Summary
In Porphyromonas gingivalis, the protein PG1660, composed of 174 amino acids, is annotated as an extracytoplasmic function (ECF) sigma factor (RpoE homologue-σ24). Because PG1660 can modulate several virulence factors and responds to environmental signals in P. gingivalis, its genetic properties were evaluated. PG1660 is co-transcribed with its downstream gene PG1659, and the transcription start site was identified as adenine residue 54-nucleotides upstream of the ATG translation start codon. In addition to binding its own promoter, using the purified rPG1660 and RNAP core enzyme from Escherichia coli with the PG1660 promoter DNA as template, the function of PG1660 as a sigma factor was demonstrated in an in vitro transcription assay. Transcriptome analyses of a P. gingivalis PG1660-defective isogenic mutant revealed that under oxidative stress conditions 176 genes including genes involved in secondary metabolism were downregulated more than two-fold compared with the parental strain. The rPG1660 protein also showed the ability to bind to the promoters of the highly downregulated genes in the PG1660-deficient mutant. As the ECF sigma factor PG0162 has a 29% identity with PG1660 and can modulate its expression, the cross-talk between their regulatory networks was explored. The expression profile of the PG0162PG1660-deficient mutant (P. gingivalis FLL356) revealed that the type IX secretion system genes and several virulence genes were downregulated under hydrogen peroxide stress conditions. Taken together, we have confirmed that PG1660 can function as a sigma factor, and plays an important regulatory role in the oxidative stress and virulence regulatory network of P. gingivalis.
Keywords: oxidative stress, periodontitis, Porphyromonas gingivalis, RpoE
1 INTRODUCTION
Multiple signaling pathways are known to enable bacteria to alter their gene expression in response to environmental signals. This gene expression is mainly controlled at the transcriptional level, and the regulation includes extracytoplasmic function (ECF) sigma factors, a large group of alternative sigma factors that regulate gene expression in response to cell envelope stresses or environmental stimuli.1 In Gram-negative bacteria, some ECF sigma factors are also under the control of the cell-surface signaling system, which includes an outer membrane receptor in the signal transduction pathway.1,2
The ECF sigma factors belong to subfamily 4 of the sigma 70 class.2 This diverse group of small proteins contains conserved regions σ4 and σ2, which are necessary for interaction with the RNA polymerase core enzyme and recognition of promoter motifs (–35 and –10, respectively).3,4 ECF sigma factors receive and respond to extracytoplasmic stresses, which implies that their function is associated with sensor or transporter proteins located on/in the outer membrane. In most cases, the ECF sigma factors are regulated by their cognate anti-sigma factor, or occasionally by an anti-anti-sigma factor.2,5 Other characteristics of the ECF sigma factors are their autoregulation and their ability to modulate relatively small regulons.2,6
To date, the regulatory mechanism of ECF sigma factor RpoE (σ24) from Escherichia coli is well studied and clearly illustrated.7 In E. coli, the RpoE (σ24) gene is co-transcribed with its cognate anti-sigma factor RseA and its co-anti-sigma factor RseB.8 Without an inducing signal, RseA tethers the σ24 to the inner membrane, and the binding of RseB to the periplasmic domain of RseA is known to strengthen the inhibition of σ24.9 Under stress conditions, the RseB is disassociated from RseA by an unknown mechanism. Simultaneously, DegS, a serine protease, degrades the extracytoplasmic domain of RseA while the RseP metalloprotease degrades the transmembrane domain of RseA to release the cytoplasmic domain of RseA and σ24.10-12 The last step of the regulated intramembrane proteolysis involves cleavage of the cytoplasmic domain of RseA by ClpXP resulting in the release of σ24 for association with the RNAP core enzyme.13
Periodontitis is a chronic inflammatory condition of an infectious nature involving the tissues supporting the teeth.14 Because of the chronic inflammation and other prevailing conditions, including fluctuations in nutrient availability, temperature, pH and oxygen tension, the periodontal pathogens must have properties that will allow them to colonize and survive in the periodontal pocket under stress conditions. During this encounter, extracytoplasmic stress can result from the effects of the hostile changing environmental conditions on the membrane architecture of the bacteria. Porphyromonas gingivalis, a black-pigmented, Gram-negative anaerobe, is considered as an important etiologic agent of periodontal disease.15 To date, little is known about the relationship between the regulation of adaptive mechanisms and ECF sigma factors in P. gingivalis. A total of six ECF sigma factors were found in the P. gingivalis W83 genome – PG0162, PG0214, PG0985, PG1318, PG1660 and PG1827.16 A PG0162-defective mutant showed reduced gingipain activity;17 a PG1318-defective mutant showed a mutator phenotype;18 a PG1827-defective mutant showed more sensitivity to oxidative stress;19 and PGN_0274 and PGN_1740 mutants showed increased biofilm formation in P. gingivalis ATCC 33277.20 A PG1660-deficient mutant showed more sensitivity to 0.25 mM of hydrogen peroxide and gingipain activity reduction compared with the wild-type.17 In this study, we show that PG1660, an RpoE homologue, is a sigma factor that can respond to environmental stress signals and regulate the expression of genes by binding to their promoter regions. The likelihood that the PG1660 modulated genes may be part of a yet-to-be described oxidative stress resistance pathway is further discussed.
2 MATERIAL AND METHODS
2.1 Bacterial strains, plasmids, and culture conditions
Strains and plasmids used in this study are listed in Table 1. The P. gingivalis strains were cultured as described previously.21 In brief, P. gingivalis was grown in brain–heart infusion (BHI) medium supplemented with yeast extract (0.5%), hemin (5 μg mL−1), vitamin K (0.5 μg mL−1) and cysteine (0.1%), and incubated in an anaerobic chamber in 10% H2, 10% CO2 and 80% N2 at 37°C.
TABLE 1.
Strains and plasmids used in this study
| Strains and plasmids | Relevant characteristics | References |
|---|---|---|
| Strains | ||
| Porphyromonas gingivalis | ||
| W83 | Wild-type | 17 |
| ATCC33277 | Wild-type | 21 |
| FLL350 | ΔPG0162∷ermF | 17 |
| FLL354 | ΔPG1660∷ermF | 17 |
| FLL356 | ΔPG0162∷ermF-ΔPG1660∷tetQ | This study |
| FLL398 | APGN_0450∷ermF | This study |
| E. coli | ||
| FLL387 | BL21 with pET102-1660 | This study |
| Plasmids | ||
| pT-COW | Apr, tetQ | 22 |
| pVA2198 | Ermr | 23 |
| pET102-TOPO | Apr, His-tag | Life Technology, InC |
| pET102-1660 | Apr, PG1660 | This study |
2.2 Bioinformatics analyses
The secondary structure prediction and modeling of the proteins were performed using the online software package I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/).22,23 Multiple sequence alignment was performed using the online CLUSTALW2 software (www.ebi.ac.uk/Tools/msa/clustalw2) and the online NCBI BLAST tools (blast. ncbi.nlm.nih.gov/Blast.cgi). The motif logo was created by using the online LOGO software (weblogo.berkeley.edu).
