Abstract
Varenicline (VAR) is approved to aid in smoking cessation that has been shown to be effective for reducing alcohol consumption in heavy drinkers. Little is known, however, about treatment moderators that may influence efficacy. The current study reanalyzed data from a human laboratory study (Verplaetse et al., 2016) to determine whether VAR was more effective at reducing alcohol use among drinkers reporting symptoms of depression. Participants were 60 adults meeting DSM-IV criteria for alcohol use disorders (n = 60) who were randomly assigned to receive VAR (1 mg/day, 2 mg/day) or placebo. Following 7 days of medication pretreatment, participants attended a laboratory testing session. They provided self-reported ratings of alcohol craving and performed an ad libitum alcohol consumption task after receiving a priming dose of alcohol (target blood alcohol concentration = 0.030 g/dL). Higher blood VAR plasma levels were associated with less alcohol craving and less drinking among participants with more depressive symptoms. Among participants with fewer depressive symptoms, VAR was associated with more drinking during the ad libitum drinking task. These findings show that depression symptoms may be a moderator of VAR efficacy in alcohol users and provides evidence for the role of nAChRs in depression and alcohol use.
Keywords: varenicline, depression, treatment moderators, laboratory, alcohol use disorder
Introduction
Varenicline (VAR) (Chantix; Pfizer, New York) is a partial agonist at α4β2* (where * indicates potential additional subunits) nicotinic acetylcholine receptors (nAChR) that was developed as a smoking cessation aid (Gonzales et al., 2006). Our group (Verplaetse et al., 2016) recently completed a human laboratory study testing the efficacy of VAR in a group of adult drinkers meeting criteria for alcohol use disorders. We found evidence that VAR effectively reduced drinking risk in this cohort, as indicated by reductions in alcohol craving and ad libitum alcohol consumption during a laboratory drinking task. These findings are consistent with results of both preclinical studies and clinical trials conducted by our and other research groups showing VAR can reduce alcohol consumption among problem drinkers (Steensland et al., 2007; Litten et al., 2013; McKee et al., 2009; Fucito et al., 2011).
In understanding pathways by which VAR could reduce alcohol use, it is important to consider the role of nAChR in alcohol use behavior. Preclinical studies have shown that alcohol-induced mesolimbic dopamine release is mediated in part by the direct effects of alcohol on nAChR (Soderpalm et al., 2000). As dopamine release in this region is critical in the reinforcing effects of alcohol, the partial agonist effects of VAR may block or limit the reinforcing effect of alcohol. Indeed, other studies have shown that mecamylamine, an nAChR antagonist, can reduce rates of alcohol consumption in rats (Lě et al., 2000) and reduce pleasurable subjective alcohol effects in humans (Blomqvist et al., 2002). As a partial agonist, VAR may reduce craving via its agonist properties as well as reduce acute alcohol reinforcement by blocking action at key nAChR subtypes.
Identifying patient characteristics that predict treatment efficacy can improve treatment outcomes (Kraemer et al., 2006). Falk and colleagues (2015) recently reanalyzed data from a Phase III clinical trial that used VAR to treat alcohol use disorder and identified several treatment moderators (i.e., treatment goal, years drinking regularly, age, and reduction in cigarettes smoked). The potential moderators in this study related primarily to patient’s demographic and substance use characteristics. Other potential treatment moderators include comorbid psychopathology, particularly symptom clusters that share pathophysiological characteristics with alcohol use disorder (Hutchison, 2010). One condition that often coexists with alcohol use disorder and also is associated with dysregulation of the nAChR system is depression (Saricicek et al., 2012; Mineur et al., 2013). Drugs targeting nAChR receptors can reduce depressive symptoms in nonhuman animals (Mineur et al., 2007). Evidence for this relationship also comes from studies on the effects of nicotine on mood. Smokers show higher rates of depression than the general population (Breslau et al., 1993) and tobacco withdrawal can precipitate depressive symptomatology (Cook et al., 2015), potentially due to the sudden removal of nicotine from the adapted cholinergic system.
The current study examined depressive symptoms as a treatment moderator in a human laboratory study examining the effect of VAR in reducing alcohol consumption among heavy drinkers. There is significant heterogeneity among individuals with alcohol use disorder, and it is important to identify patient characteristics that predict responses to different pharmacotherapies. Considering the overlap in the neurobiological substrates of alcohol use disorder and depression, it is possible that VAR may be particularly effective at reducing drinking among individuals who also report symptoms of depression. This is also an important question clinically because alcohol use disorder frequently co-occurs with depression (Kessler et al., 1997), and depression can worsen the prognosis of the alcohol use disorders (Sullivan et al., 2005).
