Neuroscientific work has elucidated the importance of social contexts for brain development in adolescence, a time of enormous biological and social change (Davey, Yucel, & Allen, 2008; Nelson & Guyer, 2011; Schriber & Guyer, 2016; Somerville, 2013). One such context is the school setting (Eccles & Roeser, 2011). Ideally, schools provide a supportive environment for fostering youths’ academic and social competencies. Conversely, experiencing hostility at school may sensitize youths to social threat and hinder their development. School violence, including criminal and delinquent acts ranging from vandalizing school property to school shootings, contributes to unsafe feelings at school (Mijanovich & Weitzman, 2003) and antisocial behavior (O’Keefe, 1997). Likewise, discrimination, a form of hostility often along racial/ethnic lines, generates feelings of negativity, alienation, and aggression (Hoskin, 2013). Despite a wealth of evidence that stressful experiences alter the brain (Hertzman, 2012; McEwen & Gianaros, 2011), little is known about how stressors from a hostile school environment (HSE) impinge on neural mechanisms involved in responding to social threat. Thus, the present study examined how HSE exposure predicted neural responses to social threat and increases in social deviance in a sample of Mexican-origin adolescents, for whom HSE exposure is likely.
Indeed, precarious is the position of Mexican-origin adolescents living in the United States. Like other ethnic minority youths, they stand not only at the crossroads of childhood and adulthood, but also at that of two cultures. In each case, they need to negotiate their place entre dos mundos – between two worlds – across a variety of social settings. Due to the concentration of poverty among people of Mexican origin, Mexican-origin youths are at greater risk for attending schools beset by violence (Clauss-Ehlers & Levi, 2002). Such settings also feature problems with ethnic discrimination (Cartledge & Johnson, 2004), a “symbolic violence” (Henry, 2000) that can critically affect minority youths’ self-views (Fisher, Wallace, & Fenton, 2000). These school-based risk factors compound each other (Soriano & Soriano, 1994). Their co-occurrence can elicit antisocial behavior at the same time that it is socialized. Despite the contribution of HSE factors to maladjustment in Mexican-origin youths (e.g., Stone & Han, 2005), little attention has been paid to neurobiological mechanisms. No work to date has examined how HSE exposure relates to neural sensitivity to social exclusion, a particularly aversive experience in adolescence (Sebastian, Viding, Williams, & Blakemore, 2010) that for Mexican-origin youths may signal the greater potential for encountering disrupted social bonds.
In the present study, we aimed to identify the neurobiological pathways by which HSE exposure is detrimental to adolescent development. At the theoretical level, experiencing a HSE is likely to affect adolescents’ identity formation, peer relations, academic achievement, occupational goals, mental health, and physiological functioning (Eccles, Early, Fraser, Belansky, & McCarthy, 1997). We thus examined whether HSE exposure affects how adolescents engage with their social environments, including at the neurobiological level. We further investigated how potential buffers of neurobiological risk, such as positive family influences (Fuligni & Telzer, 2013), operate. At the applied level, examining how brain function is shaped by HSE exposure and prospestively predicts outcomes (Berkman & Falk, 2013) can inform the development of interventions aimed at combatting the negative effects of a HSE.
Neural responses to social exclusion
Social exclusion threatens our fundamental need to belong (Baumeister & Leary, 1995). Research on neural responses to social exclusion has largely used functional magnetic resonance imaging (fMRI) paired with the Cyberball task (Eisenberger, 2003), an ecologically-valid measure in which participants are included or excluded from a simulated ball-tossing game. Studies using Cyberball have implicated social-affective regions (e.g., subgenual anterior cingulate cortex, subACC; anterior insula, AI) in the subjective distress of being excluded and regulatory regions (e.g., ventrolateral and dorsolateral prefrontal cortex, vlPFC and dlPFC) in the top-down control of this distress (Eisenberger, 2003; Masten et al., 2009).
The subACC, in particular, has emerged as a key region for understanding responses to social exclusion in adolescence. Greater subACC reactivity to exclusion is seen in adolescents (versus adults) that reflects greater distress over being excluded (Masten et al., 2009) and predicts increases in depressive symptoms over one year (Masten et al., 2011). This sensitivity also prospectively predicts adolescents’ susceptibility to peer influence on their risk-taking behaviors (Falk et al., 2014). Involvement of subACC in social exclusion makes sense given this region’s role in negative affect (Bush et al., 2000); emotion conflict (Etkin et al., 2006), including in the context of higher neuroticism (Haas et al., 2007); self-appraisal (Rosen et al., 2010); and social learning (Behrens et al., 2008). These findings highlight the subACC as a mechanism of (1) affective processing, contributing to the “sting” of social exclusion, and (2) social susceptibility, signaling misalignment with the group and a possible need to conform. These ideas converge on the hypothesis that HSE exposure promotes social deviance through subACC response to social exclusion, such as due to rejection-aggression links that inherently involve hostility (Leary, Twenge, & Quinlivan, 2006) and rejection-susceptibility links that could socialize hostility if it is normative (Carter-Sowell, Chen, & Williams, 2008).
Social-contextual stressors and neural responses to social exclusion
Social stressors like those from a HSE are rarely studied with regard to brain-behavior relations. Only one study in adults examined neural responses to perceived discrimination as it took place (Masten, Telzer, & Eisenberger, 2011). In this study, Black participants believed that they were playing Cyberball with White participants. Those attributing social exclusion to racial bias showed less activity in dorsal ACC (dACC) and more in rostral ACC in an area close to subACC, in ventral ACC (vACC). Using a different social stress paradigm – performing difficult tasks in front of critical observers – another cross-sectional study found that ethnic minority status and more perceived discrimination in everyday life were positively related to activation of and connectivity with pregenual ACC, also in vACC (Akdeniz et al., 2014). Ventral, as opposed to dorsal, ACC is highly interconnected with the limbic system (Bush et al., 2000). This suggests that discriminatory experiences alter how emotionally charged information is neurally processed. Of note, no work to date has examined how school violence relates to these neural sensitivities.
In addition, only a few studies have examined how neural responses to social threat are shaped by one’s history in social contexts. Two studies conducted across developmental periods that included adolescence showed effects of social context on dACC response. This portion of ACC, more prominent in the adult social pain literature, is deemed part of the brain’s “alarm system” that aids detection of and responses to predicted versus actual outcomes (Alexander & Brown, 2011). Will et al. (2016) found that adolescents who were chronically rejected versus accepted in childhood showed heightened responses in dACC and anterior PFC during social exclusion. Masten et al. (2012) found that more time spent with friends in high school predicted, in young adulthood, dampened responses to social exclusion in dACC and AI. These results suggest that the social pain system is sensitized over time by social adversity but also shielded from it by past experiences of social support. Given the sensitivity of this neural system to social context, it is likely to track and reflect the nature of one’s social ecology across development.
The framework that guides the present study is “biological embedding” (Hertzman, 2012). Biological embedding refers to the process by which stressful experiences get “under the skin” to affect outcomes. Systematic differences in experience lead to systematic differences in biological states; these alterations stabilize; and long-term changes in health and behavior result. Accordingly, HSE exposure may attune the subACC to social threat, affecting social behavior.
Social-contextual stressors, neural responses to social exclusion, and deviant behavior
At the behavioral level, greater HSE exposure may promote social deviance, here defined as increases in externalizing behaviors and affiliations with deviant peers. We were interested in both types of behaviors as relatively self-directed, proximal reactions to hostility perceived at the school level. Externalizing problems are fueled by negative affect and impaired self-regulation, both often triggered by the experience of social exclusion and other hurtful acts (Baumeister, DeWall, Ciarocco, & Twenge, 2005; Chow, Tiedens, & Govan, 2008). Moreover, the limited routes for achieving status or dominance often encountered by minority youths may render more deviant means for doing so appealing (Thornberry, 1997). In pursuing these means, youths often collude with others. Deviance “spreads” among peers (Dishion, Patterson, & Griesler, 1994), especially in socially disorganized settings (Chung & Steinberg, 2006). Indeed, in these settings, deviant peer affiliations may be adaptive. Delgado, Updegraff, Roosa, and Umaña-Taylor (2011) found that perceived discrimination predicted having deviant peers, “who may represent a family-type unit that can provide protection (outside the actual family home)” (p. 135). Because deviant affiliations and behaviors are interconnected through adolescence into young adulthood (Monahan, Steinberg, & Cauffman, 2009), we examined them together as a function of a HSE.
