Abstract
Rhesus macaque is an important animal model for studies testing interventions like antibody therapeutics, as such knowledge of inter-individual variations in function of genes affecting antibody recycling is important for optimal experimental design. Neonatal Fc receptor (FcRn), a heterodimer composed of FCGRT and β2-m chains, plays critical role in extending catabolic half-life of IgG. We studied genomic polymorphisms in rhesus macaque FcRn and asked if they are functional by assessing correlations with serum IgG or β2-m levels. We tested 75 animals and report the presence of a VNTR polymorphism in promoter of FcRn as well as a single nucleotide polymorphism in the signal peptide of β2-m. A VNTR minor allele was associated with lower levels of serum IgG. This polymorphism may account for inter-animal variation in antibody levels and has relevance for effective design of rhesus macaque studies investigating vaccine induced antibody responses and passive immunizations.
Introduction
The neonatal Fc receptor (FcRn) is a heterodimer of a transmembrane MHC class I like protein (α-chain, encoded by FCGRT gene) and β2 microglobulin (β2-m). FcRn plays critical roles in IgG and albumin homeostasis, IgG transcytosis across polarized epithelium, and antigen presentation. Within the acidic environment of endosomes, FcRn binds the Fc region of endocytosed monomeric IgG and recycles it back to the extracellular space via the exocytic pathway, thereby protecting IgG from intracellular degradation in lysosomes (Reichert 2005). This property of FcRn to temporarily protect IgG from harsh circulatory conditions imparts IgG1 with a long serum half-life of 7–21 days.
Therapeutic use of monoclonal antibody (mAb) products is being explored for various cancers, autoimmune diseases, and infectious diseases including HIV (Nissim and Chernajovsky 2008; Reichert 2005; Reichert et al. 2005). Passive transfer of neutralizing antibodies has shown protection against HIV infection in the rhesus macaque challenge model (Moldt et al. 2011) (Mascola et al. 1999) and is a potential treatment or prophylaxis option. The effectiveness of such antibody treatments will depend on the bioavailability of these compounds in serum. IgG affinity for FcRn determines it’s serum half-life as shown by studies in which amino acid substitutions in the Fc domain of IgG modify the affinity to FcRn and change the serum half-life of the IgG (Dall’Acqua et al. 2006; Freiberger et al. 2010a; Hinton et al. 2006; Mascola et al. 1999; Petkova et al. 2006; Vaccaro et al. 2005; Yeung et al. 2009). In an intravenous immunoglobulin (IVIG) therapy trial of common variable immunodeficiency (CVID) patients, the expression levels of FcRn inversely correlated with the rate of IgG decline (Freiberger et al. 2010a), showing a role for FcRn in IVIG catabolism. FcRn also impacts the magnitude and duration of the humoral response to external immunogens. In mice, over-expression of FcRn was shown to result in a potent and long lasting antigen specific humoral response to a weak immunogen (Vegh et al. 2011). Interestingly, such over-expression of FcRn also resulted in expansion of antigen specific B cells and plasma cells (Cervenak et al. 2011; Vegh et al. 2011; Vegh et al. 2012). In the same transgenic model, Influenza vaccination produced a 2-fold increase in the amount of virus-specific antibody production in FcRn transgenic animals, and the antibodies were more efficient in a hemagglutination inhibition assay (Cervenak et al. 2011). Variations in FcRn expression or function are thus likely to influence the fate of antibodies whether passively transferred or vaccine induced.
