Abstract
Several modifiable health behaviors and health factors that comprise the Life’s Simple 7, a cardiovascular health metric, have been associated with hypertension risk. We determined the association between cardiovascular health and incident hypertension in the Jackson Heart Study, a cohort of African-Americans. We analyzed participants without hypertension or cardiovascular disease at baseline (2000–2004) who attended ≥1 follow-up visit in 2005–2008 or 2009–2012 (n=1878). Body mass index, physical activity, diet, cigarette smoking, blood pressure, total cholesterol and fasting glucose were assessed at baseline and categorized as ideal, intermediate or poor using the American Heart Association’s Life’s Simple 7 definitions. Incident hypertension was defined at the first visit wherein a participant had systolic blood pressure≥140 mmHg, diastolic blood pressure≥90 mmHg or self-reported taking antihypertensive medication. There percentage of participants with ≤1, 2, 3, 4, 5 and 6 ideal Life’s Simple 7 components was 6.5%, 22.4%, 34.4%, 25.2%, 10.0% and 1.4%, respectively. No participants had 7 ideal components. During follow-up (median: 8.0 years), 944 (50.3%) participants developed hypertension, including 81.3% with ≤1 and 11.1% with 6 ideal components. The multivariable-adjusted hazard ratios (95% confidence interval) for incident hypertension comparing participants with 2, 3, 4, 5 and 6 versus ≤1 ideal component were 0.80 (0.61–1.03), 0.58 (0.45–0.74), 0.30 (0.23–0.40), 0.26 (0.18–0.37) and 0.10 (0.03–0.31), respectively (p-trend<0.001). This association was present among participants with baseline systolic blood pressure<120 mmHg and diastolic blood pressure<80 mmHg and, separately systolic blood pressure 120–139 or diastolic blood pressure 80–89 mmHg. African-Americans with better cardiovascular health have lower hypertension risk.
Keywords: African American, incident hypertension, Life’s Simple 7, cardiovascular health
Compared with whites, African Americans have higher blood pressure (BP) levels beginning in childhood and a higher incidence and prevalence of hypertension across the lifespan.1–4 Although antihypertensive medication is effective in lowering BP and cardiovascular disease (CVD) risk, it does not lower risk to the same level as someone without hypertension.5, 6 Preventing hypertension is a central component for reducing CVD among African Americans.7, 8
In 2010, the American Heart Association (AHA) announced a strategic impact goal for the year 2020 “to improve cardiovascular health in all Americans by 20%, while reducing deaths from CVD and stroke by 20%”.1, 9 The concept of cardiovascular health was described to help promote health behaviors and highlight the importance of preventing CVD risk factors from developing and sustaining the absence of CVD throughout the life-course.9 The AHA identified 4 health behaviors (i.e., body mass index [BMI], physical activity, diet, cigarette smoking) and 3 health factors (i.e., BP, total cholesterol and fasting glucose levels) that can be modified to reduce the number of CVD and stroke events occurring in the United States.9 Termed Life’s Simple 7, these components are being used by the AHA to monitor the cardiovascular health of the US population.9 Each Life’s Simple 7 component has been defined using poor, intermediate and ideal levels in order to monitor the full spectrum of cardiovascular health.9
Studies have shown that having ideal levels of more Life’s Simple 7 components is associated with a lower incidence of CVD.1, 10–13 Data on interventions that prevent hypertension in African Americans are limited.7, 8 Determining the benefits of ideal cardiovascular health on the risk for hypertension may provide evidence to develop multifaceted prevention approaches tailored for African Americans.1 Therefore, we evaluated the association of cardiovascular health defined by the Life’s Simple 7 with incident hypertension among African Americans. We hypothesized that better cardiovascular health would be associated with a lower risk for developing hypertension. Results from the current study may provide evidence that the AHA’s Life’s Simple 7 cardiovascular health metric designed to monitor CVD risk is a practical, population-level approach for surveillance of hypertension risk in African Americans.
Methods
Study population
The Jackson Heart Study (JHS), a community-based prospective cohort study, was designed to evaluate CVD risk among African Americans.14 Between 2000 and 2004, the JHS enrolled 5,306 non-institutionalized African Americans aged ≥20 years from the Atherosclerosis Risk in the Community (ARIC) study site in Jackson, Mississippi (n=1,626), and a representative sample of urban and rural Jackson, Mississippi metropolitan tri-county (Hinds, Madison and Rankin counties) residents, volunteers, randomly contacted individuals and secondary family members of participants (n=3,680).15 Participants from the ARIC study were eligible for the JHS if they lived in the Jackson, Mississippi metropolitan tri-county area, completed the fourth ARIC study exam in 1996–1998 and trained recruiters determined they were physically and mentally competent in order to increase the probability of retention.16 The current analysis was restricted to JHS participants with complete information on SBP, DBP and antihypertensive medication use at the baseline exam. Participants with hypertension, defined below, at baseline were excluded from the current analyses. As the concept of cardiovascular health emphasizes preventing CVD, participants who self-reported a physician diagnosis of myocardial infarction (MI) or stroke at baseline were excluded. Among participants who were enrolled in the ARIC study, history of MI and stroke also included adjudicated events that occurred following the ARIC baseline study visit and prior to their enrollment in the JHS. Participants who did not attend Exam 2 (2005–2008) or Exam 3 (2009–2012) and did not have complete data on SBP, DBP and use of antihypertensive medication from at least one of these exams were excluded because we were not able to determine if they developed incident hypertension. After applying these criteria, we analyzed data from 1,878 JHS participants. Institutional Review Boards at the University of Mississippi Medical Center, Jackson State University and Tougaloo College approved the JHS protocol. The Institutional Review Board at the University of Alabama at Birmingham approved the current analysis of de-identified data. All participants provided written informed consent.
Data Collection
Baseline data were collected during an in-home interview and clinic examination. Trained research staff administered questionnaires to collect self-reported information on age, sex, education, annual household income, marital status, history of MI and stroke, parental history of hypertension, antihypertensive, lipid-lowering and anti-diabetes medication use, cigarette smoking, diet and physical activity. Also, the pill bottles for prescription and over the counter medications taken in the two weeks prior to the study exam were reviewed. The names of medications were recorded and categorized by class. Trained research staff also measured height, weight and BP and collected blood and urine samples.