At the neurobiological level, no studies to our knowledge have examined exclusion-related neural predictors of antisocial behavior in youths. However, relevant studies in adults again highlight the dACC. For example, Chester et al. (2013) found that elevated dACC (and AI) responses to social exclusion predicted greater retaliation (i.e., issuing noise blasts to rejecters) in participants lower in executive functioning. These results are consistent with the view that antisocial acts stemming from social exclusion are “reactive,” elicited by hypersensitivity to social threat and dysregulated negative affect (Blair, 2004). Because exclusion-related distress likewise predicts antisocial outcomes among adolescents (Sandstrom et al., 2003) and because the subACC uniquely tags this distress and predicts subsequent behaviors in social contexts (Falk et al., 2014), we reasoned that subACC sensitivity to social exclusion would mediate the HSE-deviance link. We also examined dACC to assess the specificity of our effects and because of the attention dACC has gained in the literature, including in what have so far been separate looks into the developmental precursors and behavioral effects of neural responses to social exclusion.
Moderation by family connectedness
Finally, due to the role of the family in strengthening social bonds (Hastings, Miller, & Troxel, 2015), we examined whether links among HSE exposure, neural sensitivity to social exclusion, and social deviance would be moderated by family connectedness. Familism, a Mexican cultural value centered on family love and closeness (Rodriguez, Mira, Paez, & Myers, 2007), protects against maladjustment in Mexican-origin youths (e.g., Germán, Gonzales, & Dumka, 2009). These salubrious effects appear to take root in the brain. For example, Mexican-origin youths with greater family obligation values showed less reward-driven ventral striatal activation and more control-related dlPFC activation, both neural response patterns that predicted less risk-taking behaviors (Telzer, Fuligni, Lieberman, & Galván, 2013). We examined sense of family connectedness across the same period as HSE exposure. This allowed us to take the unique approach of jointly considering the developmental impact of two salient and important social contexts, home and school, on behavioral outcomes. Guided by multi-system biopsychosocial models of developmental psychopathology (Cicchetti & Curtis, 2007; Hastings, 2015), in which the interaction of biological and social influences on adjustment is focal, we expected stronger family ties to buffer the extent to which neural responses to social exclusion would reflect past HSE exposure, predict later social deviance, or both.
The present research
In the present study, we examined whether adolescents’ neural responses to social exclusion were related to past exposure to a HSE and increasing levels of social deviance. We also tested whether family connectedness would buffer against this neurobiologically mediated path, if found. We examined these questions in a sample of Mexican-origin adolescents studied longitudinally across high school (grades 9–12). This longitudinal design allowed us to test our hypotheses that (1) HSE exposure would predict increases in social deviance, (2) subACC responses to social exclusion would mediate the HSE-deviance link, and (3) this brain-mediated link would be moderated by family connectedness as a protective factor.
Method
Participants
Two-hundred twenty-nine adolescents (49.3% female; M age at MRI scan = 17.16 years, SD = .41, range = 16.24–17.98 years) were recruited from a 10-year, prospective, longitudinal study of 674 Mexican-origin youths and their families. Most youths (73.8%) were born in the United States (56.3% first-generation; 17.5% second-generation), and 26.2% were born in Mexico. Most (81.1%) reported Spanish as their first language; all were fluent in English. Families were originally recruited based on having a child in grade 5 (age 10) randomly selected from school rosters of the 2006–2007 and 2007–2008 academic years. At the time of recruitment for the current sub-study, youths were in grade 9 (age 14) and distributed across 42 schools. Nine schools had 6 or more participants each (range = 6–35 participants) and were attended by 73% of the recruited sample. The remaining 33 schools were attended by less than 5 participants each (range = 1–4, mode = 1). School enrollments were similarly represented at each wave.
The sub-study was designed to examine neurobiological mechanisms of depression and attempted to oversample youths with elevated levels. Counts of self-reported depressive symptoms in grade 9 (age 14) on the Computerized Diagnostic Interview Schedule for Children-IV (C-DISC, Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000, see below) were used, as well as scores on the General Distress and Anhedonic Depression subscales of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson & Clark, 1991). The proportions of adolescents from the recruited as compared to remaining sample who scored above the median on these measures were as follows: MASQ General Distress, 50.2% vs. 44.5% (median = 1.30); MASQ Anhedonic Depression, 48.3% vs. 48.2% (median = 1.67); and C-DISC MDD symptom counts, 46.5% vs. 42.3% (median = 3). A dichotomous recruitment status variable (1 = scored above the median on any recruitment measure, 0 = scored below the median on all measures) was included as a covariate in analyses due to the focus of the current study on social deviance.
Of the 229 recruited adolescents, ten were ineligible for scanning (e.g., had contraindicated dental ware, history of epilepsy, discomfort with the scanner), and two had unavailable neuroimaging data due to scanner malfunction, resulting in 217 youths who provided neuroimaging data. Of these, 36 were omitted from final analyses for reasons of data quality: 35 had neuroimaging data showing artifact (e.g., motion, ghosting), and one did not understand the task. Another 15 were omitted because their neuroimaging data were collected after the outcome variable (see below), resulting in a final sample of 166 youths (54.4% female; M age at MRI scan = 17.18 years, SD = .41, range = 16.24–17.98 years) from 40 schools. All parents provided informed consent and youths gave their assent. Participants were compensated for participating in this study, which was approved by the Institutional Review Board.
Procedure
The main independent variable, past exposure to a hostile school environment, was assessed annually across grades 9, 10, and 11 (Waves 1, 2, and 3). The potential moderator, past family connectedness, was measured in grades 9 and 11 (Waves 1 and 3). An average of 6.09 months (SD = 3.56) after Wave 3, in grades 11 or 12, neural responses to social exclusion, a potential mediator, were measured during a visit involving an MRI scan. Finally, an average of 4.52 months (SD = 3.56) after the MRI visit, in grade 12 (Wave 4), our outcome variable, later social deviance, was measured. Baseline social deviance was measured at Wave 1 to control for initial levels. Because the Wave 4 assessment preceded the MRI visit for 14 participants and was missing for another, these 15 participants were excluded from final analyses. The remaining 166 youths had a Wave 4 assessment an average of 5.37 months (SD = 2.77) after the MRI visit.
Measures
Hostile school environment
Past HSE exposure was represented by two constructs that focused on peers as a source of hostility at school. One was perceived discrimination, reported by adolescents about the extent to which peers discriminate against Mexicans/Mexican-Americans at school. We used Johnston and Delgado’s (2004) measure based on the Racism in the Workplace Scale (Hughes & Dodge, 1997) and Schedule of Sexist Events (Klonoff & Landrine, 1995). Five items (e.g., “Kids at school dislike Mexicans/Mexican-Americans”) were rated on a 4-point scale (1=Not at all true to 4=Very true) and averaged. To measure perceived discrimination across high school, we averaged scores across grades 9–11. Reliability was adequate (αs = .62–.77), and scores were moderately stable (rs = .41–.44, p < .001).
The second construct, school violence, was assessed as the prevalence of criminal and delinquent peer behaviors observed at school using the Violence, Gangs, and Crime in Schools Scale, adapted from the Neighborhood Criminal Events Scale (Aneshensel & Sucoff, 1996). Ten items (e.g., “How often are there groups of kids hanging around who make you feel unsafe?”) were rated on a 4-point scale (1=Almost never or never to 4=Almost always or always) and averaged. We averaged school violence scores across high school in grades 9–11. Reliability was high (αs = .87–.88), and scores were moderately stable (rs = .42–.57, p < .001).
Indices of perceived discrimination and school violence were significantly positively correlated (r = .36, p < .001). Due to the relevance of both to Mexican-origin youths and to limit the number of tests, they were averaged to derive a single measure of HSE exposure. Across all schools with at least 2 participants and at each wave (15–17 schools per wave), low intraclass correlation coefficients (ICCs = .00–.12) indicated low between-school variance in HSE ratings, suggesting that HSE exposure was more of an individual than a school-level phenomenon.
