Abstract
Patent ductus arteriosus (PDA) is a congenital cardiovascular defect in which a fetal connection between the aorta and pulmonary artery does not spontaneously close shortly after birth. If left uncorrected serious complications and even death can occur. Surgical ligation is the traditional treatment method; however, it is an invasive procedure, that motivates development of a minimally invasive option. Shape memory polymer (SMP) foams are unique materials that hold promise in the field of minimally invasive occlusion devices. In this work, a prototype nitinol foam cage (NFC) incorporating SMP foams has been designed and evaluated in multiple mechanical and in vitro verification tests. The NFC demonstrated acceptable fatigue resistance in a preliminary strut integrity test, withstanding one million cycles without complete strut fracture. Radial force analysis of both thick- and thin-walled prototype variations generated less vessel distension and wall tension in a vessel mimic compared to a commercial device. The NFCs exhibited negligible in vitro migration, comparable to that of a commercial device, using simplified, ideal models of PDA. Deployment characteristics of the prototypes were evaluated and compared to that of a commercial device when delivered into physiological models of PDA. During mock deployments, a veterinary cardiologist noted that, while deliverable, the thin-walled NFC prototype exhibited poor deployment characteristics, however the thick-walled NFC had deployment characteristics comparable to that of a commercial device. The promising results of this study warrant further investigation of the NFC device for canine PDA closure.
Keywords: Patent ductus arteriosus, ACDO, Shape memory polymer
Graphical abstract

1. Introduction
Patent ductus arteriosus (PDA) occurs when a normal fetal connection between the aorta and pulmonary artery does not close shortly after birth, leading to a continuous left-to-right side shunting of blood. When left uncorrected, the PDA leads to many complications including, congestive heart failure and even death (Buchanan, 2001; Nguyenba and Tobias, 2008)
PDA is the most common congenital cardiovascular defect that occurs in canines, manifesting in 6.8 out of 1000 live births and comprising approximately 28% of all congenital cardiovascular defects (Patterson, 1968). PDA has been traditionally treated via surgical ligation, in which the chest cavity of the dog is opened via thoracotomy and the ductus is ligated (Birchard et al., 1990; Eyster, 1976; Jackson and Henderson, 1979; Nguyenba and Tobias, 2008). While effective, surgical ligation is invasive, and carries non-trivial operative risks including, nonfatal hemorrhage, pneumothorax, and chylothorax as reported in 11–15% of cases (Bureau et al., 2005; Goodrich et al., 2007; Stanley et al., 2003; Van Israel et al., 2002) and operative mortality in up to 11% of cases (Birchard et al., 1990; Bureau et al., 2005; Eyster, 1976; Goodrich et al., 2007; Stanley et al., 2003; Van Israel et al., 2002). These complications motivate the development of improved minimally invasive closure techniques (Tobias and Stauthammer, 2010).
Many endovascular devices intended for human PDA and peripheral occlusion, such as embolization coils, the Amplatzer™ duct occluder (ADO), and the Amplatzer™ vascular plug (AVP), have been investigated as options to treat the canine PDA. While there has been moderate success using human devices within the canine anatomy, limitations such as incompleteness of occlusion, device migration, delivery system size, and ease of delivery have prevented widespread use (Achen et al., 2008; Campbell et al., 2006; Glaus et al., 2002; Gordon and Miller, 2005; Hogan et al., 2006; Singh et al., 2012; Sisson, 2003; Tanaka et al., 2007; Tobias and Stauthammer, 2010) and led to the development of the Amplatz® canine ductal occluder (ACDO) (Nguyenba and Tobias, 2007, 2008). The ACDO is very successful in PDA closure, yet is still limited by cost and its relatively large delivery system profile. While some advances have been made to reduce the profile (Stauthammer et al., 2015), the smallest sheath that current commercial devices can be delivered through is a 4 Fr sheath, restricting the range of dogs that can be treated.
