Data resource basics
The Network for Analysing Longitudinal Population-based HIV/AIDS data on Africa (ALPHA Network) was established in 2005 and aims to: (i) broaden the evidence base on HIV epidemiology for informing policy; (ii) strengthen analytical capacity for HIV research; and (iii) foster collaboration between study sites. 1 All of the study sites participating in the ALPHA Network are independently managed and have their own scientific agendas and tailored research methodologies, but share a common interest in HIV epidemiology and its interactions with the socio-demographic characteristics of the populations they cover. The ALPHA Network study sites and their institutional affiliations are described in Table 1 , and their geographical distribution is shown in Figure 1 . Several of the ALPHA Network study sites have published site-specific profiles that contain more detail. 2–10 Most of the ALPHA Network study sites are also members of the INDEPTH Network of demographic surveillance sites [ http://www.indepth-network.org/ ].
Table 1.
Description of the ALPHA Network study sites
| Name | Short Name | Institutional affiliation URL | Location, Country | Demographicsurveillance:start/frequency b | Serological survey: start/frequency b | Verbal autopsy: start date | Linked facility data: start date |
|---|---|---|---|---|---|---|---|
| Kyamulibwa General Population Cohort | Masaka |
|
Kalungu District (formerly Masaka), Uganda |
|
|
1990 |
|
| Rakai Community Cohort Study | Rakai |
|
Rakai District, Uganda |
|
|
1999 |
|
|
Kisesa |
|
Magu District (Mwanza Region), Tanzania |
|
|
1994 |
|
| Karonga Health and Demographic Surveillance System | Karonga |
|
Karonga District, Malawi |
|
Annual survey from 2007 to 2011. New residents and individuals with long test interval since 2012 | 2002 |
|
| Manicaland HIV/STD Prevention Project | Manicaland |
|
Manicaland Province, Zimbabwe |
|
|
1998 | N/A |
| Africa Centre Demographic Information System (ACDIS) | uMkhanyakude |
|
uMkhanyakude (formely Hlabisa) Discrict, South Africa |
|
|
2000 | Hlabisa HIV Treatment and Care Programme: 2005 |
|
Agincourt |
|
Ehlanzeni District, South Africa |
|
|
1992 |
|
| Nairobi Urban Health and Demographic Surveillance System | Nairobi |
|
Viwandani and Korogocho (Nairobi), Kenya |
|
|
2003 | N/A |
|
Ifakara |
|
Kilombero District, Tanzania |
|
2012 and 2014 | 2007 | N/A |
|
Kisumu |
|
|
|
|
2002 |
|
Notes:
a The Ifakara DSS started in 1997 and covered a total of 25 villages in the Kilombero and Ulanga districts. In 2007, the DSS was extended with 5 urban areas of Ifakara town. This cohort profile pertains to the urban DSS only. HIV serosurveillance is done in two of five of the urban areas
b In many instances the fieldwork is spread over multiple calendar years. We only report the start date.
c Record linkage with HIV treatment facilities coincided with the rollout of ART. Prior to 2005, record linkage was done for TB-related work.
Figure 1.
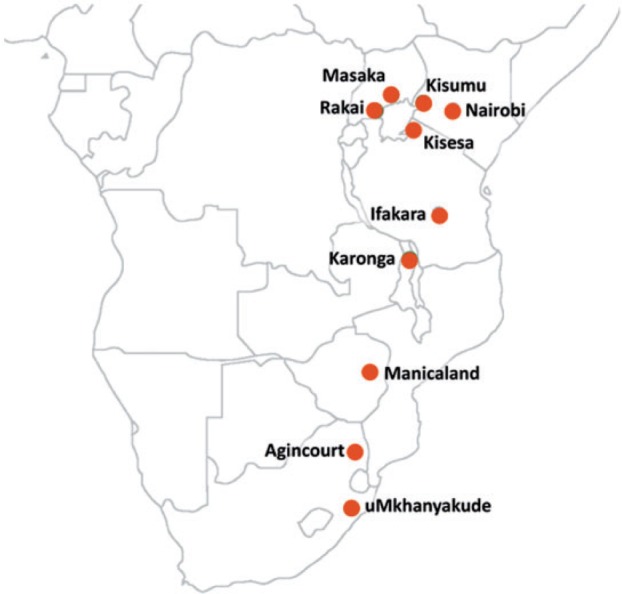
Location of the ALPHA member study sites.