Host genetic polymorphisms in the FCGRT gene can influence the expression of FcRn or binding of this receptor to IgG Fc. Several single nucleotide polymorphisms in a Japanese population were described (Ishii-Watabe et al. 2010), however, the variants studied had similar IgG recycling efficiency in in vitro assays. Other polymorphisms are known to influence antibody binding and can thus influence immune responses. A variable number of tandem repeats (VNTR) polymorphism in the human FcRn gene was shown to affect FcRn expression levels and total IgG binding (Sachs et al. 2006). Such a variation can influence the pharmacokinetics of therapeutic antibodies. Anti-TNF antibodies administered to IBD patients with a VNTR2/VNTR3 genotype had lower induction concentrations than in VNTR3 homozygote patients (Billiet et al. 2016). However, this polymorphism was not shown to influence maternal IgG transfer across the placenta (Freiberger et al. 2010b). Other single nucleotide polymorphisms in FCGRT showed association with serum IgG concentration in new born calves (Laegreid et al. 2002) and influenced IgG levels in bovine colostrum (Zhang et al. 2009). Polymorphisms in β2M are less studied, however, a rare single nucleotide polymorphism was identified in signal peptide sequence of β2-m, which results in hypercatabolic hypoproteinemia in humans by disrupting the function of β2-m (Wani et al. 2006).
It is not known if functional genetic variations within FcRn exist in rhesus macaques that can influence the inter-individual variation in antibody levels generated by these approaches. Here we studied polymorphisms in the two genes encoding heterodimeric FcRn in rhesus. We identified variations with potential functional implications that were not reported earlier and observed an association of VNTR alleles with serum IgG levels. Further studies are needed to confirm if this polymorphism affects antibody catabolism as this may impact design of animal studies investigating either vaccine induced antibody levels or fate of passively transferred antibodies.
Materials and methods
Genomic DNA isolation
Peripheral blood mononuclear cell samples from 75 Indian rhesus macaques were used as source of genomic DNA. The animals were part of past projects at the Institute of Human Virology that were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine. Frozen PBMC and serum/plasma samples from animals were available to us for the present study.
DNA was isolated from PBMC using EZ DNA isolation kit from ZymoResearch (Irvine, CA, USA) according to the manufacturer’s instructions.
PCR and Sequencing
The sequence of rhesus FcRn gene is available at NCBI (Gene ID: 718859, NCBI Reference Sequence: NC_027911.1) and was used for primer design. FCGRT exons 2–6 and β2-m exons 1–3 were amplified and then sequenced directly using the primers used for amplification. Primers for non-synonymous and synonymous SNPs in FCGRT and β2-m are the same as described previously by others (Uno et al. 2014). PCR was carried out in 25 μl reaction volume containing 10 ng genomic DNA, 1x PCR buffer, 10 mM dNTPs, 0.5 pMol primers and 1 unit AmpliTaq Gold DNA polymerase (Thermo Fisher Scientific). Cycling conditions used were initial denaturation at 95 °C for 5 min, 35 cycles at 95 °C for 30s, 60 °C for 30s and 72 °C for 1 min with 10 minutes final extension at 72 °C. The VNTR polymorphism shown in human FCGRT promoter (Sachs et al. 2006) has not been reported previously in macaques. In order to test for this polymorphism, we designed primers 5′-CTGAAGGGAACGTGAGCCGA-3′ and 5′-CTCGGTCCAGACTGACAACAA-3′ which amplify FcRn promoter region homologous to the VNTR polymorphism region reported for humans. HotStarPlus MasterMix kit (Qiagen) containing HotStarTaq Plus DNA Polymerase was used with cycling conditions of initial denaturation at 95 °C for 5 min, 35 cycles at 94 °C for 30s, 60 °C for 30s and 72 °C for 1 min with 7 minutes final extension at 72 °C. This polymorphism is present as variable number of tandem repeats in the amplified region and appears as bands of varying sizes in animals with different alleles. In order to sequence this polymorphism, individual bands were gel excised and purified (Qiaquick gel extraction kit, Qiagen), followed by cloning in Topo TA Cloning vector (Thermo Fisher Scientific) using standard procedures and sequenced using M13 forward and reverse primers.