Family connectedness
Feelings of closeness, love, and support in the family were reported by adolescents on a measure of familism (Villarreal, Blozis, & Widaman, 2005), a widely upheld value in Hispanic culture emphasizing the centrality of the family. Five items (e.g., “Your family is always there for you in times of need”) were rated on a 4-point scale (1=Strongly disagree to 4=Strongly agree) and averaged. To measure family connectedness across high school, concurrent to HSE exposure, we averaged scores from grades 9 and 11. Reliability was good (αs = .81 and .84), and scores were moderately stable (r = .57, p < .001).
Social deviance
Social deviance was assessed as youths’ involvement with deviant peers and their own deviant behaviors. Peer deviance was measured with the Prosocial and Problem Behaviors Scale (PPBS; Jacobs, Vernon, & Eccles, 2004). Twenty-three items (e.g., “How many of your friends sold drugs?”) that concerned the proportion of peers engaging in risky and antisocial behaviors were rated on a 4-point scale (1=None to 4=Most or all). Nine items on peer prosociality were excluded. Because peer deviance scores were positively skewed with a modal response of 0, they were normalized with an inverse hyperbolic sine transformation (i.e., log(yi+(yi2+1)1/2; Burbidge, Magee, & Robb, 1988). Grade 9 scores provided a measure of baseline peer deviance, and grade 12 scores, of later peer deviance. Reliability was excellent (αs = .94 and .92), and scores were moderately stable (r = .53, p < .001).
Adolescents’ own deviant behaviors were measured as conduct disorder and oppositional defiant disorder symptom counts from the disruptive behavior module of the C-DISC (Shaffer et al., 2000). The C-DISC is a highly structured diagnostic instrument that assesses 34 common psychiatric diagnoses (e.g., major depressive disorder, posttraumatic stress disorder) by determining the presence or absence of symptoms according to diagnostic criteria specified by the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000). The reliability and validity of the C-DISC is well supported (Shaffer et al., 2000). Because these symptom counts were positively skewed with a modal response of 0, they were normalized with an inverse hyperbolic sine transformation (Burbidge et al., 1988). Grade 9 scores provided a measure of baseline own deviance, and grade 12 scores, of later own deviance. Scores were moderately stable (r = .48, p < .001).
Measures of peer and own deviance were significantly positively correlated (r = .48, p < .001, at grade 9; r = .37, p < .001, at grade 12). To capture the extent of youths’ deviance across these tightly interconnected proximal contexts, z-scores of peer and own deviance, respectively, were created then averaged to derive single measures of social deviance at grades 9 and 12.
Family economic status
Because HSE exposure and social deviance are predicted by poverty and its correlates (e.g., location of school in a disadvantaged community), we computed an income-to-needs ratio by dividing total annual family income reported by mothers in grade 9 by the official poverty threshold in 2010 for the given household size. A ratio of 1 or less signified poverty status, and a ratio above 1, the extent of being over the poverty line. This measure was examined in relation to key variables for potential inclusion as a covariate.
Neural responses to social exclusion
Neural responses to social exclusion were assessed in the scanner via the Cyberball fMRI task (Eisenberger, 2003), a widely-used, ecologically valid measure in which participants are included or excluded from a simulated ball-tossing game. Participants were familiarized with the task and practiced lying still in a mock scanning environment. They were told that they would play a virtual ball-tossing game with two computerized players and asked to imagine, as vividly as possible, that they were playing with other kids their age. On the screen, participants saw cartoon figures and usernames representing two other players of no apparent gender or race/ethnicity, their cartoon “hand” controlled via button-box, and username, chosen during this mock scan session. During the game, the ball was thrown back and forth among all three players. Participants chose the recipient of their throws using the button-box, and throws of the two other players were selected by computer.
During the scan, participants played 12 rounds of Cyberball, six rounds of Inclusion and six rounds of Exclusion, always presented in the same pseudorandom order: Inclusion, Exclusion, Inclusion, Inclusion, Exclusion, Inclusion, Exclusion, Inclusion, Exclusion, Exclusion, Exclusion, and Inclusion. Throughout the Inclusion round, the other players were equally likely to throw the ball to the participant or each other. However, during Exclusion, near the beginning of the round, the other players stopped throwing the ball to the participant and continued throwing it only to each other. Each round lasted 36 sec, being comprised of a fixation point (4 sec), “Begin Match!” notification (2 sec), and 10–11 ball tosses of game play (22–23 sec) that included all relevant players’ ball tosses, followed by a short reloading screen (7–8 sec). There were also Instructions (8 sec) at the start and a “Thank you!” (3 sec) at the end signifying completion of the task. The functional scan lasted 7 min 23 sec implemented in one run.
After the scan, subjective distress to being excluded was assessed with the Need Threat Scale (Van Beest & Williams, 2006). Threats to four basic human needs (self-esteem, belonging, meaningfulness, control) were rated on 12 items (e.g., “I felt like an outsider”) using a 5-point scale (1=Not at all to 5=Very much so) with ratings averaged. Reliability was excellent (α = .91). In addition, different affective states were rated on four items (bad-good; sad-happy; relaxed-tense; unfriendly-friendly) with a 7-point scale anchored by oppositely valenced terms. Both measures were collected approximately 20 minutes after Cyberball, after an unrelated fMRI task.
Data Acquisition, Processing, and Analysis
fMRI data acquisition
Imaging data were collected using a Siemens 3T Tim Trio scanner with a 32-channel head coil. Adolescents were given extensive instructions to decrease head motion, which was also limited with foam padding and surgical tape. Whole-brain high-resolution structural images were acquired using a T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan collected in the sagittal plane (TR = 2500 ms; TE = 4.33 ms; slices = 208; flip angle = 7°; field of view (FOV) = 243 mm; image matrix = 243 * 243 mm; voxel size = 0.9 * 0.9 * 0.9 mm; slice thickness = .95 mm). Whole-brain functional images were acquired using T2*-weighted echo-planar images (TR = 2000 ms; TE = 27 ms; slices = 246; echo time = 49 ms; flip angle = 80°; interleaved slice geometry; FOV = 224 mm, image matrix = 224 * 224 mm; voxel size = 3.5 * 3.5 * 3.5 mm; slice thickness = 3.5 mm). The first two volumes were discarded to allow for equilibration of T1 saturation effects.
fMRI data analysis
All preprocessing and data analyses were conducted using Analysis of Functional NeuroImaging (AFNI: www.afni.nimh.nih.gov/afni, version AFNI_16.2.09; Cox, 1996). Preprocessing of functional data consisted of several stages, starting with interleaved slice timing correction, image realignment to the third volume using rigid body motion correction with 6 degrees of freedom, and co-registration of functional data with brain-extracted structural images normalized to Montreal Neurological Institute (MNI) stereotaxic space. Alignment was visually confirmed for all participants. Subsequently, spatial smoothing with a 6 mm Gaussian kernel, full-width at half-maximum, was conducted to increase the signal-to-noise ratio and adjust for individual variability in anatomy. Volumes with head motion greater than 1 mm from the previous volume were censored during further processing.
For first-level processing, Cyberball was modeled as a block design consisting of Inclusion or Exclusion conditions. The time series of 246 image volumes were deconstructed into five regressor types, each included in the design matrix. These consisted of Inclusion, Exclusion, instructions, “Begin match!”, and button presses (mostly during Inclusion) to control for motor activity. The last three were regressors of no interest. Exclusion and Inclusion were modeled as boxcar functions with an amplitude of 1 using AFNI’s duration modulation (dmBLOCK) to account for duration variability due to reaction time differences. Other regressors of interest were modeled using gamma functions. All were convolved with a canonical haemodynamic response function. The six motion parameters were modeled as effects of no interest to account for variance due to head movement. Finally, the beginning fixation point, end reloading screen, and final “Thank you!” were not modeled to maintain an implicit baseline. Linear contrasts were calculated for the Exclusion > Inclusion comparison for each participant.