A prototype nitinol foam cage (NFC) device that utilizes shape memory polymer (SMP) foams has been developed as previously described in Wierzbicki et al (Wierzbicki et al., 2016) with design modifications to the shape for improved stability and increased foam capacity. The NFC prototype utilizes an SMP polyurethane foam, rather than a dense nitinol mesh to occlude flow. SMPs are a unique class of materials that can be heated above a characteristic glass transition temperature (Tg) and when deformed and cooled below the Tg, hold a secondary shape. The polymer can then be actuated back to its original configuration through an entropy driven process by increasing the temperature of the polymer above its Tg via an external stimulus such as heat or contact with body temperature blood (Lendlein and Kelch, 2002). The unique mechanical and embolic properties of these materials have led to the investigation of multiple biomedical embolic device applications for endovascular occlusion (Maitland et al., 2007; Small et al., 2010). Multiple in vitro and in vivo studies have been conducted demonstrating the favorable occlusion and biocompatible properties of these materials, as shown through occlusion within two minutes once implanted (Rodriguez et al., 2014b) and minimal inflammation characterized by dense tissue growth and collagen deposition at 90 days post-implant, prompting investigation for their use in other embolization applications (Rodriguez et al., 2014a).
The prototype NFC discussed herein is constructed from one continuous nitinol conduit comprising three sections: the proximal cage, waist, and distal cage, and an SMP foam. In endovascular deliveries, the distal cage is advanced out of the catheter first and pulled against the pulmonary artery wall, serving as an anchor point and landmark for the device and clinician, respectively. The waist is designed to be 2× oversized relative to the minimal ductal diameter (MDD), such that it will apply an outward force to the ductus, aiding in stability. The proximal cage expands to apply pressure to the ampulla wall. The SMP foam is anchored within the proximal cage and spans all three sections of the frame to volumetrically fill the PDA.
Through discussions with clinicians, multiple design criteria were defined to determine device success. First, the device must be deliverable through a 4 Fr sheath having an internal diameter of 1.3 mm or smaller, as larger sheaths and catheters exclude small dogs, an important subset of the canine population that cannot currently be treated with minimally invasive devices. Additionally, the prototype must be retractable into the sheath in the event of an improper deployment or sizing, be visible under fluoroscopy following delivery, and remain stable once deployed at physiological and elevated pressures. Fabricated NFC prototypes were tested against ACDO performance in multiple verification tests to evaluate strut integrity and nitinol frame radial forces, as well as stability and deployment characteristics in simplified and physiological PDA models, respectively, under experimental flow conditions.
2. Methods
2.1 Device Fabrication
NFC prototypes were fabricated in a similar way to those described in Wierzbicki et al (Wierzbicki et al., 2016); however, two variations in wall-thickness were used to assess differences regarding the effects of strut stiffness on the prototype’s deployment characteristics. Briefly, the NFC prototypes were fabricated by cutting ten equally spaced slots radially into a straight, superelastic, nickel titanium alloy (nitinol) tubing using an excimer laser (Resonetics, Nashua, NH). The thin-walled prototypes (NFCTn) were constructed using 1.12 mm (0.044”) outer diameter (OD) and 1.04 mm (0.041”) inner diameter (ID) (NDC, Fremont, CA) tubing (0.038 mm (0.0015”) wall thickness), while the thick-walled prototypes (NFCTk) were constructed using 1.12 mm OD and 1.00 mm ID (Vascotube, Birkenfeld, Germany) tubing (0.06 mm wall thickness). The austenite finish temperature (Af) of the thick-walled material was approximately −11°C prior to shape setting. The Af of the thin-walled material following shape setting was approximately ~11°C as determined by a bend and free recovery test based upon ASTM F2082 in which the NFCTn prototypes were submerged within a −20°C water/ethanol solution, elongated to a maximal length and imaged in 0.5–1°C intervals as the solution was slowly heated. Axial device length was measured from the images and plotted against temperature. The Af was estimated from the generated curves. The laser cut nitinol tubes were deburred using abrasive paper and sonicated in isopropyl alcohol. Custom fixturing compressed the tubing, while allowing the struts to expand to the desired shape with a waist of 6 mm OD, shown in Figure 1. All material deformations when preparing the devices for shape setting were conducted at room temperature, when the nitinol was superelastic. The final shape was defined by annealing the nitinol frame and fixture within a furnace held at 550°C for twenty-five minutes, followed by quenching in water. A threaded release mechanism was laser welded to the proximal end of the device and an 8 mm OD, 5 mm length SMP foam was compressed to a minimal diameter and epoxied into the lumen of the proximal portion of the device. Figure 2 illustrates a comparison between the ACDO and the NFCTn prototype with a compressed and expanded foam insert. Additionally, a subset of thick-walled prototypes (NFCTk) were electropolished (NFCTkEP) (Able Electropolishing, Chicago, Il) to a wall thickness of approximately 0.038 mm (0.0015”) to evaluate how electropolishing influenced frame durability and strut integrity.