In this study, we tested whether VAR was efficacious for reducing alcohol self-administration and group of adults with alcohol use disorder. This study is a reanalysis of data from a human laboratory experiment testing the effects of 1 and 2 mg/day VAR on well-validated indicators of alcohol use risk, including alcohol self-administration following a priming dose of alcohol and self-reported alcohol craving (Verplaetse et al., 2016). These outcome measures were chosen for their previously established sensitivity to the effects of VAR (Childs et al., 2012; McKee et al., 2009). The primary purpose of this reanalysis was to determine whether participants’ self-reported levels of depression symptoms acted as a moderator of treatment efficacy.
The primary reanalyses will examine the effects of blood plasma levels of VAR (VAR plasma) on alcohol self-administration and craving was moderated by participants’ levels of depression. In our prior publication from this human laboratory study (Verplaetse et al., 2016), we found significant variability in VAR plasma levels, even among participants who received identical doses of the drug. Moreover, VAR plasma level was a stronger predictor of craving and ad libitum drinking than was oral VAR dose, suggesting that VAR plasma may provide a better estimate of treatment efficacy than would analyzing outcomes according to dosing condition. The current reanalysis will build on our previous findings (Verplaetse et al., 2016) demonstrating that VAR plasma levels show a negative relationship with craving and ad libitum drinking, such that individuals with higher levels of VAR plasma report less alcohol craving and consume less alcohol. We predicted that this effect would be moderated by participants’ self-reported depressive symptoms, such that participants reporting more depressive symptoms would show a greater reduction in drinking when VAR plasma levels were high.
Method
Participants
Participants were eligible if they were ≥ 21 years of age and were able to read and speak English. All participants met DSM-IV criteria for past 6-month alcohol abuse or alcohol dependence. They also met criteria for heavy drinking (binge drinking at least once per week, which was defined as ≥ +4/+5 per episode for females/males, respectively). Exclusion criteria included illicit drug use (except for occasional cannabis use), past 30-day use of psychoactive drugs, treatment-seeking for alcohol or tobacco use, current suicidal or homicidal ideation, pregnancy or nursing, or medical conditions contraindicating alcohol use (e.g., liver enzymes ≥ 3× normal) or VAR administration (e.g., known allergy to VAR). Volunteers diagnosed with serious axis I disorders other than alcohol or tobacco dependence (e.g., psychotic spectrum disorders, bipolar disorder) were not invited to participate. Those reporting depression were allowed to participate unless they were currently prescribed medication to manage their depression or were found to be high risk for suicide. Those who were deemed likely to exhibit clinically significant alcohol withdrawal, as evidenced by elevated scores on the CIWA-Ar (Sullivan et al., 1989) were not invited to participate.
Design
The study was a double-blind, placebo-controlled, parallel-group design. Additional methodological details concerning study design, procedures, and materials are reported in a previous publication from this laboratory study (Verplaetse et al., 2016). Randomization of participants to VAR (1 or 2 mg/day) or a matching placebo (0 mg/day) groups were stratified by sex and smoking status. This study is registered with ClinicalTrials.gov, NCT00580645 (Study 1c). The Human Investigation Committee of Yale University approved this study and written informed consent was obtained.
Procedures
Eligibility screening
The human investigation committee of Yale University approved this study and written informed consent was obtained. Participants underwent an extensive eligibility screening, including physical examination, electrocardiogram, urine toxicology, pregnancy test, and basic blood chemistries.
Medication
VAR and matching placebo were provided by Pfizer (NY), and were overencapsulated with riboflavin added to monitor compliance. Varenicline was titrated to steady-state levels over 7 days. Medication compliance was monitored with pill counts and riboflavin marker on days 5 and 8 (Del Boca et al., 1996). Plasma levels were assessed at the start of the laboratory session on day 8.
Priming dose
The alcohol priming drinks were administered from 3:00 to 3:05 PM and consisted of 1 part 80-proof liquor of the participants’ choosing and 3 parts mixer chosen from a selection of equally caloric, non-caffeinated, noncarbonated beverages. Dosing was designed to produce a blood alcohol concentration (BAC) of 0.030 g/dL based on Watson’s (1989) formula that takes into account participants’ body weight, height, sex, and age to estimate total body water. The average dose of absolute alcohol administered was 21.29 mL (SD = 4.27 mL) and the average total beverage volume was 212.90 mL (SD = 42.67 mL).
Alcohol self-administration
Participants completed two 1-hour ad libitum drinking periods during which they were permitted to drink up to 4 alcoholic beverages (8 drinks total over the entire 2-hour self-administration session; each calculated to raise BAC 0.015 g/dL). On average, each drink contained 10.65 mL (SD = 2.14 mL) absolute alcohol. The average total volume of each beverage was 106.40 mL (SD = 22.28). They were given an initial drinking tab ($24) and told that they could purchase up to 4 drinks each session. Participants kept $3 for each drink they did not consume which incentivized their abstinence during this task and modeled a situation in which reducing alcohol use was a desirable outcome for the drinker. They were given free access to their drinks and their drinking tab was totaled after the end of the ad libitum drinking periods.