The population perspective offered by the study sites complements the evidence from HIV clinical cohorts and health facility-based studies; their longitudinal character is what sets them apart from cross-sectional serological surveys. The activities of the Network revolve around a series of thematic workshops that lay the foundations for both site-specific and pooled analyses. Topics that have been studied in the past include HIV incidence, 11 sexual behaviour, 12 orphaning and children’s living arrangements, 13 and fertility. 14 The monitoring of HIV-associated mortality has been and continues to be one of its focus areas. 15–17 The Network is also well positioned to evaluate the population-level effects of antiretroviral therapy (ART) scale-up, and member sites have recently extended their efforts to collect more and better data on the uptake of HIV diagnostic and AIDS care services.
The ALPHA Network is a regular contributor to the United Nations Programme on HIV/AIDS (UNAIDS) Reference Group on Estimates, Modelling and Projections [ http://www.epidem.org ], which oversees the data and methods used for producing HIV estimates for most countries in the world, and to the modelling community through an agreement with the HIV Modelling Consortium [ http://www.hivmodelling.org/ ].
Data collected
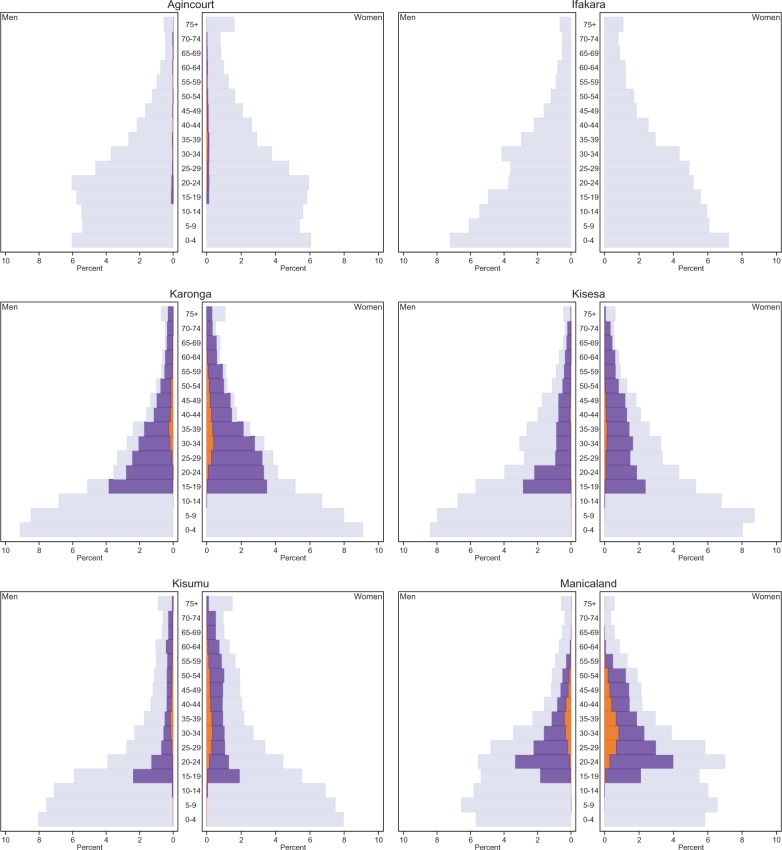
The ALPHA Network pools and harmonizes data from member sites that conduct demographic surveillance in populations that range from approximately 20 000 to 220 000 in size. Most study sites cover the entire population in one contiguous area, but some members conduct surveillance in multiple areas to represent distinct socioeconomic zones (e.g. Manicaland). Surveillance is generally conducted via household visits and interviews with one of the household members—sometimes referred to as proxy-respondents—who report on births, deaths and in- and out- movements in the household in the intercensal period. The Karonga demographic surveillance is an exception, and is done by means of village informants with an annual census to check on reporting completeness. 2 In most study sites, the enumerated population includes all resident members of households in the surveillance area. The two South African study sites (uMkhanyakude and Agincourt) are characterized by high levels of temporary or circulatory migration, and both resident and non-resident members of households are enumerated (non-resident household members are included in the ALPHA Network datasets for Agincourt only). 18,19 With the exception of the study sites in Nairobi and Ifakara, the ALPHA Network member sites are predominantly rural, and they all have relatively young age structures ( Figure 2 ). In some of the settings, the population age structure is marked by high levels of out-migration (e.g. Masaka and uMkhanyakude).
Figure 2.
Relative age distribution by study site, sex and HIV status, 2011.
