Abstract
Compelling evidence suggests that transplantation of neural stem cells (NSCs) from multiple sources ameliorates motor deficits after stroke. However, it is currently unknown to what extent the electrophysiological activity of grafted NSC progeny participates in the improvement of motor deficits and whether excitatory phenotypes of the grafted cells are beneficial or deleterious to sensorimotor performances. To address this question, we used optogenetic tools to drive the excitatory outputs of the grafted NSCs and assess the impact on local circuitry and sensorimotor performance. We genetically engineered NSCs to express the Channelrhodopsin-2 (ChR2), a light-gated cation channel that evokes neuronal depolarization and initiation of action potentials with precise temporal control to light stimulation. To test the function of these cells in a stroke model, rats were subjected to an ischemic stroke and grafted with ChR2-NSCs. The grafted NSCs identified with a human-specific nuclear marker survived in the peri-infarct tissue and coexpressed the ChR2 transgene with the neuronal markers TuJ1 and NeuN. Gene expression analysis in stimulated versus vehicle-treated animals showed a differential upregulation of transcripts involved in neurotransmission, neuronal differentiation, regeneration, axonal guidance, and synaptic plasticity. Interestingly, genes involved in the inflammatory response were significantly downregulated. Behavioral analysis demonstrated that chronic optogenetic stimulation of the ChR2-NSCs enhanced forelimb use on the stroke-affected side and motor activity in an open field test. Together these data suggest that excitatory stimulation of grafted NSCs elicits beneficial effects in experimental stroke model through cell replacement and non-cell replacement, anti-inflammatory/neurotrophic effects.
Keywords: Neural stem cells (NSCs), Neural transplantation, Optogenetic stimulation, Sensorimotor behavior, Stroke
INTRODUCTION
Stroke is one of the leading causes of death and long-term disability worldwide (19). Neural stem cell (NSC)-based therapy has demonstrated efficacy in improving motor deficits in experimental models of stroke. To better understand and direct the functional repair process, the mechanisms mediating stem cell functional recovery in stroke-damaged brain tissue need to be elucidated.
The ability of NSCs grafted into the stroke-damaged brain to modulate local circuits is not fully understood. The electrophysiological properties of grafted neural progeny in the stroke-damaged tissue have been investigated using mouse embryonic stem cells (mESCs) (3) and neural precursors derived from the developing brain that ubiquitously express enhanced green fluorescent protein (6). Patch clamp recordings demonstrated that 27.7% of grafted cells expressed action potentials and voltage gated Na+ and K+ currents typical of the neuronal phenotype. Graft-derived neurons showed spontaneous postsynaptic current between 3 and 7 weeks posttransplant, demonstrating that they were receiving synaptic inputs (6). Similarly, electrophysiological recordings from neural progeny derived from mESCs grafted into the middle cerebral artery occlusion (MCAO) stroke model demonstrated synaptic connections with the host and behavioral improvement in the fear-conditioning test (12). These data suggest that cell replacement could support functional recovery in an animal model of stroke. However, the capacity of grafted NSCs to innervate stroke-damaged host parenchyma and drive the neural circuit involved in sensorimotor function is unknown. We utilized the high spatial and temporal precision of optogenetic stimulation by modifying NSCs with the genetically encoded photosensitive membrane protein Chlamydomonas reinhardtii Channelrhodopsin-2 (ChR2). This enabled a targeted approach to probe the role of grafted NSCs in driving compromised neural circuits and sensorimotor function in an experimental stroke model.
MATERIALS AND METHODS
Generation of the NSC-Expressing ChR2
NSCs were derived as we previously described (8) from human embryonic stem cells (hESCs; H9; WiCell Research Institute, Madison, WI, USA), approved by Stanford University Stem Cell Research Oversight (SCRO) Committee. Lentiviral vectors carrying the ChR2 gene fused with enhanced yellow fluorescent protein (ChR2-EYFP) under the EF1a promoter were produced, as previously described (31,32). Maps and clones are available at http://www.stanford.edu/group/dlab/optogenetics. Then the NSCs were transduced with the lenti-ChR2 or with lenti-YFP and isolated using the fluorescence activated cell sorting (FACS) Vantage SE cell Sorter (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA), as previously reported (7). The NSCs were cultured in a chemically defined medium composed of DMEM/F12 (1:1) supplemented with 1% N2, 2% B27, epidermal growth factor (EGF) (20 ng/ml) (all from Life Technologies, Carlsbad, CA, USA); basic fibroblast growth factor (bFGF; 10 ng/ml, from R&D Systems, Minneapolis, MN, USA); and leukemia inhibitory factor (LIF; 10 ng/ml; from Millipore, Temecula, CA, USA). Dissociated NSCs were plated at a density of 100,000 cells/ml in Corning T25 or T75 (Invitrogen, Carlsbad, CA, USA) culture flasks. For differentiation, dissociated NSCs were plated at a density of 106 cells/ml in the absence of growth factors and supplemented with 1% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) on poly-L-ornithine-coated (15 mg/ml; Sigma-Aldrich) glass coverslips in 24-well Nunclon culture dishes (Thermo Fisher Scientific, Waltham, MA, USA) for 1 week and processed for immunocytochemistry.