Laboratory session
On day 8, each participant completed a 14-hour laboratory session conducted at the Yale Center for Clinical Investigation, New Haven, Connecticut. The laboratory procedures were similar to those used in our previous alcohol self-administration studies (McKee et al., 2009), which conform to guidelines for alcohol administration (National Advisory Council on Alcohol Abuse and Alcoholism, 2005).
The timing of the laboratory session was as follows: Participants arrived at the laboratory at 8:00 AM and their final dose of medication was provided at 9:00 AM. Alcohol craving was assessed three times throughout this period, first when they arrived at the laboratory and again each hour until receiving the priming beverage at 3:00 PM. Alcohol craving was measured in 10 minute intervals after participants finished the priming beverage. Participants started the first and second hour of alcohol self-administration 50 and 120 minutes after consuming the priming drink, respectively.
Materials and Measurement
Alcohol use
Participants’ drinking habits were assessed using the Timeline Follow-back (TLFB) procedure (Sobell and Sobell, 1992). This protocol uses a structured calendar anchored with holidays and other notable events to assist participants in recording their drinking behavior. The TLFB yielded two measures, including (1) average number of drinks per drinking session, and (2) proportion of heavy drinking days (i.e., proportion of drinking days consuming 4/5 [female/male] or more drinks). Participants also completed the alcohol use disorders identification test (AUDIT) as a measure of alcohol use problem severity (Saunders et al., 1993). These measures were used to assess for baseline differences between groups in drinking habits.
Alcohol craving
Alcohol craving was measured using the Alcohol Urge Questionnaire (AUQ; Bohn et al., 1995). This 12-item questionnaire assesses alcohol craving using a 100-point visual analogue scale. The AUQ was administered three times before and four times after the priming beverage was administered. Tonic alcohol craving was measured as the average score of AUQs administered before the priming beverage. Craving after alcohol prime was measured as the average score of AUQs administered between 10 and 180 minutes after the priming beverage.
Depressive symptoms
The Center for Epidemiologic Studies Depression Scale (CESD) is a 20-item self-report scale that measures depressive symptoms (Radloff, 1977). Responses are provided on a four-point scale based on the frequency of occurrence of the symptom. The CESD showed sufficient internal consistency in the current sample (α = .83).
Varenicline plasma levels
Human plasma containing VAR and the internal standard, Varenicline-15N2D2 was extracted using a solid-phase extraction method for the determination of VAR in human K2-EDTA plasma by Worldwide Clinical Trials, Austin, TX. The range of quantitation was 0.0500 to 10.0 ng/mL based on the analysis of 0.500 mL of plasma.
Data Analyses
The outcome variables of interest were VAR plasma levels, CESD scores, ad libitum alcohol consumption, alcohol craving before drinking (i.e., tonic craving), and craving after alcohol prime.
Primary analyses were designed to test whether blood plasma levels of VAR were more or less effective at reducing alcohol self-administration and craving at different levels of depression. All variables for these analyses were z transformed to minimize unnecessary multicollinearity. Effects of VAR on alcohol use variables were analyzed using two step hierarchical regression analyses with VAR plasma levels and CESD scores entered in the first step and a VAR plasma level X CESD score interaction term entered in the second step. Significant interaction terms were probed using the PROCESS macro for SPSS 22 (Hayes, 2013). Simple slopes were calculated based on standard deviations within our sample. These simple slopes determined whether VAR plasma was associated with the primary outcome variables at “low” depression (CESD = 2.04 [M – 1 SD]), “moderate” depression (CESD score = 8.25 [M]), and “high” depression (CESD = 14.45 [M + 1 SD]). These scores are consistent with conventional strategies for probing at the mean value of the moderator and 1 SD above and below the mean (Preacher et al., 2006). Low and moderate depression were well below the recommended clinical cutoff score of the CESD (i.e., 16), and high depression approached this cutoff score, suggesting the presence of clinically significant symptoms. The majority of the sample scored below the recommended cut score indicating a likely depressive disorder (n = 53) although some scored above (n = 7). These regression analyses were conducted with ad libitum alcohol consumption, tonic craving, and craving after alcohol prime as dependent variables.