The ALPHA Network assembles data from different sources, including demographic surveillance, verbal autopsy interviews, serological and sexual behaviour surveys and individually-linked data from medical facilities. The starting date and intervals of data collection are summarized in Table 1 . Demographic surveillance generates data about residence episodes and the starting (birth and in-migration) and terminating (migration and death) events of residence episodes. Most study sites routinely follow up the report of deaths in the household with a verbal autopsy (VA) interview for determining the underlying and immediate causes of death. VA instruments were developed independently, and have over the years converged towards the standard VA questionnaire proposed by the World Health Organization (WHO). 20 VA questionnaires typically include retrospective questions about the HIV status of the deceased, and many study sites now also enquire about HIV services use.
Along with the demographic surveillance, ALPHA Network study sites organize repeated population-based HIV serosurveys, using either home visits or temporary HIV testing centres to which residents are invited. HIV testing eligibility criteria vary across sites, and some of the study sites have restricted testing to a sample of the adult population (Nairobi and Agincourt).
HIV testing protocols and participation rates vary between sites and over time. Prior to the availability of rapid HIV tests, test results were optionally returned to participants at the household or temporary clinic a few weeks later. In recent serosurveys, residents are usually given an opportunity to receive standard HIV testing and counselling (HTC) during the visit when specimens for a research test are collected. The Manicaland study has offered free HIV tests for diagnostic purposes at a local antenatal clinic. Some of the ALPHA Network members have published studies describing the HIV serosurveys, non-response in the HIV serosurveillance and associated bias in HIV prevalence estimates. 21–25 The serosurveys also create an opportunity to administer an individual interview on sexual behaviour, health services utilization etc.
A few of the ALPHA Network sites have established record linkage between the demographic surveillance and medical facility data via a unique (set of) identifier(s) (e.g. Karonga, uMkhanyakude, Rakai and Masaka) and others, including Kisesa, Kisumu and Agincourt, have piloted probabilistic record linkage. 26 A more recent initiative, currently implemented in Agincourt and Kisesa, is to conduct the record linkage in the presence of the patient at the time that he or she visits the health facility. This protocol has the advantage that the patient can confirm his or her identity, which ensures better-quality matches. In addition, the presence of the patient offers an opportunity to seek informed consent for linking medical information to the demographic surveillance database. Linked medical facility data are used to retrieve information on HIV status, care and treatment services use, and in some cases also clinical markers of disease progression and viral suppression.
Essential (input) data for estimating mortality and HIV incidence and prevalence are the residence episodes and HIV test results. These data are updated on a regular basis and, to that end, we have developed a series of metadata templates that are used to guide the study sites on the structure and attributes of the data they contribute to the ALPHA Network. Metadata templates have also been developed for parent-child links, fertility, respondent background characteristics, verbal autopsy data and self-reported and clinic data on the use of HIV care and treatment services. Metadata templates are available through the ALPHA Network website (see below).
The allocation of person-time to HIV status (and treatment) categories is study-specific. In analyses of mortality, we generally classify time prior to the first recorded HIV test as HIV status unknown. Failure to do so would introduce downward bias in mortality estimates as only survivors can be tested. The time following a positive test remains positive until censoring or death. Studies often also allow for exposure time following a negative test in order to estimate mortality among HIV-negative individuals, but the period is kept sufficiently short to ensure that elevated mortality among seroconvertors does not introduce upward bias. Similar rules for imputing and stale-dating information are used in studies focusing on other personal attributes (e.g. marital status).
Table 2 summarizes the person-years of exposure and number of deaths by HIV status and, for people living with HIV (PLHIV), the stage on the HIV care and treatment cascade. All sites measure HIV infection, ART initiation and death. Some of the study sites can also distinguish between seroconversion and the receipt of an HIV-positive diagnosis, between PLHIV who are in care and those who are not, and between those who ever interrupted treatment and those who have been on ART continuously.
Table 2.