Cell Transplantation
Sprague–Dawley adult male rats (275–310 g; Charles River Laboratories, Wilmington, MA, USA) were subjected to transient 65-min MCAO as previously reported (5). One week later, rats were immunosuppressed with IP injections of cyclosporine A (20 mg/ml, Sandimmune; Novartis Pharmaceuticals, East Hanover, NJ, USA) and were transplanted with lenti-ChR2- or lenti-YFP-NSCs (2 × 105) or vehicle into the ischemic boundary zone in the striatum, as previously described (5,8). All animals underwent baseline motor behavioral assessment before and after the ischemic lesion and at 4 and 8 weeks after cell transplantation. The animals were euthanized at 8 weeks posttransplantation survival time and the brains processed for immunohistopathology or gene expression analysis. Animal experiments were conducted in accordance with protocols approved by the Stanford Institutional Animal Care and Use Committee (IACUC).
Optogenetic Stimulation
Rats were implanted with a fiber-guiding cannula stabilized to the scull using cranioplastic cement (10). The stereotaxic implantation coordinates were the same as those used for cell transplantation. An optical patch cable coupled to a 473-nm laser (OEM Laser Systems, Bluffdale, UT, USA) was attached to the implanted guiding cannula. The intensity of the light (1–2 mW) was verified before each use by a light sensor (S130A; Thor Labs, Newton, NJ, USA). The optical fiber 200 μm (external size) (Thor Labs) was lowered along the fiber guide to the grafted NSCs. Optogenetic stimulation was delivered at 10 Hz, 12 mW, 20-ms pulses and controlled by a waveform generator (Agilent Technologies, Santa Clara, CA, USA). The animal received continuous stimulation three times for 30 s, with a 1-min rest period in between. Stimulation was administered daily for 4 weeks starting after transplantation (Fig. 1).
Figure 1.
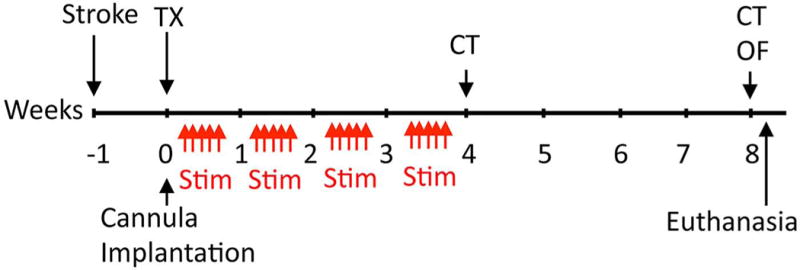
Experimental paradigm. Abbreviations: TX: transplantation of NSCs; Stim: optogenetic stimulation delivered at 10 Hz, 12 mW, 20-ms pulses, the animal received continuous stimulation three times for 30 s, with a 1-min rest period in between. Stimulation was administered daily 5 days a week for 4 weeks starting after transplantation; CT: cylinder test; OF: open field test.
Electrophysiological Recording
In Vitro Recording
NSCs were cultured on a glass coverslip and whole-cell voltage-clamp and current-clamp recordings were obtained from visually identified YFP+ cells. In both current- and voltage-clamp recordings, the light power density used was 5 mW/mm2, and NSCs expressing lenti-ChR2 or lenti-YFP were held at −65 mV during optical stimulation (475 nm).
In Vivo Recording
Simultaneous in vivo optical stimulation and electrophysiological recording from the NSC grafts was performed through the fiber-guiding cannula in an intact rat using an extracellular tungsten electrode (1 MOhm, 125 μm; A-M Systems, Carlsborg, WA, USA) attached to an optical fiber (200 μm outer diameter; ThorLabs). The recorded activity was filtered between 0.3 and 10 kHz bandpass, amplified (A-M Systems), digitized (Molecular Devices, Sunnyvale, CA, USA), and recorded with Clampex software (Molecular Devices). The optical stimulation parameters were 3 × 30 s, 10 Hz, 2.35 V (~5 mW) (OEM Laser Systems).
Behavioral Tests
The cylinder test assessed spontaneous forelimb use during lateral exploration movements (21), as previously described (6,8). Rats were placed in a transparent acrylic cylinder (20 cm diameter) for 5 min. The cylinder encouraged use of the forelimbs for vertical exploration. A mirror was placed behind the cylinder so that the forelimbs could be viewed at all times. Testing sessions were videotaped, and forelimb use was scored by a blinded operator. Animals were tested at baseline, after stroke, and 4 and 8 weeks after cell transplantation.
To measure the locomotor activity in response to optical stimulation, animals were placed in an open field box equipped with overhead cameras and a computer, allowing behavioral data to be recorded via the automated tracking system EthoVision. The test was performed 2 months after transplantation.