A supplemental set of analyses were conducted to examine the effects of VAR on primary outcome variables according to oral dosage. VAR plasma is not commonly measured, so analyses by oral dose were intended to facilitate integration our findings with the broader literature and facilitate replication. For these analyses, high and low depressive symptom groups were formed based on whether participants fell above or below the cutoff score of 9. This cutoff score was recommended by Weissman et al. (1977) to distinguish between people with no depressive symptoms and those reporting at least some clinically significant depressive symptoms. These analyses were intended to inform future research regarding dose selection by separating groups by oral dose of VAR (placebo, 1 mg/day, 2 mg/day) and using a clinical cutoff score on the CESD. Participants with scores of 9 or higher were included in the depression symptoms group and those with scores lower than 9 were included in the no depression group. These analyses were 3 (dose: placebo, 1 mg/day VAR, 2 mg/day VAR) X 2 (no depression versus depression symptoms) between-subject ANOVAs. A priori t-tests were used to probe the effects of medication within each group and a second set of these tests were used to examine differences between depression groups within each medication condition.
Finally, supplemental analysis examined whether smoking status had a significant effect on our pattern of findings. As results demonstrated that smoking status did not moderate findings, it was not included in the final models presented below.
Results
Demographics
Demographic information and baseline alcohol use characteristics are reported in Table 1. As seen in this table, the groups did not differ substantially in terms of demographic characteristics. A 2 (depression group) × 3 (VAR dose) ANOVA tested for group differences in demographic and alcohol use variables. There were no significant main effects or interactions for these demographic variables (ps ≥ 0.338), nor were there significant effects related to AUDIT scores or self-reported drinking on the TLFB prior to randomization (ps ≥ 0.091).
Table 1.
Baseline Characteristics by Group
| No Depression | Depressive Symptoms | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo | 1 mg VAR | 2 mg VAR | Placebo | 1 mg VAR | 2 mg VAR | |
|
|
|
|||||
| n = 12 | n = 13 | n = 13 | n = 8 | n = 7 | n = 7 | |
| Age (years) | 34.25 (8.68) | 33.4 (8.51) | 34.2 (11.60) | 34.13 (11.29) | 31.71 (7.89) | 39.43 (11.39) |
| Sex (% male) | 8 (67) | 7 (54) | 10 (77) | 6 (75) | 5 (71) | 5 (71) |
| Race | ||||||
| White | 6 (50) | 7 (54) | 10 (77) | 5 (63) | 3 (43) | 5 (71) |
| Other | 6 (50) | 6 (46) | 3 (23) | 3 (38) | 4 (57) | 2 (29) |
| Education | ||||||
| ≤ high school | 5 (42) | 6 (46) | 2 (15) | 5 (63) | 2 (29 | 1 (14) |
| ≥ college | 7 (58) | 7 (54) | 11 (85) | 3 (38) | 5 (71) | 6 (86) |
| Marital status | ||||||
| Not married | 10 (83) | 10 (77) | 12 (92) | 5 (63) | 6 (86) | 7 (100) |
| Married | 2 (17) | 3 (23) | 1 (8) | 3 (38) | 1 (14) | 0 (0) |
| Smoking Status | ||||||
| Smoker (%) | 7 (58) | 8 (62) | 10 (77) | 5 (63) | 5 (71) | 2 (29) |
| Non-smoker (%) | 5 (42) | 5 (39) | 3 (23) | 3 (38) | 2 (29) | 5 (71) |
| CESD | 5.66 (1.23) | 7.20 (5.78) | 7.95 (6.35) | 15.50 (6.84) | 13.86 (4.49) | 14.71 (5.94) |
| Alcohol use | ||||||
| Weekly (Q) | 27.53 (13.73) | 31.26 (11.46) | 27.36 (16.40) | 41.15 (33.85) | 29.67 (15.72) | 28.85 (23.50) |
| Weekly (F) | 4.36 (1.53) | 4.36 (1.53) | 4.39 (1.44) | 5.34 (1.50) | 4.50 (1.49) | 4.50 (1.74) |
| Weekly Binge Day | 3.54 (2.04) | 3.50 (1.91) | 2.79 (1.59) | 3.75 (2.25) | 2.89 (1.53) | 3.07 (2.23) |
| AUDIT | 11.45 (5.88) | 13.85 (4.72) | 10.15 (3.24) | 17.75 (6.58) | 12.71 (5.82) | 11.86 (5.05) |
Note. Parenthetical values are standard deviations for ordinal variables and percentage of total sample for categorical variables. There were no significant differences between groups based on 2 (depressive symptom group) × 3 (VAR dose) ANOVA or chi-square when appropriate. CESD is the Center for Epidemiologic Studies Depression Scale. Weekly (Q) is average number of drinks consumed each week. Weekly (F) is average number of drinking sessions each week. Weekly Binge Days is the average number of days each week where women/men report drinking 4/5 or more drinks, respectively.
Adverse Events
Adverse event data were reported in the previous publication from this the parent project (Verplaetse et al., 2016). There were no reported instances of suicidal thoughts or attempts in any group.
VAR Trough Plasma and Medication Compliance
All participants were at least 80% compliant with pill counts and urine florescence. Mean trough plasma levels were as follows: 1 mg/day VAR = 2.25 ng/mL (SD = 1.23 ng/mL); and 2 mg/day VAR = 4.60 ng/mL (SD = 1.86 ng/mL).
