control, but the effects weakened as neural cognitive control became exceedingly worse. It seems that for adolescents with the poorest levels of cognitive control, the beneficial effects of PA are no longer effective as they may be “overwhelmed” by severely impaired prefrontal functioning. Indeed, this finding is in line with a phenomenon commonly observed in risk and resilience research: individuals’ adjustment outcomes may deteriorate despite the presence of protective factors when the benefits are outweighed by risk factors (e.g., Zielinski & Bradshaw, 2006).
Our data revealed moderating effects of neural indicators of cognitive control, but not behavioral cognitive control. Previous neuroimaging work on adolescent neurocognitive functioning has found similar discrepancies in behavioral versus neural indicators, such as pronounced differences in neural processing between substance abusers and non-users, even in the absence of differences in behavioral performance (Galvan, Poldrack, Baker, McGlennen, & London, 2011; Wetherill, Squeglia, Yang, & Tapert, 2013). Neural indicators may better capture the general state of neurobiological vulnerability compared to behavioral indicators sampled during a laboratory task, which may be limited in representing real-life behaviors (Richards, Plate, & Ernst, 2013). We also note that past research based exclusively on questionnaire data failed to detect an interaction between positive and negative emotion and emotional control (e.g. Kim et al., 2007). Therefore, our findings present preliminary evidence suggesting that the moderating effects of cognitive control may be more sensitively detected by neural markers, which may not be observable at a behavioral level.
Our cross-sectional findings support previous research that implicates the important role of affect in the development of internalizing and externalizing symptomatology. Consistent with prior findings indicating powerful predictions of NA on children and adolescents’ internalizing and externalizing symptomatology (Eisenberg et al., 2009; Kim et al., 2007; Langhinrichsen-Rohling et al., 2004; Silk et al., 2003), we found concurrent associations between higher NA and higher internalizing and externalizing symptomatology at Time 1. However, there was no moderating effect of cognitive control, suggesting that the main effects of NA on symptomatology were uniformly strong regardless of neurocognitive vulnerability. This pattern was also true for the concurrent effect of PA on internalizing symptomatology; higher PA was associated with less internalizing symptomatology, regardless of level of cognitive control. Adhering to Luthar et al.’s (2000) conceptualization of risk and resilience, in the current findings NA can be understood as a “vulnerable-stable” factor that has consistent disadvantageous effects regardless of risk level (i.e., cognitive control), whereas PA can be understood as a “protective-stabilizing” factor that has consistent advantageous effects despite increasing risk level.
The current findings should be interpreted in the context of study limitations. First, our measures of affect and internalizing and externalizing symptomatology were based on adolescent self-report. Self-report measurement can be revealing for symptomatic behaviors related to private or internal experience such as emotion assessment (e.g., Kendall, Cantwell, & Kazdin, 1989) and thus may be especially important for our understanding of affect and internalizing symptomatology. However, it is possible that our reliance on self-report may suffer from within-subject bias and method variance, and would benefit from multiple informants and multiple methods. Second, future studies should extend our longitudinal findings to investigate the joint contributions between affect and cognitive control to psychopathology beyond middle adolescence as PFC functioning continues to develop throughout late adolescence and young adulthood (Ordaz et al., 2013). Finally, we note that we used a median split of the cognitive control factors (i.e., high versus low) and there is a concern regarding dichotomizing continuous variables (e.g., MacCallum, Zhang, Preacher, & Rucker, 2002) particularly due to reduced power by simplifying the variance. However, we chose to use the multiple group SEM approach because it allowed us to systematically test where moderating effects of cognitive control were significant by imposing equality constraints one path at a time and examining changes in model fit using indices that were not influenced by sample size.
Despite these limitations, there are several strengths to the current study that offer meaningful contributions to the literature on the development of adolescent psychopathology. We tested theoretically-based moderation effects using both behavioral and neural indicators of cognitive control allowing us to systematically examine differential roles of task-based blood oxygenation level—dependent (BOLD) responses versus manifest behaviors. Furthermore, our measurement of neural cognitive control was based on latent factor scores using BOLD responses from multiple ROIs that were correlated with behavioral responses and empirically demonstrated to be neural substrates of cognitive control in prior research, thus providing a comprehensive indicator of prefrontal function.