For second-level processing, we performed a structural region-of-interest (ROI) analysis to explore the effects of a HSE on blood-oxygen level-dependent (BOLD) responses to Exclusion > Inclusion in subACC and, for comparison, dACC. Because laterality in subACC function has been found in past work (Guinjoan et al., 2010; Teasdale et al., 1999), right and left subACC (rsubACC, lsubACC) ROIs were created from right and left Brodmann Area (BA) 25 masks as defined by the Talairach-Tournoux database within AFNI, transformed to MNI space using the tta2mni function, then modified to include only areas of BA 25 that were under the genu of the corpus callosum posterior to y = 30 and identifiable, including through using AFNI’s whereami function in MNI space, as “cingulate cortex.” The resultant ROI was similar to significant clusters of activation reported in Cyberball studies on adolescents (Masten et al., 2009; 2011; Sebastian et al., 2011). Right and left dACC (rdACC, ldACC) ROIs were constructed using the “cingulate cortex” mask in the MNI database and modified to use a rostral boundary of y = 32 consistent with criteria established by Vogt, Berger, & Derbyshire (2003) and a caudal boundary of y = 0 given that most social pain studies find activations anterior to that coordinate (see Supporting Figure 1 for ROI masks). Within each mask and for each participant, we extracted average beta values for the linear contrast of Exclusion > Inclusion; these contrast beta values were correlated with our variables of interest.
Statistical analyses using all variables of interest and ROIs were performed with IBM SPSS Statistics version 23.0 (SPSS Inc., Chicago, IL) and PROCESS for SPSS (Hayes, 2013). Preliminary analyses indicated that age-at-scan and income-to-needs ratio were not associated with any variables of interest; thus, they were not included as covariates in our models. For our key analyses, zero-order correlations and hierarchical linear regressions using the ROI-extracted data were conducted. First, we examined zero-order correlations among all variables of interest. Second, we tested for unique relations among past HSE, right or left subACC responses to social exclusion, and later social deviance using multiple regression. Third, a mediation analysis was conducted to test whether subACC response to social exclusion was a mechanism linking past HSE exposure to later social deviance. Fourth, we examined whether past family connectedness moderated the link between past HSE and later social deviance at the behavioral level. Fifth, if moderation was found, we tested for moderated mediation to assess the moderating role of past family connectedness on the paths between (1) past HSE and subACC response and/or (2) subACC response and later social deviance. Several covariates were included: sex, baseline depression (Wave 1), baseline social deviance (Wave 1), and later depression (Wave 4); their inclusion did not introduce multicollinearity issues. Analyses were replicated with the dACC ROI to determine whether any effects were specific to subACC or also involved dACC.
Next, exploratory whole-brain, group-level, random-effects analyses were conducted to supplement ROI analyses to assess brain areas showing BOLD differences for the Exclusion > Inclusion contrast that related to either past HSE exposure or later social deviance. Past HSE exposure and later social deviance, respectively, were entered as covariates in AFNI’s 3dttest++ in two separate analyses. Based on AFNI’s recently updated 3dClustSim program (see Eklund et al., 2016), which uses Monte Carlo simulations to determine appropriate cluster sizes, a voxel-wise threshold of t = 2.843, p = 0.005, and cluster-extent threshold of 23 voxels were needed to produce an overall alpha of < 0.05. The filter width for simulation was determined using 3dFWHMx. Given concerns about previous versions of 3dClustSim (Eklund et al., 2016), we also applied the false discovery rate (FDR) procedure (Genovese et al., 2002) using AFNI’s 3dFDR to control the proportion of false positives among the significantly activated voxels at q < 0.05. All reported activations passed both cluster-extent and FDR thresholds. Finally, to parallel the ROI-based mediation analyses at the whole-brain level, we ran a conjunction analysis (Nichols et al., 2005) to test for areas of overlap in the above maps as these areas might link past HSE exposure to later deviance. We created an intersection map of past HSE exposure ∩later social deviance.
Results
Correlations and descriptive statistics
Table 1 shows the means and standard deviations of variables in raw units. Table 2 shows intercorrelations among these variables. As predicted, greater past HSE exposure was significantly related to greater later social deviance. Also consistent with our hypotheses, although we made no predictions about laterality, rsubACC responses to social exclusion were significantly related to both aspects of HSE exposure (perceived discrimination, school violence) and to both aspects of later social deviance (peer and own deviance); lsubACC responses were related only to later peer deviance. Thus, subsequent analyses focused only on rsubACC. Girls reported significantly higher levels of depression, discrimination, and own deviance. In addition, depressive symptoms were related to rsubACC responses to social exclusion and later social deviance, consistent with past work involving subACC and the known comorbidity between externalizing and internalizing symptoms. Thus, sex, baseline social deviance, and baseline and later depression were included as covariates in subsequent analyses.
Table 1.
Descriptive Statistics and Timing of Demographic, Neural Response, and Behavioral Measures
| Measure | Wave (and Corresponding Grade) of Assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1 (9th) | 2 (10th) | 3 (11th) | Scan (11th/12th) | 4 (12th) | ||||||
|
|
|
|
|
|
||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Age (years) | 14.20 | .46 | 15.25 | .49 | 16.18 | .44 | 17.14 | .40 | 17.20 | .41 |
| Income-to-needs ratio | 1.13 | .77 | - | - | - | - | - | - | - | - |
| Depression | ||||||||||
| Baseline (recruitment status) | .78 | .42 | - | - | - | - | - | - | - | - |
| Later (MDD symptom counts) | - | - | - | - | - | - | - | - | 3.40 | 3.49 |
| Past HSE exposure | ||||||||||
| Discrimination | .28 | .37 a | .21 | .31 b | .31 | .38 a | ||||
| School violence | .57 | .50 a | .54 | .50 a | .54 | .48 a | - | - | - | - |
| Past family connectedness | 2.42 | .41 a | - | - | 2.36 | .44 a | - | - | - | - |
| Neural responses to social exclusion | ||||||||||
| rsubACC | - | - | - | - | - | - | .03 | .23 | - | - |
| lsubACC | - | - | - | - | - | - | .03 | .21 | - | - |
| Social deviance | ||||||||||
| Peer deviance | .29 | .36 a | - | - | - | - | - | - | .26 | .32 a |
| Own deviance | ||||||||||
| CD symptom counts | .97 | 1.55 a | - | - | - | - | - | - | 1.28 | 1.75 b |
| ODD symptom counts | 2.59 | 2.70 a | - | - | - | - | - | - | 1.63 | 2.00 b |
Note: MDD = Major Depressive Disorder. rsubACC or lsubACC = Right or left subgenual anterior cingulate cortex. HSE = Hostile school environment. CD = Conduct disorder. ODD = Oppositional defiant disorder. All brain data were collected between Waves 3 and 4. Superscripts indicate significantly different means for a given measure.
Table 2.
Zero-Order Correlations Among Demographic, Neural Response, and Behavioral Measures
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | - | |||||||||||||||
| 2. Income-to-needs | −.03 | - | ||||||||||||||
| 3. Baseline depression | .22** | −.07 | - | |||||||||||||
| 4. Later depression | .32** | −.08 | .27** | - | ||||||||||||
| 5. Past HSE exposure | .12 | .05 | .33** | .10 | - | |||||||||||
| 6. Discrimination | .23** | .08 | .30** | .08 | .74** | - | ||||||||||
| 7. School violence | .01 | .01 | .26** | .09 | .89** | .36** | - | |||||||||
| 8. Past family connectedness | .06 | .02 | −.24** | −.17* | −.12 | −.05 | −.14† | - | ||||||||
| 9. rsubACC | .07 | −.04 | .12 | .21** | .25** | .19* | .23** | .00 | - | |||||||
| 10. lsubACC | .02 | −.11 | .01 | .18* | .10 | .07 | .09 | .00 | .81** | - | ||||||
| 11. Baseline social deviance | .15† | −.01 | .41** | .17* | .45** | .16* | .51** | −.26** | .07 | .02 | - | |||||
| 12. Peer deviance | −.01 | −.11 | .32** | .04 | .41** | .11 | .50** | −.29** | .09 | .04 | .86** | - | ||||
| 13. Own deviance | .26** | .11 | .38** | .25** | .36** | .17* | .38** | −.15† | .03 | −.02 | .86** | .48** | - | |||
| 14. Later social deviance | .14† | .10 | .29** | .32** | .43** | .22** | .45** | −.11 | .31** | .19* | .52** | .44** | .46** | − | ||
| 15. Peer deviance | −.03 | .05 | .19* | .11 | .41** | .22** | .43** | −.16* | .29** | .20** | .46** | .53** | .27** | .83** | - | |
| 16. Own deviance | .26** | .11 | .30** | .42** | .30** | .15† | .31** | −.03 | .22** | .11 | .40** | .24** | .48** | .83** | .37** | - |
Note. Baseline = 9th Grade. Later = 12th Grade. rsubACC or lsubACC = Right or left subgenual anterior cingulate cortex response to social exclusion (vs. inclusion). HSE = Hostile school environment. Age, which had low variability in our sample and is not shown here, was not associated with any of the above variables.