Breath Alcohol Concentrations
Peak BACs for the priming dose were achieved 10 minutes after the priming beverage (M = 0.028 g/dL, SD = 0.014). Peak BAC following the priming dose was not correlated with CESD scores, r (56) < 0.01, p = 0.974, or VAR plasma levels, r (56) = −0.13, p = 0.337. Peak BACs during the ad libitum phase were achieved 100 minutes following the first ad libitum drinking session (M = 0.060 g/dL, SD = 0.081) and was not associated with CESD scores, r (56) = 0.15, p = 0.256, or VAR plasma levels, r (56) = 0.095, p = 0.480.
Ad Libitum Drinking
Hierarchical regression analyses examining the effect of VAR plasma on ad libitum drinking are presented in Table 2. In the first step, neither VAR plasma nor CESD scores showed a significant association with number of drinks consumed. In the second step, however, the VAR plasma X depression score interaction term was significant. Simple slope analyses found that when depression levels were moderate, there was no association between VAR plasma and number of drinks consumed. At high levels of depression, there was a significant negative relation between VAR plasma and number of drinks consumed, such that participants with higher concentrations of VAR plasma consumed fewer alcohol beverages. For low depression, however, there was a significant positive association between VAR plasma and number of drinks consumed, such that participants with higher levels of VAR plasma consumed more drinks than those with lower plasma levels.
Table 2.
Hierarchical regression analyses of effects of VAR plasma and CESD on ad libitum alcohol consumption
| Dependent Variable | Simple Slopes | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| B | ΔF | ΔR2 | Low Depression | Moderate Depression | High Depression | ||
| Drinks Consumed | |||||||
| Step 1 | 0.68 | .02 | |||||
| CESD | 0.09 | ||||||
| VAR Plasma | −0.12 | ||||||
| Step 2 | 11.86** | .17 | |||||
| CESD X VAR Plasma | −0.92* | 2.13 (0.42)* | −0.86 (−0.10) | −3.31 (−0.63) ** | |||
Simple slopes report t values (parenthetical values are B weights).
p < 0.05,
p < 0.01
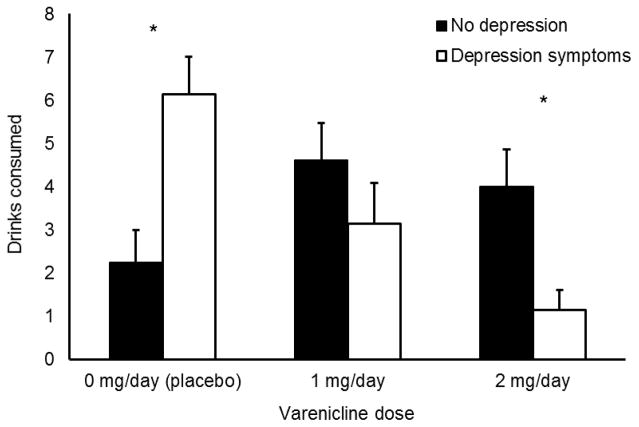
The ANOVA of oral VAR dose and depression group on ad libitum drinking found no significant main effect of VAR dose or depression group (ps > 0.05), but there was a significant medication X depression group interaction, F (2, 54) = 8.20, p = 0.001. As seen in Figure 1, VAR reduced the number of drinks consumed among participants with depression symptoms. Among those with no depression symptoms, however, VAR appeared to increase rates of drinking. A priori t-tests probed the effects of medication within each depression group. Among those in the depression symptoms group, participants receiving placebo consumed more alcohol than those receiving 1 mg/day VAR, t (13) = 2.33, p = 0.037. d = 1.29, and those receiving 2 mg/day VAR, t (13) = 4.83, p < 0.001. d = 2.68. Those receiving 2 mg/day VAR drank less than those receiving 1 mg/day VAR, although the difference only approached significance, t (12) = 1.92, p = 0.079. d = 1.07. Within the no depression symptoms group, those receiving placebo drank less than those receiving 1 mg/day VAR, t (23) = −2.07, p = 0.050, d = 0.86, but did not differ from participants receiving 2 mg/day VAR, t (23) = −1.52, p = 0.142, d = 0.63. There was no significant difference between those receiving 1 and 2 mg/day VAR, t (24) = 0.51, p = 0.616. d = 0.21.
Figure 1.
Effects of VAR on ad libitum drinking in no depression and depressive symptoms groups. Capped bars are SEM. * indicates significant difference between depression groups.
The second set of a priori t-tests found that among participants receiving placebo, those with depression symptoms drank more than those with no depression symptoms, t (18), = −3.32, p = 0.004. There was no significant difference between depression group among those receiving 1 mg/day VAR. Among participants receiving 2 mg/day VAR, those with depression symptoms drank less than those with no symptoms, t (18) = 2.31, p = 0.033.