Descriptive statistics of the pooled ALPHA dataset, 1990–2011 a
|
1990–2003
|
2009–2011
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Population | Individuals b | Person-years | Deaths | Deaths with VA | Death rate (per 1000) | 95%-CI | Death rate (per 1000) | 95%-CI |
| All | 1 338 983 | 6 469 798 | 72 949 | 43 280 | 10.41 | (10.28–10.55) | 10.34 | (10.19–10.49) |
| Chidren (≤ 15 years) | 692 747 | 2 682 163 | 14 558 | 1 497 | 5.01 | (4.87–5.16) | 4.94 | (4.77–5.11) |
| Adults (>15 years) | 831 404 | 3 787 634 | 58 391 | 41 783 | 14.43 | (14.22–14.64) | 14.00 | (13.77–14.23) |
| HIV status unknown c | 802 467 | 2 921 897 | 45 423 | 33 055 | 14.38 | (14.15–14.62) | 14.05 | (13.77–14.33) |
| Known HIV- c | 222 261 | 718 949 | 5 544 | 3 406 | 6.80 | (6.44–7.18) | 9.57 | (9.21–9.95) |
| Known HIV+ c | 46 038 | 146 789 | 7 424 | 5 322 | 74.52 | (71.22–77.96) | 31.71 | (30.38–33.1) |
| Diagnosed HIV+ c | 31 618 | 84 633 | 4 076 | 3 213 | 59.79 | (54.77–65.27) | 32.96 | (31.43–34.56) |
| Ever treated HIV+ c | 10 126 | 21 108 | 947 | 751 | – | – | 37.39 | (34.47–40.55) |
| Ever interrupted ART c | 614 | 898 | 93 | 79 | – | – | 66.46 | (50.64–87.22) |
Notes:
a Based on pooled ALPHA data from December 2014.
b An individual can contribute to more than one population subgroup as he or she ages, is tested, or moves to the next stage on the HIV treatment cascade. Counts of individuals may be inflated by incomplete reconcilliation of internal migrations, but it will not affect the person-years of exposure or the number of deaths .
c A person with a known HIV status is someone for whom the study has a (recent) record of a negative or positive HIV test. Persons with an unknown HIV status may be aware of their own HIV status but their test could have taken place at a facility not linked to the study and never had a chance (or did not want) to report their status. Similarly, diagnosed or ever treated HIV positive persons are those with a study record of HTC or treatment initiation, respectively.
The breakdown of the study populations by HIV status is shown in Figure 2 . This illustrates that the coverage of the HIV status information differs considerably across study sites. These differences result from variation in the eligibility criteria for HIV testing, frequency of testing, and HIV testing participation rates. In addition, some study sites retrieve HIV status information through record linkage with treatment facilities (see Table 1 ). The latter also explains the small number of known HIV-positive children in uMkhanyakude even though they were not eligible for participation in the serosurveys. Record linkage with treatment facilities can only identify HIV-positive residents, and that tends to inflate HIV prevalence in the dataset. Point prevalence of HIV is therefore more appropriately estimated from the serological surveys (ideally with a correction for non-response bias). 21
Data resource use
Early studies from ALPHA sites documented the severe impact of HIV on adult mortality. 27–30 Later, data from the ALHA Network’s members established that the survival of HIV-positives in the absence of ART was similar to that in high-income countries, including the shorter survival of people infected at older ages, and the absence of gender differences in the survival post infection once the age at infection was taken into account. 15,31 These estimates of the survival of PLHIV who are not receiving treatment are incorporated in the Spectrum model, 32–34 which is the tool used by UNAIDS for generating national and global estimates of the HIV/AIDS epidemic. Similarly, long-term follow-up of children has allowed the ALPHA Network to contribute evidence on the survival patterns of infected and uninfected children of HIV-positive mothers 35 and has demonstrated that a mother’s death can have as important an impact on a child’s short-term probability of dying as the mother’s HIV infection. 36
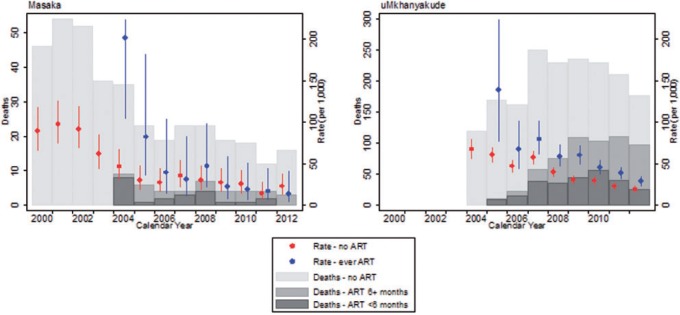
More recently, the ALPHA Network member sites have registered important population-level mortality reductions following the introduction of ART. 16,37–39,40 Crude death rates among adult PLHIV across all study sites have more than halved, from 74.52 per 1000 in 1990–2003 [95% confidence interval (CI): 71.22–77.96] to 31.71 in 2009–11 (95% CI: 30.38–33.1) ( Table 2 ). Continued improvements in the timing of ART initiation have not only reduced the mortality of PLHIV receiving treatment, but also reduced mortality of those who have not (yet) started ART because they are increasingly selected for early-stage disease. 38Figure 3 further illustrates that the mortality rates of PLHIV on ART were often very high as treatment was just being rolled out, because a backlog of patients with severe immunosuppression were quickly ushered onto treatment as it became locally available. As ART programmes matured, the mortality rates of PLHIV on ART declined and so did the fraction of deaths among PLHIV during the first 6 months of treatment ( Figure 3 ). Both of these statistics are indicative of improvements in the timing of treatment initiation and the clinical management of patients. The bar graph in Figure 3 also suggests, however, that a large fraction—the majority in Masaka—of the deaths to PLHIV occur among men and women without a record of treatment initiation. Such deaths are not usually captured in clinic-based studies, and that is where the ALPHA study sites fill an important information gap.
