Abstract
Objective
The examination of lung cancer by histology type is important for monitoring population trends that have implications for etiology and prevention, screening and clinical diagnosis, prognosis and treatment. We provide a comprehensive description of recent histologic lung cancer incidence rates and trends in the U.S. using combined population-based registry data for the entire nation.
Materials and Methods
Histologic lung cancer incidence data was analyzed from CDC’s National Program of Cancer Registries (NPCR) and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program. Standardized rates and trends were calculated for men and women by age, race/ethnicity, and U.S. Census region. Rate ratios were examined for differences in rates between men and women, and annual percent change was calculated to quantify changes in incidence rates over time.
Results
Trend analysis demonstrate that overall rates have decreased, but incidence has remained stable for women aged 50 or older. Adenocarcinoma and squamous cell carcinoma were the two most common histologic subtypes. Adenocarcinoma rates continued to increase in men and women, and squamous cell rates increased in women only. All histologic subtype rates for white women exceeded rates for black women. Histologic rates for black men exceeded those for white men, except for small cell carcinoma. The incidence rate for Hispanics was nearly half the rate for blacks and whites.
Conclusion
The continuing rise in incidence of lung adenocarcinoma, the rise of squamous cell cancer in women, and differences by age, race, ethnicity and region points to the need to better understand factors acting in addition to, or in synergy with, cigarette smoking that may be contributing to observed differences in lung cancer histology.
Keywords: Histologic type, lung cancer, time trend, incidence, tumor registry, surveillance
INTRODUCTION
Lung cancer, the leading cause of cancer deaths in the United States and the second most common cancer type for both men and women (1), is more than one disease. For reporting purposes, lung cancer is typically classified by two major histologic types: small cell lung cancer and non-small cell lung cancer (NSCLC). Adenocarcinoma, squamous cell carcinoma, and large cell carcinoma are the three main NSCLC subtypes. In the 1990s, pathologists tended not to report subtypes of non-small cell carcinomas since treatment and prognoses were considered similar. With the evolution of new advances in subtype classification and treatment, the classification of lung cancer by histology type became important for prognosis and choice of targeted therapies. The distinction between lung cancer histologic types also has implications for etiology and prevention, screening and clinical diagnosis, prognosis, and choice of treatment options (2–6).
In the United States, lung cancer incidence rates and trends by histologic type differ by sex and have shifted over time (7–11). From 1950 to early 1980, squamous cell carcinoma was most prevalent in men, and the most common type of lung cancer reported in U.S. tumor registries (9). Declining rates for squamous cell carcinoma were first noted in the mid-1980s, and this decline paralleled a decrease in smoking rates in the United States (8, 11). National data from 2004 to 2006 indicate squamous cell carcinoma rates were more than twice as high for men than for women (12). Studies based on data from selected cancer registries noted an increase in the incidence of adenocarcinoma in both men and women beginning in the 1960s and 1970s (8, 10, 13). By the 1980s, the incidence rate of adenocarcinoma had surpassed that of squamous cell carcinoma (11). Data from the 1980s suggested a decline in adenocarcinoma incidence rates (14), especially among men younger than age 65 years, white women younger than age 45 years, and black women younger than age 55 years (11). A more recent analysis suggested a peak in adenocarcinoma incidence rates at the end of the 1990s (15). Since then, adenocarcinoma has remained the most common histologic subtype in men and women (12).
This study used 2004–2009 cancer data from population-based cancer registries for the entire U.S. population to provide a comprehensive description of recent lung cancer incidence rates with sufficient numbers to examine trends by histologic type by sex, age, race/ethnicity, and geographic region.
METHODS
We analyzed data from CDC’s National Program of Cancer Registries (NPCR) and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program. Together, these two programs collate cancer incidence data for the entire U.S. population and the data are combined to create the United States Cancer Statistics (USCS) data set. In each U.S. state and the District of Columbia, cancer incidence data are collected from patient records at medical facilities (i.e., hospitals, physicians’ offices, therapeutic radiation facilities, freestanding surgical centers) and pathology laboratories and reported to NPCR or SEER central cancer registries. State-level incidence data must meet USCS high quality standards prior to publication: case ascertainment is 90% or more complete; no more than 5% of cases are ascertained solely on the basis of a death certificate; no more than 3% of cases are missing information on sex; no more than 3% of cases are missing information on age; no more than 5% of cases are missing information on race; and at least 97% of the registry’s records passed a set of single-field and inter-field computerized edits. Because data from Wisconsin were suppressed at the state’s request during 2004–2009, this report covers 98% of the U.S. population.
We restricted our analysis to lung and bronchus cancer cases diagnosed during 2004–2009 because cancer stage is not comparable for cases diagnosed prior to 2004 (16). Initially, we identified 1,260,271 cases by using site codes C34.0–C34.9 from the International Classification of Diseases for Oncology, Third Edition (ICD-0-3) (17). We excluded cases without microscopic confirmation (12%), those identified through autopsy or death certificate only (0.1%), and those with a diagnosis code of sarcomas or other specific or unspecified cancer types (1.2%). We used ICD-0-3 morphology codes to define lung cancer histologic types for this study (see footnote of Table 2).
Table 2.
