Abstract
An expanded hexanucleotide repeat in C9orf72 causes amyotrophic lateral sclerosis and frontotemporal dementia (c9FTD/ALS). Therapeutics are being developed to target RNAs containing the expanded repeat sequence (GGGGCC); however, this approach is complicated by the presence of antisense strand transcription of expanded GGCCCC repeats. We found that targeting the transcription elongation factor, Spt4, selectively decreased production of both sense and antisense expanded transcripts, as well as their translated dipeptide repeat (DPR) products, and also mitigated degeneration in animal models. Knockdown of SUPT4H1, the human Spt4 ortholog, similarly decreased production of sense and antisense RNA foci as well as DPR proteins in patient cells. Therapeutic targeting of a single factor to eliminate c9FTD/ALS pathological features offers advantages over approaches that require targeting sense and antisense repeats separately.
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are devastating neurodegenerative diseases. The C9orf72 mutation (1, 2) is a common cause of both ALS and FTD (c9FTD/ALS), and intense efforts are exploring disease mechanisms and developing therapeutic strategies (3, 4). The C9orf72 gene harbors a hexanucleotide repeat, GGGGCC, located in the first intron. In c9FTD/ALS patients, this hexanucleotide repeat tract is expanded to hundreds or even thousands of repeats. Several hypotheses have emerged to explain how C9orf72 mutations cause neurodegeneration. RNAs transcribed from the GGGGCC repeats that accumulate as foci in the nucleus and cytoplasm could cause disease by sequestering RNA-binding proteins and splicing factors via an RNA toxicity mechanism (5-7). The transcribed nucleotide repeats are also substrates for an unconventional form of translation, termed repeat-associated non-ATG (RAN) translation (8-10). The resulting dipeptide repeat (DPR) proteins could cause disease through proteotoxic mechanisms (11). Lastly, the expanded repeats reduce C9orf72 mRNA expression (12, 13), which may cause a loss of C9orf72 function. A therapeutic approach that uses antisense oligonucleotides (ASOs) to target GGGGCC repeat-containing RNA transcripts for degradation is being pursued (5, 14, 15). However, RNA foci containing GGCCCC repeats transcribed in the antisense direction also accumulate abundantly in c9FTD/ALS (14, 16). Thus, it will be important to consider strategies that target both sense and antisense repeat-containing RNAs.
Spt4 is a highly conserved transcription elongation factor that, together with its binding partner Spt5, regulates RNA polymerase II processivity (17-20). A recent genetic screen in yeast revealed that mutation of Spt4 selectively reduced the transcription of long CAG trinucleotide repeats associated with Huntington’s disease (21). Inhibiting the mammalian ortholog of Spt4, Supt4h, also decreased production of expanded CAG-derived polyQ aggregates in murine striatal neurons. Remarkably, lowering levels of Spt4 did not affect expression of proteins containing short CAG repeat regions. Moreover, RNA-seq analysis found only a limited effect of SPT4 deletion on normal gene expression throughout the transcriptome (21). Thus, Spt4 acts as a specialized transcription factor selectively required for the expression of long trinucleotide repeat-containing transcripts.
Because Spt4 inhibition lowers the expression of disease-associated trinucleotide repeat expansions (21), we hypothesized that Spt4 inhibition might reduce expression of expanded sense and antisense hexanucleotide repeats in mutant C9orf72. If so, it could overcome the current hurdles of designing and optimizing two strategies to target sense and antisense repeats separately. To address this hypothesis, we began by testing the effect of SPT4 deletion in yeast (Saccharomyces cerevisiae) expressing C9orf72 hexanucleotide repeats. We transformed wild type (WT) yeast cells with a galactose-inducible expression construct harboring either short (2) or expanded (66) sense GGGGCC repeats or antisense GGCCCC repeats lacking an ATG start codon. While yeast expressing expanded sense or antisense repeats formed cytoplasmic and perinuclear RNA foci, neither the short nor expanded repeat constructs were toxic, as assessed by serial dilution growth analysis of yeast strains (Fig. S1A–D). Using quantitative reverse transcription polymerase chain reaction (RT-PCR), we next evaluated the effect of SPT4 deletion (spt4Δ) on transcription of sense and antisense repeats. Whereas spt4Δ had no effect on short, non-expanded repeats, we observed a significant decrease in expression of (GGGGCC)66 or (GGCCCC)66 transcripts in spt4Δ cells relative to WT (Fig. 1A). Moreover, SPT4 deletion completely blocked RNA foci formation in yeast expressing (GGGGCC)66 or (GGCCCC)66 (Figs. 1B–D). Expressing Spt4 from a plasmid in spt4Δ cells restored sense and antisense RNA foci formation (Figs. 1B–D), confirming the requirement of Spt4 for the production of transcripts containing expanded C9orf72 repeats and the subsequent accumulation of these RNA transcripts into foci.