2.3 Creation of PGN_0450-deficient mutant FLL398
Polymerase chain reaction (PCR) -based fusion of several fragments was performed as previously described.17 The primers used are listed in Table 2. The promoter containing erythromycin-resistant cassette, ermF, was fused to 1 kb upstream and 1 kb downstream fragments of PGN_0450.24 The fused fragment was used to transform P. gingivalis ATCC33277 as previously reported.25 The transformant was plated on a BHI agar plate containing 10 μg mL−1 of erythromycin and incubated at 37°C for 10 days. The correct gene replacement in the erythromycin-resistant mutant was confirmed by PCR and DNA sequencing.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| RT-PCR | |
| PG1659-RT_F | GACTCAAACAACGCTTCGACGTGAT |
| PG1659-RT_R | CGATGCTTCGTGTTGCGATATAGTT |
| PG1660-RT_F | GTGGAGCGATACAGCGACATGCT |
| PG1660-RT_R | GTGGAGCTTGACCTTCACGTTGGA |
| Overexpression of PG1660 in Escherichia coli | |
| PG1660_ET_F | CACCATGGAGAAGCAATCCGACA |
| PG1660_ET_R | TTTTCTCATCATTTTATAGAGTTTGGT |
| For mutants construction | |
| PG0162 PG1660-deficient mutant | |
| PG1660_F1 | CCCATGATAGAGACCATCTCCCCTT |
| PG1660_TetQ_R1 | CAAATTATTCCTCCTAGTTAGTCATAGCTGTCGGATTGCTTCTCCATCT |
| PG1660_TetQ_F3 | AAAATAATACCTGGAGGGAATAATCTGATGAGAAAATGAAACAGAATATA |
| PG1660_R3 | CTCGCGTAAGGAGATACAGCTTGAGA |
| TetQ_pro_F | TGACTAACTAGGAGGAATAATTTGTTAGATATATTTTTTTGTGTA |
| Tet_Q_R | GATTATTCCCTCCAGGTATTATTTTGATGACATTGATTTTGGA |
| PGN_0450-deficient mutant | |
| PGN_0450_F1 | CCCATGATAGAGACCATCTCCCCTT |
| PGN_0450_R1 | TACCTTATTCCTCCTAGTTAGTCATAGCTGTCGGATTGCTTCTCCATCT |
| PGN_0450_F3 | TTCGTAGTACCTGGAGGGAATAATCTGATGAGAAAATGAAACAGAATATA |
| PGN_0450_R3 | GAACATGAAGAACTCCCTGATGCATC |
| Real-time quantitative PCR | |
| PG1459-realtime_F | GTGGAAGATTTGGAGATGGAAAAGAT |
| PG1459-realtime_R | GATAGCTTCACACACGGGGCT |
| PG0844-realtime_F | GAAAACAAGCCGAGCCCTTTGG |
| PG0844-realtime_R | CCCAGAAATGCCGATAGCCTATAA |
| PG0655-realtime_F | GACGACAGAGAACAGAGAGAAGA |
| PG0655-realtime_R | TCAATAAGAGGCTGTACTCGTGGT |
| PG1511-realtime_F | GCAGGACGGGAACTGGGATTT |
| PG1511-realtime_R | TATAATCGGTGCAGGGGTTGGA |
| PG1167-realtime_F | AAGCTGTCTTTCGGATCGGAG |
| PG1167-realtime_R | CCGCTTCTTTTTACCCCCTTATTT |
| PG1663-realtime_F | GAACTCATCCGCAATCTCTCCGA |
| PG1663-realtime_R | GTATTCCATGGCCATCTCATTGCGT |
| PG1664-realtime_F | TCAGACGATGCCATGAAGAGAAAA |
| PG1664-realtime_R | GGACAGATTTACGTATTTCCCTATG |
| PG2110-realtime_F | CACAGATCGGATGGTTCGGCA |
| PG2110-realtime_R | CATGAGCAGCCAGTCTATAGGT |
| PG2111-realtime_F | GGACTGGCTGCCCTCTTG |
| PG2111-realtime_R | AGGTGGCTTGGATGATCAGGA |
| PG1545-realtime_F | CGCTGACTTGAATACCATCGTACA |
| PG1545-realtime_R | GGACGGAACTGAGTGAAATAGAGA |
| PG1659-realtime_F | CGATCTTTCCGCAATCCCTTCGT |
| PG1659-realtime_R | GGACAGGAAGATCAGAGGTGCT |
| 16S_Realtime_F | CGAGAGCCTGAACCAGCCAAGT |
| 16S_Realtime_R | GATAACGCTCGCATCCTCCGTATTA |
| For 5′-RACE | |
| 5′ RACE inner primer | GAAGTAGATGGTGGGCAGGAAGAT |
| PG1660-RT_R | GTGGAGCTTGACCTTCACGTTGGA |
| For in vitro transcription | |
| PG1660_pro_F | GTATCGTTGGTTACCCTCTTCAAGA |
| PG1660_pro_R | GGCGATTCGGTAGATCCATGT |
| For electrophoretic mobility shift assay (EMSA) | |
| PG1660_EMSA_F | Same as PG1660_pro_F |
| PG1660_EMSA_R | GGTTACACGGACATACCACCTG |
| PG0844_EMSA_F | GAAGAGTCGGGCGAGTCCTGA |
| PG0844_EMSA_R | CTTTCCTAGGCTATTCGGGCGT |
| PG1459_EMSA_F | CGGTAATGCGAGGAGGAGTTATTT |
| PG1459_EMSA_R | CTTCGGGTAAAGTGTCAAGGCCT |
| PG0374_EMSA_F | GTAGTGAAGGATATCGGCATCATTT |
| PG0374_EMSA_R | CTATATCGGCAATTAGCGGCACA |
| PG1511_EMSA_F | CTTTCGAGGGCTTGAGAGAC |
| PG1511_EMSA_R | GCTTCGCATTAAAGAATTACAGCAG |
| PG2111_EMSA_F | TGACCGACGTACCTTCAGCTT |
| PG2111_EMSA_R | CATATCAATCGGGTAATGGGTTTG |
2.4 Creation of PG0162-PG1660 deletion mutant FLL356
A promoter containing tetracycline-resistant cassette tetQ was PCR amplified from pT-COW,26 and fused to the 1-kb upstream and 1-kb downstream fragments of PG1660. The fused fragment was used to transform the PG0162-deficient mutant strain P. gingivalis FLL350 by electroporation.17 The cells were plated on BHI agar containing 10 μg mL−1 of erythromycin and 3 μg mL−1 of tetracycline, and then incubated at 37°C for 10 days. The correct gene replacement in the tetracycline-and erythromycin-resistant mutant was confirmed by PCR and DNA sequencing.
2.5 Hydrogen peroxide stress and growth study with NaCl
The hydrogen peroxide stress study was performed as previously reported.21 Briefly, aliquots of an overnight P. gingivalis culture were used to inoculate fresh pre-warmed BHI broth, which was further incubated at 37°C under anaerobic conditions. At an optical density at 600 nm (OD600) of approximately 0.2, hydrogen peroxide was added to the testing cultures to a concentration of 0.25 mM. The growth of the cultures was monitored spectrophotometrically (OD600) at regular intervals after the addition of the hydrogen peroxide. BHI broth was used as a blank control. The tolerance of P. gingivalis to NaCl was performed by inoculating fresh pre-warmed BHI broth containing 0.4 M NaCl and monitor its growth spectrophotometrically (OD600) as described above. The cultures incubated in regular BHI broth at 37°C under anaerobic conditions were used as controls. For the transcriptome study of P. gingivalis exposed to oxidative stress, the cells were collected after exposure to 0.25 mM of hydrogen peroxide for 15 min at the OD600 ≈ 0.8.
2.6 Biofilm assay
Porphyromonas gingivalis cells were harvested from overnight grown culture, and then washed twice with 1× PBS buffer. The cell pellet was resuspended in BHI/PBS (1/2) and adjusted to a cell density at OD600 ≈ 0.2~0.3. Aliquots (100 μL) of the cell suspension were distributed in 96-well polyvinyl chloride microplates and incubated overnight anaerobically at 37°C. The bound cells were stained with 100 μL of a crystal violet solution for 30 min at room temperature, and then washed four times with 1× PBS buffer. The bound cells were de-stained in 100 μL of 95% ethanol for 30 min and then 500 μL of ddH2O was added. Absorbance of crystal violet from the micro-plate was measured by using a spectrophotometer at 550 nm.
2.7 DNA microarray
For RNA isolation, the P. gingivalis cells were grown to OD600 of 0.8 and collected by centrifugation at 10 000 g for 10 min at 4°C. Total P. gingivalis RNA was isolated using the SV Total RNA Isolation kit (Promega, Madison, WI). Complementary DNA (cDNA) was synthesized by using the High Fidelity cDNA synthesis kit according to the manufacturer’s protocol (Roche, Indianapolis, IN). DNA microarray was performed by using ROCHE NIMBLEGEN customer arrays (100910_CW_P_ging_w83_expr_HX12) according to the standard NIMBLEGEN procedure (NimbleGen Arrays User’s Guide: Gene Expression Analysis v5.1) as previously reported.27
2.8 RNA sequencing
The RNA sequencing was performed as previously reported.28 Briefly, ribosomal RNA was removed from 2 μg of total RNA using a RiboMinus Transcriptome Isolation Kit (Life Technologies, Carlsbad, CA). The first-strand and second-strand cDNA synthesis, the RNA-sequencing barcode ligation, and other steps of libraries construction were performed using a Nextflex RNA-Seq kit according to the protocol of the manufacturer (Bioo Scientific, Austin, TX). The PCR-amplified cDNA samples were sequenced using a NextSeq 550 sequencing system (Illimina Inc., San Diego, CA), with 75-bp reads and at least 10 million reads per sample. The microarray and RNA-seq data were submitted to the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo, access number: GSE103839).