ELISA
To understand any influence of identified polymorphisms on serum IgG levels, Monkey IgG ELISA kit (IGG-3 Life Diagnostics, Inc) was used in accordance with the manufacturer’s instructions. The β2-m polymorphism (G>C) is located in the signal peptide of β2-m. This polymorphism has potential relevance for transport of β2-m across golgi and hence its extracellular concentration. To test this, β2-m levels in plasma were measured with Monkey β2-m ELISA kit (My Biosource) using manufacturers instructions. Association between levels of IgG or β2m with genotype was tested using Kruksal-Wallis test and Mann-Whitney tests.
Phenotype assessment of β2-m signal peptide polymorphism
The polymorphism in β2-m signal peptide (G>C) might influence efficacy of β2-m extracellular transport and hence its concentration on cell surface. This was tested by comparing the ability of signal peptide variants for trafficking GFP across golgi/ER. β2-m signal peptide cDNA sequence with either the G or C allele was amplified and cloned upstream of GFP. Signal peptide gene sequences were synthesized into pEF-GFP vector through Gen script and cloned in XL-2 competent cells. DNA from colonies was sequenced to test for presence of desired signal peptide sequence and colonies were amplified in LB broth. HEK 293 T cells were transfected with individual signal peptide DNA using the Lipofectamine 2000 reagent (Invitrogen). Localization of GFP in golgi/ER of 293T cells was studied by confocal microscopy to test whether signal peptide variants have different subcellular localization of GFP. The efficiency of protein expression and secretion may vary depending on the sequence of the signal peptide. Flow cytometry of transfected 293 T cells was used to compare the intensity of GFP expression in constructs with either variant of signal peptide.
Results and Discussion
A VNTR polymorphism in the promoter of rhesus FcRn promoter
The promoter region of human FcRn contains a 37 bp VNTR polymorphism known to influence IgG binding efficiency by transcriptionally changing expression levels of FcRn (Sachs et al. 2006). As rhesus macaques are routinely used to test vaccination approaches like passive immunization with monoclonal antibodies (Gautam et al. 2016; Mascola et al. 1999), a similar variation in these animals can significantly impact interpretation of such studies. It is not known if this animal specie has a similar variation. We genotyped FCGRT from rhesus macaques and found a similar repeat region in FCGRT promoter region like present in humans with 70% similarity to the homologous region from human.
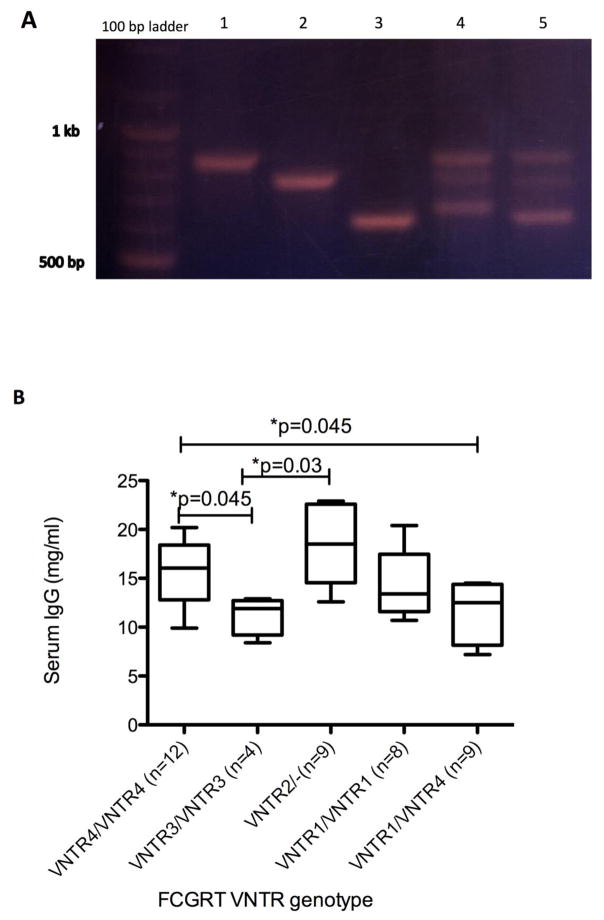
The PCR bands corresponding to different variants were gel excised and sequenced. Sequencing results show the polymorphism in rhesus has differences with the one reported in humans. Instead of 1–5 repeats of 37 bp reported for humans, corresponding rhesus region shows between 1–7 repeats of 37 bp region. These are described as VNTR1 through VNTR4: VNTR1 is 634 bp region in PCR gel with 1 repeat, VNTR2 is 671 bp with 2 repeats, VNTR3 is 782 bp with 5 repeats and VNTR4 is 856 bp region with 7 repeats (Figure 1A). The GenBank accession numbers for the sequences are: MF157529-MF157532.