Immunocytochemistry
Cultured NSCs and brain sections were rinsed in phosphate-buffered saline (PBS; Life Technologies) for 3 × 5 min then incubated for 2 h (cultures) or overnight (brain sections) with the appropriate primary antibodies for multiple labeling. Secondary antibodies raised in the appropriate hosts and conjugated to FITC, RITC, AMCA, CY3, or CY5 chromogenes (Jackson ImmunoResearch, West Grove, PA, USA) were used. Cells and sections were counterstained with the nuclear marker 4¢,6-diamidine-2¢-phenylindole dihydrochloride (DAPI; from Roche Molecular Biochemicals, Indianapolis, IN, USA). Positive and negative controls were included in each run. Immunostained sections were coverslipped using fluorsave (Calbiochem, Temecula, CA, USA) as the mounting medium. The following antibodies were used: anti-human STEM121 (1:100; StemCells Inc., Hayward, CA, USA), anti-TuJ1 (monoclonal 1:100; Covance, San Leandro, CA, USA; polyclonal 1:200; Aves Labs, Tigard, OR, USA); anti-YFP (polyclonal 1:200; Aves Labs); anti-synaptophysin (Millipore); and anti-NCAM (monoclonal 1:250; Santa Cruz Biotechnology, Dallas, TX, USA). Fluorescence was detected, analyzed and photographed with a Zeiss LSM550 laser scanning confocal photomicroscope (Pleasanton, CA, USA).
Microarray Analysis
Total RNA was extracted from tissue dissected out from the ipsilateral striatum of vehicle and NSC-treated animals (n = 2 or 3) using the RNAeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The microarray analysis was performed by the Gene Expression Core facility at Stanford University using the Affymetrix (Santa Clara, CA, USA) Rat Gene 1.0 St array. Data were normalized using the RMA algorithm in Partek Genomics Suite v6.6 and analyzed using DAVID (david.abcc.ncifcrf.gov/) and Ingenuity Pathway Analysis software.
Statistical Analysis
Outcome measurements for each experiment were reported as mean ± SEM. All data were analyzed using SPSS 11 for Mac OS X (IBM, Armonk, NY, USA). Significance of intergroup differences was determined by applying the Student’s t-test where appropriate. One-way ANOVA analysis was used to compare group differences. Differences between the groups were determined using Bonferroni’s post hoc test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
Isolation of NSCs From hESCs and Transduction With ChR2
The process of hESC cultures and NSC isolation and perpetuation were previously described (8). Primary neurospheres derived from hESCs were isolated and replated to eliminate other nonneural cells. The selectively harvested secondary neurospheres were perpetuated by passaging the cultures weekly. This process enabled us to derive a homogenous neural cell population without the presence of Oct4+ or Nanog+ pluripotent precursors (8). The NSCs were transduced with a lentiviral vector expressing ChR2-YFP (NSC-ChR2) under the control of the EF1a promoter (32) (Fig. 2A). Control NSCs were transduced with lenti-YFP. Transduced NSCs were purified based on YFP expression by fluorescent activated flow cytometry cell sorting, as previously reported (7). With this procedure, virtually all of the NSCs and differentiated progeny expressed the transgene (Fig. 2B).
Figure 2.
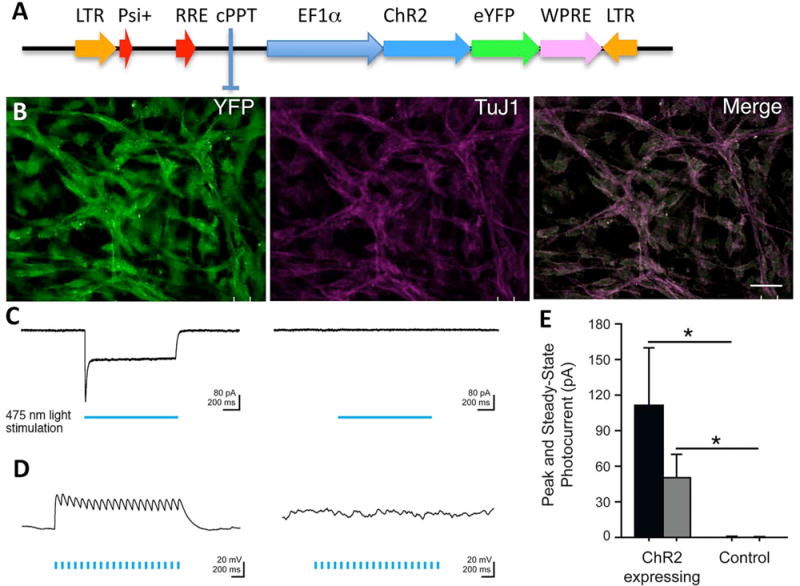
NSCs express functional ChR2 transgene. (A) Schematic representation of the lentiviral vector carrying ChR2 fused to a fluorescent protein YFP and driven by the EF1a promoter (31). (B) Photomicrograph showing transduced NSCs differentiated into TuJ1+ neurons expressing the YFP reporter gene. (C) Sample voltage-clamp recordings show that 475 nm light (indicated by blue bar) induces inward photocurrents in NSCs stably expressing ChR2 (left) but does not produce a light-evoked response in control NSCs (right). Light power density is at 5 mW/mm2; cells were held at −65 mV. (D) Sample current clamp recordings show that blue light depolarizes NSCs expressing ChR2 (left), but not control cells (right). Light power density is at 5 mW/mm2; cells were held at −65 mV. (E) Summary bar graph of peak and steady-state photocurrent size. Mean ± SEM is plotted (n = 7) for ChR2-expressing cells (n = 5) for control cells. Scale bars: 10 μm (A).