2.9 Real-time quantitative PCR
Amplifications were performed with the SYBR Green kit (Life Technologies), and real-time fluorescence was detected using the Applied Biotechnology QuantStudio™ 7 Flex Real-Time PCR System (Fisher Scientific, Tustin, CA). The PCR were performed as follows: 50°C for 2 min, 95°C for 5 min; then 40 cycles of 94°C for 15 s, 54°C for 30 s, 72°C for 30 s. Each measurement was performed in triplicate. The 16S rRNA was used as an internal control. The ΔΔCT method was used to analyze the data as described elsewhere.29
2.10 Reverse transcription PCR
The primers used to amplify the PG1660 or PG1659 gene are listed in Table 2. The PCR were performed as follows: 95°C for 5 min; 94°C for 15 s, 53°C for 30 s, 68°C for 1 min, 25 cycles. Each amplification reaction was performed in triplicate for each gene. The 16S rRNA was used as a reference in this experiment.
2.11 Overexpression and purification of recombinant PG1660
The PG1660 coding region without stop codon was amplified by PCR and then ligated to an expression vector pET102/D-TOPO (Life Technologies). The recombinant plasmid pET102-1660 was transformed into E. coli BL21 (DE3) (Life Technologies). The transformant strain carrying the pET102-1660 plasmid was designated as FLL387. Overexpression of the recombinant PG1660 (rPG1660) protein containing a hexahistidine-tag at the C-terminal was induced by 1 mM of IPTG at 16°C. The rPG1660 was purified from E. coli BL21 cell lysate by using nickel-nitrilotriacetic acid (Ni-NTA) beads according to the manufacturer’s guidelines (Qiagen, Valencia, CA).
2.12 Western blot
The overexpressed and purified rPG1660 was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and the proteins were then transferred to Bio-Trace nitrocellulose membranes at 15 V for 30 min using a Semi-Dry Trans-blot apparatus (Bio-Rad, Hercules, CA). The Western-blot was first probed with primary antibody (mouse) against the His-tag, and then probed with the horseradish peroxidase conjugated secondary goat anti-mouse antibody (Zymed Laboratories, San Francisco, CA). Immunoreactive proteins were detected using the Western Lightning Chemiluminescence Reagent Plus Kit (Perkin-Elmer Life Sciences, Boston, MA).
2.13 RNA ligase-mediated rapid amplification cDNA end assay
The transcriptional start site (TSS) was determined by 5′-RACE using the First Choice RNA ligase mediated rapid amplification cDNA end assay (RLM-RACE) kit according to the manufacturer’s protocol (Ambion, Austin, TX). The PCR fragments were ligated into the pCR2.1-TOPO vector (Life Technology, Carlsbad, CA), and the ligated DNA was transformed into E. coli Top10 competent cells. The insert was confirmed by DNA sequencing (Eton Bioscience, San Diego, CA).
2.14 Electrophoretic mobility shift assay
DNA fragments containing the promoter regions of the PG1660 and the highly downregulated genes in FLL354 were PCR amplified, respectively, and then labeled with the Biotin 3′ end DNA labeling kit (Thermo Scientific/Pierce Bio, Rockfold, IL). The electrophoretic mobility shift assay (EMSA) was performed according to the manufacturer’s guidelines (Thermo Scientific). Briefly, 0.5 (or 1) pmol of the purified rPG1660 protein was mixed with 10 fmol of biotin-labeled DNA in binding buffer (10 mM Tris–HCl, 50 mM KCl, 1 mM dithiothreitol; pH 7.5), and incubated at room temperature for 30 min. The samples were resolved using a 5% polyacrylamide non-denaturing gel and analyzed using a chemiluminescence nucleic acid detection kit (Thermo Scientific).
2.15 In vitro transcription
In vitro transcription assay was performed using the E. coli RNA polymerase core enzyme (Epicentre, Madison, WI), and the purified rPG1660 protein. The DNA fragment carrying the PG1660 promoter was used as template. The primers used are listed in Table 2. The reactions were incubated at 37°C for 2 h in buffer containing 40 mM Tris–HCl (pH 7.5), 150 mM KCl, 10 mM MgCl2, 0.1 mM dithiothreitol, 0.01% Triton X-100, 2.5 mM of NTP mix, ~1 mCi [α-32P]-ATP (MP Biomedicals, Solon, OH). The samples were analyzed on an 8% polyacrylamide denaturing gel and quantified using a phosphor-imaging system (Molecular Dynamics, Sunnyvale, CA).
3 RESULTS
3.1 Protein modeling of PG1660
PG1660 is 525-bp in length and codes for a 174-amino-acid protein. Protein modeling shows that the PG1660 protein has two sigma-70 subdomains at amino acid positions 24–91 (σ2) and 116–168 (σ4), respectively (Figure 1A, B, C), and these two subdomains contain Helix-Coil-Helix motifs that could bind to DNA (Figure 1D). The modeling also shows that PG1660 has motifs that could bind to peptides/proteins (Figure 1E). The amino acid sequence blast showed that PG1660 is 44% homologous to sigma-24 of Bacteroides coprocola (E value: 4e-45), 41% homologous to sigma-24 of Paludibacter propionicigenes WB4 (E value: 3e-39), and 29% homologous to RpoE of E. coli K-12 (E value: 1e-17).
FIGURE 1.

Structure prediction of PG1660 showed that PG1660 has two sigma 70 sub-domains. (A) Model of PG1660. (B) Structure model of subdomain-2. (C) Structure model of subdomain-4. (D) PG1660 model shows the subdomains bind DNA. Green color line represents the DNA; blue color represents the amino acids binding to DNA and the pink represents the positions of amino acids in PG1660. (E) PG1660 model shows that the two domains may bind to protein/peptides. Green color represent the peptides to which PG1660 binds; blue color represent the amino acids binding to peptides and the pink color represents the positions of amino acids in PG1660
3.2 The TSS of the PG1660 is mapped to an adenine residue
The TSS of PG1660 was determined by using 5′-RACE. As shown in Figure 2(A), a 600-bp length DNA fragment was PCR amplified. An adenine (A) residue which locates 54-nucleotides upstream of the predicted translation start codon ATG was identified as the TSS by DNA sequencing (Figure 2B). Putative –35 “GTCTCA” and –10 “TGTAAG” elements were observed upstream of the TSS (Figure 2B).
FIGURE 2.

(A) Polymerase chain reaction amplification of PG1660 promoter by using RNA as template in 5′-RACE. (B) Sequence of the PG1660 promoter, and the transcription initiate from an adenine 54 nucleotides upstream of translation start codon ATG. (C) Western blot of rPG1660 (bottom half) after overexpression and purification (top half). (D) In vitro transcription analysis of PG1660 promoter. L: φX174 DNA/Hinf I Markers; 1, PG1660 promoter DNA without rPG1660; 2, PG1660 promoter DNA with sigma factor rPG1660 and RNAP Core enzyme; 3, PG1660 promoter DNA with sigma factor rPG1660 and RNAP Core enzyme with addition of RNaseA; 4, PG1660 promoter DNA with sigma factor rPG1660 and RNAP Core enzyme with addition of DNaseI
3.3 The protein rPG1660 function as sigma factor
Most ECF sigma factors are autoregulated.2 To test if rPG1660 can initiate its own gene transcription, a 636-bp (−347 to +289) DNA fragment predicted to carry the promoter region of the PG1660 gene was used as a template in in vitro run-off transcription reconstitution assays. The rPG1660 protein, overexpressed and purified from E. coli, was first confirmed by Western blot using anti-His tag antibody (Figure 2C) and then used in in vitro transcription assay. As shown in Figure 2(D), when the promoter region of the PG1660 was used as a template, a fragment approximately 289 nucleotides in length was observed in the presence of the E. coli core RNAP and the rPG1660 protein. In the control experiments, when the rPG1660 protein was absent, or with the presence of RNaseA, there were no transcription products detected (lane 3, Figure 2D). These results indicate that the P. gingivalis rPG1660 protein may function as a sigma factor and can initiate transcription from its own promoter. PG1660 is designated RpoE (sigma-24).