Figure 1. Spt4 is required for C9orf72 mutant strand transcription, RNA foci formation, and RAN translation in Saccharomyces cerevisiae.
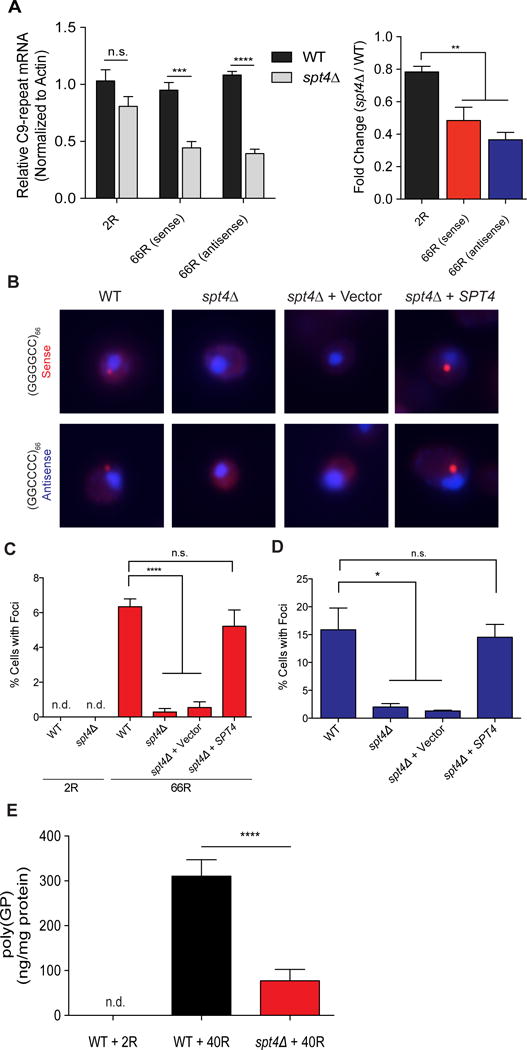
Yeast were transformed with plasmids expressing sense (GGGGCC)n or antisense (GGCCCC)n C9orf72 repeats of varying lengths (2R or 66R) but lacking ATG translation start codons under the control of a galactose-inducible promoter. (A) qRT-PCR analysis of C9-repeat RNA levels in WT and spt4Δ yeast after six hour galactose inductions. C9-repeat RNA levels were normalized to levels of yeast actin, then quantified relative to WT yeast (two-tailed unpaired t-tests, ****p < 0.0001, *** p < 0.001, n.s. = not significant). Data expressed as relative fold changes, spt4Δ/WT (one-way ANOVA with Dunnett’s multiple comparisons test, ** p < 0.01). (B) Sense and antisense RNA foci were detected by fluorescence in situ hybridization. Percentages of yeast cells containing sense (C) and antisense (D) foci were quantified across genotypes (blue = DAPI, red = C9-repeat LNA probe; one-way ANOVA with Dunnett’s multiple comparisons test, **** p < 0.0001, * p < 0.05, n.s. = not significant, n.d. = not determined). (E) Sandwich immunoassay detection of RAN translated poly(GP) in WT and spt4Δ yeast (two-tailed unpaired t-test **** p < 0.0001).