Albumin and creatinine were quantified from a 24-hour urine collection or from a spot urine sample using the nephelometric immunoassay and enzymatic methods, respectively.17 Serum creatinine was measured using enzymatic methods and calibrated to Isotope Dilution Mass Spectroscopy (IDMS) traceable creatinine.18 Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.19 CKD was defined as urinary-albumin-to-urinary-creatinine ratio ≥30 mg/g and/or eGFR <60 ml/min/1.73 m2.
Life’s Simple 7
The AHA’s Life’s Simple 7 components are comprised of 4 health behaviors (BMI, physical activity, diet and cigarette smoking) and 3 health factors (BP, total cholesterol and fasting glucose). Definitions have been published for categorizing each Life’s Simple 7 component as being ideal, intermediate and poor (Table S1).5
Health behaviors (BMI, physical activity, diet, cigarette smoking)
Height and weight were measured during the baseline study visit. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Using a modified Baecke questionnaire that was validated in the JHS using pedometers and accelerometers, the weekly duration and annual frequency of participation in sports/exercises over the previous year were recorded and summed to calculate a total number of minutes per week spent in moderate or vigorous physical activity.20, 21 Dietary intake of fruits, vegetables, fish, whole grains, sodium and sugary beverages were assessed using a 158-item food frequency questionnaire (FFQ) that was validated in a subset of the JHS participants using 24-hour dietary recall repeated over four consecutive months.22 Cigarette smoking status was defined using the same questions as the ARIC study including “Have you smoked more than 400 cigarettes in your lifetime?” and “Do you now smoke cigarettes?”.23
Health factors (BP, total cholesterol, fasting glucose)
Clinic BP was measured at each exam following a standardized protocol.12 After participants had sat for at least 5 minutes in an upright position with their back and arms supported, feet flat on the floor, legs uncrossed and an appropriate cuff size was fitted, trained staff conducted two BP measurements in their right arm.14, 24 Cuff size was determined from an arm circumference measurement. One minute elapsed between the two measurements. A random-zero sphygmomanometer (Hawksley and Sons Ltd) was used at baseline and Exam 2. A semi-automatic oscillometric device (Omron HEM-907XL, Omron Healthcare Inc., Lake Forest, IL) was used for all participants at Exam 3. A BP comparability sub-study was performed in 2,115 participants who had their BP measured simultaneously with a random-zero sphygmomanometer and the oscillometric device using a Y-connector at Exam 2.25 Since semi-automated devices are commonly used in clinical practice, the random-zero BP measurements were calibrated to the semi-automated device by modeling the differences in BP measurements between devices as a function of the random-zero sphygmomanometer. Detailed information related to the calibration is provided in the Supplemental Materials. Figure S1 shows Bland-Altman plots comparing systolic and, separately diastolic BP measurements using the random-zero sphygmomanometer and oscillatory device.
BP was measured by technicians who were trained and certified to follow the research protocol. A study coordinator or BP supervisor conducted training. Instruction on BP measurement using the random-zero sphygmomanometer included listening to recordings of Korotkoff sounds and observing BP measurements. Certification to measure BP required staff to pass a test in which the technician and instructor simultaneously measured BP using a Y-tube stethoscope. The JHS Coordinating Center conducted quality control by monitoring digit preference, comparing mean BP measurements within and between technicians, providing feedback during regular meetings and through re-certification testing every six months. SBP and DBP were defined as the mean of the two measurements at each exam. Normal BP was defined as SBP <120 mm Hg and DBP < 80 mm Hg.6 Prehypertension was defined as SBP between 120 and 139 mm Hg with DBP < 90 mm Hg or DBP between 80 and 89 mmHg with SBP < 140 mm Hg.6
Blood samples collected after an overnight fast (≥8 hours) were used to quantify total cholesterol, hemoglobin A1c (HbA1c) and blood glucose.9
Incident hypertension
At each exam, participants were categorized as taking antihypertensive medication if they self-reported use of BP lowering medication for high BP in the two weeks prior to their clinic examination. Incident hypertension was defined as the first follow-up exam at which a participant had SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg or self-reported antihypertensive medication use.
Statistical analysis
The distribution of ideal Life’s Simple 7 components was calculated for participants included in the current analysis. Baseline characteristics and the cumulative incidence of hypertension during follow-up were calculated overall and by number of ideal Life’s Simple 7 components. Since there were few participants with 0 ideal Life’s Simple 7 components, those with 0 or 1 component were grouped together. Also, no participants had all 7 ideal Life’s Simple 7 components. The cumulative incidence of hypertension and 95% confidence intervals (CI) was calculated among participants with ≤1, 2, 3, 4, 5 and 6 ideal components. Hazard ratios (HR) and 95% CI for incident hypertension associated with 2, 3, 4, 5 and 6 versus ≤1 ideal component were calculated using interval-censored regression. Interval-censored regression was used because the exact date when participants developed hypertension during follow-up was unknown, only that it occurred between two study visits.26 Participants who attended the baseline exam and at least one follow-up exam but did not develop hypertension were censored at the last exam they attended. The interval-censored regression model accounted for the time since baseline at which each event occurred and the censoring of participants who did not have complete follow-up. We selected variables for adjustment a priori based on their known associations with the Life’s Simple 7 components (exposure) and incident hypertension (outcome). An initial model included adjustment for age, sex, education, income and marital status. A second model included further adjustment for CKD and parental history of hypertension. Analyses were repeated for participants with normal BP and prehypertension, separately, and after excluding BP as a Life’s Simple 7 component. Also, the association between number of ideal health behaviors (i.e., BMI, physical activity, diet, smoking status) and factors (i.e., BP, cholesterol, glucose), separately, was calculated. The association for the levels (i.e., intermediate and ideal versus poor) of individual Life’s Simple 7 components with incident hypertension was calculated. Next, a Life’s Simple 7 score was determined for each participant by assigning individual components 2 points for an ideal level, 1 point for an intermediate level and 0 points for a poor level. The composite score was calculated as the sum of assigned values (possible range: 0 to 14 points) with higher scores indicating better cardiovascular health. Participants were categorized by Life’s Simple 7 composite scores into similarly sized groups (i.e., Life’s Simple 7 scores ≤6, 7, 8, 9, 10, ≥11). The cumulative incidence of hypertension and adjusted HRs for incident hypertension associated with the Life’s Simple 7 score were calculated, overall, and for participants with normal BP and prehypertension. In a sensitivity analysis, we required self-reported antihypertensive medication use to be confirmed by the presence of one or more classes of antihypertensive medication on the pill bottle review. In this analysis, incident hypertension was defined as the first follow-up visit where a participant had SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg or self-reported antihypertensive medication use with one or more classes of antihypertensive medication present on the pill bottle review. For the above analyses, missing data were imputed with 10 data sets using chained equations.27 The number and percentage of participants with missing data for each variable included in this analysis is reported in Table S2. In a sensitivity analysis, we also repeated the main analyses after excluding participants missing relevant data (i.e., a complete case analysis). P-values <0.05 were considered statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC) or Stata/IC version 12.1 (Stata Inc., College Station, TX).