Figure 3.
Deaths and death rates of adult PLHIV (ages 15–65) by treatment status, Masaka (UGA) and uMkhanyakude (RSA).
Despite sizeable declines, mortality among PLHIV remains relatively high: in 2009–11, the crude death rate among adult PLHIV (31.71 per 1000, 95% CI: 30.38–33.1) was still more than three times higher than the death rate of known HIV-negative adults (9.57 per 1000, 95% CI: 9.21–9.95, Table 2 ). Verbal autopsy studies from the ALPHA Network sites have demonstrated that HIV and tuberculosis (TB) continue to account for a large share of deaths among PLHIV, which suggests that HIV services use remains sub-optimal. These studies also suggest, however, that PLHIV have elevated mortality rates from a wide range of causes, including some that are not immediately suggestive of HIV. 41–42
Mortality reductions among PLHIV have generally been larger for women than for men, 38 and that is thought to be associated with women’s greater engagement with HIV services. However, the remaining burden of HIV-related mortality—as measured by the adult life-years lost to HIV—remains higher for women than for men. Women’s higher HIV prevalence, younger ages at infection and lower mortality rates from other causes all contribute to this phenomenon. 43
ALPHA network study sites have also provided direct evidence about HIV incidence in community-based, non-trial settings, and these measurements have been used to validate indirect estimation methods based on models 11 and methods based on incidence assays. 44 Contrary to the expectations raised by studies of discordant couples, 45 so far only one ALPHA site has shown a population-level decline in HIV incidence associated with ART roll-out. 46
A comprehensive list of publications is maintained on the ALPHA Network website ( http://alpha.lshtm.ac.uk ).
Strengths and weaknesses
The ALPHA Network assembles anonymized data from large population-based HIV surveillance sites in sub-Saharan Africa and is an important resource for studying the epidemiology of HIV. Because demographic surveillance sites cover relatively small populations, however, their findings cannot be easily extrapolated to entire countries or regions. This critique applies in varying degrees to the ALPHA Network study sites, but their justification stems from a conscious decision to prioritize data quality and repeated measurement over national representativeness. Generalizability of findings can be compromised further if study sites are used to embed trials or are serviced by superior medical facilities. 48 Pooling of the data from across the ALPHA Network overcomes some of the aforementioned critiques, as findings are more convincing whenever they can be reproduced in distinct locations with dissimilar epidemiological and socioeconomic profiles and different fieldwork practices. The ALPHA Network’s added value is fully brought to bear in analyses that require the statistical power of a pooled dataset. Examples include the estimation of HIV-associated maternal mortality 49 and a study on the risks of acquiring HIV infection during pregnancy. 14
Because the ALPHA Network brings together data from various demographic surveillance sites, it shares many of their challenges. These include high population mobility (internal and external, and both temporary migration and population movements with a more permanent character), the limitations arising from proxy respondent reporting, and survey fatigue. Challenges particular to a network of surveillance sites are the standardization and documentation of datasets. Data extraction and transformation to the ALPHA meta-data specifications rely on the correct interpretation of the data specifications and an understanding of the site’s contemporary and historical data collection procedures and instruments. Personnel and study protocols often change over time, and transforming these diverse data to a standard format is both challenging and time consuming. Work is ongoing to document these data extraction and transformation procedures in detail.
The integration of demographic surveillance with health facility data is an important and relatively recent enhancement of the ALPHA Network’s data repository for policy-relevant analyses, but it is a logistically and ethically challenging enterprise. For example, most of the health facilities in the study sites do not keep electronic patient records and do not have centralized patient administration. In addition, most health facilities are independently managed and the interface between research and health services provision has to be carefully negotiated with respective authorities.