In addition to RNA foci, another pathological feature of c9FTD/ALS is the presence of DPR proteins produced by RAN translation (8, 9, 22). To investigate whether RAN translation occurs in yeast expressing expanded GGGGCC repeats, we used an immunoassay to detect expression of a DPR protein produced from the Glycine-Proline (GP) reading frame. Poly(GP) was not detected in lysates of yeast cells expressing 2 or 66 GGGGCC repeats from a low copy (CEN) expression plasmid; however, robust poly(GP) expression was observed in lysates of yeast cells in which 40 GGGGCC repeats were expressed at higher levels from a high copy (2-micron) expression plasmid (Fig. 1E, Fig. S1C). These data are consistent with the prior finding that the abundance of poly(GP) proteins correlate with levels of repeat-containing transcripts (11, 12). Importantly, deleting SPT4 in (GGGGCC)40-expressing yeast significantly decreased levels of RAN-translated poly(GP) (Fig. 1E). This effect was specific to the expanded nucleotide repeat construct (as in Fig. 1A), because SPT4 deletion did not suppress toxicity of three nonrepeat containing disease-associated proteins [TDP-43, FUS/TLS, and poly(PR)] (Fig. S2A). Further, SPT4 deletion did not affect expression of GFP under the control of the same galactose-inducible promoter or expression of the endogenous yeast GAPDH protein (Fig. S2B). Thus, two key components of c9FTD/ALS pathology (RNA foci and DPR proteins) are recapitulated in yeast models and are mitigated by Spt4 depletion.
We next tested the effect of modulating Spt4 levels in vivo using animal models. Using the synaptobrevin (snb-1) promoter to drive neuronal expression of (GGGGCC)66 (Fig. S3A), we developed a nematode (Caenorhabditis elegans) model of the C9orf72 repeat expansion characterized by RNA foci formation (Fig. 2A, S3B,C), DPR protein production (Fig. 2C), and a significant reduction in lifespan (Fig. 2D, S3G). To determine the impact of altered Spt4/SUPT4H1 expression levels, (GGGGCC)66-expressing worms were crossed to worms expressing human SUPT4H1 also driven by the snb-1 promoter (Fig. 2A–D, S3D–F) or fed RNAi to knockdown endogenous Spt4 (Fig. 2E–H). We observed an increase in GGGGCC repeat RNA and poly(GP) expression, as well as enhanced toxicity in (GGGGCC)66 worms in the presence of exogenous SUPT4H1 (Fig. 2B–D, Fig. S3E–G). Conversely, reduction of endogenous Spt4 levels decreased both GGGGCC repeat RNA and poly(GP) levels, and also improved the survival of (GGGGCC)66 worms (Fig. 2E–H, S3G). Similar modulation of toxicity was observed in transgenic Drosophila engineered to express 6, 29 or 49 pure GGGGCC repeats under the control of the gmr-GAL4 driver. Consistent with previous reports (22-26), expression of (GGGGCC)49 caused severe eye degeneration (Fig. 2I, Fig. S4), while the shorter expanded repeat, (GGGGCC)29, caused a mild phenotype with a modest disruption of the external eye and significant thinning of the retinal ganglia. The unexpanded repeat (GGGGCC)6 caused no observable effect in the retina. Spt4 RNAi partially suppressed the degenerative phenotype of the external and internal eye in (GGGGCC)49-expressing flies, and almost completely suppressed the retinal thinning normally observed in (GGGGCC)29 expressing flies (Fig. 2J, Fig. S4B–D). Like in yeast, the effect of Spt4 depletion was specific to expression of expanded GGGGCC repeats because the expression of a control β-galactosidase transgene was unaffected (Fig. S4E). Furthermore, reducing the expression of Spt4 significantly increased the lifespan of adult flies ubiquitously expressing (GGGGCC)49 repeats (Fig. 2K). Thus, the level of Spt4 expression influences the pathogenicity of C9orf72 GGGGCC repeats in the nervous systems of both Caenorhabditis elegans and Drosophila.
Figure 2. Spt4 modulation influences pathogenicity of GGGGCC repeats in C.elegans and mitigates neurodegeneration in a Drosophila model of c9FTD/ALS.