Lung Cancer Incidence and Percent by Histology Type—United States, 2004–2009
| Histology Type | Men
|
Women
|
Rate Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Ratea | 95% CI | N | % | Ratea | 95% CI | ||
| Small Cell | 82,363 | 14% | 9.8 | (9.7–9.9) | 80,898 | 16% | 7.9 | (7.8–8.0) | 1.3* |
| Non-Small Cellb | 513,411 | 86% | 62.5 | (62.3–62.6) | 419,604 | 84% | 40.9 | (40.8–41.0) | 1.5* |
| Squamous | 153,408 | 26% | 18.8 | (18.7–18.9) | 87,035 | 17% | 8.5 | (8.4–8.6) | 2.1* |
| Adenocarcinoma | 201,918 | 34% | 24.5 | (24.4–24.6) | 205,189 | 41% | 20.0 | (19.9–20.1) | 1.3* |
| Large Cell | 20,135 | 3% | 2.4 | (2.4–2.5) | 14,855 | 3% | 1.5 | (1.4–1.5) | 1.6* |
| Other non-small cell | 137,950 | 23% | 16.8 | (16.7–16.9) | 112,525 | 22% | 10.9 | (10.9–11.0) | 1.5* |
Abbreviations: CI = confidence interval
Small cell and non-small cell = 100%; non-small cell subcategories = 86%
Data are from population-based registries that participate in the National Program of Cancer Registries and/or the Surveillance, Epidemiology, and End Results and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population.
Lung cancer histology groups were defined using International Classification of Diseases for Oncology version 3 (ICD-0-3): non-small cell carcinoma (8010-8015, 8020-8022, 8030-8040, 8046, 8050-8052, 8070-8084, 8090-8110, 8120-8131, 8140-8156, 8160-8162, 8170-8175, 8180, 8190-8221, 8230-8231, 8240-8263, 8270-8280, 8290-8337, 8340-8347, 8350-8390, 8400-8562, 8570-8576, 8580-8671, 8940-8941), small cell carcinoma (8041-8045). Non-small cell carcinomas were further categorized as squamous cell (8051-8052, 8070-8084, 8120-8131), adenocarcinoma (8050, 8140-8149, 8160-8162, 8190-8221, 8250-8263, 8270-8280, 8290-8337, 8350-8390, 8400-8560, 8570-8576, 8940-8941), large cell carcinoma (8011-8015), and Other non-small cell carcinoma (8010, 8020-8022, 8030-8040, 8046, 8090-8110, 8150-8156, 8170-8175, 8180, 8230-8231, 8240-8249, 8340-8347, 8561-8562, 8580-8671).
Rates are per 100,000 and age-adjusted to the 2000 U.S. Standard Population (19 age groups—Census P25-1130) standard
Percentages for NSCLC subcategories equal 100% of the total NSCLC
Rate for men is significantly different than the rate for women (referent group) (p<0.05)
Incidence rates and temporal trends were calculated with SEER*Stat version 8.0.1 software (18). Because of sex differences in smoking habits and temporal trends for lung cancer, separate analyses were conducted for men and women. Incidence rates were standardized to the U.S. 2000 standard population, with population denominators estimated by SEER from the 2000 Census. Rates and percentages for men and women are presented by age group (<40 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, and ≥80 years), race/ethnicity (non-Hispanic white, non-Hispanic black, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, and Hispanic), U.S. Census region (Northeast, West, South, and Midwest), and histologic type (small cell carcinoma, squamous cell carcinoma, adenocarcinoma, large cell carcinoma, and other non-small cell carcinoma). Rate ratios were calculated to examine differences in lung cancer rates between men and women by demographic characteristics. Trends were examined by histologic type, and annual percent change (APC) was calculated to quantify the change in incidence rates over time, with a P value cutoff point of 0.05.
RESULTS
During 2004–2009, a total of 1,096,276 lung cancer cases were diagnosed and reported in the United States. The average annual incidence rate was 73.3 per 100,000 population among men and 49.4 among women. Among those with newly diagnosed lung cancer, 54% were men and 46% were women. Adenocarcinoma (44%) and squamous cell carcinoma (26%) accounted for the largest proportions of cases. Rates by patient characteristics—including age, race/ethnicity, and U.S. Census region—for men and women are summarized in Table 1. The incidence of lung cancer was 1.5 times higher for men than for women. Rates for women younger than age 50 years were higher than the rates for men in this age group.
Table 1.
Lung Cancer Incidence Rates for Men and Women by Age, Race/Ethnicity, and Census Region—United States, 2004–2009
| Characteristic | Men | Women | Rate Ratio | ||
|---|---|---|---|---|---|
|
| |||||
| Ratea | 95% CI | Ratea | 95% CI | ||
| Total | 73.3 | (72.1–72.5) | 49.4 | (48.6–48.9) | 1.5* |
| Age | |||||
| < 40 | 0.7 | (0.7–0.8) | 0.8 | (0.8–0.9) | 0.88* |
| 40–49 | 19.7 | (19.5–19.9) | 20.2 | (20.0–20.5) | 0.99* |
| 50–59 | 85.1 | (84.6–85.6) | 65.5 | (65.0–65.9) | 1.3* |
| 60–69 | 266.5 | (265.3–267.8) | 191.4 | (190.4–192.4) | 1.4* |
| 70–79 | 468.9 | (466.8–471.0) | 303.0 | (301.6–304.5) | 1.6* |
| 80+ | 399.9 | (397.4–402.5) | 201.2 | (199.8–202.5) | 2.0* |
| Race/Ethnicity | |||||
| NH, White | 75.6 | (75.4–75.8) | 53.2 | (53.1–53.3) | 1.4* |
| NH, Black | 89.8 | (89.0–90.5) | 46.6 | (46.2–47.0) | 1.9* |
| NH, AI/AN | 51.8 | (49.7–54.0) | 38.6 | (37.0–40.3) | 1.3* |
| NH, A/PI | 44.6 | (43.8–45.4) | 25.4 | (24.9–25.9) | 1.8* |
| Hispanic | 40.1 | (39.5–40.6) | 23.6 | (23.3–24.0) | 1.7* |
| Census Regionb | |||||
| Northeast | 71.9 | (71.5–72.3) | 51.8 | (51.5–52.2) | 1.4* |
| Midwest | 77.6 | (77.2–78.0) | 52.4 | (52.1–52.7) | 1.5* |
| South | 81.9 | (81.6–82.3) | 50.6 | (50.4–50.8) | 1.6* |
| West | 55.5 | (55.2–55.9) | 41.8 | (41.5–42.1) | 1.3* |
Abbreviations: CI = confidence interval; NH = non-Hispanic; AI/AN = American Indian/Alaska Native; A/PI = Asian/Pacific Islander
Data are from population-based registries that participate in the National Program of Cancer Registries and/or the Surveillance, Epidemiology, and End Results and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population.