p < .10;
p < .05;
p < .01
subACC responses as a mediator between HSE and social deviance
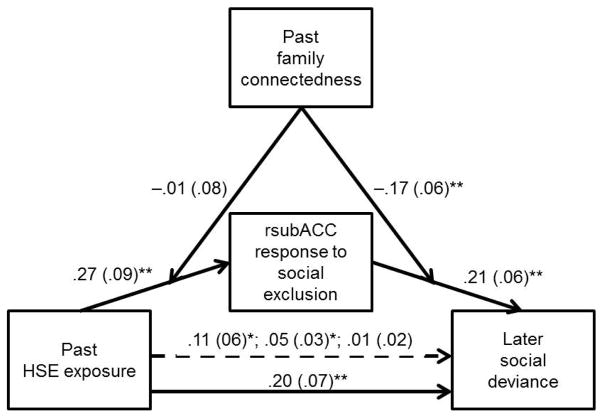
To evaluate rsubACC responses to social exclusion as a mechanism linking past HSE exposure to later social deviance, we tested for mediation using bootstrapping (10,000 resamples; Table 3 and Figure 1). We began by establishing three relations among our key variables, controlling for all covariates (sex, baseline depression and social deviance, later depression): First, we established the positive relation between past HSE exposure and later social deviance (total effect; β = .24, SE = .07, t(160) = 3.29, p < .01). Second, we confirmed the positive relation between past HSE exposure and rsubACC responses to social exclusion (β = .27, SE = .09, t(160) = 3.14, p < .01). The third relation we established was between rsubACC responses and later social deviance (β = .23, SE = .06, t(160) = 3.63, p < .001). Finally, to test for mediation, we simultaneously entered past HSE exposure and rsubACC responses to social exclusion (plus all covariates) as predictors of later social deviance. This model showed that rsubACC responses continued to significantly predict later social deviance and that the predictive value of past HSE, as indicated by the direct effect, dropped in magnitude. The indirect effect of past HSE on later social deviance through rsubACC responses was significant (β = .05, SE = .02, p < .05).
Table 3.
Mediation and Moderated Mediation Models
| rsubACC as a mediator between past HSE exposure and later social deviance | |||
|---|---|---|---|
|
| |||
| Predictors | Total effect of HSE on later social deviance | rsubACC (mediator) | Later social deviance (outcome) |
|
|
|
|
|
| β (SE) | β (SE) | β (SE) | |
|
|
|
|
|
| Sex (0=male, 1=female) | −.02 (.07) | −.01 (.08) | −.01 (.07) |
| Baseline depression | .01 (.07) | .01 (.09) | .01 (.07) |
| Baseline social deviance | .37 (.07)*** | −.08 (.09) | .39 (.07)*** |
| Later depression | .24 (.07)*** | .19 (.08)* | .20 (.07)* |
| Past HSE exposure | .24 (.07)** | .27 (.09)** | .18 (.07)* |
| rsubACC (mediator) | – | – | .19 (.06)* |
|
| |||
| R2 | .37 | .10 | .40 |
| F statistic | 18.85 | 3.67 | 17.94 |
| Indirect effects | .05 (.02)* | ||
|
| |||
| Past family connectedness as a moderator of the mediating pathways | |||
|
| |||
| Predictors | rsubACC (mediator) | Later social deviance (outcome) | |
|
|
|
||
| β (SE) | β (SE) | ||
|
|
|
|
|
| Sex (0=male, 1=female) | −.02 (.08) | −.02 (.07) | |
| Baseline depression | .02 (.09) | −.01 (.07) | |
| Baseline social deviance | −.07 (.09) | .38 (.07)*** | |
| Later depression | .20 (.08)* | .22 (.07)** | |
| Past HSE exposure | .27 (.09)** | .20 (07)** | |
| Past family connectedness | .05 (.08) | .05 (.06) | |
| Past HSE exposure x Past family connectedness | −.01 (.08) | – | |
| rsubACC (mediator) | – | .20 (.06)** | |
| rsubACC x Past family connectedness | – | −.17 (.06)** | |
|
| |||
| R2 | .11 | .43 | |
| F statistic | 2.67 | 14.98 | |
| Indirect effects at levels of past family connectedness | ≤ 1 SD .11 (.06)*; M .05 (.03)*; ≥ 1 SD .01 (.02) | ||
Note. rsubACC = Right subgenual anterior cingulate cortex responses to social exclusion (vs. inclusion); HSE = Hostile school environment. β = Standardized coefficient; SE = Standardized error. M = Mean. A dash indicates that a predictor was not entered in the given model.
p < .05;
p < .01;
p < .001.
Figure 1.
Values are standardized coefficients from ordinary least squares regression and standard errors are represented in parentheses. Values on the dashed line represent the indirect effect of past exposure to a hostile school environment (HSE) on later social deviance through right subgenual anterior cingulate cortex (rsubACC) response to social exclusion (vs. inclusion). Specifically, the values indicate the indirect effects estimated at each moderating level of family connectedness: lower-than-average (≤ 1 standard deviation below mean), average (within 1 standard deviation of mean), and higher-than-average (≥ 1 standard deviation above mean), respectively.
* p < .05. ** p < .01.
Family connectedness as a moderator of the mediation model
We extended the above mediation model by assessing family connectedness as a possible moderator. First, we tested for moderation at the behavioral level, finding that past HSE exposure significantly interacted with past family connectedness (β = −.13, SE = .06, t(158) = 2.09, p < .05) in the prediction of later social deviance from past HSE exposure, past family connectedness, their interaction, plus all covariates (R2 = .39, F(7, 158) = 13.90, p < .001). This interaction was interpreted by analyzing the simple regression lines estimated for adolescents at varying levels of family connectedness. For adolescents reporting high (≥ 1 SD), average (within 1 SD of mean), and low (≤ 1 SD) levels of family connectedness, equations were used to plot values of later social deviance at these levels of past HSE exposure. As predicted, slopes were significantly different from zero for adolescents reporting low (β = .38, SE = .11, t(158) = 3.31, p < .01) and average (β = .25, SE = .08, t = 3.10, p < .001) but not high (β = .12, SE = .09, t = 1.35, ns) family connectedness, suggesting a familial buffering of the HSE-deviance link behaviorally.
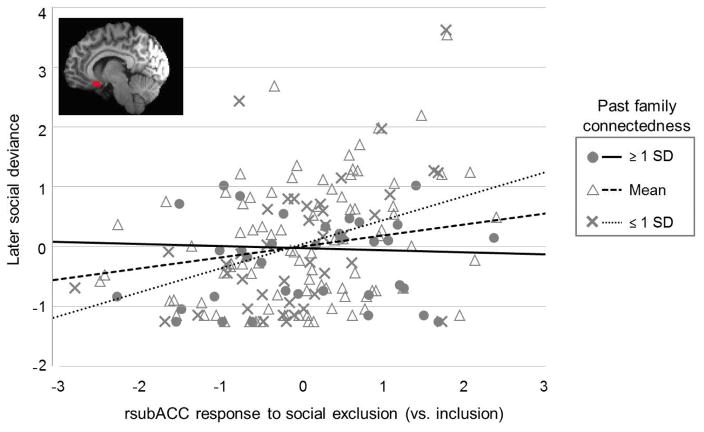
If found at the neurobiological level, this familial buffering could operate along two different paths: (1) from greater HSE exposure to greater exclusion-related rsubACC responses, and/or (2) from greater exclusion-related rsubACC responses to greater later social deviance. We tested both possibilities. Results suggested that family connectedness did not moderate the first path but did the second (Table 3 and Figure 2). Specifically, in predicting later social deviance, the conditional indirect effects for the HSE-deviance link that was mediated by rsubACC were significant for adolescents reporting low (β = .11, SE = .06, p < .01) and average (β = .05, SE = .03, p < .05) but not high (β = .01, SE = .02, ns) levels of family connectedness. This again suggested family connectedness as a protective factor, specifically between brain and behavior.