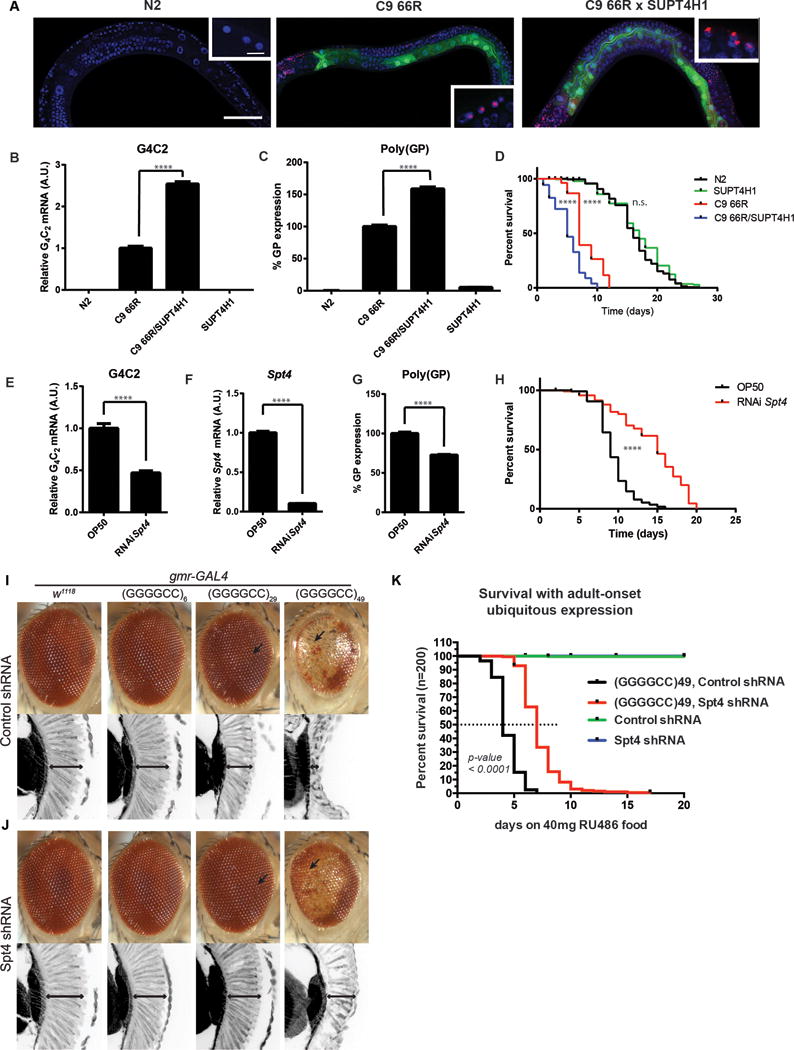
(A) RNA FISH was performed to evaluate GGGGCC RNA foci formation (red) in normal (N2) worms, (GGGGCC)66-expressing worms (C9 66R), and worms expressing both (GGGGCC)66 and human SUPT4H1 (C9 66R × SUPT4H1). The intestinal marker (green) denotes the presence of the (GGGGCC)66 transgene, and DAPI (blue) was used to label nuclei (scale bar: low magnification 50 μm, high magnification 5 μm). (B,C) Expression of GGGGCC RNA levels quantified by qPCR relative to levels in (GGGGCC)66-expressing worms (two-tailed unpaired t-test **** p < 0.0001) (B) and poly(GP) levels evaluated by immunoassay (two-tailed unpaired t-test **** p < 0.0001) (C). (D) A lifespan assay was performed to assess the impact of (GGGGCC)66 and/or SUPT4H1 overexpression on survival (N2 n=145, C9 66R n=160, C9 66R × SUPT4H1 n= 122, SUPT4H1 n=144; log-rank (Mantel-Cox) test **** p < 0.0001, n.s. = not significant. See also Fig. S3G). (E–H) qPCR was performed to measure GGGGCC (E) and Spt4 mRNA (F) levels in (GGGGCC)66-expressing worms fed control (OP50) or RNAi targeting Spt4 (two-tailed unpaired t-test **** p < 0.0001). (G) An immunoassay was utilized to evaluate the percent change in poly(GP) levels following RNAi-mediated reduction of Spt4 (two-tailed unpaired t-test **** p < 0.0001). (H) The ability of Spt4 RNAi to improve survival in (GGGGCC)66 worms was assessed by a lifespan survival assay (OP50 n=119, RNAi Spt4 n=120; log-rank (Mantel-Cox) test **** p < 0.0001. See also Fig. S3G). (I,J) External and internal retinal structures of normal flies or flies expressing (GGGGCC)n transgenes, with co-expression of (I) a control (luciferase) shRNA or (J) spt4 shRNA. A gmr-GAL4 eye specific driver was used to express UAS-transgenes. (I) Control animals with driver alone (w1118) or a short GGGGCC repeat (GGGGCC)6 have normal eye structure. Animals expressing GGGGCC29 show a mild disruption to the highly precise external ommatidial structure, and thinning and gaps in the internal retina (arrows), whereas animals expressing GGGGCC49 have severely degenerate tissue both externally and internally. (J) Reduction in spt4 has no effect on normal or GGGGCC6 expressing animals, and resulted in reduced toxicity in both the external and internal retinal structures to flies bearing 29 or 49 GGGGCC repeats. (K) Reducing the expression of spt4 in (GGGGCC)49 animals significantly increases the life expectancy for animals expressing