Electrophysiological Characterization of ChR2 Expression by the NSCs
To determine the specific high temporal resolution of the optogenetic stimulation of the NSCs, we plated NSC-ChR2 and control NSC-YFP on glass coverslips coated with poly-L-ornithine for a period of 7 days in vitro (8). Voltage-clamp recordings from the differentiated neurons showed that stimulation with blue light that specifically drives ChR2 induced inward photocurrents in NSCs that stably express ChR2 but did not produce light-evoked responses in control NSCs that stably express YFP (Fig. 2C). Current clamp recordings show that blue light depolarizes NSC-ChR2 but not control NSC-YFP (Fig. 2D).
Transplantation and Chronic Optogenetic Stimulation of NSCs in an Experimental Model of Ischemic Stroke
To determine whether the grafted NSC-ChR2 survived and expressed a functional transgene, we stimulated and recorded directly from the grafted NSCs in vivo in anesthetized animals, as previously described (Fig. 3A) (10). The in vivo recording demonstrated that the NSC-ChR2 responded to the stimulation, confirming the functional expression of the genetically encoded ChR2 (Fig. 3B). We then perfused the animals and looked at the survival of the graft and the expression of the transgenes. Detection of the grafted cells using the human-specific marker STEM-121 demonstrated a robust survival (Fig. 3C). The expression of ChR2 in the grafted NSCs was confirmed by the YFP immunocytochemistry showing a dispersion of the grafted NSCs in the parenchyma and expression of neural cell adhesion molecule (NCAM) (Fig. 3D) and TuJ1 (Fig. 3E) by the neuronal progeny with neuritic outgrowth. Synaptophysin expression was present in the grafted zone (Fig. 3F), suggesting the presence of synaptic contacts with host neurons, as we previously reported (7).
Figure 3.
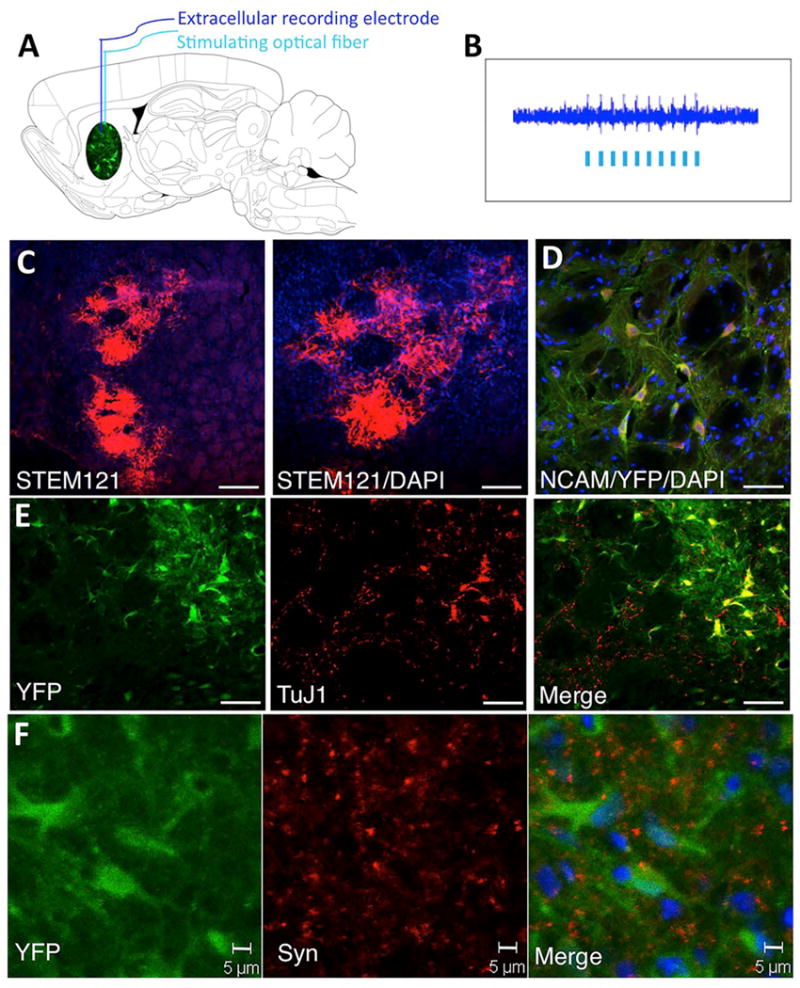
In vivo optical stimulation recording and characterization of NSC grafts. (A) Schematic drawing of a sagittal section through the rat brain illustrating the simultaneous in vivo optical stimulation and recording from the NSC grafts. Both the stimulating fiber optic (200 μm) and the extracellular tungsten recording electrode (1 MOhm, ~125 μm) were inserted through the guiding cannula used for chronic stimulation. (B) Electrophysiological recording of the extracellular activity driven by the optogenetic stimulation. (C) Low magnification microphotos taken from frontal sections through the graft in the striatum showing grafted NSCs expressing the human marker STEM121. (D) ChR2-expressing grafted cells identified by the expression of YFP (green) coexpress the neural marker NCAM. (E) Grafted cells expressing ChR2 (YFP, green) coexpress the neuronal marker TuJ1. (F) Grafted cells expressing ChR2 (YFP, green) appear decorated by synaptophysin+ in synaptic puncta (red). Scale bars: (C) 100 μm; (NCAM, TuJ1) 20 μm; (Syn) 5 μm.