Figure 2.
Plot of simple slopes showing the interaction of adolescents’ right subgenual anterior cingulate cortex (rsubACC) responses to social exclusion (vs. inclusion) with levels of past family connectedness in the prediction of later social deviance, consistent with our moderated mediation model. Slopes were significant only for adolescents with lower-than-average (“≤ 1 SD,” or ≤ 1 standard deviation below mean) and average (“Mean,” or within 1 standard deviation of mean) levels of family connectedness.
Subjective distress to social exclusion
Because rsubACC responses to social exclusion linked past HSE exposure to later social deviance, we wanted to better understand their significance in terms of subjective experience. Contrary to expectation, greater rsubACC responses did not relate to being more distressed over being excluded (r = −.04, ns). Moreover, greater rsubACC responses predicted feeling more, not less, “friendly” (r = .24, p < .01) after the scan. Neither subjective measure was related to past HSE exposure or later social deviance. Given our findings that family connectedness moderated the brain-behavior relation, we also tested whether distress over being excluded was an interactive product of rsubACC responses and past family connectedness, as different brain-emotion relations depending on levels of past family connectedness might emerge. Their interaction was not significant, however (β = .09, SE = .36, t(158) = .24, ns).
Specificity analysis of subACC vs. dACC
Replicating our foregoing analyses using left and right dACC as the ROIs yielded largely null results. Despite a significantly positive relation between dACC and subACC responses to social exclusion (rs = .43–.48, ps < .001), only own later deviance (rs = .15–.16, p < .05) and, accordingly, later social deviance (rs = .16–.17, p < .05) were related to dACC responses. Thus, rsubACC responses appeared to be unique in mediating the past HSE-deviance link.
Post-hoc whole-brain analyses
Finally, whole-brain analyses revealed one set of brain activations from the Exclusion > Inclusion contrast related to HSE exposure (e.g., angular gyrus, middle frontal gyrus), and another related to deviance (e.g., inferior frontal gyrus, precuneus) (Supporting Table 1). Both sets included clusters within superior parietal lobule, middle temporal gyrus, precentral gyrus, and middle occipital gyrus. Testing for overlap using conjunction analysis showed significant convergence in right superior parietal lobule (corrected for multiple comparisons using cluster-wise and FDR thresholds, Supporting Figure 2). Although these analyses were exploratory, greater involvement of these regions during social exclusion as opposed to inclusion would be expected given their links to social reasoning and attentional orienting, as discussed below.
Discussion
The present study showed that adolescents’ neural responses to social exclusion were related to their past exposure to HSE and increases in their socially deviant behaviors. Indeed, adolescents’ neural responses to social exclusion served as a mediator of the positive association between past HSE exposure and escalation in social deviance. Moreover, family connectedness moderated this brain-mediated link. Although all adolescents showed effects of past HSE exposure on neural sensitivity to being excluded, only adolescents with weaker, as compared to stronger, family ties expressed this sensitivity behaviorally. Thus, family connectedness appeared to protect adolescents from a HSE-conferred neurobiological risk for social deviance.
Our results were specific to subACC, a brain region found in past research to be responsive to social exclusion, particularly in adolescence (e.g., Masten et al., 2009). Aside from tracking adolescent-specific distress over being excluded, subACC responses during this event have forecasted increases in adolescent depressive symptoms (Masten et al., 2011). In the wider literature, the subACC is well-known for its role in depression (Drevets et al., 2008). Of special relevance here, research has found that depressive symptoms mediate the link between exposure to adversity and later delinquent behaviors, such as through a lack of future orientation (Allwood, Baetz, DeMarco, & Bell, 2012). Still, controlling for depressive symptoms let us establish a HSE-deviance link mediated by subACC that was free from overlap with depression. Below, we consider three related routes by which subACC may promote deviance within a HSE.
First, HSE exposure may attune the subACC to breaks in social bonds, enabling their perception even in ambiguous contexts. In this way, if subACC tracks the likelihood of hostility, it may become sensitized to hostility when that likelihood is high. Although our results did not replicate with dACC, our interpretation is consistent with findings on dACC, whose responses to exclusion in adolescence were found to be higher given a history of childhood rejection (Will et al., 2016). Research that differentiates the functions of subregions of ACC supports the idea that dACC serves more “cold,” cognitive functions (e.g., discrepancy detection) whereas the subACC serves more “hot,” affective ones (e.g., emotion and its regulation) (Bush et al., 2000). Moreover, the subACC shows heightened activity to the vicarious experience of social and physical pain (Novembre, Zanon, & Silani, 2014), suggesting that it can become sensitized to hostility even as a bystander within a HSE. Future work should examine the role of cognition in this sensitization process, given that hostile attribution biases predict growths in social deviance (Dodge, 1991).
Second, and relatedly, heightened subACC responses to social exclusion may promote hostility through increases in negative emotion. That is, HSE exposure may sensitize youths to hostility in the very neural region that gives rise to its toxic “sting.” Although subACC responses did not vary with self-reported distress in our study, it is noteworthy that our findings were localized to the right side, which is more greatly implicated in negative affect and psychopathology (Guinjoan et al., 2010; Teasdale et al., 1999). In probing the type of negative affect involved, one study linking subACC activity during emotion conflict to neuroticism, or trait-level negative affect, found that subACC activity was uniquely related to its anxious, not depressive, form (Haas et al., 2007). In linking this pattern to behavior, these findings could suggest a role for subACC in reactive aggression, conceptualized as a fear-based, irritable, and affect-laden defensive response (Dodge, 1991). Interestingly, subACC activity has been found to predict approach, not withdrawal, behaviors in a fear-eliciting circumstance (Nili et al., 2010).
Third, subACC responses to social exclusion may operate as a mechanism of social susceptibility. Greater susceptibility and attempts to affiliate have been documented following social exclusion (Baumeister & Leary, 1995; Carter-Sowell et al., 2008). In our study, greater subACC responses during social exclusion predicted how “friendly” youths felt after the scan. Indeed, subACC responses have been associated with affiliative tendencies and the desire to take corrective action after perceiving oneself to have violated a standard, as seen in experiences of guilt (Zahn, Oliveira-Souza, Bramati, Garrido, & Moll, 2009). In addition, some have suggested that subACC is implicated in similar prediction error signaling as dACC, signifying conflict with the group via a sense of discomfort and encouraging social conformity (Swieten & Pijnenburg, 2012). While counterintuitive, it may be that a neurobiologically facilitated tendency to “get along” may foster social deviance in settings where deviance is normative – where the disruption of social bonds is both modeled and accepted, even encouraged, by peers (Monahan et al., 2009).
Family connectedness acted as a buffer that protected youths from the behavioral effects of HSE exposure. At the neurobiological level, this familial moderation operated not on the first path, from greater past HSE exposure to greater subACC response, but on the second path, from greater subACC response to greater later social deviance. Thus, how much such external input was neurobiologically processed, or sensitivity, was not affected by the strength of one’s family bonds, which instead limited the behavioral output of this processing, or responsivity (Pluess, 2015). Perhaps when the brain is re-oriented to peers in adolescence (Nelson et al., 2016), the family is less likely to shape the neural sensitivities that are maturing in salient social contexts like school. However, by scaffolding and even motivating the development of emotional and social competencies, the family may guide how these sensitivities are controlled and expressed. Future work might examine what mediates the moderating influence of family connectedness.
Results of whole-brain analyses revealed brain areas whose exclusion-related activity was related to past HSE exposure and later social deviance. Several of these regions (e.g., angular gyrus, inferior frontal gyrus, precuneus) have been shown to facilitate “mentalizing,” the ability to infer others’ thoughts and feelings (Decety & Moriguchi, 2007). In the context of social exclusion, for example, mentalizing would enable the perception that one is being excluded on purpose. In addition, consistent with our idea that social susceptibility promotes the HSE-deviance link, Peake, Dishion, Stormshak, Moore, & Pfeifer (2013) found that greater recruitment of mentalizing regions while being excluded predicted greater risk-taking among peers for youths lower in resistance to peer influence. Still, the common neural correlate of HSE exposure and later deviance involved right superior parietal lobule, whose activity has been linked to spatial attention driven endogenously (Yantis, 2000). Taken together, findings suggest that youths exposed to a HSE and/or increasing in social deviance engage in excess social reasoning and attentional shifting, perhaps in states of hypervigilance, frustration, or avoidance.