Figure 1.
VNTR polymorphism in promoter of rhesus macaques FCGRT. A. Lanes with different VNTR alleles are as follows. 1: VNTR4/VNTR4; 2: VNTR3/VNTR3; 3: VNTR1/VNTR1; 4: VNTR2/VNTR4; 5: VNTR1/VNTR4. B. Average serum IgG levels in animals with major VNTR genotypes in our study are shown. Number of animals tested in each genotype is given in parentheses. VNTR2 heterozygotes are denoted as VNTR2/-.
The frequency of VNTR alleles in our animals are: VNTR1 (26%), VNTR2 (13.2%), VNTR3 (8.8%) and VNTR4 (52%).
In humans, the receptor with 3 repeats of a 37 bp region (VNTR3) is associated with a 1.66 fold increase in FcRn expression compared to one with 2 repeats (VNTR2) and this results in increased IgG binding by VNTR3 homozygotes compared with VNTR2/3 heterozygotes. Our ELISA results showed that animals with VNTR2 heterozygous and VNTR4/VNTR4 genotypes had significantly higher IgG levels compared with those carrying VNTR3 alleles (p<0.05) (Figure 1B). While this difference is only marginally significant, nevertheless it has potential to impact data interpretation from studies, especially those using small numbers of animals per experimental group.
Non-synomous polymorphisms in FCGRT and β2-m
We confirmed the presence of several single nucleotide polymorphisms in FCGRT exons 2–6 and in β2-m (exon 1–3); these were reported earlier in cynomolgus and rhesus macaques (Uno et al. 2014) and data from those is not shown here. Similar to that previous study, we did not find any non-synonymous polymorphisms in FCGRT gene in rhesus macaques, which were present in cynomolgous macaques from that study. The synonymous SNPs are not very likely to influence FCGRT function and hence IgG levels, nevertheless they are also under selection pressure like non-synonymous alleles and can potentially impact protein function (Hunt et al. 2009). To address the functional significance of these, we tested for correlations between variant alleles (Uno et al. 2015) and serum IgG or β2-m levels that were determined by ELISA. None of these polymorphisms were associated with levels of IgG or β2-m in animal sera (data not shown).