Results
Participant characteristics
The percentage of participants with 0 or 1, 2, 3, 4, 5 and 6 ideal Life’s Simple 7 components is shown in Figure 1. Participants with more ideal Life’s Simple 7 components were younger, less likely to be men, have less than a high school education, household income < $25,000 annually and CKD (Table 1). Also, participants with more ideal Life’s Simple 7 components had lower mean SBP and DBP at baseline.
Figure 1.
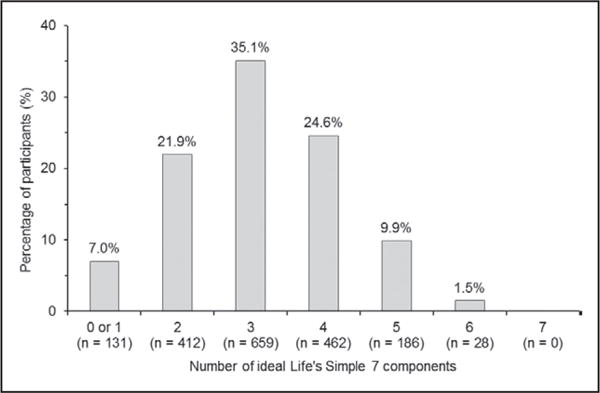
Distribution of the number of ideal Life’s Simple 7 components among Jackson Heart Study participants without hypertension at baseline (n=1878).
Table 1.
Baseline characteristics of Jackson Heart Study participants without hypertension by number of ideal Life’s Simple 7 components.
| Characteristic | Overall (n=1878) |
Number of Ideal Life’s Simple 7 Components* | p-trend | |||||
|---|---|---|---|---|---|---|---|---|
| 0 or 1 (n=131) |
2 (n=412) |
3 (n=659) |
4 (n=462) |
5 (n=186) |
6 (n=28) |
|||
| Age, years | 49.0 ± 11.9 | 54.2 ± 10.9 | 53.2 ± 11.2 | 49.5 ± 11.6 | 46.1 ± 11.3 | 43.0 ± 11.7 | 37.4 ± 9.8 | <0.001 |
| Men | 39.0% | 44.3% | 42.2% | 38.2% | 35.3% | 41.4% | 28.6% | 0.058 |
| Less than a high school education | 11.7% | 22.1% | 17.2% | 10.3% | 7.4% | 9.1% | 0.0% | <0.001 |
| Household income < $25,000 annually | 29.1% | 38.2% | 29.9% | 30.2% | 25.5% | 26.9% | 21.4% | 0.005 |
| Married | 58.6% | 61.1% | 60.2% | 57.5% | 61.7% | 50.0% | 57.1% | 0.195 |
| Chronic kidney disease | 7.3% | 19.8% | 11.2% | 6.4% | 4.1% | 1.6% | 7.1% | <0.001 |
| Parental history of high blood pressure | 68.3% | 66.4% | 65.3% | 68.3% | 70.3% | 71.5% | 67.9% | 0.112 |
| Systolic blood pressure, mmHg | 118.6 ± 10.6 | 125.8 ± 8.0 | 124.3 ± 8.7 | 119.8 ± 10.3 | 113.6 ± 9.6 | 110.7 ± 8.1 | 108.5 ± 6.1 | <0.001 |
| Diastolic blood pressure, mmHg | 74.1 ± 7.3 | 75.3 ± 7.2 | 76.4 ± 7.3 | 74.6 ± 7.3 | 72.2 ± 6.7 | 71.1 ± 6.4 | 70.6 ± 6.7 | <0.001 |
| Life’s Simple 7 individual components | ||||||||
| Ideal body mass index | 18.6% | 3.8% | 3.4% | 13.2% | 25.5% | 53.2% | 96.4% | <0.001 |
| Ideal physical activity | 23.1% | 1.5% | 5.3% | 17.9% | 32.5% | 61.3% | 100.0% | <0.001 |
| Ideal diet | 0.6% | 0.0% | 0.0% | 0.5% | 0.9% | 2.2% | 3.6% | 0.001 |
| Ideal smoking status | 85.9% | 56.5% | 79.9% | 86.5% | 93.3% | 97.3% | 100.0% | <0.001 |
| Ideal blood pressure level | 45.2% | 2.3% | 11.9% | 38.2% | 74.5% | 93.0% | 100.0% | <0.001 |
| Ideal total cholesterol level | 51.6% | 3.8% | 20.1% | 50.7% | 74.5% | 94.1% | 100.0% | <0.001 |
| Ideal fasting blood glucose level | 87.3% | 23.7% | 79.4% | 93.0% | 98.9% | 98.9% | 100.0% | <0.001 |
Numbers reported in table are mean ± standard deviation or percentages.
The definition for each ideal Life’s Simple 7 component is shown in Table 1.
No participants had 7 ideal Life’s Simple 7 components.