A number of limitations must be acknowledged. First, because neural responses were measured only once, we cannot determine the direction of the relation between HSE exposure and subACC responses. Greater subACC responsivity may have preceded HSE exposure and even contributed to youths’ perceptions of school as hostile. Future work should use longitudinal designs with at least two time points of brain data to assess transactional relations and change over time. Second, although we assessed change in social deviance by controlling for baseline levels, our study was not designed to test relations between the brain and different trajectories of social deviance. Doing so may help identify the etiological factors, including neural mechanisms, that contribute to distinct developmental courses of antisocial behavior (e.g., adolescence-limited versus life-course persistent; Moffit, 1993). Third, although the current study provided much-needed findings on an understudied ethnic group, important within-group differences (e.g., ethnic pride) were not considered and would be informative to examine. Relatedly, although including only one ethnic group allowed us to inherently control for ethnicity, it remains to be seen whether the present findings would generalize to other minority groups.
The results of our study have implications for prevention and intervention efforts. First, results underscore the need to create a supportive school environment. Interventions can occur at the schoolwide level, with the priority of socializing a sense of community and respect, and at the individual level, with the aim of altering the experiences and behaviors of students at-risk for or showing disruptive behaviors (Andreou, 2016). As past HSE exposure may affect neural mechanisms involved in the surge of social processing in adolescence, interventions can target this circuity by helping adolescents manage their threat appraisals, self-esteem, impulsivity, and perspective-taking, to the betterment of the peer milieu. This includes cultivating cultural sensitivity (Cartledge & Johnson, 2004), as our findings suggest that not respecting others, such as due to their seeming differences, might have a lasting impact on their brain function.
Second, our work points to the family as a pivotal point of intervention, as family connectedness buffered the link between risk and maladaptation. From a family resilience perspective (Masten, 2007), the family offers both family- and individual-level resources that enable resilience in youths by facilitating their positive adaptations to adversity. For example, in the face of adversity, multiple regulatory processes distributed across the family (e.g., meaning making, communication, parental sensitivity and monitoring) can strengthen youths’ regulatory abilities (MacPhee et al., 2015), including at the physiological level (Bai & Repetti, 2015). Many of these processes are bolstered by family-based programs that target the functioning of families and/or youths who are under stress or likely to be. Even before adversity such as discrimination is encountered, parents can help combat its effects by socializing youths to be proud of their ethnic group (Hernández, Conger, Robins, Bacher, & Widaman, 2014). Youths taught to respond proactively to derogatory behavior enjoy greater self-efficacy and self-esteem (Phinney & Chavira, 1995). Ultimately, by keeping youths feeling connected, supported, and loved, the family can diminish links between biological risk and maladjustment forged outside the home.
In sum, we provided evidence that youths who perceive a HSE are more neurally sensitive to social threat and likely to manifest deviant behaviors, unless they feel connected to their families. Our results thus offer clues about who faces risk for developing deviant behaviors as a function of what social contexts and through which neural circuits. Although research on biology-environment interplay has escalated rapidly, few such studies include fMRI measures of brain responses to salient social cues and fewer use these measures to predict behavior (Boyce, 2016; Schriber & Guyer, 2016). Thus, an important contribution of this report is its examination of brain-behavior relations in the context of HSE exposure and family connectedness. Further, by investigating both social contexts, we pieced together the impact of two salient developmental settings, home and school, in a biopsychosocial study of social exclusion and its consequences.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Grants R01MH098370 (A. Guyer, P. Hastings) and R01DA017902 (R. Robins, R. Conger); William T. Grant Foundation Mentoring Award #182606 (A. Guyer, R. Schriber) and Scholar Award #180021 (A. Guyer); National Science Foundation #1327768 (M. Page, P. Hastings, K. Conger, A. Guyer); University of California, Davis, Behavioral Health Center of Excellence Award (A. Guyer); and U. S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Analysis #1H79AE000100-1 to University of California, Davis, Center for Poverty Research (A. Stevens, M. Page). We thank Nicole Welindt, Timothy Bell, Lucia Leon, and Nilsen Gomez-Tabal for assisting with recruitment and data collection.
References
- Akdeniz C, Tost H, Streit F, Haddad L, Wüst S, Schäfer A, … Meyer-Lindenberg A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority–associated environmental risk. JAMA Psychiatry. 2014;71(6):672. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood MA, Baetz C, DeMarco S, Bell DJ. Depressive symptoms, including lack of future orientation, as mediators in the relationship between adverse life events and delinquent behaviors. Journal of Child & Adolescent Trauma. 2012;5(2):114–128. [Google Scholar]
- Andreou E. School violence prevention: The youth development perspective. British journal of education, society and behavioural science. 2015;5(4):389–395. [Google Scholar]
- Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. Journal of Health and Social Behavior. 1996;37(4):293–310. [PubMed] [Google Scholar]
- Bai S, Repetti RL. Short-term resilience processes in the family. Family Relations. 2015;64(1):108–119. doi: 10.1111/fare.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. Journal of Personality and Social Psychology. 2005;88(4):589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456(7219):245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB. Beyond brain mapping: Using neural measures to predict real-world outcomes. Current Directions in Psychological Science. 2013;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Boyce WT. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology. 2016;41(1):142–162. doi: 10.1038/npp.2015.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge JB, Magee L, Robb AL. Alternative transformations to handle extreme values of the dependent variable. Journal of the American Statistical Association. 1988;83(401):123–127. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter-Sowell AR, Chen Z, Williams KD. Ostracism increases social susceptibility. Social Influence. 2008;3(3):143–153. [Google Scholar]
- Cartledge G, Johnson CT. School violence and cultural sensitivity. In: Conoley JC, Goldstein AP, editors. School violence intervention: A practical handbook. 2. New York: Guilford; 2004. pp. 441–482. [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Richman SB, Bushman BJ, DeWall CN. The interactive effect of social pain and executive functioning on aggression: An fMRI experiment. Social Cognitive and Affective Neuroscience. 2013:nst038. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RM, Tiedens LZ, Govan CL. Excluded emotions: The role of anger in antisocial responses to ostracism. Journal of Experimental Social Psychology. 2008;44(3):896–903. [Google Scholar]
- Chung HL, Steinberg L. Relations between neighborhood factors, parenting behaviors, peer deviance, and delinquency among serious juvenile offenders. Developmental Psychology. 2006;42(2):319–331. doi: 10.1037/0012-1649.42.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ. Multilevel perspectives on pathways to resilient functioning. Development and Psychopathology. 2007;19(3):627–629. doi: 10.1017/s0954579407000314. [DOI] [PubMed] [Google Scholar]
- Clauss-Ehlers CS, Levi LL. Violence and community, terms in conflict: An ecological approach to resilience. Journal of Social Distress and the Homeless. 2002;11(4):265–278. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. BioPsychoSocial Medicine. 2007;1:22. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MY, Updegraff KA, Roosa MW, Umaña-Taylor AJ. Discrimination and Mexican-origin adolescents’ adjustment: The moderating roles of adolescents’, mothers’, and fathers’ cultural orientations and values. Journal of Youth and Adolescence. 2011;40(2):125–139. doi: 10.1007/s10964-009-9467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Patterson GR, Griesler PC. Peer adaptations in the development of antisocial behavior: A confluence model. In: Heusmann LR, editor. Current perspectives on aggressive behavior. New York, NY: Plenum Press; 1994. pp. 61–95. [Google Scholar]
- Dodge KA. The structure and function of reactive and proactive aggression. In: Pepler DJ, Rubin KH, editors. The development and treatment of childhood aggression. Hillsdale, NJ: Erlbaum; 1991. pp. 201–218. [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JS, Early D, Fraser K, Belansky E, McCarthy K. The relation of connection, regulation, and support for autonomy to adolescents’ functioning. Journal of Adolescent Research. 1997;12(2):263–286. [Google Scholar]