Taken together, the current findings make several important contributions to our understanding of emotional risk factors in conjunction with prefrontal functioning in adolescence. First, we reinforce previous findings that implicate PA as a protective factor that promotes trajectories of adaptation. Using neuroimaging data, we offer novel evidence that PA is not universally protective against externalizing symptomatology, but may be more specifically beneficial to individuals with poor prefrontal functioning. In particular, we have elucidated these processes during a developmental period that is characterized by dramatic changes in emotion and prefrontal functioning, thereby identifying possible targets for timely intervention. That is, the prospective benefit of PA does not manifest in adolescents with strong prefrontal functioning and is germane to early adolescents who exhibit neurobiological vulnerability related to cognitive control. Recently, “positive interventions” have been developed in order to increase positive emotion and foster a sense of well-being to optimize psychological and physical health. Quoidbach, Mikolajczak, and Gross (2015) offer a conceptual framework for implementation of such interventions, and our findings indicate that efforts would be most effective in vulnerable populations with poor neural cognitive control, together laying the groundwork for effective intervention strategies in adolescents with externalizing symptomatology. Knowing that adolescents with exceedingly poor neural cognitive control did not benefit from the PA effect, paying attention to potential mechanisms for improvement in prefrontal functioning related to cognitive control is also important. More broadly, our findings provide promising insights for preventive intervention efforts to impede trajectories of maladaptation by considering how emotion and cognitive control interface with each other during the emotionally salient period of adolescence.
Acknowledgments
This work was supported by grants awarded to Jungmeen Kim-Spoon and Brooks King-Casas from the National Institute on Drug Abuse (DA036017). Funding source had no involvement in study design, data collection and analysis, or writing of the report/submission for publication. We are grateful to the adolescents and parents who participated in this study.
References
- Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: Department of Psychiatry, University of Vermont; 2001. [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Erlbaum; 1996. pp. 243–277. [Google Scholar]
- Arbuckle JL. Amos (Version 23.0) [Computer Program] Chicago: IBM SPSS; 2014. [Google Scholar]
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the National Comorbidity Survey-- adolescent supplement: Prevalence, correlates, and treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54:37–44. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen, Long S, editors. Testing structural equation models. Beverly Hills: Sage; 1993. pp. 136–162. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive. Science. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: Validation study with fMRI in individual subjects. Molecular Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galván A, Somerville LH. Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling. 2002;9:233–255. doi: 10.1207/S15328007SEM0902_5. [DOI] [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70:6–20. doi: 10.1037/0022-006X.70.1.6. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social - affective engagement and goal flexibility. Nature. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis M, Suveg C. Focusing on the positive: A review of the role of child positive affect in developmental psychopathology. Clinical Child and Family Psychology Review. 2014;17:97–124. doi: 10.1007/s10567-013-0162-y. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/S0954579497001375. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, et al. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity, and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JP, Kim-Spoon J. The development of adolescent self-regulation: Reviewing the role of parent, peer, friend, and romantic relationships. Journal of Adolescence. 2014;37:433–440. doi: 10.1016/j.adolescence.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Perkins SC, Angstadt M, Johnson T, Stern ER, Welsh RC, Taylor SF. The development of performance-monitoring function in the posterior medial frontal cortex. Neuroimage. 2010;49:3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. Cultivated emotions: Parental socialization of positive emotions and self-conscious emotions. Psychological Inquiry. 1998;9:279–281. doi: 10.1207/s15327965pli0904_4. [DOI] [Google Scholar]
- Galvan A, Poldrack RA, Baker CM, McGlennen KM, London ED. Neural correlates of response inhibition and cigarette smoking in late adolescence. Neuropsychopharmacology. 2011;36:970–978. doi: 10.1038/npp.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala - prefrontal circuitry. Journal of Neuroscience. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.344612.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KE. The neglected role of positive emotion in adolescent psychopathology. Clinical Psychology Review. 2012;32:467–481. doi: 10.1016/j.cpr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Vogt A. Joint Statistical Meetings. San Diego, CA: American Statistical Association; 2012. Outliers: An evaluation of methodologies; pp. 3455–3460. [Google Scholar]
- Guyer J, Silk EE, Nelson AE. The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews. 2016;70:74–85. doi: 10.1016/j.neubiorev.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanish LD, Eisenberg N, Fabes RA, Tracy L, Ryan P, Schmidt S. The expression and regulation of negative emotions: Risk factors for young children’s peer victimization. Development and Psychopathology. 2004;16:335–353. doi: 10.1017/S0954579404044542. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/S0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Kim-Spoon J, Deater-Deckard K. Executive function and social interaction: The role of peer stress. Journal of Abnormal Child Psychology. 2016;44:31–42. doi: 10.1007/s10802-015-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JM, Oatley K. Psychopathology and short-term emotion: The balance of affects. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41:463–72. doi: 10.1017/S0021963000005709. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Cantwell DP, Kazdin AE. Depression in children and adolescents: Assessment issues and recommendations. Cognitive Therapy Research. 1989;13:109–146. [Google Scholar]