Alcohol Craving
Tonic craving
Results of the hierarchical linear regression analyses examining the relation among VAR plasma, CESD scores, and tonic craving are presented in Table 3. As seen in this table, there was a significant VAR plasma X depression interaction. Simple slope analyses found that at low levels of depression, there was no significant relation between VAR plasma and drinking amount. At moderate levels of depression, higher levels of VAR plasma were associated with less self-reported craving. At high levels of depression, VAR plasma showed a strong negative association with self-reported craving.
Table 3.
Hierarchical regression analyses of effects of VAR plasma and CESD on alcohol craving
| Dependent Variable | Simple Slopes | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| B | ΔF | ΔR2 | Low Depression | Moderate Depression | High Depression | ||
| Tonic Craving | |||||||
| Step 1 | 6.33** | .19 | |||||
| CESD | 0.28* | ||||||
| VAR Plasma | −0.32* | ||||||
| Step 2 | 6.03* | .08 | |||||
| CESD X VAR Plasma | −0.36* | 0.23 (0.05) | −2.70 (−0.32)** | −3.65 (−0.69) *** | |||
| Craving after Alcohol Prime | |||||||
| Step 1 | 2.67 | .09 | |||||
| CESD | .24 | ||||||
| VAR Plasma | −.16 | ||||||
| Step 2 | 1.79 | .03 | |||||
| CESD X VAR Plasma | −.18 | 0.26 (0.06) | −1.25 (−0.16) | −1.85 (−0.38) | |||
Simple slopes report t values (parenthetical values are B weights).
p < 0.05,
p < 0.01,
p < 0.001
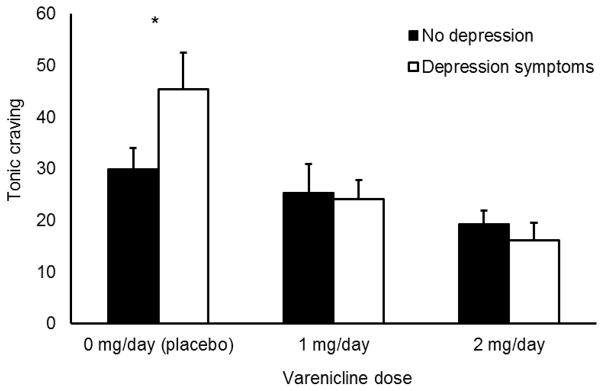
Tonic alcohol craving is graphed in Figure 2. The 2 × 3 ANOVA found a significant main effect of medication condition, F (2,52) = 3.32, p = 0.005, but no main effect of depression group, F (1, 52) = 1.60, p = 0.212. The medication X depression group interaction approached significance, F (2, 52) = 2.99, p = 0.059. A priori t-tests probed this interaction. These tests found that within the depression symptoms group, participants receiving placebo reported greater tonic craving than those receiving 1 mg/day VAR, t (12) = 2.47, p = 0.029, d = 1.43, and 2 mg/day VAR, t (11) = 3.33, p = 0.007. d = 2.00. There was no significant difference in craving between 1 and 2 mg/day VAR in this group, t (11) = 1.60, p = 0.138. d = 0.96. Among those in the no depression symptoms group, there was no significant difference in tonic craving among those receiving placebo and 1 mg/day VAR, t (23) = 0.07, p = 0.944. d = 0.03, or 2 mg/day VAR, t (23) = 1.35, p = 0.191. d = 0.56. There was no significant difference in tonic craving between participants receiving 1 or 2 mg/day VAR in this group, t (24) = 1.12, p = 0.273. d = 0.47. The second set of a priori t-tests found that among participants receiving placebo, those with depression symptoms reported more tonic craving than those with no depression symptoms, t (17), = −2.52, p = 0.022. There was no significant difference in tonic craving between depression group among those receiving 1 or 2 mg/day VAR, ts ≤ 0.57, ps ≥ 0.591.
Figure 2.
Effect of VAR on tonic craving as measured on a visual analogue scale in no depression and depressive symptoms groups. Capped bars are SEM. * indicates significant difference between depression groups.
Craving after alcohol prime
As seen in Table 3, hierarchical linear regression analyses found no significant relation among VAR plasma, CESD scores, and alcohol craving following priming drink. Ratings of alcohol craving after the priming drink were as follows: placebo (M = 40.25, SD = 15.95), 1 mg/day VAR (M = 36.55, SD = 18.27), and 2 mg/day VAR (M = 32.20, SD = 18.27). The 2 × 3 ANOVA found no significant main effect of depression group or VAR dose (ps > 0.05). The depression group × VAR dose interaction was not significant (p = 0.546).