Rates are per 100,000 and age-adjusted to the 2000 U.S. Standard Population (19 age groups—Census P25-1130) standard
Categorized by US census region
Rate for men is significantly different than the rate for women (referent group) (p<0.05)
In addition to differences by sex, rate differences also were observed by race/ethnicity. Rates for non-Hispanic (NH) black men were about 20% higher than NH white men. Among both men and women, the rate for Hispanics was nearly half the rate for NH blacks and NH whites. Men had higher incidence rates than women for race and ethnic groups, but the relative size of this difference by sex was higher for NH blacks, NH Asian/Pacific Islanders and Hispanics compared with NH whites. The relative difference in incidence rates between men and women was slightly lower for NH American Indian/Alaska Native (AI/AN) populations than for whites. Differences between men and women living in the West were less pronounced compared to those living in other regions (Table 1).
During 2004–2009, NSCLC made up 86% of lung cancers in men and 84% of lung cancers in women (Table 2). Age-adjusted incidence rates for all histologic types were significantly higher for men than for women, but adenocarcinoma represented a larger proportion of lung cancers in women (41%) than in men (34%). The sex differences in rates of adenocarcinoma and small cell carcinoma were less pronounced than the differences observed for other histologic types (Table 2).
Tables 3 and 4 present incidence rates by histologic type for men and women by age, race/ethnicity, and U.S. Census region. Among men, the incidence of squamous cell carcinoma and adenocarcinoma were higher for NH blacks than for NH whites; among women, differences by race/ethnicity were less pronounced. Histologic incidence rates for NH AI/AN men and women were lower compared to NH white and NH black. Rates for small cell carcinoma in NH AI/AN women exceeded those for NH black women (Table 4). Data stratified by region showed a higher incidence of adenocarcinoma for men than for women in all regions, but differences in rates were smaller between men and women living in the West.
Table 3.
Men Lung Cancer Incidence Rates by Age, Race/Ethnicity, and Census Region—United States, 2004–2009
| Characteristics | Small Cell
|
Squamous Cell
|
Adenocarcinoma
|
Large Cell
|
Other Non-Small Cell
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratea | 95% CI | Ratea | 95% CI | Ratea | 95% CI | Ratea | 95% CI | Ratea | 95% CI | |
| Age | ||||||||||
| < 40 | 0.1 | (0.1–0.1) | 0.1 | (0.1–0.1) | 0.3 | (0.3–0.3) | 0.0 | (0.0–0.0) | 0.3 | (0.2–0.3) |
| 40–49 | 2.8 | (2.8–2.9) | 3.4 | (3.3–3.5) | 7.2 | (7.0–7.3) | 0.8 | (0.8–0.9) | 5.2 | (5.1–5.3) |
| 50–59 | 13.4 | (13.2–13.6) | 18.8 | (18.5–19.0) | 28.9 | (28.6–29.2) | 3.1 | (3.0–3.2) | 20.1 | (19.8–20.3) |
| 60–69 | 39.4 | (38.9–39.9) | 69.6 | (68.9–70.2) | 87.8 | (87.1–88.5) | 9.0 | (8.8–9.2) | 58.0 | (57.4–58.6) |
| 70–79 | 59.7 | (58.9–60.4) | 128.0 | (127.0–129.1) | 156.2 | (155.1–157.4) | 15.0 | (14.6–15.4) | 104.2 | (103.2–105.2) |
| 80+ | 43.6 | (42.8–44.5) | 105.7 | (104.4–107.0) | 133.0 | (131.5–134.5) | 12.0 | (11.5–12.4) | 96.6 | (95.4–97.9) |
| Race/Ethnicity | ||||||||||
| NH, White | 10.7 | (10.6–10.7) | 19.4 | (19.3–19.5) | 25.0 | (24.9–25.2) | 2.5 | (2.5–2.5) | 17.0 | (16.9–17.1) |
| NH, Black | 8.6 | (8.4–8.8) | 25.2 | (24.8–25.6) | 29.1 | (28.7–29.5) | 3.1 | (2.9–3.2) | 22.4 | (22.1–22.8) |
| NH, AI/AN | 7.5 | (6.7–8.3) | 15.1 | (13.9–16.3) | 14.2 | (13.1–15.3) | 2.0 | (1.6–2.4) | 12.1 | (11.1–13.1) |
| NH, A/PI | 4.3 | (4.0–4.5) | 8.9 | (8.5–9.2) | 19.4 | (18.9–20.0) | 1.2 | (1.1–1.3) | 10.2 | (9.8–10.6) |
| Hispanic | 4.7 | (4.6–4.9) | 9.1 | (8.9–9.4) | 14.3 | (14.0–14.6) | 1.2 | (1.1–1.3) | 9.9 | (9.7–10.2) |
| Census Regionb | ||||||||||
| Northeast | 9.1 | (9.0–9.3) | 18.0 | (17.8–18.2) | 27.1 | (26.8–27.3) | 1.8 | (1.8–1.9) | 15.1 | (14.9–15.3) |
| Midwest | 11.0 | (10.8–11.1) | 20.4 | (20.2–20.6) | 25.4 | (25.1–25.6) | 2.6 | (2.5–2.6) | 17.2 | (17.0–17.4) |
| South | 11.2 | (11.1–11.3) | 21.9 | (21.7–22.1) | 25.2 | (25.0–25.4) | 3.2 | (3.1–3.2) | 19.3 | (19.1–19.4) |
| West | 6.8 | (6.7–6.9) | 12.5 | (12.3–12.7) | 20.1 | (19.9–20.3) | 1.5 | (1.5–1.6) | 13.6 | (13.5–13.8) |
Abbreviations: CI = confidence interval
Data are from population-based registries that participate in the National Program of Cancer Registries and/or the Surveillance, Epidemiology, and End Results and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population.