Figure 1.
Shape setting sequence for nitinol foam cage prototype fabrication. A: Laser cut nitinol tube. Gauge pin placed within lumen across the length of the tube. B: Laser cut tube placed within shape setting clamps (top to bottom: proximal, waist, distal) with struts expanded to accommodate proximal spacer (Top half of clamps not shown. Spacer shown sectioned in half). C: Spacer advanced into proximal clamp. D: Waist clamp butted against proximal clamp. E: Distal clamp butted against waist clamp. F: Shape set nitinol cage following heat treatment, quench, and removal from the fixture.
Figure 2.
Nitinol foam cage (NFC) prototype comparison to Amplatz® canine ductal occluder (ACDO). A: ACDO, B: NFC with foam compressed, C: NFC with foam expanded. Cage regions defined in right most column.
2.2 PDA Model Fabrication
While the PDA morphology varies from animal to animal, there are three basic morphologies that the ductus arteriosus may manifest which vary in size and shape (Miller et al., 2006). Simplified thin-walled silicone models were fabricated with shapes based on those described by Miller et al (Miller et al., 2006), including the cylindrical (IIA) and elliptical ampulla (IIA ovular), conical ampulla (IIB), and a wide ampulla (WA) models with 3 mm and 5 mm minimal ductal diameters (MDD) (Figure 3). Dimensions of all simplified models are detailed in Table 4.1. The WA model simulates conditions in which the device struts do not contact the ampulla walls, as could occur shortly after occlusion due to systemic hypertension and a reflex bradycardia (Branham reflex) (Achen et al., 2008; Franks, 2004). The 6 mm waist of the NFC prototype is designed to be twice the diameter of the 3 mm PDA MDD. Model MDDs were designed such that the prototypes would evaluate 2× and 1.2× oversizing to serve as a safety factor for clinician device sizing. Additionally, elliptical morphology dimensions were applied for the IIA ovular model to account for irregularly shaped PDAs that occur in vivo (Saunders et al., 2010).
Figure 3.
Simplified patent ductus arteriosus (PDA) morphologies. Schematics of each simplified thin-walled model used in experimental flow stability studies. IIA and IIB morphologies are the most common PDA shapes. The wide ampulla model evaluates performance under conditions in which the ampulla enlarges following occlusion (Franks, 2004).
Table 1.
Dimensions of simplified, flexible, thin-walled, patent ductus arteriosus models used in experimental flow stability studies. Device waist oversizing is the ratio of the waist diameter of the device to the minimal ductal diameter. An oversize factor of 2× is used clinically.
| Model | Ampulla (mm) |
Diameter | Minimal Ductal Diameter (mm) |
Device Waist Oversizing |
|---|---|---|---|---|
| IIA | 8 | 3 | 2× | |
| 8 | 5 | 1.2× | ||
| IIA ovular | 6 × 8 | 3 × 4 | ~2× | |
| IIB | 10 | 3 | 2× | |
| 10 | 5 | 1.2× | ||
| Wide Ampulla | 12 | 3 | 2× | |
| 12 | 5 | 1.2× |
Flexible, thin-walled, polydimethylsiloxane siloxane (PDMS) molds of the simplified PDA geometries were constructed by 3D printing the vessel lumen using a dissolvable material and the outer wall mold pieces using a non-dissolvable material. PDMS resin was injected into the mold to form a wall thickness of approximately 0.5 mm and then cured. The printed polymer lumen was then dissolved from the PDMS mold in a heated base bath solution.