3.4 Electrophoretic mobility shift assay showed that PG1660 is autoregulated
A 228-bp DNA fragment containing the promoter region of the PG1660 gene was PCR amplified and labeled with biotin. The EMSA shows that the purified rPG1660 protein could bind to its own promoter and result in a significant retardation in its migration compared with the control (Figure 3A). Additionally, the shift was significantly inhibited by the addition of a 200-fold concentrated non-labeled PG1660 promoter DNA fragment.
FIGURE 3.
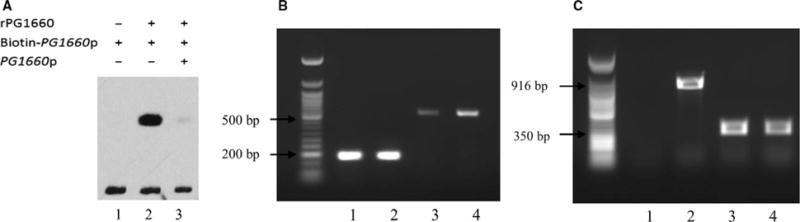
(A) Electrophoretic mobility shift assay showed that rPG1660 could bind to the promoter of PG1660. 1, reaction without rPG1660 as control; 2, reaction with addition of rPG1660; 3, reaction with addition of non-labeled promoter as competitor. (B) Reverse transcription– polymerase chain reaction (RT-PCR) of PG1660 showed that PG1660 was induced when Porphyromonas gingivalis was exposed to hydrogen peroxide. 1, 16S gene amplified by using cDNA of W83; 2, 16S gene amplified by using cDNA of W83 stress; 3, PG1660 gene amplified by using cDNA of W83; 4, PG1660 gene amplified by using cDNA of W83 stress. (C) RT-PCR showed that PG1660 and PG1659 are in the same operon. 1, PG1659-1660 gene amplified by using W83 RNA; 2, PG1659-1660 gene amplified by using W83 cDNA; 3, PG1659 gene amplified by using FLL354 cDNA; 4: PG1659 gene amplified by using W83 cDNA
3.5 The PG1660 gene can be modulated by hydrogen peroxide-induced stress
Porphyromonas gingivalis FLL354, a PG1660-defective isogenic mutant, is more sensitive to hydrogen peroxide compared with the wild-type W83 strain.17 RT-PCR was used to analyze the expression of the PG1660 gene in P. gingivalis W83 under both anaerobic and oxidative stress conditions. As shown in Figure 3(B), the expression of the PG1660 gene was induced when P. gingivalis was exposed to 0.25 mM of hydrogen peroxide. RT-PCR was also used to determine if PG1660 and PG1659 were in the same operon as there is a four-nucleotide overlap between these two genes. As shown in Figure 3(C), the PG1660-PG1659 fragment was amplified from the W83 cDNA, which indicated that PG1660 and PG1659 are part of the same transcriptional unit.
3.6 ECF sigma factor can modulate biofilm formation in P. gingivalis
ECF sigma factors in other bacteria including P. gingivalis can modulate biofilm formation as part of their adaptation to changing environmental conditions.30-32 To evaluate the role of PG1660 in biofilm formation, an isogenic mutant defective in PGN_0450 (PG1660 homologue) was constructed in P. gingivalis ATCC33277. As reported, P. gingivalis W83 is a poor biofilm forming strain.30 Similar to the wild-type, the PGN_0450-deficient mutants showed black pigmented colonies on blood BHI plates. One colony randomly chosen and designated FLL398 was used for further studies. Porphyromonas gingivalis FLL398 (ΔPGN0450∷ermF) had a similar growth rate compared with the wild-type strain (Figure 4A). The FLL398 isogenic strain showed more than 50% reduction of biofilm formation compared with the wild-type ATCC33277 strain (Figure 4B). These results indicated that the PG1660 (RpoE) may be involved in the regulation of biofilm formation in P. gingivalis. It is noteworthy that P. gingivalis FLL398 is more sensitive to hydrogen peroxide compared with the wild-type P. gingivalis ATCC33277 strain (data not shown).
FIGURE 4.

(A) Growth study showed that inactivation of PGN_0450 did not affect the growth of ATCC33277. (B) PGN_0450 mutant showed 50% reduction of biofilm formation. All the results shown as the average of at least three replicates, and the asterisks indicated the significant difference between activity of mutant compared with the wild-type (P < 0.005, two tailed t-test)
3.7 Transcriptome analysis of FLL354 under anaerobic and oxidative stress conditions
To identify the genes and regulons regulated by the PG1660 (RpoE), total RNA from P. gingivalis FLL354 (ΔPG1660∷ermF) and W83 under both anaerobic and hydrogen peroxide stress conditions were isolated and subjected to cDNA microarray analysis. Approximately 3.7% (85 genes) of the P. gingivalis genome displayed altered expression in the PG1660-deficient mutant under normal anaerobic conditions (fold-change ≥ 1.5, P-value ≤ .05, FDA≤ 0.05; see Supplementary material, Table S1). Thirty-two genes were downregulated and 53 genes were upregulated in P. gingivalis FLL354 compared with the wild-type. Among the differentially expressed genes, 38 (17 downregulated; 21 upregulated) were annotated as hypothetical or of unknown function (see Supplementary material, Table S1). Two group of genes differentially expressed are involved in gene expression and transporter systems (see Supplementary material, Table S1). All five genes downregulated more than two-fold are predicted to encode hypothetical proteins, including PG1459, PG0844, PG0655, PG1511 and PG0374. The genes upregulated more than two-fold include PG0555 encoding a putative histone-like family DNA binding protein, PG0906 encoding G/U mismatch-specific DNA glycosylase with PG0554, PG1085 and PG1268 encoding hypothetical proteins.
Under hydrogen peroxide stress conditions, approximately 865 genes (including 468 genes that were downregulated and 397 that were upregulated more than 1.5-fold) were differentially expressed in P. gingivalis FLL354 compared with the wild-type strain. Several of the genes most highly downregulated in FLL354 are functionally associated with metabolism, transport and transposons (Figure 5A; Table 3; see Supplementary material, Table S2, S3 and S4). Notably, genes involved in thiamine and menaquinone synthesis were downregulated (Figure 5B, C; see Supplementary material, Table S3), which indicated that PG1660 (RpoE) could be involved in regulation of secondary metabolism.
FIGURE 5.

(A) Category of genes downregulated in FLL354 under hydrogen peroxide stress conditions. (B) Genes involved in thiamine synthesis were downregulated. (C) Genes involved in menaquinone synthesis were downregulated
TABLE 3.
Comparison of differentially expressed genes in FLL354 and W83 under hydrogen peroxide stress
| Gene ID | Annotation | Fold-change (FLL354H2O2/W83H2O2) | Fold-change (W83H2O2/W83) |
|---|---|---|---|
| Genes were upregulated in W83 H2O2 stress 10 or 15 min but downregulated in FLL354 H2O2 stress 15 min | |||
| PG0174 | Pyridine nucleotide-disulphide oxidore-ductase family protein | −2.0 | 3.0 |
| PG0562 | Potassium uptake protein TrkA, putative | −2.0 | 5.0 |
| PG0851 | Conserved hypothetical protein | −1.9 | 2.1 |
| PG1148 | Hypothetical protein | −1.5 | 2.4 |
| PG1345 | Glycosyl transferase, group 1 family protein | −1.9 | 4.1 |
| PG1458 | Hypothetical protein | −2.0 | 2.1 |
| PG1474 | Conjugative transposon protein TraO | −2.8 | 2.7 |
| PG1480 | Conjugative transposon protein TraI | −2.1 | 2.2 |
| PG1495 | DNA topoisomerase III | −2.5 | 6.0 |
| PG1522 | Mandelate racemase/muconate lactonizing enzyme family protein | −1.9 | 3.9 |
| PG1564 | Membrane protein, putative | −2.0 | 2.1 |
| PG1601 | Biotin-acetyl-CoA-carboxylase ligase | −1.6 | 2.4 |
| PG1632 | Aldose 1-epimerase | −1.5 | 2.2 |
| PG1711 | Alpha-1,2-mannosidase family protein | −1.7 | 2.1 |
| PG1733 | Hypothetical protein | −1.9 | 2.4 |
| PG1899 | TonB-dependent receptor, putative | −1.6 | 2.1 |
| PG1968 | Hypothetical protein | −2.1 | 2.1 |
| PG1999 | Conserved hypothetical protein | −1.8 | 2.2 |
| PG2099 | ATP-dependent RNA helicase, DEAD/DEAH box family | −1.5 | 3.8 |
| PG2147 | Xanthine phosphoribosyltransferase | −2.0 | 2.6 |
| PG2210 | Excinuclease ABC, A subunit | −1.5 | 6.2 |
| PG1169 | Hypothetical protein | −2.2 | 4.8 |
To confirm the DNA microarray gene expression profile, 11 genes that were observed to be highly downregulated in FLL354 (four under anaerobic conditions, and seven under hydrogen peroxide stress conditions) were selected for expression analysis by using real-time quantitative PCR. As shown in Tables 4 and 5, a similar pattern of expression was observed in these genes.