- Khazanov GK, Ruscio AM. Is low positive emotionality a specific risk factor for depression? A meta-analysis of longitudinal studies. Psychological Bulletin. 2016;142:991–1015. doi: 10.1037/bul0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Walden T, Harris V, Karrass J, Catron T. Positive emotion, negative emotion, and emotion control in the externalizing problems of school-aged children. Child Psychiatry and Human Development. 2007;37:221–239. doi: 10.1007/s10578-006-0031-8. [DOI] [PubMed] [Google Scholar]
- Kim J, Deater-Deckard K. Dynamic changes in anger, externalizing and internalizing problems: attention and regulation. Journal of Child Psychology and Psychiatry. 2011;52:156–166. doi: 10.1111/j.1469-7610.2010.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Holmes C, Deater-Deckard K. Attention regulates anger and fear to predict changes in adolescent risk-taking behaviors. Journal of Child Psychology and Psychiatry. 2015;56:756–765. doi: 10.1111/jcpp.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Kahn RE, Lauharatanahirun N, Deater-Deckard K, Bickel WK, Chiu PH, King-Casas B. Executive functioning and substance use in adolescence: Neurobiological and behavioral perspectives. Neuropsychologia. 2017;100:79–92. doi: 10.1016/j.neuropsychologia.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3. New York: Guildford Press; 2011. [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Hulle Van CA, Keenan K, Rathouz PJ, Rodgers JL. Temperament and parenting during the first year of life predict future child conduct problems. Journal of Abnormal Child Psychology. 2008;36:1139–1158. doi: 10.1007/s10802-008-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhinrichsen-Rohling J, Arata C, Bowers D, O’Brien N, Morgan A. Suicidal behavior, negative affect, gender, and self-reported delinquency in college students. Suicide & Life-Threatening Behavior. 2004;34:255–66. doi: 10.1521/suli.34.3.255.42773. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner J, Thomas E, Rudolph KD, Potter KI. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. doi: 10.1037/1040-3590.11.3.326. [DOI] [Google Scholar]
- Lee TH, Telzer EH. Negative functional coupling between the right fronto-parietal and limbic resting state networks predicts increased self-control and later substance use onset in adolescence. Developmental Cognitive Neuroscience. 2016;20:35–42. doi: 10.1016/j.dcn.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, West SG, Sandler IN. Temperament as a predictor of symptomatology in children: Addressing contamination of measures. Child Development. 1998;69:164–181. doi: 10.1111/j.1467-8624.1998.tb06141.x. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989X.7.1.19. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Karlsson S, Rieckmann A, Nyberg L, Bäckman L. Aging-related increases in behavioral variability: Relations to losses in dopamine D1 receptors. Journal of Neuroscience. 2012;32:8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski DF, van Lier PA, Branje SJ, Meeus WH, Koot HM. A 5-year longitudinal study on mood variability across adolescence using daily diaries. Child Development. 2015;86:1908–1921. doi: 10.1111/cdev.12420. [DOI] [PubMed] [Google Scholar]
- Maciejewski DF, van Lier PAC, Branje SJT, Meeus WHJ, Koot HM. A daily diary study on adolescent emotional experiences: Measurement invariance and developmental trajectories. Psychological Assessment. doi: 10.1037/pas0000312. in press. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A, van Lier PA, Frijns T, Meeus W, Koot HM. Emotional dynamics in the development of early adolescent psychopathology: A one-year longitudinal study. Journal of Abnormal Child Psychology. 2011;39:657–669. doi: 10.1007/s10802-011-9509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. Journal of Neuroscience. 2013;33:18109–18124. doi: 10.1523/jneurosci.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A, Casey BJ. The adolescent brain and the emergence and peak of psychopathology. Journal of Infant, Child, and Adolescent Psychotherapy. 2015;14:3–15. doi: 10.1080/15289168.2015.1004889. [DOI] [Google Scholar]
- Putnam SP. Positive emotionality. In: Zentner M, Shiner RL, editors. Handbook of temperament. New York, NY: Guilford Press; 2012. pp. 105–123. [Google Scholar]
- Quoidbach J, Mikolajczak M, Gross JJ. Positive interventions: An emotion regulation perspective. Psychological Bulletin. 2015;141:655–693. doi: 10.1037/a0038648. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience and Biobehavioral Reviews. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KI, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. Journal of Cognitive Neuroscience. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Salk RH, Petersen JL, Abramson LY, Hyde JS. The contemporary face of gender differences and similarities in depression throughout adolescence: Development and chronicity. Journal of Affective Disorders. 2016;205:28–35. doi: 10.1016/j.jad.2016.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental Science. 2015;18:771–784. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou K, Davey CG, Kerestes R, Whittle S, Pujol J, Yücel M, Harrison BJ. Brain functional correlates of emotion regulation across adolescence and young adulthood. Human Brain Mapping. 2016;37:7–19. doi: 10.1002/hbm.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. Journal of Personality. 2009;77:1747–1775. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118(1):117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef GJ, Whittle S, Allen NB, Lubman DI, Simmons JG, Yücel M. Cognitive control as a moderator of temperamental motivations toward adolescent risk-taking behavior. Child Development. 2016;87:395–404. doi: 10.1111/cdev.12480. [DOI] [PubMed] [Google Scholar]
- Zielinski D, Bradshaw CP. Ecological influences on the sequelae of child maltreatment: A review of the literature. Child Maltreatment. 2006;11:49–62. doi: 10.1177/1077559505283591. [DOI] [PubMed] [Google Scholar]