Data resource access
The ALPHA Network regularly provides aggregate data tables and estimates to the UNAIDS Reference Group on Estimates, Modelling and Projections, and the HIV Modelling Consortium. Individual-level demographic data from most ALPHA Network member sites are available through INDEPTH’s iShare repository [ http://www.indepth-ishare.org ]. Further, the ALPHA Network has made a commitment to deposit a subset of the individual-level data in a public repository by the end of this funding cycle, and work is ongoing to develop a mechanism to facilitate the use of data that are not open access. Meanwhile, access to micro-data is at the discretion of each participating study site, but data requests may be channelled through the network co-ordinating group at the London School of Hygiene and Tropical Medicine (LSHTM). Data requests including a short description of their purpose should be sent to [ alpha@lshtm.ac.uk ]. More information about the ALPHA Network, including a detailed description of the metadata, are available on the ALPHA Network website: [ http://alpha.lshtm.ac.uk ].
Alpha network in a nutshell:
The ALPHA Network brings together 10 population-based HIV surveillance sites in eastern and southern Africa. It was established in 2005 and aims to strengthen the analytical capacity for HIV research through collaboration and to broaden the evidence base on HIV epidemiology for policy.
Member sites monitor vital events, HIV infection and health services utilization in populations that range from approximately 20 000 to 220 000 individuals. These jointly contribute over 6 million person-years of exposure time, and over 140 000 person-years by people who are known to be HIV-positive.
The ALPHA Network is an important resource for population-level monitoring of the HIV epidemic, the impact of prevention and treatment programmes, and their downstream effects on individuals and families.
Access to microdata is at the discretion of each of the study sites but may be channelled through the network co-ordinating group at LSHTM. More information about the ALPHA Network and contact details are available on its website: [ http://alpha.lshtm.ac.uk ].
Funding
The core funding for the network comes from the Wellcome Trust (085477/Z/08/Z), and is administered by the London School of Hygiene and Tropical Medicine (LSHTM). At its inception in 2005, the network consisted of six study sites. The network is now in its second Wellcome Trust funding cycle and has grown to 10 study sites. Additional funding has been received from the Bill and Melinda Gates Foundation (2013, BMGF- OPP1082114) for monitoring HIV-related mortality in the era of ART, and the Nuffield Foundation (2012) for studying the effects of HIV on children and adolescents. UNAIDS and WHO have supported various ALPHA Network meetings and publications.
Conflict of interest : Simon Gregson holds stocks in AstraZeneca and GlaxoSmithKline, makers of pharmaceutical products.
References
- 1. Maher D, Biraro S, Hosegood V, et al. . Translating global health research aims into action: the example of the ALPHA network . Trop Med Int Health 2010. ; 15 : 321 – 28 . [DOI] [PubMed] [Google Scholar]
- 2. Jahn A, Crampin AC, Glynn JR, et al. . Evaluation of a village-informant driven demographic surveillance system in Karonga, Northern Malawi . Demogr Res 2007. ; 16 : 219 – 48 . [Google Scholar]
- 3. Tanser F, Hosegood V, Bärnighausen T, et al. . Cohort Profile: Africa centre demographic information system (ACDIS) and population-based HIV survey . Int J Epidemiol 2008. ; 37 : 956 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn K, Collinson MA, Gómez-Olivé FX, et al. . Profile: Agincourt health and socio-demographic surveillance system . Int J Epidemiol 2012. ; 41 : 988 – 1001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emina J, Beguy D, Zulu EM, et al. . Monitoring of health and demographic outcomes in poor urban settlements: evidence from the Nairobi Urban Health and Demographic Surveillance System . J Urban Health 2011. ; 88 : 200 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odhiambo FO, Laserson KF, Sewe M, et al. . Profile: the KEMRI/CDC Health and Demographic Surveillance System—Western Kenya . Int J Epidemiol 2012. ; 41 : 977 – 87 . [DOI] [PubMed] [Google Scholar]
- 7. Crampin AC, Dube A, Mboma S, et al. . Profile: the Karonga Health and Demographic Surveillance System . Int J Epidemiol 2012. ; 41 : 676 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asiki G, Murphy G, Nakiyingi-Miiro J, et al. . The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies . Int J Epidemiol 2013. ; 42 : 129 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geubbels E, Amri S, Levira F, Schellenberg J, Masanja H, Nathan R . Health and Demographic Surveillance System Profile: The Ifakara Rural and Urban Health and Demographic Surveillance System (Ifakara HDSS) . Int J Epidemiol 2015. ; 44 : 848 – 61 . [DOI] [PubMed] [Google Scholar]