Polymorphism in β2-m signal peptide
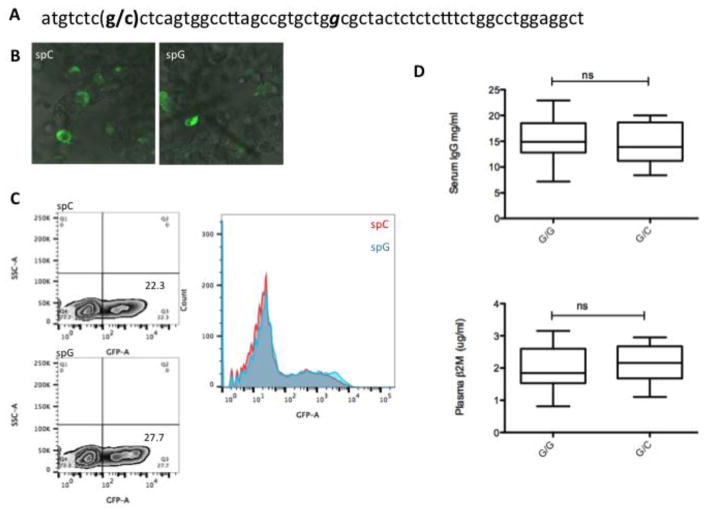
In this study, a single nucleotide polymorphism was found located in the β2-m signal peptide (Figure 2A). The allelic distribution of C versus G at the position of change was 30/70 in our rhesus macaques. Report of another polymorphism in humans in β2-m signal peptide (Wani et al. 2006) (Figure 2A) which has strong association with reduced IgG and soluble β2-m levels in serum, prompted us to investigate if this polymorphism in rhesus influences the function of signal peptide. For this, the signal peptide was cloned upstream of GFP and tested for it’s ability to modulate GFP expression as well as for trafficking of GFP in transfected HEK-293 T cells. Confocal image analysis shows similar ER/golgi distribution of constructs with either variant (Figure 2B); flow cytometry showed similar levels of GFP expression in 293T cells transfected with either construct (Figure 2C). Moreover, there was no difference in the levels of either IgG or β2-m in plasma from animals harboring either of the variant (Fig 2D). These results indicate that the observed polymorphism does not affect the expression or secretion of β2-m in rhesus macaques.
Figure 2.
Single nucleotide polymorphism in β2-m signal peptide of rhesus macaque. A. C/G polymorphism reported in this study is shown in parentheses. The human β2-m signal peptide polymorphism associated with hypercatabolic hypoproteinemia (see text) is italicized. B. Confocal images showing similar golgi/ER targeting of GFP by constructs with either signal peptide (spC or spG). GFP was cloned downstream of the signal peptide as described in methods. C. Flowcytomtery histogram overlay shows similar GFP expression in 293T cells transfected with variant constructs. D. ELISA results for serum IgG and plasma β2m levels in animals with β2-m signal peptide GC or GG alleles.
In conclusion, we report the presence of a VNTR polymorphism in rhesus macaques that has known functional significance in humans. Our observation of potential significance of this polymorphism in impacting IgG levels warrants further characterization in larger studies. Others have shown that this polymorphism does not influence maternal-fetal IgG transfer (Freiberger et al. 2010b). However, the VNTR polymorphism in humans influences IgG binding capacity and pharmacokinetics of injected antibody drugs (Billiet et al. 2016). Thus, further studies should determine if catabolism of vaccine induced IgG or of passively transferred antibody drugs is impacted by it.
Acknowledgments
This work was supported by National Institutes of Health award R03AI110229 (to B. P.)
References
- Billiet T, Dreesen E, Cleynen I, Wollants WJ, Ferrante M, Van Assche G, Gils A, Vermeire S. A Genetic Variation in the Neonatal Fc-Receptor Affects Anti-TNF Drug Concentrations in Inflammatory Bowel Disease. Am J Gastroenterol. 2016;111:1438–1445. doi: 10.1038/ajg.2016.306. [DOI] [PubMed] [Google Scholar]
- Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, Erdei A, Bosze Z, Kacskovics I. Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J Immunol. 2011;186:959–68. doi: 10.4049/jimmunol.1000353. [DOI] [PubMed] [Google Scholar]
- Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–24. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- Freiberger T, Grodecka L, Ravcukova B, Kurecova B, Postranecka V, Vlcek J, Jarkovsky J, Thon V, Litzman J. Association of FcRn expression with lung abnormalities and IVIG catabolism in patients with common variable immunodeficiency. Clin Immunol. 2010a;136:419–25. doi: 10.1016/j.clim.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Freiberger T, Ravcukova B, Grodecka L, Kurecova B, Jarkovsky J, Bartonkova D, Thon V, Litzman J. No association of FCRN promoter VNTR polymorphism with the rate of maternal-fetal IgG transfer. J Reprod Immunol. 2010b;85:193–7. doi: 10.1016/j.jri.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–9. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–56. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- Hunt R, Sauna ZE, Ambudkar SV, Gottesman MM, Kimchi-Sarfaty C. Silent (synonymous) SNPs: should we care about them? Methods Mol Biol. 2009;578:23–39. doi: 10.1007/978-1-60327-411-1_2. [DOI] [PubMed] [Google Scholar]