Two rigid PDMS molds were constructed based on reconstructions from computed tomographic angiograms (CTA) of the canine PDA. The rigid physiological PDA models were made in a similar fashion as described in Wierzbicki et al. as the complex geometry complicates flexible, thin-walled mold manufacture (Wierzbicki et al., 2016). Briefly, the surfaces of the aorta, PDA, and, MPA were extracted and sectioned using Mimics (Materialise, Belgium). Because the CTAs provided were an unusual morphology, the geometry was minimally modified to resemble a the IIA and IIB taper with a 3 mm MDD (Figure 4). The extracted volume was then 3D printed with a dissolvable material and the model was cast in PDMS in a similar manner to the simplified PDA models.
Figure 4.
Physiological patent ductus arteriosus models used to obtain feedback on deployment characteristics. Dye placed within lumen for visibility of the aorta, ampulla, main pulmonary artery (MPA), and minimal ductal diameter (narrowest portion between ampulla and MPA).
2.3 Mechanical Verification Tests
2.3.1 Prototype Strut Integrity
A preliminary analysis of fatigue characteristics was conducted by axially cycling the nitinol frame on a dynamic mechanical analysis (DMA) unit (DMA Q800, TA instruments, New Castle, DE). Three untreated, NFCTn, and three electro-polished, NFCTkEP, nitinol frames were cycled with an amplitude of 1 mm at a frequency of 2 Hz for approximately one million cycles simulating small deflections similar to those that the devices would be subject to in vivo. Untreated samples were prepared as described in Section 2.1 and were not electropolished or processed further following shape setting. The force imparted by the NFCTn and NFCTkEP devices were recorded and monitored for sudden changes and the struts were visually inspected for damage following the test.
2.3.2 Radial Force Measurements
The radial force exerted by the nitinol frames of the NFCTn and NFCTk and ACDO were measured using a Blockwise J-Crimp™ RJA62 Radial Compression Station (Blockwise Engineering LLC, Tempe, AZ) attached to an Instron 5965 Test Frame (Instron, Norwood, MA) held at 37°C. The Blockwise utilizes a linear relationship between the axial force measured by the load cell (F), and the total radial force (TRF), as shown in equation (1)
| Eq. (1) |
where, 2.712 is a conversion constant provided by Blockwise for our instrument.
Devices were first loaded into a polytetrafluorethylene (PTFE) sheath (1.35 mm ID, Cole Parmer, Vernon Hills, Il) and positioned within the bore of the Blockwise for delivery. The sheath was then retracted, allowing the devices to expand. Both distal and proximal cages contacted the straight tubular bore. The radial force of the NFCTn. NFCTk, and ACDO was measured at diameters between 4 and 12 mm within the bare surface bore of the Blockwise, denoted STn, STk, and SACDO respectively. Additionally, devices were deployed into a 5.5 mm ID, 6.5 mm OD vessel mimic (LifeLike Biotissue, London, ON) placed within the Blockwise bore to measure the constrictive force required to prevent full vessel distension due to the nitinol frame, denoted BTn, BTk, and BACDO.
As the NFC prototypes and ACDO exhibit a very different contact area, strut and constrictive force were not directly compared between device types. Instead, wall tension within the mock vessel was calculated to compare mechanical properties of the devices. The NFC radial forces (STn, STk) and Blockwise radial constrictive forces (BTn, BTk) were divided by the ten total struts, to obtain the radial force per strut (sTn, sTk) and constrictive force per strut (bTn, bTk), respectively. By measuring these two forces, the wall tension as seen in the vessel due to the NFC prototypes can be calculated as,
| Eq (2) |
where, τNFC is the wall tension in the vessel imparted by a NFC device (N/mm), sNFC is the radial force per strut (N), bNFC is the constrictive force per strut (N) of the NFCTn or NFCTk prototypes, L is the strut length contacting the vessel (mm), and α is the half angle of the ideal decagonal cross section formed by the struts. Figure 5 below illustrates the strut isolation and free body diagram for the calculation.
Figure 5.