Number of ideal Life’s Simple 7 components and incident hypertension
Over a median follow-up of 8.0 years (maximum: 11.8 years), 944 (50.3%) participants developed hypertension (618 incident cases at Exam 2 and 326 incident cases at Exam 3). The cumulative incidence of hypertension was lower among participants with more ideal Life’s Simple 7 components (p-trend<0.001; Figure 2). After multivariable adjustment, the HR (95% CI) for incident hypertension comparing 2, 3, 4, 5 and 6 versus ≤1 ideal component were 0.79 (0.62–1.02), 0.57 (0.45–0.73), 0.30 (0.23–0.40), 0.26 (0.18–0.37) and 0.10 (0.03–0.32), respectively (p-trend<0.001; Table 2, top panel). The cumulative incidence of hypertension was lower with increasing numbers of ideal Life’s Simple 7 components among participants who had normal BP and prehypertension, separately (Table 2, middle and bottom panel). Also, the cumulative incidence of hypertension was lower with more ideal Life’s Simple 7 components when the ideal number of Life’s Simple 7 was calculated without BP as a component (Table S3).
Figure 2.
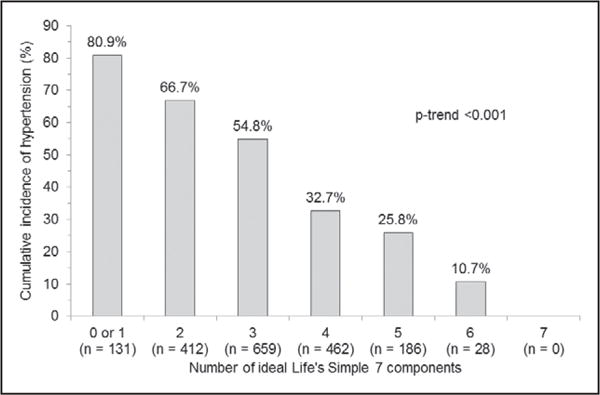
Cumulative incidence of hypertension by number of ideal Life’s Simple 7 components (n=1878).
Table 2.
Hazard ratios for incident hypertension associated with the number of ideal Life’s Simple 7 components in the overall population and for participants with normal blood pressure and prehypertension, separately, at baseline.
| Number of Ideal Life’s Simple 7 components* | N cases of incident hypertension/N at risk (% with incident hypertension) |
Hazard ratio (95% confidence interval) |
|
|---|---|---|---|
| Model 1 | Model 2 | ||
|
Overall* (n=1878) |
|||
| 0 or 1 | 106 / 131 (80.9%) | 1 (reference) | 1 (reference) |
| 2 | 275 / 412 (66.7%) | 0.78 (0.61–1.00) | 0.79 (0.62–1.02) |
| 3 | 361 / 659 (54.8%) | 0.56 (0.44–0.72) | 0.57 (0.45–0.73) |
| 4 | 151 / 462 (32.7%) | 0.30 (0.23–0.39) | 0.30 (0.23–0.40) |
| 5 | 48 / 186 (25.8%) | 0.25 (0.17–0.35) | 0.26 (0.18–0.37) |
| 6 | 3 / 28 (10.7%) | 0.10 (0.03–0.31) | 0.10 (0.03–0.32) |
| p-trend | <0.001 | <0.001 | <0.001 |
|
Normal blood pressure*† (n=911) |
|||
| 0 or 1 | 6 / 12 (50.0%) | 1 (reference) | 1 (reference) |
| 2 | 42 / 76 (55.3%) | 1.34 (0.50–3.57) | 1.34 (0.50–3.62) |
| 3 | 112 / 275 (40.7%) | 0.82 (0.32–2.07) | 0.82 (0.32–2.13) |
| 4 | 89 / 347 (25.6%) | 0.52 (0.21–1.31) | 0.53 (0.21–1.36) |
| 5 | 39 / 173 (22.5%) | 0.50 (0.19–1.30) | 0.51 (0.19–1.35) |
| 6 | 3 / 28 (10.7%) | 0.23 (0.05–1.00) | 0.24 (0.05–1.03) |
| p-trend | <0.001 | <0.001 | <0.001 |
|
Prehypertension*‡ (n=967) |
|||
| 0 or 1 | 100 / 119 (84.0%) | 1 (reference) | 1 (reference) |
| 2 | 233 / 336 (69.3%) | 0.75 (0.58–0.96) | 0.76 (0.59–0.98) |
| 3 | 249 / 384 (64.8%) | 0.69 (0.54–0.90) | 0.70 (0.55–0.91) |
| 4 | 62 / 115 (53.9%) | 0.52 (0.37–0.73) | 0.53 (0.38–0.75) |
| 5 | 9 / 13 (69.2%) | 0.87 (0.44–1.74) | 0.93 (0.47–1.87) |
| 6 | * | * | * |
| p-trend | <0.001 | <0.001 | 0.001 |
For the overall population and among participants with normal blood pressure, the maximum number of ideal components was 6. In participants with prehypertension, the maximum number of ideal components was 5 since these participants cannot have ideal or intermediate blood pressure by definition.
Normal blood pressure was defined as systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg.
Prehypertension was defined as systolic blood pressure between 120 and 139 mmHg and/or diastolic blood pressure between 80 and 89 mmHg. For participants with prehypertension, the maximum number of ideal components was 5.
Model 1 is adjusted for age, sex, education, income and marital status.
Model 2 is adjusted for the variables in Model 1 and parental history of hypertension and chronic kidney disease.
Number of ideal Life’s Simple 7 health behaviors and factors and incident hypertension
Overall, 8.1%, 59.1%, 29.3%, 3.5% of participants had 0, 1, 2 and 3 ideal behavioral components, respectively. No participants had ideal levels of all 4 health behaviors. Also, 6.0%, 28.1%, 41.7%, and 24.2% of participants had 0, 1, 2 and 3 ideal health factors, respectively. Participants with more ideal Life’s Simple 7 health behaviors and health factors had a lower incidence of hypertension before and after multivariable adjustment (Table 3).