- 10. Kishamawe C, Isingo R, Mtenga B, et al. . Health and Demographic Surveillance System Profile: The Magu Health and Demographic Surveillance System (Magu HDSS) . Int J Epidemiol 2015. ; 44 : 1851 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hallett TB, Zaba B, Todd J, et al. . Estimating incidence from prevalence in generalised HIV epidemics: methods and validation . PLoS Med 2008. ; 5 : e80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gregson S, Todd J, Zaba B . Sexual behaviour change in countries with generalised HIV epidemics? Evidence from population-based cohort studies in sub-Saharan Africa . Sex Transm Infect 2009. ; 85(Suppl 1) : i1 – 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hosegood V, Floyd S, Marston M, et al. . The effects of high HIV prevalence on orphanhood and living arrangements of children in Malawi, Tanzania, and South Africa . Popul Stud (Camb) 2007. ; 61 : 327 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marston M, Newell ML, Crampin A, et al. . Is the risk of HIV acquisition increased during and immediately after pregnancy? A secondary analysis of pooled HIV community-based studies from the ALPHA network . PLoS One 2013. ; 8 : e82219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghys PD, Zaba B, Prins M . Survival and mortality of people infected with HIV in low and middle income countries: results from the extended ALPHA network . AIDS 2007. ; 21 : S1 – S4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Todd J, Slaymaker E, Zaba B, Mahy M, Byass P . Measuring HIV-related mortality in the first decade of anti-retroviral therapy in sub-Saharan Africa . Glob Health Action 2014. ; 7 : 24787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todd J, Wringe A, Floyd S, Zaba B . Antiretroviral therapy in sub‐Saharan Africa: evidence about need, uptake and impact from community‐based cohort studies . Trop Med Int Health 2012. ; 17 : e1 – e2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collinson MA, White MJ, Bocquier P, et al. . Migration and the epidemiological transition: insights from the Agincourt sub-district of northeast South Africa . Glob Health Action 2014. ; 7 : 23514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosegood V, Benzler J, Solarsh GC . Population mobility and household dynamics in rural South Africa: implications for demographic and health research . South Afr J Demogr 2005. : 10 : 43 – 68 . [Google Scholar]
- 20. WHO . Verbal Autopsy Standards . 2015. http://www.who.int/healthinfo/statistics/verbalautopsystandards/en/ (7 April 2015, date last accessed) .
- 21. Floyd S, Molesworth A, Dube A, et al. . Underestimation of HIV prevalence in surveys when some people already know their status, and ways to reduce the bias . AIDS 2013. ; 27 : 233 – 42 . [DOI] [PubMed] [Google Scholar]
- 22. Nyirenda M, Zaba B, Bärnighausen T, Hosegood V, Newell M-L . Adjusting HIV prevalence for survey non-response using mortality rates: an application of the method using surveillance data from rural South Africa . PloS One 2010. ; 5 : e12370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez-Olive FX, Angotti N, Houle B, et al. . Prevalence of HIV among those 15 and older in rural South Africa . AIDS Care 2013. ; 25 : 1122 – 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolff B, Nyanzi B, Katongole G, Ssesanga D, Ruberantwari A, Whitworth J . Evaluation of a home-based voluntary counselling and testing intervention in rural Uganda . Health Policy Plann 2005. ; 20 : 109 – 16 . [DOI] [PubMed] [Google Scholar]
- 25. IHI . MZIMA: Longitudinal Cohort Study. Progress Report Serosurvey Round 1 June 2012 – May 2013 . Dar-es-Salaam: : Ifakara Health Institute; , 2014. . [Google Scholar]
- 26. Kabudula CW, Clark BD, Gómez-Olivé FX, Tollman S, Menken J, Reniers G . The promise of record linkage for assessing the uptake of health services in resource constrained settings: a pilot study from South Africa . BMC Med Res Methodol 2014. ; 14 : 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulder DW, Nunn A, Kamali A, Nakiyingi J, Wagner H, Kengeya-Kayondo J . Two-year HIV-1-associated mortality in a Ugandan rural population . Lancet 1994. ; 343 : 1021 – 23 . [DOI] [PubMed] [Google Scholar]
- 28. Sewankambo NK, Wawer MJ, Gray RH, et al. . Demographic impact of HIV infection in rural Rakai district, Uganda: results of a population-based cohort study . AIDS 1994. ; 8 : 1707 – 14 . [DOI] [PubMed] [Google Scholar]
- 29. Urassa M, Boerma JT, Isingo R, et al. . The impact of HIV/AIDS on mortality and household mobility in rural Tanzania . AIDS 2001. ; 15 : 2017 – 23 . [DOI] [PubMed] [Google Scholar]