Illustration of vessel mimic wall tension measurements. A: Thick-walled nitinol foam cage prototype (NFCTk) in mock vessel. Proximal (bottom) and distal (top) struts seen contacting the vessel wall at discrete points. B: Section of idealized decahedron geometry formed by struts contacting the vessel wall. C: Vessel segment looking at only one strut. D: Free body diagram of strut contact point to analyze wall tension, τ.
As the ACDO is a continuous structure and does not have discrete struts, it was more appropriate to calculate wall tension as,
| Eq (3) |
where τACDO is the wall tension generated by the ACDO, r is the radius of the Blockwise bore, PSACDO, the contact pressure applied by the ACDO, was calculated as,
| Eq (4) |
and PBACDO, the compressive pressure applied by the Blockwise to the ACDO within the mock vessel, was calculated as,
| Eq (5) |
where, SACDO is the radial force (N) applied by the ACDO within the bare surface bore, and SAcontact is the surface area (m2) of the ACDO in contact with the vessel wall, and BACDO is the radial force (N) applied by the ACDO within the mock vessel. The strut contact length and SAcontact were determined from images, similar to the one shown in Figure 5A, using Image-J™ software (http://rsb.info.bih.gov/ij/). Three NFCTn and NFCTk were measured, and one ACDO were tested, at least three times each at each diameter.
The wall tension generated by the devices was compared to the estimated wall tension at failure of the canine aorta. This was estimated by first calculating the burst pressure as,
| Eq (6) |
where PBurst is the burst pressure of the vessel, s is the ultimate material strength of the vessel, t is the wall thickness of the vessel, and d0 is the outer vessel diameter. Wall tension was calculated using Laplace’s law as,
| Eq (7) |
where τRupture is the wall tension at vessel rupture. Using values from Cohen et al. and Moriwaki et al., the estimated wall tension at vessel rupture is 0.91 N/mm (Cohen et al., 1972; Moriwaki et al., 2011).
2.4 Device Stability Testing Using Simplified in vitro models
2.4.1 Flow Setup for Simplified in vitro PDA Models
A gravity fed flow loop held at approximately 37°C was built to create a steady, pressure driven flow to subject the devices to physiological (100 mmHg) and elevated (200 mmHg and 300 mmHg) pressures. The height of the reservoir dictated the maximum pressure that the system could generate. Two pressure transducers (PX M209-1.6A10V, Omega Engineering, Inc, Norwalk, CT) were placed upstream and downstream of the model geometry. A needle valve positioned upstream of the PDA model was used to control the pressure gradient applied to the system. The pressure gradient was constantly collected using an NI USB-6009 DAQ and monitored using a custom program developed in Labview (National Instruments, Austin, TX). Figure 6 displays a schematic of the flow set up.
Figure 6.
Schematic of the steady flow, gravity fed flow loop used in device stability studies. The maximum pressure that could be generated by the system was set by the height of the reservoir relative to the model. The pressure seen by the device was modulated by the needle valve positioned upstream of the model and monitored with pressure transducers positioned upstream and downstream of the model.
2.4.2 Device Preparation and Delivery
To provide a worst-case scenario for the NFC device geometry, the NFCTn prototypes were used for comparison against the commercial ACDO device, as nitinol frames with a wall thickness smaller than of 0.038 mm (0.0015”) would not be used for this application. The NFCTn prototypes were fitted with compressed SMP foams and loaded into PTFE introducer tubes. After setting the pressure gradient across the ductus to 100 mmHg, a 4 Fr sheath (Cook Medical, Bloomington, IN) was passed across the MDD such that the tip resided in the modeled pulmonary artery. The introducers containing devices were loaded into the sheath and the devices were advanced through the sheath until the distal cage was exposed. The delivery cable and sheath were then retracted together such that the distal cage rested against the pulmonic ostium of the model. The proximal cage was then unsheathed. The delivery cable was then pushed forward to allow the proximal cage to conform to its cupped shape and to position the foam within the ampulla, MDD and pulmonary artery. Prior to release, a push-pull test was performed, in which gentle forward and backward motion was applied to the delivery cable to ensure that the device was stable and not easily pushed into the pulmonary artery (Gordon et al., 2010). The SMP foam was allowed to fully expand (~2 mins) before releasing the device by unscrewing it from the delivery cable.