Table 3.
Hazard ratios for incident hypertension associated with the number of ideal Life’s Simple 7 health behaviors and factors, separately (n=1878).
| Number of Ideal Life’s Simple 7 components | N cases of incident hypertension/N at risk (% with incident hypertension) |
Hazard ratio (95% confidence interval) |
|
|---|---|---|---|
| Model 1 | Model 2 | ||
| Health behaviors* | |||
| 0 | 98 / 152 (64.5%) | 1 (reference) | 1 (reference) |
| 1 | 601 / 1109 (54.2%) | 0.73 (0.59–0.91) | 0.74 (0.59–0.92) |
| 2 | 224 / 551 (40.7%) | 0.53 (0.42–0.68) | 0.54 (0.43–0.69) |
| 3 | 21 / 66 (31.8%) | 0.39 (0.24–0.63) | 0.40 (0.25–0.64) |
| p-trend | <0.001 | <0.001 | <0.001 |
| Health factors† | |||
| 0 | 91 / 113 (80.5%) | 1 (reference) | 1 (reference) |
| 1 | 351 / 527 (66.6%) | 0.73 (0.57–0.94) | 0.74 (0.57–0.96) |
| 2 | 378 / 783 (48.3%) | 0.48 (0.37–0.62) | 0.49 (0.38–0.63) |
| 3 | 124 / 455 (27.3%) | 0.24 (0.18–0.32) | 0.25 (0.18–0.33) |
| p-trend | <0.001 | <0.001 | <0.001 |
Health behaviors: body mass index, physical activity, diet and smoking status. There were no participants with all 4 ideal health behaviors.
Health factors: blood pressure, total cholesterol and fasting blood glucose.
Model 1 is adjusted for age, sex, education, income and marital status.
Model 2 is adjusted for the variables in Model 1 and parental history of hypertension and chronic kidney disease.
Life’s Simple 7 individual components and incident hypertension
The percentage of participants with ideal Life’s Simple 7 components was 18.6% for BMI; 23.1% for physical activity, 0.6% for diet, 85.9% for smoking status, 45.2% for BP, 51.6% for total cholesterol and 87.3% for fasting blood glucose. The incidence of hypertension was lower for intermediate and ideal versus poor levels of BMI, physical activity, diet and fasting blood glucose, ideal compared with poor smoking status and ideal compared with intermediate BP before and after multivariable adjustment (Table 4). Cholesterol was not associated with incident hypertension after adjustment.
Table 4.
Hazard ratios for incident hypertension associated with the levels of cardiovascular health for individual Life’s Simple 7 components (n=1878).
| Level of cardiovascular health | N cases of incident hypertension/N at risk (% with incident hypertension) |
Hazard ratio (95% confidence interval) |
|
|---|---|---|---|
| Model 1 | Model 2 | ||
| Body mass index | |||
| Poor | 496 / 869 (57.1%) | 1 (reference) | 1 (reference) |
| Intermediate | 310 / 659 (47.0%) | 0.74 (0.64–0.86) | 0.75 (0.65–0.87) |
| Ideal | 138 / 350 (39.4%) | 0.60 (0.49–0.72) | 0.61 (0.50–0.74) |
| P-trend | <0.001 | <0.001 | <0.001 |
| Physical activity | |||
| Poor | 437 / 776 (56.3%) | 1 (reference) | 1 (reference) |
| Intermediate | 324 / 668 (48.5%) | 0.87 (0.75–1.00) | 0.86 (0.74–0.99) |
| Ideal | 183 / 434 (42.2%) | 0.76 (0.64–0.91) | 0.76 (0.63–0.90) |
| P-trend | <0.001 | 0.002 | 0.001 |
| Diet | |||
| Poor | 646 / 1268 (50.9%) | 1 (reference) | 1 (reference) |
| Intermediate | 295 / 598 (49.3%) | 0.89 (0.77–1.03) | 0.89 (0.77–1.03) |
| Ideal | 3 / 12 (25.0%) | 0.30 (0.10–0.95) | 0.32 (0.10–0.99) |
| P-trend | 0.267 | 0.031 | 0.032 |
| Cigarette smoking | |||
| Poor | 147 / 246 (59.8%) | 1 (reference) | 1 (reference) |
| Intermediate | 9 / 19 (47.4%) | 0.91 (0.46–1.82) | 0.92 (0.46–1.82) |
| Ideal | 788 / 1613 (48.9%) | 0.74 (0.61–0.89) | 0.74 (0.62–0.90) |
| P-trend | 0.018 | 0.001 | 0.002 |
| Blood pressure* | |||
| Poor* | * | * | * |
| Intermediate | 693 / 1029 (67.3%) | 1 (reference) | 1 (reference) |
| Ideal | 251 / 849 (29.6%) | 0.32 (0.28–0.37) | 0.33 (0.28–0.38) |
| P-trend | <0.001 | <0.001 | <0.001 |
| Total cholesterol | |||
| Poor | 154 / 267 (57.7%) | 1 (reference) | 1 (reference) |
| Intermediate | 337 / 642 (52.5%) | 0.97 (0.79–1.20) | 1.00 (0.81–1.23) |
| Ideal | 453 / 969 (46.7%) | 0.95 (0.77–1.18) | 0.98 (0.80–1.22) |
| P-trend | 0.001 | 0.655 | 0.855 |
| Fasting blood glucose | |||
| Poor | 68 / 85 (80.0%) | 1 (reference) | 1 (reference) |
| Intermediate | 101 / 153 (66.0%) | 0.70 (0.51–0.97) | 0.71 (0.51–0.98) |
| Ideal | 775 / 1640 (47.3%) | 0.48 (0.37–0.63) | 0.49 (0.37–0.65) |
| P-trend | <0.001 | <0.001 | <0.001 |
Participants with poor blood pressure (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or self-reported antihypertensive medication) were excluded from the analysis based on the primary outcome being incident hypertension. The intermediate category is the reference for the blood pressure component of the Life’s Simple 7.
Model 1 is adjusted for age, sex, education, income and marital status.
Model 2 is adjusted for the variables in Model 1 and parental history of hypertension and chronic kidney disease.