TABLE 4.
Real-time quantitative polymerase chain reaction (PCR) analyzed differentially expressed genes in FLL354 under normal anaerobic conditions
| Gene | Real-time PCR | DNA microarray | ||
|---|---|---|---|---|
| Fold-change (FLL354/W83) | Standard deviation | Fold-change (FLL354/W83) | P-value | |
| PG0655 | −2.46 | 0.41 | −2.42 | .001224 |
| PG0844 | −5.62 | 1.08 | −2.63 | 6.94E-07 |
| PG1459 | −2.14 | 0.73 | −3.60 | 6.25E-06 |
| PG1551 | −3.51 | 0.37 | −2.41 | .025567 |
TABLE 5.
Real-time quantitative polymerase chain reaction (PCR) analyzed differentially expressed genes in FLL354 under hydrogen peroxide stress conditions
| Gene | Real-time PCR | DNA microarray | ||
|---|---|---|---|---|
| Fold-change (FLL354/W83) | Standard deviation | Fold-change (FLL354/W83) | P-value | |
| PG1167 | −4.27 | 0.69 | −9.14 | 7.47E-07 |
| PG1545 | 5.74 | 0.65 | 3.07 | .000271 |
| PG1659 | 1.97 | 0.48 | 2.08 | .00499 |
| PG1663 | −2.77 | 0.68 | −1.39 | .002257 |
| PG1664 | −2.70 | 0.81 | −2.53 | 4.81E-09 |
| PG2110 | −1.38 | 0.20 | −2.22 | 3.61E-13 |
| G2111 | −2.80 | 0.77 | −2.08 | 3.23E-10 |
3.8 PG1660 regulates expression of PG0844, PG00374, PG1511, PG2111 (thiS) and PG1459
PG0844, PG1459, PG0374, PG1511 and PG2111 (thiS) were highly downregulated in FLL354 under both anaerobic and hydrogen peroxide stress conditions (see Supplementary material, Tables S1, S3 and S4). To determine if the regulation of these genes was directly associated with the PG1660 protein, the predicted promoter region of these genes carrying their putative “–35” and “–10” elements were PCR amplified and labeled with biotin. In the presence of the purified rPG1660, the mobility shift assay shows a significant retardation in the migration of the promoter DNA fragments of these genes compared with the control (Figure 6). The shift was inhibited by the addition of a 200-fold excess amount of the unlabeled DNA fragments (Figure 6). A comparative analysis of the DNA sequence of the regulatory region of the genes revealed consensus –35 (kTCrsA) and –10 (bdTAAT) elements that can directly bind the PG1660 ECF sigma factor (Figure 6F).
FIGURE 6.

Electrophoretic mobility shift assay showed that rPG1660 could bind to the promoter of PG0844 (A), PG1459 (B), PG0374 (C), PG1511 (D), and PG2111 (E). For A anad B: 1, reaction without rPG1660 as control; 2, reaction with addition of rPG1660; 3, reaction with addition of non-labeled promoter as competitor. For C, D and E: 1, no protein; 2, 0.5 pmol of rPG1660; 3, 1 pmol of rPG1660; 4, 5 pmol of rPG1660; 5, 1 pmol of rPG1660 and non-labeled promoter DNA. (F) The conservative sequences of promoter region of the genes been regulated by PG1660 containing –35 “kTCrsA” and –10 “bdTAAT” (k:G or T; r: A or G; s: C or G; b: not A; d: not C) motifs
3.9 Characterization of PG0162-PG1660 mutant (FLL356)
The ECF sigma factor PG0162 has 29% identity with PG1660 (Figure 7A), and was shown to be involved in virulence regulation in P. gingivalis W83.17,33 To investigate any “cross-talk” between these two systems, the impact of their combined function on virulence regulation in P. gingivalis was evaluated. A P. gingivalis isogenic mutant defective in both the PG0162 and PG1660 genes was created and further characterized. On BHI blood agar plate in the presence of erythromycin and tetracycline, the PG0162-PG1660-defective mutants showed black pigmented colonies. One randomly chosen colony designated FLL356 (ΔPG0162∷ermF-ΔPG1660∷tetQ) was used for further studies. The FLL356 strain showed a lower growth rate compared to the wild-type (Figure 7B), and more sensitivity to 0.25 mM of hydrogen peroxide or 0.4 M of NaCl as compared with the wild-type. The gingipain activity for FLL356 was decreased by 60% and 30% for Rgp and Kgp, respectively, compared with the wild-type W83 (Figure 7C).
FIGURE 7.
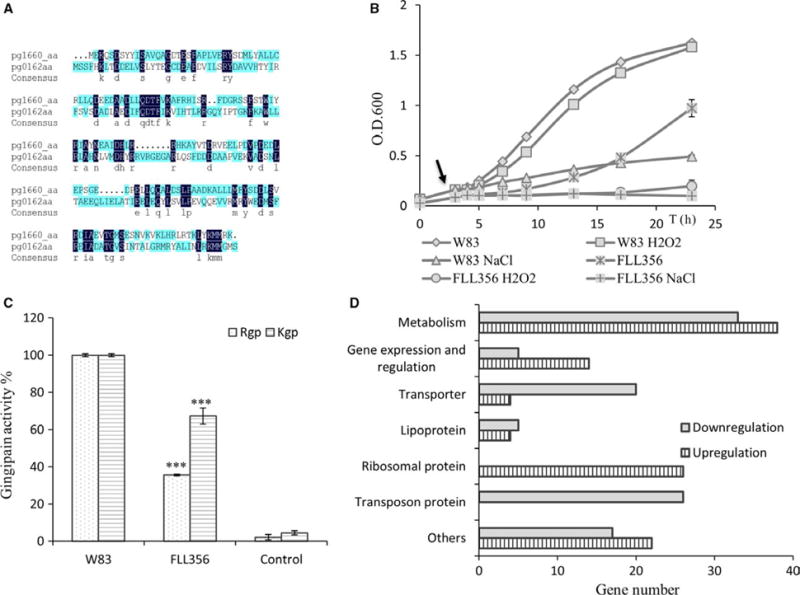
(A) Alignment of the amino acid sequences of PG0162 and PG1660. (B) Growth study showed that FLL356 (ΔPG0162-ΔPG1660) has a lower growth rate than the wild-type, and also showed more sensitivity to hydrogen peroxide and NaCl compared with the wild-type. Arrow shows the time-point of addition of hydrogen peroxide. (C) Gingipain activity assay showed that both Rgp and Kgp activity decreased in FLL356. All the results shown as the average of at least three replicates, and the asterisks indicated the significant difference between activity of mutant compared with the wild-type (P < 0.005, two-tailed t-test). (D) Category of genes differentially expressed in FLL356 under anaerobic conditions
3.10 Transcriptome analysis of PG0162-PG1660-deficient mutant FLL356
To investigate the combined effect of PG0162 and PG1660 on the modulation of genes in P. gingivalis, expression profiling by DNA-microarray analyses was performed. Under anaerobic conditions four genes were downregulated more than two-fold and 165 genes were downregulated 1.5~2.0-fold; 23 genes were upregulated more than two-fold and 150 genes were upregulated 1.5~2.0-fold in FLL356 compared with the wild-type (see Supplementary material, Table S5). Among the differentially expressed genes, 130 were annotated as unknown functional/hypothetical proteins, including 67 that were upregulated and 63 that were downregulated (see Supplementary material, Table S6).
PG0290 (porM) and PG0291 (porN), encoding PorSS (Por Secretion System) proteins, were downregulated more than two-fold; PG0287 (porP), PG0288 (porK), PG0289 (porL), PG1947 (porW), PG0809 (sov) and PG0810, encoding PorSS proteins, were downregulated more than 1.5-fold. PG1663, PG1665, PG1666 and PG1667, encoding ABC transporter system proteins along with PG1664 that encode for a permease, were downregulated more than 1.5-fold (see Supplementary material, Table S6). Interestingly, all the differentially expressed ribosomal proteins were upregulated, and all the differentially expressed transposons were downregulated (Figure 7D).