transgenes using a ubiquitous, drug-inducible driver, da-GS. Expression was induced in adult animals to avoid effects of expressing transgenes during development. Expression of the control (luciferase) shRNA or Spt4 shRNA alone had no effect on the lifespan when expressed alone. Statistical analysis was done using a log-ranked test comparing (GGGGCC)49 animals expressing the control shRNA versus the Spt4 shRNA.
The experiments in yeast, nematodes, and flies are consistent with a role for Spt4 in regulating expression of C9orf72 hexanucleotide repeat expansions. These experiments all relied on expression of GGGGCC or GGCCCC repeats from plasmids or transgenes. To extend our findings, we tested the ability of SUPT4H1 to regulate expression of endogenous C9orf72 repeats in cells obtained from humans afflicted with ALS. We first analyzed cultured fibroblast cells derived from healthy subjects or from ALS patients with or without C9orf72 repeat expansions (Fig. S5A). Foci of sense and antisense repeat-containing RNA (Fig. S5B) and poly(GP) proteins (Fig. S5C) were selectively detected in c9ALS fibroblasts. Consistent with previous findings in brain tissues from c9FTD/ALS patients (11), the abundance of poly(GP) proteins correlated with mRNA levels of C9orf72 variant 3 (Fig. S5G) but not of variant 1, variant 2 or total C9orf72 mRNA (Fig. S5D–F) or with repeat length (Fig. S5H).
Having established the occurrence of RAN translation and RNA foci formation in c9ALS fibroblasts, we evaluated whether depletion of SUPT4H1 and/or its binding partner, SUPT5H, mitigates these pathological hallmarks. Treating cultured fibroblasts from three c9ALS patients with siRNAs against SUPT4H1 and SUPT5H (siSUPT4H1, siSUPT5H respectively) simultaneously decreased both SUPT4H1 and SUPT5H mRNA and protein levels (Fig. 3A,B). Exposing fibroblasts to siSUPT4H1 alone significantly decreased SUPT4H1 mRNA and protein expression, and also caused statistically significant, albeit less drastic, decreases in SUPT5H abundance (Fig. 3A,B). Upon treatment of c9ALS fibroblasts with siSUPT5H alone, we observed the predicted reductions in SUPT5H mRNA and protein. Depletion of SUPT5H also led to a significant decrease in SUPT4H1 protein abundance although it had little effect on SUPT4H1 mRNA (Fig. 3A,B).
Figure 3. Reduction of SUPT4H1 or SUPT5H in c9ALS fibroblasts inhibits production of C9orf72 variant 3 mRNA, sense and antisense RNA foci and DPR proteins.