Life’s Simple 7 score and incident hypertension
The cumulative incidence of hypertension was lower among participants with higher Life’s Simple 7 scores (Table S4, top panel). After multivariable adjustment, the HR (95% CI) for developing hypertension associated with Life’s Simple 7 scores of 7, 8, 9, 10 and ≥11 versus ≤6 were 0.96 (0.78–1.18), 0.72 (0.59–0.89), 0.60 (0.49–0.74), 0.35 (0.27–0.46) and 0.29 (0.21–0.40), respectively. Higher Life’s Simple 7 scores were associated with a lower risk for hypertension among participants with normal BP and prehypertension, analyzed separately (Table S4, middle and bottom panel).
Sensitivity analyses
The results were consistent with the main analyses when incident hypertension was defined as SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg or self-reported antihypertensive medication use with one or more classes of antihypertensive medication identified during the pill bottle review. During follow-up, 50.4% of participants met the definition for hypertension used in the sensitivity analysis. Better cardiovascular health and, separately, better health behaviors and health factors were associated with lower risk for hypertension (Tables S5 and S6). Also, better levels of BMI, physical activity, diet and fasting blood glucose were associated with lower risk for hypertension (Table S7). Ideal compared with poor smoking status and ideal compared with intermediate BP were also associated with lower risk for hypertension. Cholesterol was not associated with incident hypertension. Results of the complete cases analyses were consistent with the main results (Tables S8, S9 and S10).
Discussion
In this community-based study of African Americans, two-thirds of participants had three or fewer ideal Life’s Simple 7 components and no participants had all seven. There was a strong graded association between having more ideal Life’s Simple 7 components and a lower incidence of hypertension. This association was consistent among participants with normal BP and prehypertension. Also, having more ideal health behaviors and health factors were each associated with a progressively lower incidence of hypertension.
Prior studies have demonstrated an association between having more ideal Life’s Simple 7 components and a lower incidence of CVD in African Americans.9, 11, 28–33 Among 3,107 African American participants of the ARIC study who were followed for a median 18.7 years, the age-sex adjusted CVD incidence rates per 1,000 person years (95% CI) for participants with 0, 1, 2, 3, 4 and ≥5 ideal Life’s Simple 7 components were 40.4 (32.0–51.5), 25.3 (22.4–28.6), 16.9 (15.0–19.1), 14.2 (12.2–16.5), 8.7 (6.6–11.5) and 3.3 (1.5–7.4), respectively.29 Having more Life’s Simple 7 components in the ideal range has also been associated with a lower risk for incident cognitive impairment34, 35, venous thromboembolism36, vascular and non-vascular death,28 end-stage renal disease37 and CKD38 in African Americans.
Several individual Life’s Simple 7 components have been associated with an increased risk for hypertension, including higher BMI, physical inactivity, diet, cigarette smoking and higher BP, cholesterol and fasting glucose levels.39 The presence of multiple Life’s Simple 7 components may accelerate the development of hypertension. Systematic reviews report that ideal cardiovascular health behaviors (i.e., ideal BMI, physical activity, diet, cigarette smoking status) are associated with lower levels of health factors (i.e., BP, cholesterol and glucose).1, 9, 29, 40 The results from the current study indicate that having more ideal Life’s Simple 7 components overall and, separately, a higher number of health behaviors and health factors are associated with lower hypertension risk.
The percentage of US adults ≥20 years old who have ideal levels of all Life’s Simple 7 components was <1% in the 2011–2012 National Health and Nutrition Examination Survey (NHANES).1 Prior studies indicate that African Americans are less likely to have ideal cardiovascular health compared with whites.1, 9, 11, 28–31 Only one-third of JHS participants without hypertension at baseline had four or more ideal Life’s Simple 7 components highlighting a need for interventions that improve cardiovascular health in African Americans to lower the incidence of hypertension and, ultimately, CVD risk.
Antihypertensive medication use lowers CVD risk in adults with hypertension.41 However, CVD risk remains higher among people with controlled BP on antihypertensive medication compared with adults with similar BP but without hypertension.42 This highlights the benefits of maintaining low BP levels across the lifespan.5, 6, 42 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) recommends lifestyle modification to lower BP and reduce CVD risk, but evidence for their use to prevent hypertension in African Americans is limited.6–8 In a systematic review of intervention studies (e.g., increasing physical activity, improving diet/nutrition and weight and stress reduction) for lowering BP or achieving BP targets, 27 studies were identified that included at least 50% enrollment of African-American participants or at least 50% of African-American participants in subgroup analyses. Health behavior modification including increasing physical activity, improving diet/nutrition and weight and stress reduction was associated with lower BP levels, improved BP control and lower incident hypertension.7, 8 More evidence was available for limiting sodium intake compared with using physical activity, weight loss and stress reduction approaches to lower BP levels.7 The current study of 1,878 African Americans suggests that improving poor and intermediate cardiovascular health to an ideal level may lower the risk for developing hypertension. Furthermore, it provides evidence that the Life’s Simple 7, an established cardiovascular health metric, may be a practical, population-level approach for surveillance of hypertension risk in African Americans.
The current study has several strengths. The JHS represents one of the largest cohort studies of African Americans with standardized and longitudinal data collection for evaluating CVD risk. BP was measured following a standardized protocol at each exam. Despite these strengths, there are also limitations. The JHS may be considered small relative to some other cohort studies or studies using administrative claims data.43, 44 However, administrative data rarely include all of the Life’s Simple 7 components. Also, the high rate of hypertension in the JHS provided enough power to detect small differences in the outcome across categories of the number of ideal Life’ Simple 7 components. Physical activity, diet and cigarette smoking were self-reported and objective measures of these factors were not available. BP was measured only two times at each study visit. Also, BP was measured using different devices at baseline and during follow-up. However, we were able to calibrate the BP measurements across study exams. While the median follow-up of 8 years may be considered short, the high incidence of hypertension in African Americans provided a large number of outcomes for this analysis.