Discussion
The current study was the first to examine whether depressive symptoms moderated the effectiveness of varenicline at reducing risk of drinking among individuals with an alcohol use disorder. The results supported our hypotheses in that participants who reported heightened levels of depression showed the largest reduction in ad libitum drinking and alcohol craving under active doses of varenicline. This effect was evident when data were analyzed both by blood plasma and oral dose. These identify a specific subpopulation of individuals with alcohol use disorders for whom varenicline is particularly effective for reducing rates of drinking. This builds on previous work attempting to identify factors that moderate the efficacy of varenicline for reducing rates of drinking (Falk et al., 2015).
Specifically, results demonstrated that there was a dose-response effect of varenicline on reduced drinking in those with depressive symptoms. Conversely, those without depressive symptomatology had the lowest levels of consumption, and both doses of varenicline increased consumption. Although it is not entirely clear why varenicline was more effective at reducing drinking in participants reporting depressive symptoms, we can speculate regarding potential mechanisms explaining this moderation effect. One possibility is that varenicline blocks alcohol-induced negative affect relief (Baker et al., 2004) due to its partial antagonism of nAChR, or alternatively varenicline may itself have improved mood and reduced alcohol motivation among depressed drinkers (Philip et al., 2009). We were unable to test these possibilities as we did not track potential changes in depressive symptoms throughout the titration phase. The presence of depression in drinkers also may signal a subtype of alcohol use disorder with cholinergic involvement that is more responsive to pharmacological intervention targeting nAChRs, although evidence for alcohol use disorder and depression being explained by a shared etiological mechanism is mixed (Swendsen and Merikangas, 2000).
A third possibility is that varenicline had a differential effect on drinking due to depression-related differences in cholinergic tone. Neuroimaging studies have found that people reporting depressive symptoms show higher occupancy of cholinergic receptors relative to their non-depressed peers (Hannestad et al., 2013; Saricicek et al., 2012), and therefore appear to have higher levels of cholinergic activity in many brain areas (Esterlis et al., 2013). Evidence that increased cholinergic signaling is associated with depressive symptoms also comes from preclinical studies demonstrating that pharmacologically increasing acetylcholine levels by decreasing its breakdown produces depression-like behavior in mice (Mineur et al., 2013). Based on this work, it is possible that participants in the current study differed in terms of cholinergic tone based on their depressive symptoms, which may explain the differential effects of varenicline on their drinking behavior. A partial agonist, by definition, decreases the effect of an endogenous agonist while acting as a less effective agonist in its absence. Thus, among participants with elevated cholinergic tone (i.e., those reporting depressive symptoms), varenicline may have reduced nAChR activity due to its partial agonist properties, attenuating alcohol-induced dopamine release and decreasing the rewarding effects of alcohol in the same manner as nAChR antagonists (Ericson et al., 1998). This hypothesis is appealing because it also explains why non-depressed participants receiving varenicline drank more than those receiving placebo. Specifically, nAChR activity may have been low among these non-depressed drinkers. As a partial agonist, varenicline may have increased nAChR activity above baseline, resulting in increased stimulation of the ventral tegmental area and increasing alcohol reward via a similar pathway as nAChR full agonists (Tizabi et al., 2002).
Although this is the first study to test whether depressive symptoms moderate the efficacy of varenicline for reducing drinking, prior research has examined the efficacy of varenicline as a smoking cessation aid in smokers with a likely history of major depression. A large clinical trial found that varenicline is equally effective in reducing smoking in smokers with a lifetime history of depression (McClure et al., 2009), although it is worth noting that depressed and non-depressed smokers did not differ in terms of nicotine dependence severity or number of cigarettes smoked at baseline. In our study, participants with depressive symptoms reported higher rates of drinking and craving compared to those with no depressive symptoms. One possibility for this difference is that participants in the current study showed less severe depressive symptoms, although participants in the study by McClure and others did not necessarily endorse current depressive symptoms. Another possibility is that varenicline affects alcohol and nicotine use through different neurobiological pathways, and its effects on alcohol use may involve pathways that are associated with mood regulation (Heinz et al., 2001).
Tonic craving levels, collected prior to alcohol consumption, mirrored the ad libitum consumption results in the placebo group only. As has been demonstrated previously, varenicline reduced tonic alcohol-related craving (Fucito et al., 2011), but this effect was not moderated by depression status. Similarly, there were no effects of depressive symptoms on changes in craving following consumption of a low-dose alcohol beverage. While there are many examples of non-overlapping craving and drinking behavior (Tiffany & Conklin, 2000), further mechanistic work will be necessary to understand how depressive symptoms influence varenicline’s effects on craving responses.