2.4.3 Device Stability Tests
Three unique NFCTn prototypes and one 6 mm waist ACDO were tested in all simplified models three times each. The pressure gradient across the model was set to 100 mmHg prior to delivery of the devices. Both the NFCTn and ACDO were delivered into the simplified PDA models following the protocol described in 2.4.2. Following device deployment, the pressure gradient was readjusted to 100 mmHg, as the pressure across the PDA increases following deployment, and held at 100, 200, and 300 mmHg for five minutes each consecutively, while images were captured in one minute intervals using a digital camcorder (Panasonic HDC-HS80, Newark, NJ). Devices in the all 3 mm and 3×4 mm MDD models were subjected to all three pressures, but the devices, when implanted into the 5 mm MDD models, were only subjected to 100 and 200 mmHg. The flow system could not develop a pressure gradient of 300 mmHg with devices (NFC or ACDO) deployed, as the devices did not generate a high enough resistance to flow. Following the tests, migration/dislocation of the distal tip, relative to the initial deployed position was measured using ImageJ™ software.
2.5 Deployment Performance in Physiological in vitro Models
2.5.1 Device Preparation and Delivery
NFC prototypes were fabricated using methods described in Section 2.1. The delivery characteristics of 6 mm NFCTn and NFCTk prototypes and a 6 mm ACDO were evaluated in the two different rigid PDMS models of a physiologically representative PDA as shown in Figure 4. Two NFCTn and NFCTk prototypes, and one ACDO were prepared and loaded into PTFE introducers. Before delivery, the pressure drop across the ductus was set to 120 mmHg, and the flow rate on the pump was constant for the entire test. The water in the flow loop was maintained at approximately 37°C. One of each type of NFC was deployed by a veterinary cardiologist into each physiological model, in a comparable manner to that described in Section 2.4.2 using fluoroscopic guidance. The same ACDO was used in both deliveries. Deployment characteristics such as ease of advancement through the catheter, deployment, positioning, and retraction were recorded.
2.5.3 Device Retraction and Repositioning
Following deployment and after allowing the foam to fully expand, retraction was attempted by advancing the catheter to the proximal struts and pulling on the delivery cable and retracting them back into the sheath. Following recapture, the device was redeployed by the veterinary cardiologist following the previously described delivery protocol. Retraction and redelivery characteristics and veterinary cardiologist recommendations were recorded.
2.6 Statistics
Two-sided analysis of variance (ANOVA) with Tukey multiple comparison analysis, was conducted to compare the differences in mean migration of the NFCTn and ACDO devices within each idealized PDA model type at varying pressure gradients. All runs for each device type at a specific model and pressure were averaged together and the migration values of all replicates were included in the standard deviations for statistical analysis. In instances in which negative average migration was observed, the average was increased to zero for statistical and presentation purposes, but the standard deviations were not altered. For each case, significance was determined with a 95% (p<0.05) confidence level. All statistics were performed using Graphpad Prism 6 (Graphpad Software, Inc, La Jolla, CA).
3. Results and Discussion
3.1 Prototype Strut Integrity
All six devices maintained strut integrity, withstanding one million fatigue cycles. The NFCTn (n=3) and NFCTkEP (n=3) devices produced a constant load of 0.019 ± 0.001 N and 0.013 ± 0.001 N, respectively, throughout the testing. Sharp decreases in the load would have indicated that a strut had completely fractured. There were no grossly visible fractured struts, on either the NFCTn or NFCTkEP, indicating that the nitinol frames should withstand implantation even with minimal post processing done on the laser cut nitinol. The NFCTkEP frames did feel softer compared to the untreated samples when loading them into the DMA clamps. Additionally, the NFCTkEP samples also exhibited a lower force during testing and exhibited permanent elongation, the device did not fully recover to its programmed shape, following cyclic testing, indicating that the treatment could have affected the material’s strength. The lower force could also be attributed to the reduced strut width of the NFCTkEP (0.229 mm, 0.009”) compared to the strut width of the NFCTn, (0.279 mm, 0.011”). From Duerig et al, stiffness of a stent can be estimated as,
| Eq (8) |
where, kθ is the stiffness per unit length, E is the elastic modulus of the material, w is the strut width, ts is thickness of the strut, n is the number of struts, and Ls is the strut length (Duerig et al., 2000). This relation shows that strut width has a cubic influence on material stiffness, and the thinner struts of the NFCTKEP samples would produce a lower reaction force compared to the NFCTn prototypes.