RNA-sequencing was used to analyze the expression profile of FLL356 under oxidative stress conditions (see Supplementary Figure S1). In all, 195 genes were downregulated for more than 1.5-fold including 49 genes coding for hypothetical proteins; 221 genes including 49 encoding hypothetical proteins were upregulated for more than 1.5-fold (Table 6; Supplementary Tables S7, S8 and S9). Eight PorSS genes were downregulated more than two-fold including porY, porP, porK, porL, porM, sov, porR, PG1058; porN was downregulated 1.98-fold, porW and porT were downregulated for 1.70-fold. Gingipain-encoding genes rgpA, rgpB and kgp were downregulated between 1.5-and 2-fold. Similar results were observed by using quantitative real-time PCR (Table 7). Thirteen CRISPR-associated proteins were downregulated in FLL356 under hydrogen peroxide stress conditions (Table 6). It is noteworthy that three ECF sigma factor PG0214, PG0985 and PG1827 were upregulated more than two-fold in FLL354 under hydrogen peroxide stress conditions (see Supplementary material, Table S8).
TABLE 6.
Genes were downregulated in FLL356 as compared to the wild-type under hydrogen peroxide stress conditions
| Gene code | Annotation | Fold change | Q-value |
|---|---|---|---|
| T9SS | |||
| PG0052 | Sensor histidine kinase, PorY | –2.34 | 0.000221 |
| PG0287 | Membrane protein, PorP | –2.09 | 0.000221 |
| PG0288 | Gliding motility-associated lipoprotein GldK/PorK | –2.71 | 0.000221 |
| PG0289 | Gliding motility protein GldL/PorL | –2.74 | 0.000221 |
| PG0290 | Gliding motility protein GldM/PorM | –2.53 | 0.000221 |
| PG0291 | Gliding motility-associated protein GldN/PorN | –1.98 | 0.000221 |
| PG0751 | PorT protein | –1.70 | 0.0008013 |
| PG0809 | Gliding motility protein, Sov | –2.19 | 0.000221 |
| PG1058 | Membrane protein | –2.11 | 0.000221 |
| PG1138 | Pigmentation and extracellular proteinase regulator PorR | –2.42 | 0.000221 |
| PG1947 | TPR domain-containing protein, PorW | –1.74 | 0.000221 |
| Virulence | |||
| PG0441 | Collagen-binding protein | –1.61 | 0.000221 |
| PG0506 | Gingipain R2, RgpB | –1.51 | 0.000221 |
| PG0884 | Glycosyltransferase group 1 family protein, VimF | –1.91 | 0.0234997 |
| PG1837 | Hemagglutinin A | –1.86 | 0.000221 |
| PG1844 | Peptidase C25, Kgp | –1.96 | 0.000221 |
| PG2024 | Peptidase C25, RgpA | –2.00 | 0.000221 |
| CRISPR | |||
| PG1982 | CRISPR-associated endonuclease Cas1 | –2.14 | 0.000221 |
| PG1983 | Type III-B CRISPR module RAMP protein Cmr6 | –1.86 | 0.000221 |
| PG1985 | Type III-B CRISPR module RAMP protein Cmr4 | –1.86 | 0.0021017 |
| PG1986 | Type III-B CRISPR module-associated protein Cmr3 | –2.09 | 0.000221 |
| PG1987 | Type III-B CRISPR-associated protein Cas10/Cmr2 | –1.90 | 0.000221 |
| PG2013 | CRISPR-associated endonuclease Cas2 | –1.95 | 0.0401059 |
| PG2014 | Subtype I-B CRISPR-associated endonuclease Cas1 | –2.31 | 0.000221 |
| PG2015 | CRISPR-associated protein Cas4 | –2.00 | 0.000221 |
| PG2016 | CRISPR-associated helicase/endonuclease Cas3 | –2.24 | 0.000221 |
| PG2017 | Type I-PGING CRISPR-associated protein Cas7/Csp1 | –1.60 | 0.000221 |
| PG2018 | Type I-PGING CRISPR-associated protein Cas8c/Csp2 | –2.10 | 0.000221 |
| PG2020 | CRISPR-associated endoribonuclease Cas6 | –2.35 | 0.000221 |
| PG2109 | Type I-PGING CRISPR-associated protein Cas5 | –2.43 | 0.000221 |
TABLE 7.
Confirmation of genes differentially expressed in FLL356 under hydrogen peroxide stress conditions
| Gene | RNA-Sequencing | QRT-PCR | ||
|---|---|---|---|---|
| Fold-change | Q-value | Fold-change | Std | |
| PG2024 (rgpA) | −2 | 0.000221 | −3.04 | 0.097 |
| PG0506 (rgpB) | −1.51 | 0.000221 | −2.26 | 0.333 |
| PG1844 (kgp) | −1.96 | 0.000221 | −2.15 | 0.202 |
| PG0052 (porY) | −2.34 | 0.000221 | −1.78 | 0.266 |
| PG0288 (porK) | −2.71 | 0.000221 | −4.37 | 0.839 |
| PG0289 (porL) | −2.74 | 0.000221 | −3.45 | 0.51 |
| PG0809 (sov) | −2.19 | 0.000221 | −2.51 | 0.253 |
| PG1138 (porR) | −2.42 | 0.000221 | −3.3 | 0.613 |
| PG0412 (mutL) | −1.57 | 0.009979 | −2.17 | 0.211 |
| PG2100 | −8.77 | 0.000221 | −14.74 | 0.532 |
| PG2102 | −12.97 | 0.000221 | −29.46 | 0.816 |
4 DISCUSSION
Oxidative stress resistance via its protection against damage to DNA, protein, vital cell components and other macromolecules is critical for the survival of P. gingivalis exposed to oxygen, hydrogen peroxide and reactive oxygen species) in the periodontal pocket.34 This is believed to be initiated by regulators that facilitate signal transduction in response to these specific environmental signals. In E. coli, the transcriptional regulator OxyR and the sigma factor RpoS are known to play a significant role as sensor and/or response regulator in a protective mechanism(s) against hydrogen peroxide-induced oxidative stress.35-37 Although the OxyR in P. gingivalis is not a sensor to hydrogen peroxide, it can activate transcription of oxidative stress-related genes,38 which raises the question of other transcriptional regulators/sensors that would be involved in a protective mechanism against hydrogen peroxide-induced stress. Two recent reports demonstrate that ECF sigma factors PG1660 and PG1827 in P. gingivalis can be response regulators to hydrogen peroxide.17,19
In the present study we provide further evidence of the ECF sigma factor PG1660 and its role in hydrogen peroxide-induced stress resistance in P. gingivalis. In addition to its ability to be modulated by hydrogen peroxide-induced stress, its function(s) including its autoregulation was confirmed by its ability to interact with the RNA core polymerase from E. coli, the binding and initiation of transcription from its promoter. The predicted –35 promoter element recognized by PG1660 is different from the traditionally –35 element (TTGACA) in Gram-negative bacteria,39,40 and the consensus promoter motif recognized by other P. gingivalis ECF sigma factors previously reported.19,33 In most cases, the ECF sigma factor is regulated by its cognate anti-sigma factor protein, which is encoded by a downstream gene on the same transcriptional unit.2,5 It is likely that the ECF sigma factor PG1660 could have a similar regulatory mechanism as the PG1660 and PG1659 (predicted anti-sigma factor) genes that were shown to be part of the same transcriptional unit.
Usually, the ECF sigma factor only controls a small regulon in response to environmental stress conditions.1,41 In our study only a few genes were downregulated in the PG1660-deficient strain (FLL354) under anaerobic conditions. This is in contrast to the large group of genes that were downregulated in the same strain under hydrogen peroxide stress conditions, so indicating that PG1660 plays an important role in response to the environmental stress. Due to the evidence that most of the genes differentially modulated by PG1660 encode for hypothetical proteins or proteins with unknown function, this could raise the possibility that an important component(s) of this oxidative stress protection mechanism is still undefined. This is further supported by the observation that the increased sensitivity of P. gingivalis FLL354 to hydrogen peroxide did not correlate with the increased expression of several of the genes – PG1545 (sodB) encoding superoxide dismutase, PG0618 encoding the C subunit of alkyl hydroperoxide reductase, PG0034, PG0275 and PG0616 encoding thioredoxin that is known to be associated with oxidative stress resistance in P. gingivalis.34,42 Taken together, it is likely that an ECF sigma factor PG1660-specific gene or regulon has a high relative significance in oxidative stress resistance. Moreover, because none of the genes related to oxidative stress in P. gingivalis such as PG1545 (sodB), PG0618/9 (ahpC-F), PG0880 (bcp), PG0270 (oxyR), PG0616 (trx) and PG1089 (rprY) were downregulated in FLL354 under both anaerobic and hydrogen peroxide stress conditions, it indicates that the sigma factor PG1660 could be involved in the regulation of a yet-to-be defined oxidative stress resistance pathway.