- 30. Gregson S, Anderson RM, Ndlovu J, Zhuwau T, Chandiwana SK . Recent upturn in mortality in rural Zimbabwe: evidence for an early demographic impact of HIV‐1 infection? AIDS 1997. ; 11 : 1269 – 80 . [DOI] [PubMed] [Google Scholar]
- 31. Todd J, Glynn JR, Marston M, et al. . Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy . AIDS 2007. ; 21 : S55 – S63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stover J, Walker N, Grassly NC, Marston M . Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package . Sex Transm Infect 2006. ; 82(Suppl 3) : iii45 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stover J, Johnson P, Zaba B, Zwahlen M, Dabis F, Ekpini RE . The Spectrum projection package: improvements in estimating mortality, ART needs, PMTCT impact and uncertainty bounds . Sex Transm Infect 2008. ; 84(Suppl 1) : i24 – i30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stover John . AIM: A Computer Program for Making HIV/AIDS Projections and Examining the Demographic and Social Impacts of AIDS. Washington, DC: Futures Group International, Health Policy Initiative, Task Order 1. 2009. http://data.unaids.org/pub/Manual/2009/20090414_aim_manual_2009_en.pdf , last accessed 12 Jan 2016 . [Google Scholar]
- 35. Marston M, Zaba B, Salomon JA, Brahmbhatt H, Bagenda D . Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics . J Acquir Immune Defic Syndr 2005. ; 38 : 219 – 27 . [DOI] [PubMed] [Google Scholar]
- 36. Zaba B, Whitworth J, Marston M, et al. . HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi . Epidemiology 2005. ; 16 : 275 – 80 . [DOI] [PubMed] [Google Scholar]
- 37. Slaymaker E, Todd J, Marston M, et al. . How have ART treatment programmes changed the patterns of excess mortality in people living with HIV? Estimates from four countries in East and Southern Africa . Glob Health Action 2014. ; 7:22789 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reniers G, Slaymaker E, Nakiyingi-Miiro J, et al. . Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA) . AIDS 2014. ; 28(Suppl 4) : S533 – 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jahn A, Floyd S, Crampin AC, et al. . Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi . Lancet 2008. ; 371 : 1603 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asiki G, Reniers G, Newton R, et al. . Adult life expectancy trends in the era of antiretroviral treatment in rural Uganda (1991–2012) . AIDS 2016. ; 30 : 487 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Byass P, Calvert C, Miiro-Nakiyingi J, et al. . InterVA-4 as a public health tool for measuring HIV/AIDS mortality: a validation study from five African countries . Glob Health Action 2013. ; 6:2448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calvert C, Li Z, McCormick T, et al. . HIV-related Causes of Death in the Era of Antiretroviral Therapy: Analysis of Verbal Autopsy Data . Annual Conference on Retroviruses and Opportunistic Infections Seattle, 23–26 February 2015 . San Francisco, CA: CROI, 2015 . [Google Scholar]
- 43. Reniers G, Eaton J, Nakiyingi-Miiro J, et al. . The Impact of Antiretroviral Therapy on Adult Life Expectancy in sub-Saharan Africa . Annual Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, 23–26 February 2015 . San Francisco, CA: CROI, 2015 . [Google Scholar]
- 44. Mullis CE, Munshaw S, Grabowski MK, et al. . Differential specificity of HIV incidence assays in HIV subtypes A and D-infected individuals from Rakai, Uganda . AIDS Res Hum Retroviruses 2013. ; 29 : 1146 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cohen MS, Chen YQ, McCauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy . N Engl J Med 2011. ; 365 : 493 – 505 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML . High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa . Science 2013. ; 339 : 966 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zaba B, Slaymaker E, Nakiyingi-Miiro J, et al. . HIV incidence trends: lifetime infection risk in six community-based longitudinal studies in sub-Saharan Africa in the era of ART roll-out . XX International AIDS conference, Melbourne 20–24 July 2014 . Geneva: AIDS 2014 International Secretariat . [Google Scholar]
- 48. Ye Y, Wamukoya M, Ezeh A, Emina JB, Sankoh O . Health and demographic surveillance systems: a step towards full civil registration and vital statistics system in sub-Sahara Africa? BMC Public Health 2012. ; 12 : 741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zaba B, Calvert C, Marston M, et al. . Effect of HIV infection on pregnancy-related mortality in sub-Saharan Africa: secondary analyses of pooled community-based data from the network for Analysing Longitudinal Population-based HIV/AIDS data on Africa (ALPHA) . Lancet 2013. ; 381 : 1763 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]