Perspectives
There was a strong graded association between having more ideal Life’s Simple 7 components and a lower risk for hypertension. This association was present among participants with normal BP and prehypertension. Having more ideal health behaviors and, separately, ideal health factors, were each associated with a lower risk for hypertension. The current study suggests that even modest improvements in cardiovascular health behaviors and risk factors may lower the risk for hypertension in African Americans. Additionally, the Life’s Simple 7 is an established metric that can be used to monitor hypertension risk in African Americans.
Supplementary Material
Novelty and Significance.
What’s new?
-
-
Few data are available on global cardiovascular health and hypertension risk in African Americans.
-
-
Determining the benefits of ideal cardiovascular health on incident hypertension among African Americans may provide evidence for using the Life’s Simple 7 to monitor this high-risk population for hypertension.
What is relevant?
-
-
The majority of African Americans had less than 3 ideal Life’s Simple 7 components and no participants had all 7.
-
-
Hypertension developed in 81.3% of participants with ≤ 1 ideal component compared to 11.1% of participants with 6 ideal components.
-
-
There was a strong graded association between higher numbers of ideal Life’s Simple 7 components and lower hypertension risk after multivariable adjustment.
Summary
-
-
African Americans with better cardiovascular health had a lower incidence of hypertension.
-
-
The Life’s Simple 7 metric may be a useful framework for monitoring hypertension risk in African Americans.
Acknowledgments
receives research support through grant F31 HL129701 (PI: JNBIII) from the National Heart, Lung and Blood Institute (NHLBI) at the National Institutes of Health (NIH); MA: receives research support through grant HL117323-02S2 (PI: MA) from the NHLBI at the NIH; RMT: None; KMD: receives research support through grant R01 HL116470-02S1 from the NHLBI at the NIH; SGB: None; GST: receives research support through grant 5T32 HL00745733 from the NHLBI at the NIH; AC: receives research support through grants P60MD002249 from the National Institute on Minority Health and Health Disparities (NIMHD) at the NIH and 14CVGPS2000 from the American Heart Association; MS: receives research support through grants P60MD002249 and U54MD008176 from the NIMHD and 1R01HL116446 from the NHLBI at the NIH and 15SFDRN26140001 and P50HL120163 from the American Heart Association; AB: receives research support through grant 1K01HL133468-01 from the NHLBI at the NIH and Novartis; TMS: receives research support through grants U54NS081765 from the National Institute of Neurological Disorders and Stroke (NINDS) at the NIH and 16SFRN28850003 from the American Heart Association; DS: receives research support through grants R01 HL117323-01 and K24-HL125704 through the NHLBI at the NIH; PM: receives research support through grant R01 HL117323-01 from the NHLBI at the NIH and the American Heart Association’s Strategically Focused Hypertension Research Network grant SFRN 15SFRN2390002 (PI: PM).
Funding Sources:
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staffs and participants of the JHS.
Study conception and design: JNBIII, PM; Acquisition, analysis or interpretation of data: JNBIII, MA, RMT, KMD, SGB, GST, AC, MS, APB, TMS, DS, PM; Statistical analysis: JNBIII; Drafting of the manuscript: JNBIII, PM; Critical revision of the manuscript: JNBIII, MA, RMT, KMD, SGB, GST, AC, MS, APB, TMS, DS, PM; JNBIII and PM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest / Disclosures: APB: receives research support from Novartis not related to the current project; DS: consultant for Abbott Vascular and Novartis Pharmaceuticals Corporation; PM: receives research support from Amgen, Inc. not related to the current project. All other authors do not have conflicts of interest / disclosures.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Berenson GS, Wattigney WA, Webber LS. Epidemiology of hypertension from childhood to young adulthood in black, white, and hispanic population samples. Public health reports. 1996;111(Suppl 2):3–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: The bogalusa heart study. American journal of hypertension. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 4.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: The multi-ethnic study of atherosclerosis. Hypertension. 2011;57:1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal B, MacMahon S, Chapman N, Blood Pressure Lowering Treatment Trialists C Effects of ace inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: Results of prospectively designed overviews of randomised trials. Blood pressure lowering treatment trialists’ collaboration. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart L, Blood Institute Joint National Committee on Prevention DE, Treatment of High Blood P, National High Blood Pressure Education Program Coordinating C The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA: the journal of the American Medical Association. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Davis AM, Vinci LM, Okwuosa TM, Chase AR, Huang ES. Cardiovascular health disparities: A systematic review of health care interventions. Medical care research and review: MCRR. 2007;64:29S–100S. doi: 10.1177/1077558707305416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scisney-Matlock M, Bosworth HB, Giger JN, Strickland OL, Harrison RV, Coverson D, Shah NR, Dennison CR, Dunbar-Jacob JM, Jones L, Ogedegbe G, Batts-Turner ML, Jamerson KA. Strategies for implementing and sustaining therapeutic lifestyle changes as part of hypertension management in african americans. Postgraduate medicine. 2009;121:147–159. doi: 10.3810/pgm.2009.05.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task F, Statistics C Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and cvd mortality among us adults. JAMA: the journal of the American Medical Association. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s simple 7 and risk of incident stroke: The reasons for geographic and racial differences in stroke study. Stroke; a journal of cerebral circulation. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA: the journal of the American Medical Association. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American heart association’s life’s simple 7: Avoiding heart failure and preserving cardiac structure and function. The American journal of medicine. 2015;128:970–976 e972. doi: 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in african americans: Design and methods of the jackson heart study. Ethnicity & disease. 2005;15:S6-4–17. [PubMed] [Google Scholar]
- 15.Wilson JG, Rotimi CN, Ekunwe L, Royal CD, Crump ME, Wyatt SB, Steffes MW, Adeyemo A, Zhou J, Taylor HA, Jr, Jaquish C. Study design for genetic analysis in the jackson heart study. Ethnicity & disease. 2005;15:S6-30–37. [PubMed] [Google Scholar]