Chronic Optical Stimulation and Functional Integration of the Optogenetically Engineered NSCs
To test the ability to drive in vivo the excitatory influence of the grafted NSCs and measure their functional integration, we used the MCAO model of stroke. One week after inducing the lesion, immunosuppressed animals were transplanted with NSC-ChR2 and control NSC-YFP cells into the ischemic boundary zone in the striatum, as previously described (6–8). Six animal groups were established: vehicle, vehicle with stimulation, and NSC-ChR2 and NSC-YFP transplants with or without chronic stimulation. Chronic optogenetic stimulation of the grafted cells was carried out daily after transplantation for 1 month according to the stimulation paradigm described in the Materials and Methods section (Fig. 1). Animals in all groups were tested at 1 and 2 months post-transplantation for their motor performance by measuring spontaneous forelimb use during lateral exploratory movements. The analysis demonstrated that light stimulation of animals grafted with the NSC-ChR2 enhanced sensorimotor performance and increased forelimb use on the lesioned contralateral side in comparison to NSC-YFP and vehicle animals (Fig. 4A). Transplantation of either NSC-ChR2 or NSC-YFP without stimulation showed improvement in sensorimotor performance and did not reach significance in comparison to the vehicle-treated group. In addition, animals that received the NSC-ChR2s significantly increased their motor activity and total distance during stimulation at 2 months after transplantation, as monitored by the EthoVision automated tracking system in the open field test (Fig. 4B).
Figure 4.
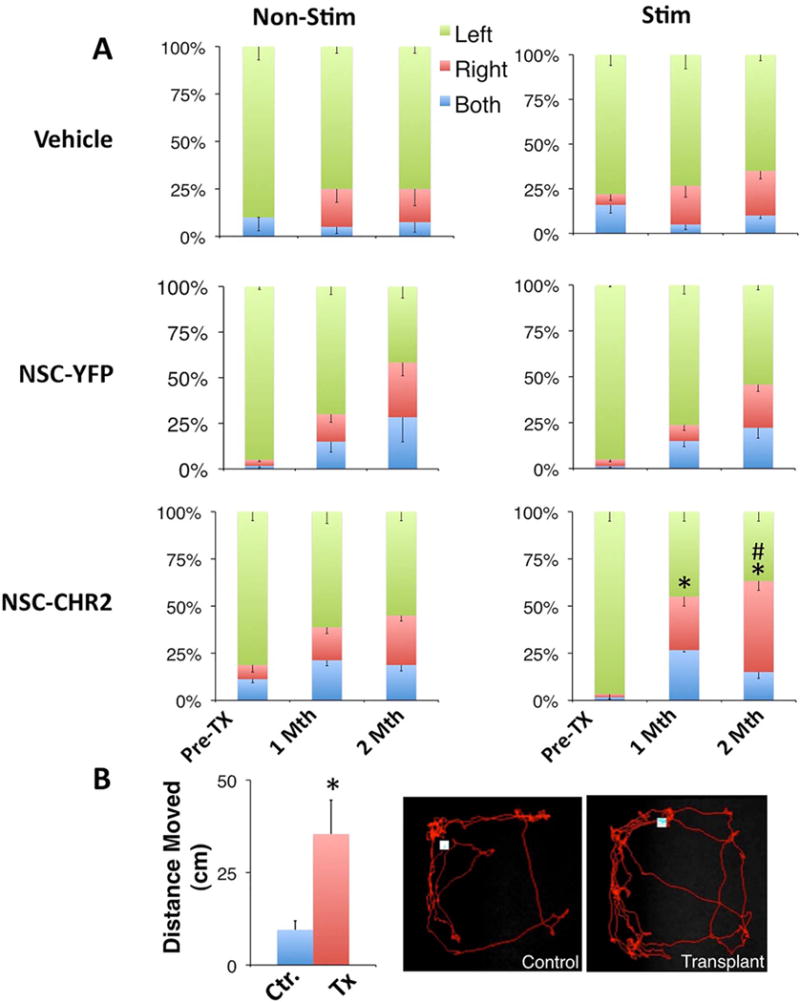
Effects of optogenetic stimulation of NSC grafts on sensorimotor function. Three animal groups (n = 16) were used: vehicle, NSCs transduced with lenti-YFP, and NSCs transduced with lenti-ChR2. Each group was split into two subgroups: stimulated and nonstimulated. We used the cylinder test to measure forelimb use during spontaneous lateral exploration (A). Animals were also subjected to locomotor activity testing in the open field test during stimulation (B). Scale bars represent percentages ± SEM of steps taken by the ipsilateral (left), contralateral (right), and both forelimbs simultaneously. *p < 0.05 versus pretransplant groups; #p < 0.05 versus nonstimulated groups.