Lung cancer histology groups were defined using International Classification of Diseases for Oncology version 3 (ICD-0-3): non-small cell carcinoma (8010-8015, 8020-8022, 8030-8040, 8046, 8050-8052, 8070-8084, 8090-8110, 8120-8131, 8140-8156, 8160-8162, 8170-8175, 8180, 8190-8221, 8230-8231, 8240-8263, 8270-8280, 8290-8337, 8340-8347, 8350-8390, 8400-8562, 8570-8576, 8580-8671, 8940-8941), small cell carcinoma (8041-8045). Non-small cell carcinomas were further categorized as squamous cell (8051-8052, 8070-8084, 8120-8131), adenocarcinoma (8050, 8140-8149, 8160-8162, 8190-8221, 8250-8263, 8270-8280, 8290-8337, 8350-8390, 8400-8560, 8570-8576, 8940-8941), large cell carcinoma (8011-8015), and Other non-small cell carcinoma (8010, 8020-8022, 8030-8040, 8046, 8090-8110, 8150-8156, 8170-8175, 8180, 8230-8231, 8240-8249, 8340-8347, 8561-8562, 8580-8671).
Rates are per 100,000 and age-adjusted to the 2000 U.S. Standard Population (19 age groups—Census P25-1130) standard
Categorized by US census region
Table 4.
Women Lung Cancer Incidence Rates by Age, Race/Ethnicity, and Census Region—United States, 2004–2009
| Characteristics | Small Cell
|
Squamous Cell
|
Adenocarcinoma
|
Large Cell
|
Other Non-Small Cell
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratea | 95% CI | Ratea | 95% CI | Ratea | 95% CI | Ratea | 95% CI | Ratea | 95% CI | |
| Age | ||||||||||
| < 40 | 0.1 | (0.0–0.1) | 0.1 | (0.0–0.1) | 0.4 | (0.3–0.4) | 0.0 | (0.0–0.0) | 0.3 | (0.3–0.3) |
| 40–49 | 3.0 | (2.9–3.1) | 1.9 | (1.8–2.0) | 9.3 | (9.2–9.5) | 0.7 | (0.7–0.8) | 5.0 | (4.9–5.2) |
| 50–59 | 12.0 | (11.8–12.2) | 8.0 | (7.9–8.2) | 28.2 | (27.9–28.5) | 2.1 | (2.1–2.2) | 14.7 | (14.4–14.9) |
| 60–69 | 34.7 | (34.3–35.1) | 32.8 | (32.4–33.2) | 76.5 | (75.9–77.1) | 5.6 | (5.4–5.7) | 40.2 | (39.7–40.6) |
| 70–79 | 46.9 | (46.3–47.5) | 61.6 | (60.9–62.2) | 117.7 | (116.8–118.6) | 8.6 | (8.3–8.8) | 64.9 | (64.2–65.6) |
| 80+ | 23.7 | (23.2–24.1) | 38.2 | (37.6–38.8) | 80.9 | (80.1–81.8) | 5.4 | (5.2–5.6) | 48.4 | (47.8–49.1) |
| Race/Ethnicity | ||||||||||
| NH, White | 9.0 | (8.9–9.1) | 9.1 | (9.1–9.2) | 21.2 | (21.1–21.3) | 1.6 | (1.6–1.6) | 11.7 | (11.6–11.8) |
| NH, Black | 5.6 | (5.5–5.8) | 9.2 | (9.0–9.4) | 18.6 | (18.3–18.9) | 1.4 | (1.4–1.5) | 11.1 | (10.9–11.3) |
| NH, AI/AN | 7.4 | (6.8–8.2) | 8.0 | (7.3–8.8) | 13.3 | (12.3–14.2) | 1.1 | (0.9–1.4) | 8.1 | (7.4–8.9) |
| NH, A/PI | 1.7 | (1.5–1.8) | 2.6 | (2.4–2.7) | 15.3 | (14.9–15.7) | 0.5 | (0.4–0.6) | 5.0 | (4.8–5.3) |
| Hispanic | 2.8 | (2.7–2.9) | 3.4 | (3.3–3.6) | 10.7 | (10.5–10.9) | 0.6 | (0.5–0.6) | 5.6 | (5.5–5.8) |
| Census Regionb | ||||||||||
| Northeast | 7.6 | (7.4–7.7) | 8.4 | (8.3–8.6) | 23.4 | (23.2–23.6) | 1.2 | (1.2–1.3) | 10.7 | (10.6–10.9) |
| Midwest | 9.1 | (9.0–9.2) | 9.4 | (9.3–9.5) | 20.4 | (20.2–20.6) | 1.5 | (1.4–1.5) | 11.5 | (11.3–11.6) |
| South | 8.6 | (8.5–8.7) | 9.2 | (9.1–9.3) | 19.0 | (18.8–19.1) | 1.8 | (1.8–1.8) | 11.4 | (11.3–11.5) |
| West | 5.8 | (5.7–5.9) | 6.3 | (6.2–6.4) | 18.2 | (18.0–18.3) | 1.0 | (1.0–1.1) | 9.9 | (9.7–10.0) |
Abbreviations: CI = confidence interval
Data are from population-based registries that participate in the National Program of Cancer Registries and/or the Surveillance, Epidemiology, and End Results and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population.