- 16.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting african-american research participation in the jackson heart study: Methods, response rates, and sample description. Ethnicity & disease. 2005;15:S6-18–29. [PubMed] [Google Scholar]
- 17.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the jackson heart study. The American journal of the medical sciences. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Young BA, Fulop T, de Boer IH, Boulware LE, Katz R, Correa A, Griswold ME. Effects of serum creatinine calibration on estimated renal function in african americans: The jackson heart study. The American journal of the medical sciences. 2015;349:379–384. doi: 10.1097/MAJ.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in african americans in atherosclerosis risk in communities. Medicine and science in sports and exercise. 2013;45:901–907. doi: 10.1249/MSS.0b013e31827d87ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smitherman TA, Dubbert PM, Grothe KB, Sung JH, Kendzor DE, Reis JP, Ainsworth BE, Newton RL, Jr, Lesniak KT, Taylor HA., Jr Validation of the jackson heart study physical activity survey in african americans. Journal of physical activity & health. 2009;6(Suppl 1):S124–132. doi: 10.1123/jpah.6.s1.s124. [DOI] [PubMed] [Google Scholar]
- 22.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA, Jr, Tucker KL. Validity and calibration of food frequency questionnaires used with african-american adults in the jackson heart study. Journal of the American Dietetic Association. 2009;109:1184–1193. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 24.Barker MH, Erlanger J, Meakins J, Schneider R, Jr, S SB, White, HUPD. Wiggers C, Wright I. Standard method for taking and recording blood pressure readings. Journal of the American Medical Association. 1939;113:294–297. [Google Scholar]
- 25.Abdalla M, Booth JN, 3rd, Seals SR, Spruill TM, Viera AJ, Diaz KM, Sims M, Muntner P, Shimbo D. Masked hypertension and incident clinic hypertension among blacks in the jackson heart study. Hypertension. 2016;68:220–226. doi: 10.1161/HYPERTENSIONAHA.115.06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelstein DM. A proportional hazards model for interval-censored failure time data. Biometrics. 1986;42:845–854. [PubMed] [Google Scholar]
- 27.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in medicine. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 28.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: The northern manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, Investigators AS Community prevalence of ideal cardiovascular health, by the american heart association definition, and relationship with cardiovascular disease incidence. Journal of the American College of Cardiology. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, Wu S, Zhao X. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke; a journal of cerebral circulation. 2013;44:2451–2456. doi: 10.1161/STROKEAHA.113.678839. [DOI] [PubMed] [Google Scholar]
- 31.Shay CM, Gooding HS, Murillo R, Foraker R. Understanding and improving cardiovascular health: An update on the american heart association’s concept of cardiovascular health. Prog Cardiovasc Dis. 2015;58:41–49. doi: 10.1016/j.pcad.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Ommerborn MJ, Blackshear CT, Hickson DA, Griswold ME, Kwatra J, Djousse L, Clark CR. Ideal cardiovascular health and incident cardiovascular events: The jackson heart study. American journal of preventive medicine. 2016;51:502–506. doi: 10.1016/j.amepre.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crichton GE, Elias MF, Robbins MA. Cardiovascular health and arterial stiffness: The maine-syracuse longitudinal study. Journal of human hypertension. 2014;28:444–449. doi: 10.1038/jhh.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The american heart association life’s simple 7 and incident cognitive impairment: The reasons for geographic and racial differences in stroke (regards) study. Journal of the American Heart Association. 2014;3:e000635. doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crichton GE, Elias MF, Davey A, Alkerwi A. Cardiovascular health and cognitive function: The maine-syracuse longitudinal study. PloS one. 2014;9:e89317. doi: 10.1371/journal.pone.0089317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson NC, Cushman M, Judd SE, McClure LA, Lakoski SG, Folsom AR, Safford MM, Zakai NA. American heart association’s life’s simple 7 and risk of venous thromboembolism: The reasons for geographic and racial differences in stroke (regards) study. Journal of the American Heart Association. 2015;4:e001494. doi: 10.1161/JAHA.114.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muntner P, Judd SE, Gao L, Gutierrez OM, Rizk DV, McClellan W, Cushman M, Warnock DG. Cardiovascular risk factors in ckd associate with both esrd and mortality. Journal of the American Society of Nephrology: JASN. 2013;24:1159–1165. doi: 10.1681/ASN.2012070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebholz CM, Anderson CA, Grams ME, Bazzano LA, Crews DC, Chang AR, Coresh J, Appel LJ. Relationship of the american heart association’s impact goals (life’s simple 7) with risk of chronic kidney disease: Results from the atherosclerosis risk in communities (aric) cohort study. Journal of the American Heart Association. 2016;5:e003192. doi: 10.1161/JAHA.116.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echouffo-Tcheugui JB, Batty GD, Kivimaki M, Kengne AP. Risk models to predict hypertension: A systematic review. PloS one. 2013;8:e67370. doi: 10.1371/journal.pone.0067370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claas SA, Arnett DK. The role of healthy lifestyle in the primordial prevention of cardiovascular disease. Current cardiology reports. 2016;18:56. doi: 10.1007/s11886-016-0728-7. [DOI] [PubMed] [Google Scholar]
- 41.Blood Pressure Lowering Treatment Trialists C. Sundstrom J, Arima H, Woodward M, Jackson R, Karmali K, Lloyd-Jones D, Baigent C, Emberson J, Rahimi K, MacMahon S, Patel A, Perkovic V, Turnbull F, Neal B. Blood pressure-lowering treatment based on cardiovascular risk: A meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 42.Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd-Jones DM. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels?: The coronary artery risk development in young adults (cardia) study and the multi-ethnic study of atherosclerosis (mesa) Journal of the American Heart Association. 2015;4:e002275. doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Booth JN, 3rd, Levitan EB, Brown TM, Farkouh ME, Safford MM, Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the reasons for geographic and racial differences in stroke study) The American journal of cardiology. 2014;113:1933–1940. doi: 10.1016/j.amjcard.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colantonio LD, Kent ST, Kilgore ML, Delzell E, Curtis JR, Howard G, Safford MM, Muntner P. Agreement between medicare pharmacy claims, self-report, and medication inventory for assessing lipid-lowering medication use. Pharmacoepidemiology and drug safety. 2016;25:827–835. doi: 10.1002/pds.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