Gingipain activity in P. gingivalis can be part of a network of mechanisms associated with oxidative stress resistance. For example, gingipain activity is known to play a role in heme accumulation on the cell surface resulting in its black pigmentation.43,44 A response to oxidative stress will involve binding of oxygen and its toxic derivatives to iron accumulated on the surface of the cell.45 The bound heme can be involved in the catalytic destruction of the toxic oxygen derivative species.46,47 Because gingipain activity can be regulated at multiple levels (including transcriptional and posttranslational), modulation occurring at any of these levels can impact oxidative resistance. Inactivation of the ECF sigma factor PG1660 gene resulted in decreased gingipain activity and increased sensitivity to hydrogen peroxide.17 There was a decrease in sialidase gene expression, the activity of which is involved in the posttranscriptional regulation of the gingipains.48 In addition, genes encoding important components of the type IX secretion system (PorSS), which is important for transportation of CTD-containing proteins including the gingipains,49 were also modulated by the ECF sigma factor PG1660.
Transposition is known to be triggered by cellular stress, which can provide an adaptive response to the local environment.50–52 The downregulation of transposon and related gene in the PG1660-defective mutant could support a role for the ECF sigma factor PG1660 in this process. In contrast to the wild-type, based on the diminished growth of the PG1660-deficient isogenic mutant under hydrogen peroxide-induced stress, the upregulation of ribosomal proteins and other proteins involved in translation was unexpected. In Bacillus subtilis, the activation of sigma B in response to the environmental stress has been shown to be mediated by a process involving ribosomal protein.53 Whether the ribosomal protein-mediated mechanism is involved in the ECF sigma factor responding to hydrogen peroxide in P. gingivalis is unknown.
ECF sigma factor, PG0162 shares a 29% homology with PG1660 (RpoE). Similar to the ECF sigma factor PG1660, PG0162 can be involved with the regulation of the type IX secretion system in P. gingivalis (PorSS).54 However, in contrast to the PG1660-deficient mutant, the PG0162-deficient mutant (P. gingivalis FLL350) showed a similar sensitivity to hydrogen peroxide-induced stress, or other environmental stress including NaCl, pH, and temperature compared with the wild-type W83 strain.33 The P. gingivalis FLL356 (ΔPG0162∷ermF-ΔPG1660∷tetQ) showed increased sensitivity to the hydrogen peroxide and NaCl compared with either FLL350 (ΔPG0162∷ermF) or FLL354 (ΔPG1660∷ermF). Gingipain activity in P. gingivalis FFL356 was now regulated both at the transcriptional and posttranscriptional levels. In addition to the downregulation of the genes involved in the type IX secretion system, the gingipain genes were also downregulated. It is noteworthy that several genes encoding CRISPR-associated proteins were downregulated in FLL356 under hydrogen peroxide stress conditions. The CRISPR-Cas system in bacteria can play a role in oxidative stress resistance.55 Together, the modulation of these systems could contribute to increased oxidative stress sensitivity consistent with their functional roles described elsewhere.56 Although we cannot rule out the regulation of other genes, it is likely that a synergy between both ECF sigma factors, PG0162 and PG1660 regulatory systems, would contribute to increased oxidative stress resistance. This would also highlight the complexity of interaction of the regulons controlled by ECF sigma factors PG0162 and PG1660 and the possibility of a more complex mechanism of oxidative stress resistance.
Other transcriptional regulators were upregulated in P. gingivalis FLL354, indicating that the PG1660 ECF sigma factor could be part of a complex regulatory signaling network in P. gingivalis. Intricate cross talks between bacterial signaling pathways triggered by stress and other environmental signals facilitate built-in redundancies that could have important physiological consequences that are vital for microbial survival/pathogenesis.57,58 A likely mechanism exists in P. gingivalis for the crosstalk/interaction between the PG1660 ECF sigma factor and other regulators.
As a pathogen, P. gingivalis must respond to and persist through the inflammatory microenvironment of the periodontal pocket. As a consequence of the hydrogen peroxide and other reactive oxygen species from polymorphonuclear leukocytes that are present in the inflamed gingival pocket, a regulatory system(s) that is responsive to specific environmental cues and that induces gene activation is vital for microbial survival/pathogenesis. We have demonstrated the functional properties of sigma factor PG1660 (RpoE) in P. gingivalis. It is likely that PG1660 may be involved in a unique and complex regulatory network that may represent a yet-to-be described oxidative stress resistance pathway in P. gingivalis. How the oxidative stress signal is sensed, the activation of the ECF sigma factor PG1660 (RpoE) and how the cognate anti-sigma factor is modulated need further study.
Supplementary Material
Acknowledgments
This work was supported by Public Health Services Grants R56DE13664, DE019730, DE019730 04S1, DE022508, DE022724 from NIDCR (to H.M.F). The RNA-sequencing in this study was supported by National Institute of Health (NIH) under award number 1S10OD019960 (to C. Wang).
Funding information
Public Health Services Grants R56DE13664, DE019730, DE019730 04S1, DE022508, DE022724 from NIDCR; NIH award number 1S10OD019960.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
ORCID
H.M. Fletcher http://orcid.org/0000-0002-7165-2159
References
- 1.Staron A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H, Mascher T. The third pillar of bacterial signal transduction: classification of the extra-cytoplasmic function (ECF) sigma factor protein family. Mol Microbiol. 2009;74:557–581. doi: 10.1111/j.1365-2958.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 2.Helmann JD. Anti-sigma factors. Curr Opin Microbiol. 1999;2:135–141. doi: 10.1016/S1369-5274(99)80024-1. [DOI] [PubMed] [Google Scholar]
- 3.Lane WJ, Darst SA. The structural basis for promoter –35 element recognition by the group IV sigma factors. PLoS Biol. 2006;4:e269. doi: 10.1371/journal.pbio.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campagne S, Allain FH, Vorholt JA. Extra Cytoplasmic function sigma factors, recent structure insight into promoter recognition and regulation. Curr Opin Struct Biol. 2015;30:71–78. doi: 10.1016/j.sbi.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Paget MS. Bacterial sigma factors and anti-sigma factors: structure, function and distribution. Biomolecules. 2015;5:1245–1265. doi: 10.3390/biom5031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A, Smollett KL, Gopaul KK, Chan BH, Davis EO. Mycobacterium tuberculosis H37Rv sigC is expressed from two promoters but is not auto-regulatory. Tuberculosis. 2012;92:48–55. doi: 10.1016/j.tube.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Ades SE. Regulation by destruction: design of the σE envelope stress response. Curr Opin Microbiol. 2008;11:535–540. doi: 10.1016/j.mib.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 8.De Las PA, Connolly L, Gross CA. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigmaE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 9.Collinet B, Yuzawa H, Chen T, Herrera C, Missiakas D. RseB binding to the periplasmic domain of RseA modulates the RseA:sigmaE interaction in the cytoplasm and the availability of sigmaE RNA polymerase. J Biol Chem. 2000;275:33898–33904. doi: 10.1074/jbc.m006214200. [DOI] [PubMed] [Google Scholar]
- 10.Cezairliyan BO, Sauer RT. Inhibition of regulated proteolysis by RseB. Proc Natl Acad Sci U S A. 2007;104:3771–3776. doi: 10.1073/pnas.0611567104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hizukuri Y, Akiyama Y. PDZ domains of RseP are not essential for sequential cleavage of RseA or stress-induced sigma(E) activation in vivo. Mol Microbiol. 2012;86:1232–1245. doi: 10.1111/mmi.12053. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Wang B, Feng L, et al. Cleavage of RseA by RseP requires a carboxyl-terminal hydrophobic amino acid following DegS cleavage. Proc Natl Acad Sci U S A. 2009;106:14837–14842. doi: 10.1073/pnas.0903289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn JM, Levchenko I, Sauer RT, Baker TA. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–752. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 16.Nelson KE, Fleischmann RD, DeBoy RT, et al. Complete genome sequence of the oral pathogenic Bacterium Porphyromonas gingivalis strain W83. J Bacteriol. 2003;185:5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dou Y, Osbourne D, McKenzie R, Fletcher HM. Involvement of extracytoplasmic function sigma factors in virulence regulation in Porphyromonas gingivalis W83. FEMS Microbiol Lett. 2010;312:24–32. doi: 10.1111/j.1574-6968.2010.02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi Y, Ohara N, Ueda O, et al. Porphyromonas gingivalis mutant defective in a putative extracytoplasmic function sigma factor shows a mutator phenotype. Oral Microbiol Immunol. 2009;24:377–383. doi: 10.1111/j.1399-302X.2009.00526.x. [DOI] [PubMed] [Google Scholar]