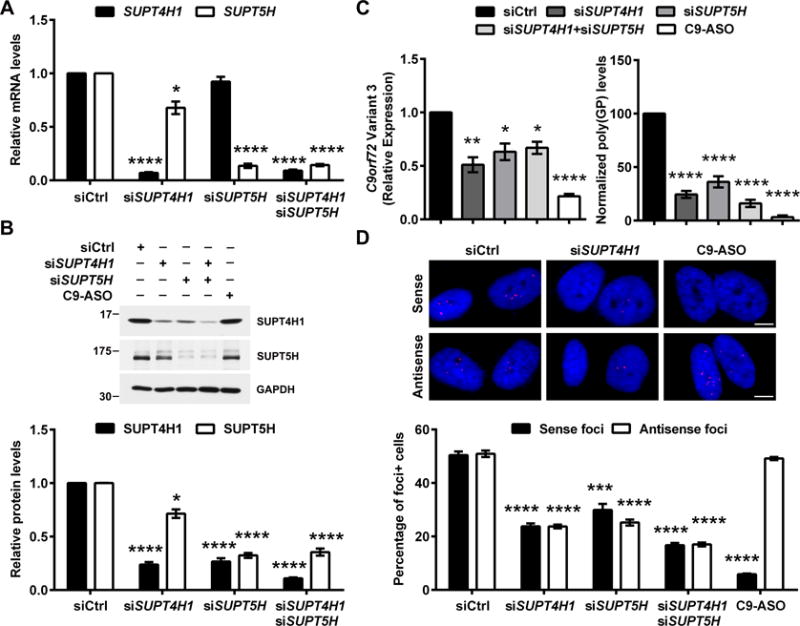
Cultured fibroblasts from three c9ALS patients were treated with a control siRNA (siCtrl), siRNA(s) directed against SUPT4H1 and/or SUPT5H (siSUPT4H1 and siSUPT5H, respectively), or a C9orf72-repeat targeting ASO (C9-ASO) for 10 days. Post-treatment, mRNA levels of SUPT4H1 and SUPT5H were determined by qPCR (A), and SUPT4H1 and SUPT5H protein expression was examined by immunoblot followed by densitometric quantification (B). (C) The effect of SUPT4H1 and/or SUPT5H depletion on C9orf72 variant 3 mRNA expression and poly(GP) protein levels was examined by qPCR and immunoassay, respectively. (D) RNA FISH with probes for GGGGCC or GGCCCC RNA was used to detect foci containing sense or antisense repeats (red) in the nucleus of cells (blue, Hoechst 33258). The percentage of cells containing foci was then determined. * p <0.05, ** p <0.01, *** p <0.001, **** p <0.0001 as assessed by one way ANOVA followed by Tukey’s post-hoc analysis.
Reduction of SUPT4H1 and/or SUPT5H significantly decreased levels of C9orf72 variant 3 mRNA, poly(GP) proteins, as well as foci formed of sense or antisense repeat RNA in c9ALS fibroblasts, but did not lead to any overt toxicity (Fig. 3C,D, Fig. S6,S7). In contrast, treatment of c9ALS fibroblasts with an ASO targeting the C9orf72 sense transcript mitigated most of these features with the key exception that foci formed of antisense GGCCCC-containing transcripts remained unaffected (Fig. 3C,D). Thus, lowering the abundance of a single gene product, SUPT4H1 or SUPT5H, decreased all three of the major pathological features of c9FTD/ALS: sense RNA foci, antisense RNA foci, and DPR proteins. Intriguingly, SUPT4H1 and SUPT5H mRNA expression levels positively correlated with levels of C9orf72 variant 3 mRNA (Fig. 4A) or poly(GP) proteins (Fig. 4B) in the cerebellum of human C9orf72 repeat expansion carriers. Partial reduction of SUPT4H1 by lentiviral shRNA in 8-week old cortical neurons differentiated from four independent lines of human induced pluripotent stem cells from c9FTD/ALS patients (13, 15) (Fig. 4C–E) resulted in decreased levels of C9orf72 variant 3 mRNA (Fig. 4D) and poly(GP) proteins (Fig. 4E). To address whether there were global transcriptional changes upon depletion of SUPT4H1, we performed RNA-sequencing on two human fibroblast lines treated with SUPT4H1 siRNA. As predicted by RNA-sequencing experiments in yeast and mouse (21), we found only a small subset of human genes that were differentially expressed after SUPT4H1 knockdown (Fig. S8), indicating that knockdown of SUPT4H1 affects expanded repeat transcripts but has a minimal role on other transcripts.
Figure 4. Pathological features of c9FTD/ALS correlate with SUPT4H1 and SUPT5H expression in patient brain tissue, and partial knockdown of SUPT4H1 in C9orf72 iPSC-derived cortical neurons reduces production of C9orf72 variant 3 mRNA and DPR proteins.