Lung cancer histology groups were defined using International Classification of Diseases for Oncology version 3 (ICD-0-3): non-small cell carcinoma (8010-8015, 8020-8022, 8030-8040, 8046, 8050-8052, 8070-8084, 8090-8110, 8120-8131, 8140-8156, 8160-8162, 8170-8175, 8180, 8190-8221, 8230-8231, 8240-8263, 8270-8280, 8290-8337, 8340-8347, 8350-8390, 8400-8562, 8570-8576, 8580-8671, 8940-8941), small cell carcinoma (8041-8045). Non-small cell carcinomas were further categorized as squamous cell (8051-8052, 8070-8084, 8120-8131), adenocarcinoma (8050, 8140-8149, 8160-8162, 8190-8221, 8250-8263, 8270-8280, 8290-8337, 8350-8390, 8400-8560, 8570-8576, 8940-8941), large cell carcinoma (8011-8015), and Other non-small cell carcinoma (8010, 8020-8022, 8030-8040, 8046, 8090-8110, 8150-8156, 8170-8175, 8180, 8230-8231, 8240-8249, 8340-8347, 8561-8562, 8580-8671).
Rates are per 100,000 and age-adjusted to the 2000 U.S. Standard Population (19 age groups—Census P25-1130) standard
Categorized by US census region
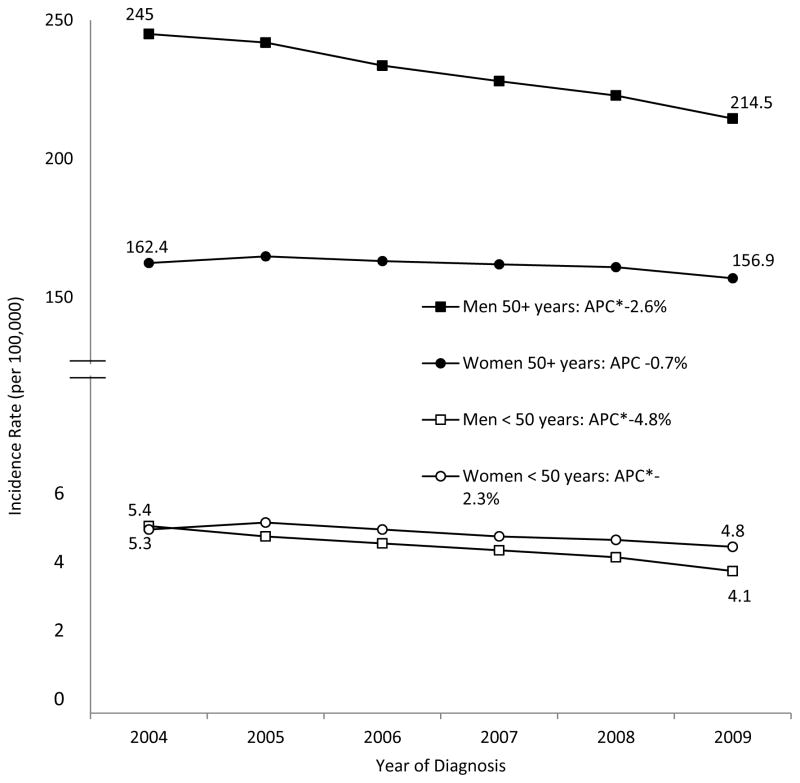
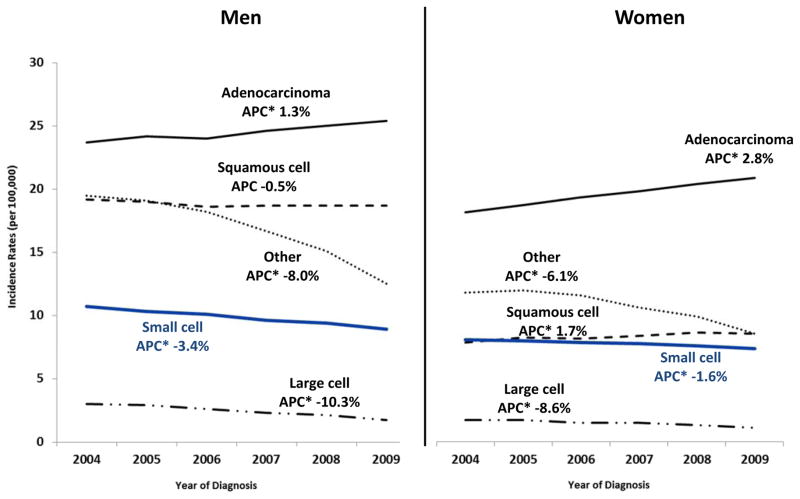
Temporal trends of total lung cancer incidence rates from 2004–2009 for men and women stratified by age (<50 years and ≥50 years) revealed a less rapid decrease in women than in men (Figure 1). Age-adjusted incidence rates for lung adenocarcinoma significantly increased for both men and women, and increased more rapidly for women than for men (Figure 2). Among adults aged 50 years or older, incidence trends for lung adenocarcinoma showed a significant decreasing trend for men (APC = −1.6%) and a significant increasing trend for women (APC = 3.2%) (data not shown). Age-adjusted incidence rates for squamous cell carcinoma increased in women but stabilized in men (Figure 2). Among those younger than age 50 years, incidence trends for squamous cell carcinoma significantly decreased in men (APC = −4.5%) but not in women (APC = −0.3%; P = 0.7), but for adults aged 50 years or older, rates significantly increased in women (APC = 2.0%) but stabilized in men (APC = −0.1%; P = 0.6) (data not shown).
Figure 1.
Lung Cancer Incidence Trends by Sex and Age—United States, 2004–2009a
Data are from population-based registries that participate in the National Program of Cancer Registries and/or the Surveillance, Epidemiology, and End Results and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population.
a Rates are per 100,000 and age-adjusted to the 2000 U.S. Std Population (19 age groups—Census P25-1130) standard
* Annual percent change (APC) is significantly different from zero (p<0.05)
Figure 2.