Overall, levels of depressive symptoms were relatively low in our sample. While participants were not specifically ruled out for a diagnosis of depression, those taking any psychoactive medications were ineligible. As a result, the mean CESD score in the sample (i.e., 8.25) was well below the recommended clinical cut score of 16, although several participants (n = 7) did score above this threshold. It is unclear whether our findings would generalize to patients meeting diagnostic criteria for depressive disorders. However, subclinical depressive symptoms are highly prevalent and a recognized risk factor for later onset of clinical depression (Cuijpers and Smit, 2004). Such subclinical depressive symptoms also are associated with current functional impairment (Johnson et al., 1992; Martin et al., 1996; Kessler et al., 1997), so the range of scores reported in the current study are likely clinically relevant.
This research provides evidence that varenicline was differentially effective at reducing drinking according to levels of depressive symptoms; however, there are limitations that should be addressed in future research. First, this manuscript reports a reanalysis of data from a previous human laboratory study. Additional research will be necessary to confirm these findings with a prospective research design with targeted recruitment to include individuals with clinically significant symptoms of depression co-morbid with an alcohol use disorder. Further, it will be important to assess participants’ depressive symptomatology continually to identify any varenicline-induced changes in these symptoms. Such information could provide insight into the mechanism of the reported treatment moderation effect. Second, we used one self-report measure of depression. Although this strategy was effective for capturing variance in depressive symptoms as a continuous symptom dimension, a more comprehensive assessment would have allowed us to make inferences related to participants’ diagnoses and recommendations for treatment based on categorical diagnoses. Related to this, our sample consisted of drinkers with mild to moderate alcohol-related problems. It is unclear whether our findings should generalize to those with severe alcohol use disorder. Third, the sample size was small, particularly in the depressive symptoms groups.
In conclusion, results of this reanalysis suggests that varenicline may be more effective at reducing drinking in people with alcohol use disorder reporting moderate or high levels of depression compared to those with few depressive symptoms. Additional research will be necessary to determine the effects of varenicline in drinkers with clinically significant symptoms of depression.
Acknowledgments
This work was supported by NIH grants R01AA017976 (SAM), UL1TR000142 (PI: Sherwin; SAM), MH077681 and DA14241 (MRP), NIDA T32DA007238 (WR), and an investigator-initiated grant from Pfizer (SAM).
References
- Baker TB, Piper ME, McCarthy DE, et al. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, et al. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin and Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin and Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression: New evidence from a prospective investigation. Arch of Gen Psychiat. 1993;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, et al. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin and Exp Res. 2012;36:906–914. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, et al. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol. 2015;124:215–225. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: A systematic review of prospective studies. Acta Psychiat Scand. 2004;109:325–331. doi: 10.1111/j.1600-0447.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, et al. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin and Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, et al. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Hannestad J, Bois F, et al. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nuclear Med. 2013;54:78–82. doi: 10.2967/jnumed.112.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Castle IJ, Ryan M, et al. Moderators of varenicline treatment effects in a double-blind, placebo-controlled trial for alcohol dependence: An exploratory analysis. J Addict Med. 2015;9:296–303. doi: 10.1097/ADM.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, et al. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA-J Am Med Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- Hannestad JO, Cosgrove KP, DellaGiola NF, et al. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5-IA SPECT. Biol Psychiat. 2013;54:768–776. doi: 10.1016/j.biopsych.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, et al. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin and Exp Res. 2001;20:487–495. [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. JAMA-J Am Med Assoc. 1992;267:1478–1483. [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiat. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Shanyang Z, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the national comorbidity survey. J Affect Disorders. 1997;45:19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: Clinical, research, and policy implications. JAMA. 2006;296:1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- Lě A, Corrigall W, Watchus J, et al. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin and Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JK, Blum SRH, Beach PM, et al. Subclinical depression and performance at work. Soc Psych Psych Epid. 1996;1:3–9. doi: 10.1007/BF00789116. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Jack L, et al. Mood, side-effects, and smoking outcomes among persons with and without probably lifetime depression taking varenicline. J Gen Intern Med. 2009;6:185–190. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiat. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. P Natl A Sci USA. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacol. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Council on Alchol Abuse and Alcoholism. National Advisory Council on Alcohol Abuse and Alcoholism’s Recommended Guidelines on Ethyl Alcohol Administration. 2005 Available at: https://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm.
- Philip NS, Carpenter LL, Tyrka AR, et al. Varenicline augmentation in depressed smokers: An 8-week, open-label study. J Clin Psychiatry. 2009;70:1026–1031. doi: 10.4088/jcp.08m04441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, et al. Persistent beta(2)*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J of Psychiat. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, et al. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Research. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. P Natl A Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: A systematic review. Am J Med. 2005;118:330–341. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conkin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95:S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, et al. Effects of combined systemic alcohol and central nicotine administration into the ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin and Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, et al. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J Addict Med. 2016;10:166–173. doi: 10.1097/ADM.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Blatt RD, editors. Human Metabolism of Alcohol. Boca Raton, FL: CRC Press; 1989. pp. 41–58. [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]