Microarray Analysis of the Grafted Striatum
To gain insight into the potential mechanism of action that mediates the improvement in the sensorimotor function, we dissected out the grafted striatal zone in the cell and vehicle-injected animals and analyzed the gene expression profiles. Stringent analysis with strict cutoff (low false discovery rate, FDR = 0.1) (p ≤ 0.05) after normalization (RMA), and ANOVA revealed upregulation of transcripts involved in neurotransmission, sodium and potassium channel functions [solute carrier family 17 (Slc17a7); potassium inwardly rectifying channel, subfamily J (KCNJ13); solute carrier family 30 (zinc transporter); member 3 (SLC30A3)], in neuronal differentiation, regeneration, axonal guidance, and synaptic plasticity [T-box brain 1 (TrB1), neurogenic differentiation 6 (NeuroD6), neurogenic differentiation 1 (NeuroD1), neurogenic differentiation 2 (NeuroD2), R-spondin 3 (RSPO3), ring finger protein 39 (RNF39), and nephroblastoma overexpressed gene (NOV)] (Fig. 5). Interestingly, all significantly downregulated genes were involved in the inflammatory response [chemokine (C-X-C motif) ligand 10 (CXCL10)], interferon-g inducible protein 47 (Ifi47), colony stimulating factor 2 receptor-b (CSF2RB), oxidized low-density lipoprotein (lectin-like) receptor 1 (OLR1), RT1 class II, locus Da (RT1-Da), Cd68 molecule (CD68), serine (or cysteine) peptidase inhibitor, clade A (Serpina3n), C-type lectin domain family 7 member A (CLEC7A), chemokine (C-C motif) ligand 2 (CCL2), lipocalin 2 (LCN2)] (Fig. 4).
Figure 5.
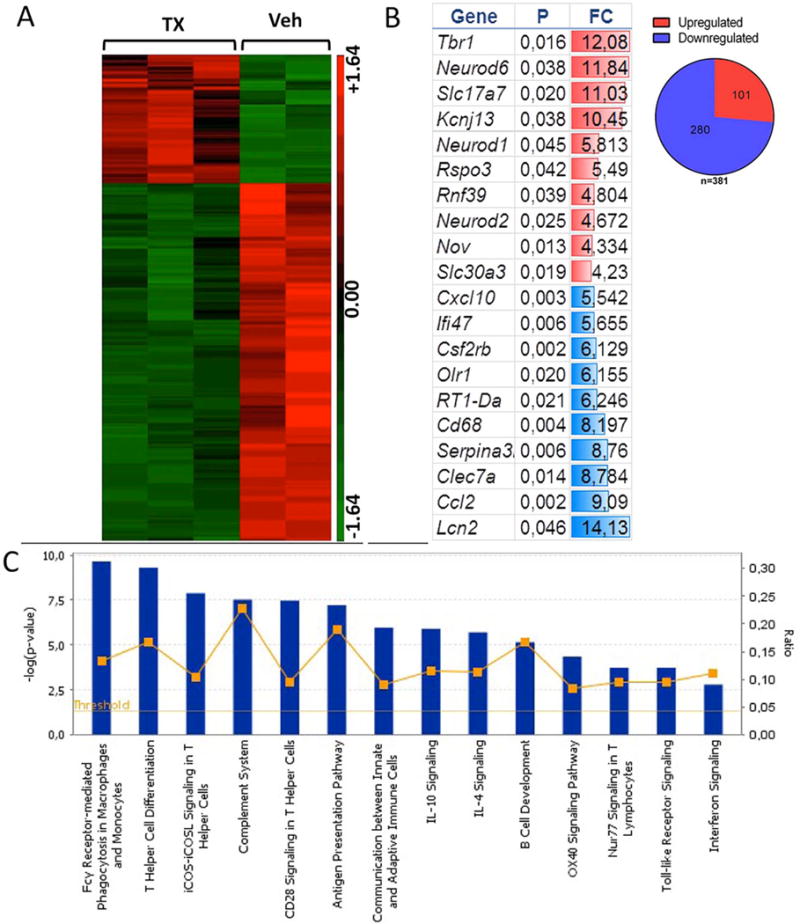
Gene expression profile analysis of the effect of graft optogenetic stimulation. Striatal tissue was freshly dissected from the graft (n = 3) or vehicle (n = 2) stimulated region and processed for RNA isolation and microarray analysis using the rat Affymetrix gene expression 1.0 ST Array. (A) Heat map of significant genes using ANOVA: red = relative increase in mRNA; green = relative decrease in mRNA expression. (B) Gene ontology analysis shows highest up- and downregulated genes, (p = p value, FC = fold change). The data show upregulation of transcripts involved in neurotransmission, neuronal differentiation, regeneration, axonal guidance, and synaptic plasticity (see Results). (C) Canonical pathways enriched among the significant genes. The blue bars correspond to the log p value, while the yellow line corresponds to the ratio between the number of focus genes in a pathway divided by the total number of genes that make up that pathway. The significance is based on Fischer’s exact test in ingenuity pathway analysis. The data show downregulation of genes involved in the inflammatory response (see Results).