Lung Cancer Incidence Trends by Sex: Small Cell vs. Non-Small Cell Lung Cancer Subtypes—United States, 2004–2009a
Data are from population-based registries that participate in the National Program of Cancer Registries and/or the Surveillance, Epidemiology, and End Results and meet high-quality data criteria. These registries cover approximately 98% of the U.S. population.
Lung cancer histology groups were defined using International Classification of Diseases for Oncology version 3 (ICD-0-3): non-small cell carcinoma (8010-8015, 8020-8022, 8030-8040, 8046, 8050-8052, 8070-8084, 8090-8110, 8120-8131, 8140-8156, 8160-8162, 8170-8175, 8180, 8190-8221, 8230-8231, 8240-8263, 8270-8280, 8290-8337, 8340-8347, 8350-8390, 8400-8562, 8570-8576, 8580-8671, 8940-8941), small cell carcinoma (8041-8045). Non-small cell carcinomas were further categorized as squamous cell (8051-8052, 8070-8084, 8120-8131), adenocarcinoma (8050, 8140-8149, 8160-8162, 8190-8221, 8250-8263, 8270-8280, 8290-8337, 8350-8390, 8400-8560, 8570-8576, 8940-8941), large cell carcinoma (8011-8015), and Other non-small cell carcinoma (8010, 8020-8022, 8030-8040, 8046, 8090-8110, 8150-8156, 8170-8175, 8180, 8230-8231, 8240-8249, 8340-8347, 8561-8562, 8580-8671).
a Rates are per 100,000 and age-adjusted to the 2000 U.S. Std Population (19 age groups—Census P25-1130) standard
* Annual percent change (APC) is significantly different from zero (p<0.05)
DISCUSSION
Our study shows that, during 2004–2009 in the U.S., the overall incidence of lung cancer significantly decreased among men and remained stable among women, and trends differed by histologic type. Increases in adenocarcinoma and squamous cell carcinoma for women were partially offset by decreases in other types of NSCLC. Consistent with findings from previous studies, we found that rates for small cell carcinoma were higher among white men, but rates for other histology types were highest among black men. Histologic rates among women were similar for black and white women; however, the rate for small cell carcinoma in white women was nearly two times higher than the rate for black women. We found that the rates for all histologic types were lowest in the West region, an area comprised of states that have the lowest percentage of smokers (20). Among men and women, the rate of adenocarcinoma was highest in the northwest region. Small cell carcinoma rates were highest in the Midwest and South regions.
The shift in lung cancer trends by histologic type that has occurred over the last several decades—specifically, adenocarcinoma becoming more prevalent than squamous cell carcinoma (8, 10) —has been hypothesized to be due to changes in cigarette composition and design (19–22). The addition of ventilated filters to cigarettes changed the way smokers smoked, causing them to inhale smoke more vigorously into the peripheral lung, where adenocarcinomas are commonly found (20, 21, 23). However, a study that examined the tumors of smokers of lower-tar cigarettes and smokers of higher-tar cigarettes failed to find an association between tar dosage and lung adenocarcinoma and squamous cell carcinoma cell types (24). Another factor could be changes in the composition of cigarettes; the levels of tobacco-specific nitrosamines, particularly potent lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), in U.S. cigarettes have increased over the past 3 decades (20, 25).
The recent trends observed in our study occurred about the same time as changes in rules for classifying lung cancer histology (2–4) and advances in lung cancer treatment that require accurate classification (5, 6). These changes may have led to fewer NSCLCs being classified as “other” and instead classified more accurately as adenocarcinoma or squamous cell carcinoma, which may partially explain the increases we observed in these histologic types.
Cigarette smoking is a causal determinant of lung cancer, and because the epidemic of tobacco use began earlier in men than in women, men historically have had higher lung cancer rates than women (26). However, because of diminishing differences in smoking patterns between men and women, the gender gap in lung cancer is also diminishing (26–28). We found that, among adults aged 50 years or older, lung cancer incidence rates for men were higher than for women; this difference was greatest among the oldest and diminished with decreasing age. However, among those younger than age 50 years, women had higher rates than men, particularly for adenocarcinoma.
A report based on data from just the SEER registries showed that lung cancer rates for all histologic types increased among women during 1969–1991 (11). Our study showed that lung adenocarcinoma and squamous cell carcinoma rates increased, but that small cell carcinoma and large cell carcinoma rates decreased during 2004–2009 among women. The increasing rates of adenocarcinoma and squamous cell carcinoma observed in this study are consistent with earlier trends reported in previous studies (8, 11). Our data for women under age 50 did not show the early signs of decline in adenocarcinoma rates that have been observed among women born after 1950 in Canada, Denmark, and Australian (29). Other authors have suggested that later uptake of cigarette smoking and less rapid decrease in smoking prevalence among women than men (27, 28, 30) might partially explain the increasing rates of squamous cell carcinoma in women. Cigarette smoking is involved in the etiology of most lung cancers, but the etiologic mechanism may differ by histologic type (31, 32). Compared with other histologic types, squamous cell lung cancers had previously been found to be more strongly associated with heavy smoking (33–35).
To date, few studies have examined age-specific sex differences in lung cancer rates by histologic type. Previous analyses have suggested that lung cancer rates in men exceed those in women for all histologic types (8, 11). However, this study found among those younger than age 50 years the rate of adenocarcinoma was higher in women than in men. In addition to cigarette smoking, several factors have been identified as potential risk factors for lung adenocarcinoma, including cooking oil fumes, hormones, menstrual cycles, and pregnancy (36–41). More recent evidence suggests other risk factors such as human papillomavirus infection (42) and indoor and outdoor air pollution (15) are associated with increased risk for lung adenocarcinoma, but etiologic mechanisms and causal associations remain unclear.