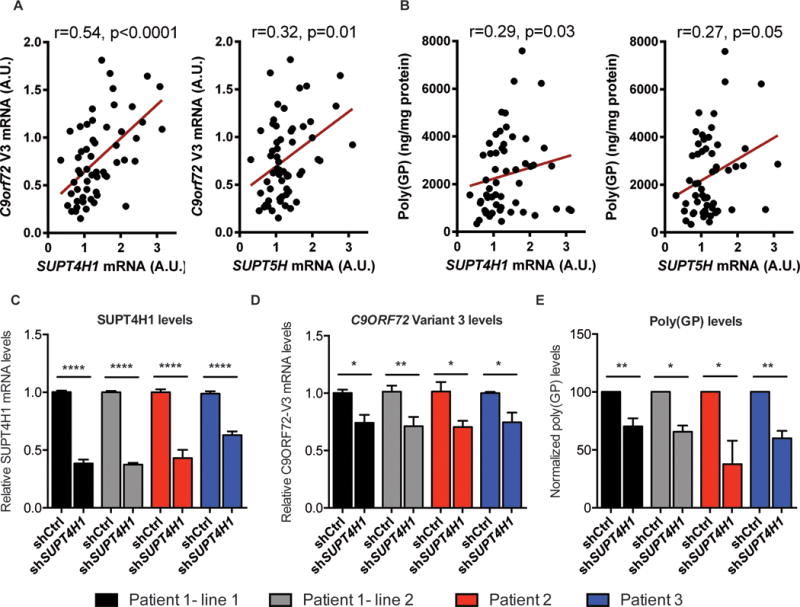
(A,B) SUPT4H1 and SUPT5H mRNA levels in cerebellar tissue from c9FTD/ALS patients were measured by qPCR and found to associate with cerebellar levels of C9orf72 variant 3 mRNA measured by qPCR (A) and with poly(GP) proteins measured by immunoassay (B) using a Spearman’s test of correlation. (C-E) Cortical neurons differentiated from each C9orf72 iPSC line were aged for 8 weeks and then transduced with lentivirus expressing either a non-coding control shRNA or an shRNA against SUPT4H1 mRNA for 6 days. Four iPSC lines derived from 3 patients with C9orf72 repeat expansions were used. Knockdown of SUPT4H1 mRNA (C) and C9orf72 variant 3 mRNA expression levels (D) were determined by qPCR. Poly(GP) protein levels was examined by immunoassay (E). *: p < 0.05, **: p < 0.01, ****: p < 0.0001 as assessed by two-tailed unpaired t-test from three independent neuronal differentiations.
Here, we found that the transcription factor Spt4/SUPT4H1 is required for the specific expression of hexanucleotide repeat expansions in the C9orf72 gene. Previous studies have shown that injection of ASOs targeting Supt4h expression or genetic reduction of Supt4h levels in mouse models of Huntington’s disease resulted in a mutant allele-specific reduction of huntingtin mRNA and protein (27). This treatment reduced the amount of polyQ aggregates, delayed motor impairment and prolonged survival (27). Our data suggest that similar strategies aimed at diminishing the function of SUPT4H1, such as ASOs or small molecules, could be pursued as an effective therapy for c9FTD/ALS.
Supplementary Material
Acknowledgments
We thank D. Cerza and Y. Zu for technical support. This work was supported by NIH grants R01NS065317 (A.D.G.), R01NS09386501 (A.D.G. and L.P.), R01NS073660 (A.D.G. and N.M.B.), R01AG026251 (L.P.), R21NS079807 (Y.-J.Z.), R21NS094489 (C.C.), R01NS063964 (L.P.), R01NS077402 (L.P.), R21NS084528 (L.P.), P01NS084974 (L.P.), R01NS088689 (L.P.), R01ES20395 (L.P.), R01NS085812 (S.N.C. and T-H.C.), R01NS079725 (F.B.G.), Mayo Clinic Foundation (L.P.), Alzheimer’s Association [NIRP-14-304425 (Y.-J.Z.)], Amyotrophic Lateral Sclerosis Association (Y.-J.Z, T.F.G., M.P., L.P., F.B.G.), the National Science Foundation Graduate Research Fellowship (N.J.K.), the Robert Packard Center for ALS Research at Johns Hopkins (L.P., A.D.G., F.B.G.), Target ALS (L.P., A.D.G., N.B., F.B.G.), Association for Frontotemporal Degeneration (S.A.), Stanford University (S.N.C.) and the Glenn Foundation (A.D.G., N.B). RNA sequencing data are deposited with NCBI Geo (####).