DISCUSSION
Grafting optogenetically engineered NSCs provides a targeted approach to probe the role of neural circuit repair in NSC-based cell therapy in experimental stroke models. Early transplantation studies reported synaptic contact between grafted neural cells and the host (4,9,13,17,24,26), and a large body of work has followed that provides evidence of cell replacement and functional integration of NSC grafts into host neural networks in various animal models of neurological disorders (1–3,6,11,14–16,18,20,22,23,25,27–30). Using slice preparations, previous studies have demonstrated that optogenetically engineered grafted NSCs participate in host neuronal network activity (18,28,29). However, the improvement of sensorimotor function by direct stimulation of grafted NSCs, to our knowledge, has not been previously reported. The pertinent finding in our study is that optogenetic manipulation of grafted NSCs positively influenced the endogenous motor network function compromised by stroke. As only ChR2-NSCs respond to blue light stimulation, our findings suggest that improved motor function is in response to driving the excitatory output of grafted NSCs. Transplantation of NSCs (either ChR2 or control YPF) without stimulation showed enhanced recovery, especially in the ability of animals to use both paws to explore the cylinder test, and it did not reach significance. The NSC-grafted animals show gradual functional recovery, which in the long term may enable the animal to use its contralateral paw independently from the ipsilateral one.
Differential gene expression analysis suggested that optogenetic stimulation of NSC grafts upregulated transcripts involved in membrane-associated ion channel functions, neuronal differentiation, axonal sprouting, and synaptic plasticity. Interestingly, all downregulated transcripts were involved in the inflammatory response. These data suggest graft neuronal activity driven by optogenetic stimulation may also reduce the inflammatory response following the stroke lesion potentially through an immunomodulatory effect, which may create a more favorable microenvironment for graft integration in neural network and tissue repair. Additional studies will shed light on how the NSC grafts modulate connectivity and the inflammatory response.
In summary, our finding suggests that optogenetic stimulation of grafted NSCs may ameliorate stroke-induced neuronal dysfunction and that the beneficial effects are mediated through multiple modalities including cell replacement, anti-inflammatory, and neurotrophic actions.
Acknowledgments
The authors thank Beth Hoyte for preparation of the figures. This work was supported in part by Stanford University School of medicine BIO-X IIP program and CIRM (California Institute for Regenerative Medicine) DR1-01480, NIH NINDS 2R01NS058784; and by Russell and Elizabeth Siegelman, and Bernard and Ronni Lacroute.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JL, Alvarez-Buylla A, Baraban SC. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26(28):7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31(12):4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129(Pt 12):3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- 4.Clarke DJ, Brundin P, Strecker RE, Nilsson OG, Bjorklund A, Lindvall O. Human fetal dopamine neurons grafted in a rat model of Parkinson’s disease: Ultrastructural evidence for synapse formation using tyrosine hydroxylase immunocytochemistry. Exp Brain Res. 1988;73(1):115–126. doi: 10.1007/BF00279666. [DOI] [PubMed] [Google Scholar]
- 5.Daadi MM, Bliss TM, Lee SH, Palmer TD, Steinberg GK. Transplantation of the medial ganglionic eminence-derived neuronal precursors into ischemic stroke-lesioned rats improves motor behavioral deficits. Exp Neurol. 2006;198(2):565–565. [Google Scholar]
- 6.Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL, Schaar B, Malenka RC, Palmer TD, Steinberg GK. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 2009;18(7):815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- 7.Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17(7):1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: Functional engraftment in experimental stroke model. PLoS One. 2008;3(2):e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freund TF, Bolam JP, Bjorklund A, Stenevi U, Dunnett SB, Powell JF, Smith AD. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: A tyrosine hydroxylase immunocytochemical study. J Neurosci. 1985;5(3):603–616. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27(52):14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, McKercher SR, Cui J, Nie Z, Soussou W, Roberts AJ, Sallmen T, Lipton JH, Talantova M, Okamoto S, Lipton SA. Myocyte enhancer factor 2C as a neurogenic and antiapoptotic transcription factor in murine embryonic stem cells. J Neurosci. 2008;28(26):6557–6568. doi: 10.1523/JNEUROSCI.0134-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahalik TJ, Finger TE, Stromberg I, Olson L. Substantia nigra transplants into denervated striatum of the rat: Ultrastructure of graft and host interconnections. J Comp Neurol. 1985;240(1):60–70. doi: 10.1002/cne.902400105. [DOI] [PubMed] [Google Scholar]