Previous studies that examined incidence trends by histologic type and by race and sex during 1969–1986 showed higher rates of squamous cell carcinoma in blacks than in other racial/ethnic groups and higher rates of adenocarcinoma in black men than in men in other racial/ethnic groups (8). In our results, rates of squamous cell carcinoma and adenocarcinoma were highest in non-Hispanic black men, and the rate of adenocarcinoma was higher in NH white women compared to women in other racial/ethnic groups. Consistent with a recent study (43) our results show that compared to NH whites, lung cancer incidence rates for Hispanics were substantially lower. Consistent with previously reported data, our results showed overall lung cancer rates for NH AI/AN were lower than rates for NH blacks and NH whites. Among women, small cell lung cancer was more common among NH AI/AN than among NH black women, and differences in cigarette use and geographical location might explain some of these observed differences (44, 45).
In 2011, a joint working group of the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society introduced a new adenocarcinoma classification to better distinguish pre-invasive, minimally invasive, and invasive adenocarcinoma (46–47). Additional research is needed to better understand the correlation between lung cancer histology and the results observed on lung cancer screening with low dose spiral computed tomography LDCT. In the initial round of screening of study participants in the the National Lung Screening Trial (NLST), lung cancer screening with low-dose computed tomography (LDCT) identified a higher proportion of adenocarcinoma than squamous cell carcinoma (48–51). Questions for future research might include the potential frequency of over diagnosis of indolent lung cancer cases that would not become clinically apparent (52) and whether LDCT screening has a uniform ability to detect all histologic types of lung cancer or a uniform effect on death and survival rates across all histologic types (53). Such research would provide information that informs recommendations for discussions with clinicians about potential limitations and harms associated with screening for lung cancer with LDCT (54, 55).
The findings in this study are subject to the following limitations. First, our results provide estimates of incidence rates and trends by histologic type for the entire U.S. population, but we were unable to examine incidence by smoking because smoking data are not consistently collected in U.S. cancer registries. Second, analyses based on race and ethnicity, particularly among NH AI/AN, might be biased if race and ethnicity were misclassified; efforts were made to ensure that this information was as accurate as possible (56, 57).
CONCLUSION
Our study provides an update on lung cancer incidence trends by histologic type and expands previous research by providing age, race/ethnicity and sex-specific trends for the entire United States. The continued examination of lung cancer by histological subtype is important because the etiology, detection, diagnosis and treatment of lung cancer may differ by type and can change over time. The variations observed by race and ethnicity, the continuing rise in lung adenocarcinoma, and the rise of squamous cell cancer in women point to important knowledge gaps in our understanding of factors that operate in addition to, or in combination with, smoking to impact lung cancer incidence rates in the United States.
Acknowledgments
We gratefully acknowledge the contributions of the state and regional cancer registry staffs for their work in collecting the data used in this study.
References
- 1.U.S. Department of Health and Human Services. United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report. 1999–2009. [cited 2013; Available from: www.cdc.gov/uscs.
- 2.Travis W, Brambilla E, Müller-Hermelink H, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC; 2004. [Google Scholar]
- 3.Travis W, Brambilla E, Van Schil P, Scagliotti G, Huber R, Sculier J, et al. Paradigm shifts in lung cancer as defined in the new IASLC/ATS/ERS lung adenocarcinoma classification. Eur Respir J. 2011;38:239–43. doi: 10.1183/09031936.00026711. [DOI] [PubMed] [Google Scholar]
- 4.Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards B. The 2007 Multiple Primary and Histology Coding Rules. Bethesda, MD: National Cancer Institute, Surveillance, Epidemiology and End Results Program; 2007. [Google Scholar]
- 5.Langer C, Besse B, Gualberto A, Brambilla E, Soria J. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:5311–20. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 6.Kulesza P, Ramchandran K, Patel J. Emerging concepts in the pathology and molecular biology of advanced non-small cell lung cancer. Am J Clin Pathol. 2011;136:228–38. doi: 10.1309/AJCPO66OIRULFNLZ. [DOI] [PubMed] [Google Scholar]
- 7.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 8.Devesa S, Shaw G, Blot W. Changing patterns of lung cancer incidence by histological type. Cancer Epidemiol Biomarkers Prev. 1991;1:29–34. [PubMed] [Google Scholar]
- 9.Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect. 1995;103:143–8. doi: 10.1289/ehp.95103s8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent R, Pickren J, Lane W, Bross I, Takita H, Houten L, et al. The changing histopathology of lung cancer: a review of 1682 cases. Cancer. 1977;39:1647–55. doi: 10.1002/1097-0142(197704)39:4<1647::aid-cncr2820390439>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Travis W, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996;77:2464–70. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Underwood J, Townsend J, Tai E, Davis S, Stewart S, White A, et al. Racial and regional disparities in lung cancer incidence. Cancer. 2012;118:1910–8. doi: 10.1002/cncr.26479. [DOI] [PubMed] [Google Scholar]
- 13.Valaitis J, Warren S, Gamble D. Increasing incidence of adenocarcinoma of the lung. Cancer. 1981;47:1042–6. doi: 10.1002/1097-0142(19810301)47:5<1042::aid-cncr2820470535>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Zheng T, Holford T, Boyle P, Chen Y, Ward B, Flannery J, et al. Time trend and the age-period-cohort effect on the incidence of histologic types of lung cancer in Connecticut, 1960–1989. Cancer. 1994;74:1556–67. doi: 10.1002/1097-0142(19940901)74:5<1556::aid-cncr2820740511>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Cole P, Bina W. Time Trend and Geographic Patterns of Lung Adenocarcinoma in the United States, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2007;16:2724–9. doi: 10.1158/1055-9965.EPI-07-0455. [DOI] [PubMed] [Google Scholar]
- 16.Young JJ, Roffers S, Ries L, Fritz AAAH, et al. SEER summary staging manual—2000: codes and coding instructions. Bethesda, MD: National Cancer Institute; 2001. Report No.: 01-4969. [Google Scholar]
- 17.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 18.Surveillance Research Program NCI. SEER*Stat software version 8.01. Available from: seer.cancer.gov/seerstat.