- Ishii-Watabe A, Saito Y, Suzuki T, Tada M, Ukaji M, Maekawa K, Kurose K, Kaniwa N, Sawada J, Kawasaki N, Yamaguchi T, Nakajima TE, Kato K, Yamada Y, Shimada Y, Yoshida T, Ura T, Saito M, Muro K, Doi T, Fuse N, Yoshino T, Ohtsu A, Saijo N, Hamaguchi T, Okuda H, Matsumura Y. Genetic polymorphisms of FCGRT encoding FcRn in a Japanese population and their functional analysis. Drug Metab Pharmacokinet. 2010;25:578–87. doi: 10.2133/dmpk.dmpk-10-rg-067. [DOI] [PubMed] [Google Scholar]
- Laegreid WW, Heaton MP, Keen JE, Grosse WM, Chitko-McKown CG, Smith TP, Keele JW, Bennett GL, Besser TE. Association of bovine neonatal Fc receptor alpha-chain gene (FCGRT) haplotypes with serum IgG concentration in newborn calves. Mamm Genome. 2002;13:704–10. doi: 10.1007/s00335-002-2219-y. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–18. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. J Virol. 2011;85:10572–81. doi: 10.1128/JVI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim A, Chernajovsky Y. Historical development of monoclonal antibody therapeutics. Handb Exp Pharmacol. 2008:3–18. doi: 10.1007/978-3-540-73259-4_1. [DOI] [PubMed] [Google Scholar]
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, Presta LG, Meng YG, Roopenian DC. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–69. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- Reichert JM. Monoclonal antibodies as innovative anti-infective agents. Discov Med. 2005;5:544–7. [PubMed] [Google Scholar]
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–8. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- Sachs UJ, Socher I, Braeunlich CG, Kroll H, Bein G, Santoso S. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor alpha-chain promoter. Immunology. 2006;119:83–9. doi: 10.1111/j.1365-2567.2006.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Matsushita A, Murayama N, Yamazaki H. Genetic polymorphism of cynomolgus and rhesus macaque CYP2C9. Drug Metab Pharmacokinet. 2015;30:130–2. doi: 10.1016/j.dmpk.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Uno Y, Utoh M, Iwasaki K. Polymorphisms of neonatal Fc receptor in cynomolgus and rhesus macaques. Drug Metab Pharmacokinet. 2014;29:427–30. doi: 10.2133/dmpk.dmpk-14-nt-033. [DOI] [PubMed] [Google Scholar]
- Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–8. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- Vegh A, Cervenak J, Jankovics I, Kacskovics I. FcRn overexpression in mice results in potent humoral response against weakly immunogenic antigen. MAbs. 2011;3:173–80. doi: 10.4161/mabs.3.2.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegh A, Farkas A, Kovesdi D, Papp K, Cervenak J, Schneider Z, Bender B, Hiripi L, Laszlo G, Prechl J, Matko J, Kacskovics I. FcRn overexpression in transgenic mice results in augmented APC activity and robust immune response with increased diversity of induced antibodies. PLoS One. 2012;7:e36286. doi: 10.1371/journal.pone.0036286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, Waldmann TA, Robinson JM, Anderson CL. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A. 2006;103:5084–9. doi: 10.1073/pnas.0600548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182:7663–71. doi: 10.4049/jimmunol.0804182. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhao Z, Zhao Y, Kacskovics I, Eijk M, Groot N, Li N, Hammarstrom L. Association of FcRn Heavy Chain Encoding Gene (FCGRT) Polymorphisms with IgG Content in Bovine Colostrum. Anim Biotechnol. 2009;20:242–6. doi: 10.1080/10495390903196448. [DOI] [PubMed] [Google Scholar]