- 14.Maisano X, Litvina E, Tagliatela S, Aaron GB, Grabel LB, Naegele JR. Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci. 2012;32(1):46–61. doi: 10.1523/JNEUROSCI.2683-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6(3):238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzocchi-Jones D, Dobrossy M, Dunnett SB. Embryonic striatal grafts restore bi-directional synaptic plasticity in a rodent model of Huntington’s disease. Eur J Neurosci. 2009;30(11):2134–2142. doi: 10.1111/j.1460-9568.2009.07006.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishino H, Ono T, Takahashi J, Kimura M, Shiosaka S, Yamasaki H, Hatanaka H, Tohyama M. The formation of new neuronal circuit between transplanted nigral dopamine neurons and non-immunoreactive axon terminals in the host rat caudate nucleus. Neurosci Lett. 1986;64(1):13–16. doi: 10.1016/0304-3940(86)90655-5. [DOI] [PubMed] [Google Scholar]
- 18.Pina-Crespo JC, Talantova M, Cho EG, Soussou W, Dolatabadi N, Ryan SD, Ambasudhan R, McKercher S, Deisseroth K, Lipton SA. High-frequency hippocampal oscillations activated by optogenetic stimulation of transplanted human ESC-derived neurons. J Neurosci. 2012;32(45):15837–15842. doi: 10.1523/JNEUROSCI.3735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullterton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matcher DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Submcommittee Executive summary: heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 20.Santos-Torres J, Heredia M, Riolobos AS, Jimenez-Diaz L, Gomez-Bautista V, de la Fuente A, Criado JM, Navarro-Lopez J, Yajeya J. Electrophysiological and synaptic characterization of transplanted neurons in adult rat motor cortex. J Neurotrauma. 2009;26(9):1593–1607. doi: 10.1089/neu.2008.0702. [DOI] [PubMed] [Google Scholar]
- 21.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, Parkinsonism and spinal cord injury. Neuropharmacology. 2000;39(5):777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen AT, Rogelius N, Lundberg C, Kokaia M. Activity-dependent long-term plasticity of afferent synapses on grafted stem/progenitor cell-derived neurons. Exp Neurol. 2011;229(2):274–281. doi: 10.1016/j.expneurol.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen AT, Thompson L, Kirik D, Bjorklund A, Lindvall O, Kokaia M. Functional properties and synaptic integration of genetically labelled dopaminergic neurons in intrastriatal grafts. Eur J Neurosci. 2005;21(10):2793–2799. doi: 10.1111/j.1460-9568.2005.04116.x. [DOI] [PubMed] [Google Scholar]
- 24.Sotelo C, Alvarado-Mallart RM. Embryonic and adult neurons interact to allow Purkinje cell replacement in mutant cerebellum. Nature. 1987;327(6121):421–423. doi: 10.1038/327421a0. [DOI] [PubMed] [Google Scholar]
- 25.Steinbeck JA, Koch P, Derouiche A, Brustle O. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell Mol Life Sci. 2012;69(3):461–470. doi: 10.1007/s00018-011-0759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonder N, Sorensen T, Zimmer J, Jorgensen MB, Johansen FF, Diemer NH. Neural grafting to ischemic lesions of the adult rat hippocampus. Exp Brain Res. 1989;74(3):512–526. doi: 10.1007/BF00247353. [DOI] [PubMed] [Google Scholar]
- 27.Tonnesen J, Kokaia M. Electrophysiological investigations of synaptic connectivity between host and graft neurons. Prog Brain Res. 2012;200:97–112. doi: 10.1016/B978-0-444-59575-1.00005-3. [DOI] [PubMed] [Google Scholar]
- 28.Tonnesen J, Parish CL, Sorensen AT, Andersson A, Lundberg C, Deisseroth K, Arenas E, Lindvall O, Kokaia M. Functional integration of grafted neural stem cell-derived dopaminergic neurons monitored by optogenetics in an in vitro Parkinson model. PLoS One. 2011;6(3):e17560. doi: 10.1371/journal.pone.0017560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weick JP, Liu Y, Zhang SC. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proc Natl Acad Sci USA. 2011;108(50):20189–20194. doi: 10.1073/pnas.1108487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, Beck H, Brustle O. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004;24(22):5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3(10):785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]