- 19.Stellman S, Muscat JE, Thompson S, Hoffmann D, Wynder E. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80:382–8. doi: 10.1002/(sici)1097-0142(19970801)80:3<382::aid-cncr5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 21.Burns D, Anderson C. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;22:13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50(4):307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Risks Associated With Smoking Cigarettes With Low Machine-Measured Yields of Tar and Nicotine. Smoking and Tobacco Control Monograph No. 13. Bethesda (MD): U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2001. NIH Publication No. 02-5047. [Google Scholar]
- 24.Brooks DR, Austin JH, Heelan RT, Ginsberg MS, Shin V, Olson SH, et al. Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:576–81. doi: 10.1158/1055-9965.EPI-04-0468. [DOI] [PubMed] [Google Scholar]
- 25.Stepanov I, Knezevich A, Zhang L, Watson CH, Hatsukami DK, Hecht SS. Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: three decades of remarkable neglect by the tobacco industry. Tob Control. 2012;21(1):44–48. doi: 10.1136/tc.2010.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jemal A, Thun M, Ries L, Howe H, Weir H, Center M, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–94. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore M. Trends in cigarette smoking in the United States. The epidemiology of tobacco use. Med Clin North Am. 1992;76:289–303. doi: 10.1016/s0025-7125(16)30354-6. [DOI] [PubMed] [Google Scholar]
- 28.Pierce J, Fiore M, Novotny T, Hatziandreu E, Davis R. Trends in cigarette smoking in the United States. Projections to the year 2000. JAMA. 1989;261:61–5. [PubMed] [Google Scholar]
- 29.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lunc cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Fiore M, Novotny T, Pierce J, Hatziandreu E, Patel K, Davis R. Trends in cigarette smoking in the United States. The changing influence of gender and race. JAMA. 1989:261. [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services. Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1989. [Google Scholar]
- 32.Wynder E, Graham E. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc. 1950;143:329–36. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 33.Harris R, Zang E, Anderson J, Wynder E. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol. 1993;22:592–9. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- 34.Hinds M, Stemmermann G, Yang H, Kolonel L, Lee J, Wegner E. Differences in lung cancer risk from smoking among Japanese, Chinese and Hawaiian women in Hawaii. Int J Cancer. 1981;27:297–302. doi: 10.1002/ijc.2910270307. [DOI] [PubMed] [Google Scholar]
- 35.Pesch B, Kendzia B, Gustavsson P, Jöckel K, Johnen G, Pohlabeln HOA, et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210–9. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metayer C, Wang Z, Kleinerman R, Wang L, Brenner A, Cui H, et al. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Cancer. 2002;35:111–17. doi: 10.1016/s0169-5002(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 37.Ko Y, Lee C, Chen M, Huang C, Chang W, Lin H, et al. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int J Epidemiol. 1997;26:24–31. doi: 10.1093/ije/26.1.24. [DOI] [PubMed] [Google Scholar]
- 38.Tsao M, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993;4:571–9. [PubMed] [Google Scholar]
- 39.Taioli E, Wynder E. Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–70. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 40.Seow A, Poh W, Teh M, Eng P, Wang Y, Tan W, et al. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int J Cancer. 2002;97:365–71. doi: 10.1002/ijc.1615. [DOI] [PubMed] [Google Scholar]
- 41.Liao M, Wang J, Wang H, Ou A, Wang X, You W. A study of the association between squamous cell carcinoma and adenocarcinoma in the lung, and history of menstruation in Shanghai women, China. Lung Cancer. 1996;14:S215–21. doi: 10.1016/s0169-5002(96)90224-x. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Tsai Y, Chen Y, Christiani D. Human papilloma virus and female lung adenocarcinoma. Semin Oncol. 2009;36:542–52. doi: 10.1053/j.seminoncol.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012 Sep-Oct;62(5):283–98. [Google Scholar]
- 44.Kelly JJ, Lanier AP, Alberts S, Wiggins CL. Differences in cancer incidence among Indians in Alaska and New Mexico and U.S. Whites, 1993–2002. Cancer Epidemiol Biomarkers Prev. 2006;15:1515–9. doi: 10.1158/1055-9965.EPI-05-0454. [DOI] [PubMed] [Google Scholar]
- 45.Bliss A, Cobb N, Solomon T, Cravatt K, Jim M, Marshall L, Campbell J. Lung Cancer Incidence Among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113(5 Suppl):1169–78. doi: 10.1002/cncr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Schil PE, Sihoe AD, Travis WD. Pathologic classification of adenocarcinoma of lung. J Surg Oncol. 2013;108(5):320–6. doi: 10.1002/jso.23397. [DOI] [PubMed] [Google Scholar]
- 47.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31(8):992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 48.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Lung Screening Trial Research Team, . Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980–91. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369(10):920–31. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976–83. doi: 10.1002/cncr.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Version 2.2014. Available at: www.nccn.org.
- 53.Pinsky P, Black B. Subset and Histological Analysis of Screening Efficacy in the National Lung Screening Trial; 2nd Joint Meeting of the National Cancer Advisory Board and Board of Scientific Directors; 2013; Bethesda, MD. [Google Scholar]
- 54.Smith RA1, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 55.Richards TB, White MC, Caraballo RS. Lung cancer screening with low-dose computed tomography for primary care providers. Prim Care Clin Office Pract. 2014;41:307–330. doi: 10.1016/j.pop.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, TMB Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113:1120–30. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 57.NAACCR Latino Research Work Group. NAACCR guideline for enhancing Hispanic/Latino identification: Revised NAACCR Hispanic/Latino identification algorithm [NHIA v2.1] Springfield, IL: North American Association of Central Cancer Registries; 2008. [Google Scholar]