- Eccles JS, Roeser RW. Schools as developmental contexts during adolescence. Journal of Research on Adolescence. 2011;21(1):225–241. [Google Scholar]
- Eisenberger NI. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. https://doi.org/10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Cascio CN, Brook O’Donnell M, Carp J, Tinney FJ, Jr, Bingham CR, … Simons-Morton BG. Neural responses to exclusion predict susceptibility to social influence. Journal of Adolescent Health. 2014;54(5, Supplement):S22–S31. doi: 10.1016/j.jadohealth.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CB, Wallace SA, Fenton RE. Discrimination distress during adolescence. Journal of Youth and Adolescence. 2000;29(6):679–695. [Google Scholar]
- Fuligni AJ, Telzer EH. Another way family can get in the head and under the skin: The neurobiology of helping the family. Child Development Perspectives. 2013;7(3):138–142. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Germán M, Gonzales NA, Dumka L. Familism values as a protective factor for Mexican-origin adolescents exposed to deviant peers. The Journal of Early Adolescence. 2009;29(1):16–42. doi: 10.1177/0272431608324475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinjoan SM, Mayberg HS, Costanzo EY, Fahrer RD, Tenca E, Antico J, … Nemeroff CB. Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(3):265–277. doi: 10.1176/jnp.2010.22.3.265. [DOI] [PubMed] [Google Scholar]
- Haas B, Constable RT, Canli T. Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience. 2007;121(2):249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Hastings PD. Introduction to the special section: Biopsychosocial processes in the etiology and development of internalizing problems. Journal of Abnormal Child Psychology. 2015;43(5):803–805. doi: 10.1007/s10802-015-9992-z. [DOI] [PubMed] [Google Scholar]
- Hastings P, Miller J, Troxel NR. Making good: The socialization of children’s prosocial development. In: Grusec J, Hastings PD, editors. Handbook of socialization: Theory and research. New York, NY: Guilford Press; 2015. pp. 637–666. [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; 2013. [Google Scholar]
- Henry S. What Is school violence? An integrated definition. The ANNALS of the American Academy of Political and Social Science. 2000;567(1):16–29. [Google Scholar]
- Hernández MM, Conger RD, Robins RW, Bacher KB, Widaman KF. Cultural socialization and ethnic pride among Mexican-origin adolescents during the transition to middle school. Child Development. 2014;85:695–708. doi: 10.1111/cdev.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hoskin AW. Experiencing prejudice and violence among Latinos: A General Strain Theory approach. Western Criminology Review. 2013;14:25–38. [Google Scholar]
- Jacobs JE, Vernon MK, Eccles JS. Relations between social self-perceptions, time use, and prosocial or problem behaviors during adolescence. Journal of Adolescent Research. 2004;19(1):45–62. [Google Scholar]
- Johnston KE, Delgado MY. Mexican American adolescents’ experiences with ethnic discrimination. Poster presented at the biennial meeting of the society for research on adolescence; Baltimore, MD. 2004. [Google Scholar]
- Klonoff EA, Landrine H. The Schedule Of Sexist Events: A measure of lifetime and recent sexist discrimination in women’s lives. Psychology of Women Quarterly. 1995;19(4):439–472. [Google Scholar]
- Leary MR, Twenge JM, Quinlivan E. Interpersonal Rejection as a determinant of anger and aggression. Personality and Social Psychology Review. 2006;10(2):111–132. doi: 10.1207/s15327957pspr1002_2. [DOI] [PubMed] [Google Scholar]
- MacPhee D, Lunkenheimer E, Riggs N. Resilience as regulation of developmental and family Processes. Family Relations. 2015;64(1):153–175. doi: 10.1111/fare.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS. Resilience in developing systems: Progress and promise as the fourth wave rises. Development and Psychopathology. 2007;19(3):921–930. doi: 10.1017/S0954579407000442. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Development and Psychopathology. 2011;23(1):283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. An fMRI investigation of attributing negative social treatment to racial discrimination. Journal of Cognitive Neuroscience. 2011;23(5):1042–1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijanovich T, Weitzman BC. Which “broken windows” matter? School, neighborhood, and family characteristics associated with youths’ feelings of unsafety. Journal of Urban Health. 2003;80(3):400–415. doi: 10.1093/jurban/jtg045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Monahan KC, Steinberg L, Cauffman E. Affiliation with antisocial peers, susceptibility to peer influence, and antisocial behavior during the transition to adulthood. Developmental Psychology. 2009;45(6):1520–1530. doi: 10.1037/a0017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience. 2011;1(3):233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, Guyer AE. Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience. 2016;17:118–127. doi: 10.1016/j.dcn.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nili U, Goldberg H, Weizman A, Dudai Y. Fear thou not: Activity of frontal and temporal circuits in moments of real-life courage. Neuron. 2010;66(6):949–962. doi: 10.1016/j.neuron.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Novembre G, Zanon M, Silani G. Empathy for social exclusion involves the sensory-discriminative component of pain: a within-subject fMRI study. Social Cognitive and Affective Neuroscience. 2015;10(2):153–164. doi: 10.1093/scan/nsu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe M. Adolescents’ exposure to community and school violence: Prevalence and behavioral correlates. Journal of Adolescent Health. 1997;20(5):368–376. doi: 10.1016/S1054-139X(97)80131-0. [DOI] [PubMed] [Google Scholar]
- Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. NeuroImage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney JS, Chavira V. Parental ethnic socialization and adolescent coping with problems related to ethnicity. Journal of Research on Adolescence. 1995;5(1):31–53. [Google Scholar]
- Pluess M. Individual differences in environmental sensitivity. Child Development Perspectives. 2015;9(3):138–143. [Google Scholar]
- Rodriguez N, Mira CB, Paez ND, Myers HF. Exploring the complexities of familism and acculturation: Central constructs for people of Mexican origin. American Journal of Community Psychology. 2007;39(1–2):61–77. doi: 10.1007/s10464-007-9090-7. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O, Rothlind J, Sturm V, Kramer JH, Weiner M, Miller BL. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. NeuroImage. 2010;49(4):3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom MJ, Cillessen ANH, Eisenhower A. Children’s appraisal of peer rejection experiences: Impact on social and emotional adjustment. Social Development. 2003;12(4):530–550. [Google Scholar]
- Schriber RA, Guyer AE. Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience. 2016;19:1–18. doi: 10.1016/j.dcn.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV) Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Somerville LH. The teenage brain sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22(2):121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano M, Soriano FI. School violence among culturally diverse populations: Sociocultural and institutional considerations. School Psychology Review. 1994;23(2):216–235. [Google Scholar]
- Stone S, Han M. Perceived school environments, perceived discrimination, and school performance among children of Mexican immigrants. Children and Youth Services Review. 2005;27(1):51–66. [Google Scholar]
- Szapocznik J, Williams RA. Brief strategic family therapy: Twenty-five years of interplay among theory, research and practice in adolescent behavior problems and drug abuse. Clinical Child and Family Psychology Review. 2000;3(2):117–134. doi: 10.1023/a:1009512719808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SCR, Checkley SA. Functional MRI study of the cognitive generation of affect. American Journal of Psychiatry. 1999;156(2):209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Meaningful family relationships: Neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience. 2013;25(3):374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry TP. Developmental Theories Of Crime And Delinquency. Transaction Publishers; 1997. [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91(5):918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Pijnenburg Y. The role of the subgenual cingulate cortex in self-appraisal. Journal of Neurology, Neurosurgery & Psychiatry. 2012 doi: 10.1136/jnnp-2012-303571. jnnp-2012-303571. [DOI] [PubMed] [Google Scholar]
- Villarreal R, Blozis SA, Widaman KF. Factorial invariance of a Pan-Hispanic Familism Scale. Hispanic Journal of Behavioral Sciences. 2005;27(4):409–425. [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. The European Journal of Neuroscience. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Will GJ, van Lier PAC, Crone EA, Güroğlu B. Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. Journal of Abnormal Child Psychology. 2015;44(1):43–55. doi: 10.1007/s10802-015-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S. Goal-directed and stimulus-driven determinants of attentional control. Attention and Performance. 2000;18:73–103. [Google Scholar]
- Zahn R, de Oliveira-Souza R, Bramati I, Garrido G, Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neuroscience Letters. 2009;457(2):107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.