- 19.Yanamandra SS, Sarrafee SS, Anaya-Bergman C, Jones K, Lewis JP. Role of the Porphyromonas gingivalis extracytoplasmic function sigma factor. SigH Mol Oral Microbiol. 2012;27:202–219. doi: 10.1111/j.2041-1014.2012.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onozawa S, Kikuchi Y, Shibayama K, et al. Role of extracytoplasmic function sigma factors in biofilm formation of Porphyromonas gingivalis. BMC Oral Health. 2015;15:4. doi: 10.1186/1472-6831-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie RM, Johnson NA, Aruni W, Dou Y, Masinde G, Fletcher HM. Differential response of Porphyromonas gingivalis to varying levels and duration of hydrogen peroxide-induced oxidative stress. Microbiology. 2012;158:2465–2479. doi: 10.1099/mic.0.056416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher HM, Schenkein HA, Morgan RM, Bailey KA, Berry CR, Macrina FL. Virulence of a Porphyromonas gingivalisW83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthiah AS, Aruni W, Robles AG, Dou Y, Roy F, Fletcher HM. In Porphyromonas gingivalis VimF is involved in gingipain maturation through the transfer of galactose. PLoS ONE. 2013;8:e63367. doi: 10.1371/journal.pone.0063367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner RG, Russell JB, Wilson DB, Wang GR, Shoemaker NB. Use of a modified Bacteroides-Prevotella shuttle vector to transfer a reconstructed β-1,4-D-endoglucanase gene into Bacteroides uniformis and Prevotella ruminicola B14. Appl Environ Microbiol. 1996;62:196–202. doi: 10.1128/aem.62.1.196-202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou Y, Aruni W, Luo T, Roy F, Wang C, Fletcher HM. Involvement of PG2212 a Zinc-finger protein in the regulation of oxidative stress resistance in Porphyromonas gingivalis W83. J Bacteriol. 2014;196:4057–4070. doi: 10.1128/JB.01907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutrin MC, Yu Y, Wang C, et al. A putative TetR regulator is involved in nitric oxide stress resistance in Porphyromonas gingivalis. Mol Oral Microbiol. 2016;31:340–353. doi: 10.1111/omi.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔCT) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Davey ME, Duncan MJ. Enhanced biofilm formation and loss of capsule synthesis: deletion of a putative glycosyltransferase in Porphyromonas gingivalis. J Bacteriol. 2006;188:5510–5523. doi: 10.1128/JB.01685-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gicquel G, Bouffartigues E, Bains M, et al. The extra-cytoplasmic function sigma factor SigX modulates biofilm and virulence-related properities in Pseudomonas aeruginosa. PLoS ONE. 2013;8:e80407. doi: 10.1371/journal.pone.0080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tettmann B, Dotsch A, Armant O, Fjell CD, Overhage J. Knockout of extracytoplasmic function sigma factor CEF-10 affects stress resistance and biofilm formation in Pseudomonas putida. Appl Environ Microbiol. 2014;80:4911–4919. doi: 10.1128/AEM.01291-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dou Y, Aruni W, Muthiah A, Roy F, Wang C, Fletcher HM. Studies of the extracytoplasmic function sigma factor PG0162 in Porphyromonas gingivalis. Mol Oral Microbiol. 2016;31:270–283. doi: 10.1111/omi.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henry LG, McKenzie RM, Robles A, Fletcher HM. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012;7:497–512. doi: 10.2217/fmb.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi H, Kim S, Mukhopadhyay P, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105:103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 36.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenstark A, Calcutt MJ, Becker-Hapak M, Ivanova A. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic Biol Med. 1996;21:975–993. doi: 10.1016/s0891-5849(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 38.Diaz PI, Slakeski N, Reynolds EC, Morona R, Rogers AH, Kolenbrander PE. Role of oxyR in the oral anaerobe Porphyromonas gingivalis. J Bacteriol. 2006;188:2454–2462. doi: 10.1128/JB.188.7.2454-2462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawley DK, McClure WR. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanaya S, Kudo Y. Specificity of promoter consensus sequences in Escherichia coli chromosome. Nucleic Acids Symp Ser. 1991;25:41–42. [PubMed] [Google Scholar]
- 41.Helmann JD. The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol. 2002;46:47–110. doi: 10.1016/s0065-2911(02)46002-x. [DOI] [PubMed] [Google Scholar]
- 42.Henry LG, Boutrin MC, Aruni W, Robles A, Ximinies A, Fletcher HM. Life in a diverse oral community-strategies for oxidative stress survival. J Oral Biosci. 2014;56:63–71. doi: 10.1016/j.job.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake DB, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JP, Dawson JA, Hannis JC, Muddiman D, Macrina FL. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J Bacteriol. 1999;181:4905–4913. doi: 10.1128/jb.181.16.4905-4913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smalley JW, Silver J, Marsh PJ, Birss AJ. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the m-oxodimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem J. 1998;331:681–685. doi: 10.1042/bj3310681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smalley JW, Birss AJ, Silver J. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the m-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett. 2000;183:159–164. doi: 10.1111/j.1574-6968.2000.tb08951.x. [DOI] [PubMed] [Google Scholar]
- 48.Aruni W, Vanterpool E, Osbourne D, et al. Sialidase and sialoglycoproteases can modulate virulence in Porphyromonas gingivalis. Infect Immun. 2011;79:2779–2791. doi: 10.1128/IAI.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato K, Yukitake H, Narita Y, Shoji M, Naito M, Nakayama K. Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol Lett. 2013;338:68–76. doi: 10.1111/1574-6968.12028. [DOI] [PubMed] [Google Scholar]
- 50.Nagy Z, Chandler M. Regulation of transposition in bacteria. Res Microbiol. 2004;155:387–398. doi: 10.1016/j.resmic.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Capy P, Gasperi G, Biemont C, Bazin C. Stress and transposable elements: co-evolution or useful parasites? Heredity. 2000;85:101–106. doi: 10.1046/j.1365-2540.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 52.Twiss E, Coros AM, Tavakoli NP, Derbyshire KM. Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol Microbiol. 2005;57:1593–1607. doi: 10.1111/j.1365-2958.2005.04794.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Scott JM, Haldenwang WG. Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor sigma(B) J Bacteriol. 2001;183:2316–2321. doi: 10.1128/JB.183.7.2316-2321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadowaki T, Yukitake H, Naito M, et al. A two-component system regulates gene expression of the type IX secretion component proteins via an ECF sigma factor. Sci Rep. 2016;6:23288. doi: 10.1038/srep23288. https://doi.org/10.1038/srep23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serbanescu MA, Cordova M, Krastel K, et al. Role of the Streptococcus mutans CRISPR-Cas systems in immunity and cell physiology. J Bacteriol. 2015;197:749–761. doi: 10.1128/JB.02333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burmistrz M, Dudek B, Staniec D, et al. Functional analysis of Porphyromonas gingivalis W83 CRISPR-Cas system. J Bacteriol. 2015;197:2631–2641. doi: 10.1128/JB.00261-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rowland MA, Deeds EJ. Crosstalk and the evolution of specificity in two-component signaling. Proc Natl Acad Sci U S A. 2014;111:5550–5555. doi: 10.1073/pnas.1317178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corrigan RM, Bowman L, Willis ER, Kaever V, Grundling A. Cross-talk between two mucleotide-signaling pathways in Staphylococcus aureus. J Biol Chem. 2015;290:5826–5839. doi: 10.1074/jbc.M114.598300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