Footnotes
References and Notes
- 1.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renton AE, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gendron TF, Belzil VV, Zhang YJ, Petrucelli L. Mechanisms of toxicity in C9FTLD/ALS. Acta Neuropathol. 2014;127:359–376. doi: 10.1007/s00401-013-1237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly CJ, et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80:415–428. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YB, et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Reports. 2013;5:1178–1186. doi: 10.1016/j.celrep.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haeusler AR, et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature. 2014;507:195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori K, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 9.Ash PE, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zu T, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Nat Acad Sci USA. 2013;110:E4968–4977. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendron TF, et al. Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathol. 2015;130:559–573. doi: 10.1007/s00401-015-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Blitterswijk M, et al. Novel clinical associations with specific C9ORF72 transcripts in patients with repeat expansions in C9ORF72. Acta Neuropath. 2015;130:863–876. doi: 10.1007/s00401-015-1480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida S, et al. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 2013;126:385–399. doi: 10.1007/s00401-013-1149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagier-Tourenne C, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Nat Acad Sci USA. 2013;110:E4530–4539. doi: 10.1073/pnas.1318835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sareen D, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Science Transl Med. 2013;5:208ra149. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendron TF, et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013;126:829–844. doi: 10.1007/s00401-013-1192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirtreiter A, et al. Spt4/5 stimulates transcription elongation through the RNA polymerase clamp coiled-coil motif. Nuc Acids Res. 2010;38:4040–4051. doi: 10.1093/nar/gkq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason PB, Struhl K. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol Cell. 2005;17:831–840. doi: 10.1016/j.molcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Rondon AG, Garcia-Rubio M, Gonzalez-Barrera S, Aguilera A. Molecular evidence for a positive role of Spt4 in transcription elongation. EMBO J. 2003;22:612–620. doi: 10.1093/emboj/cdg047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada T, et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CR, et al. Spt4 is selectively required for transcription of extended trinucleotide repeats. Cell. 2012;148:690–701. doi: 10.1016/j.cell.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Xue Y, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizielinska S, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345:1192–1194. doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freibaum BD, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–133. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew J, et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science. 2015;348:1151–1154. doi: 10.1126/science.aaa9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran H, et al. Differential Toxicity of Nuclear RNA Foci versus Dipeptide Repeat Proteins in a Drosophila Model of C9ORF72 FTD/ALS. Neuron. 2015;87:1207–1214. doi: 10.1016/j.neuron.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng HM, et al. Effects on murine behavior and lifespan of selectively decreasing expression of mutant huntingtin allele by supt4h knockdown. PLoS Genet. 2015;11:e1005043. doi: 10.1371/journal.pgen.1005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long RM, et al. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science. 1997;277:383–387. doi: 10.1126/science.277.5324.383. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen R, Nielsen PS, Jensen TH. Dramatically improved RNA in situ hybridization signals using LNA-modified probes. RNA. 2005;11:1745–1748. doi: 10.1261/rna.2139705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz TJ, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Urano F, et al. A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol. 2002;158:639–646. doi: 10.1083/jcb.200203086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweizer Burguete A, et al. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. eLife. 2015;4 doi: 10.7554/eLife.08881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, et al. Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2014;46:152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Z, et al. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron. 2014;83:1043–1050. doi: 10.1016/j.neuron.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Blitterswijk M, et al. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol. 2013;12:978–988. doi: 10.1016/S1474-4422(13)70210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prudencio M, et al. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nat Neurosci. 2015;18:1175–1182. doi: 10.1038/nn.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen X, et al. Antisense Proline-Arginine RAN Dipeptides Linked to C9ORF72-ALS/FTD Form Toxic Nuclear Aggregates that Initiate In Vitro and In Vivo Neuronal Death. Neuron. 2014;84:1213–1225. doi: 10.1016/j.neuron.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