Figure 7A–C shows a comparison of the NFCTk and NFCTkEP, and an NFCTkEP positioned within the DMA clamps. Figure 7D–F shows scanning electron microscopy (SEM) micrographs of an NFCTn post-test, an NFCTk before electropolishing, and an NFCTkEP post-test (Joel NeoScope JCM-5000 scanning electron microscope, Nikon Instruments Inc., Melville, NY). The strut thicknesses of the NFCTn and NFCTk prototypes appear much greater than that of NFCTkEP sample due to the recast that formed on the edge during laser cutting from melted nitinol. Additionally, minor defects or notches (as in Figure 7F) were present on some struts at the bends in the proximal and distal zones, but these were believed to either form during shape setting or as a defect during laser cutting as the notches only appear on strut edges and were not present throughout the entire strut width. Further, defects in the NFCTkEP struts did not appear to propagate during cyclic testing.
Figure 7.
Strut integrity test overview. A: Untreated, thick-walled nitinol foam cage prototype (NFCTk). B: Electropolished thick-walled nitinol foam cage prototype (NFCTkEP). Samples were electropolished from a wall thickness of 0.06 mm to a wall thickness of approximately (0.038 mm, 0.0015”) to provide a comparison to the untreated, thin-walled nitinol foam cage (NFCTn). C: NFCTkEP prototype loaded into DMA clamps for strut integrity testing. D–F: Scanning electron microscopy (SEM) micrographs of untreated thin-walled NFCTn (D), NFCTk (E). and NFCTkEP (F) following fatigue testing, respectively. The inset frames in D–F show the surface finish of the nitinol struts for each device. Strut fracture was not grossly observed in any devices.
While electropolishing optimization is still required, it was demonstrated that NFCTk prototypes maintained utility after being treated with a commercially relevant, light electropolish (~0.0152 mm, ~0.0006” total material removal) and both NFCTkEP and untreated NFCTn prototypes could withstand a short-term fatigue test. Electropolishing is a standard and typically a necessary processing technique used on laser cut components to improve surface finish, corrosion resistance biocompatibility and fatigue life by reducing stress concentrations and heat affected zones (MacWilliams, 2006; Paprottka et al., 2015; Stoeckel et al., 2004). Formation of recast, debris, and heat affected zones is a known issue related to laser cutting nitinol with long pulsed excimer lasers (100 Hz) (Schuessler and Strohel, 2004). Therefore, future nitinol frames should be cut using a femtosecond pulsed laser, to reduce these effects, in turn reducing required processing prior to electropolishing (MacWilliams, 2006; Schuessler and Strohel, 2004).
This pilot strut integrity test provided a preliminary evaluation of frame durability to ensure that the device would not fail on a short time scale with the proposed number of struts and strut width, prompting further evaluation of the NFCTn design.
3.2 Radial Force Measurements
The contact pressure/strut force of the ACDO, NFCTn, and NFCTk, respectively (PSACDO, sTn, sTk) was plotted against Blockwise bore diameter, as shown in Figure 8B.1–B.3. The strut force of the NFCTk was higher than that of the NFCTn at all diameters tested. Contact pressure/strut force was highest for all devices in the smallest bore diameters and tended to decrease as the bore diameter increased, allowing the NFC cage diameter to increase, until the bore diameter was approximately the same diameter as the original NFC cage design. The large standard deviation in some tests is due to variations in handling during device deployment. Since the devices were delivered into a straight tube and not into the PDA geometry, as they are intended to fit, the cages would sometimes angle, torque, or adjacent struts would entangle struts, influencing the measured forces. These effects would be minimized during delivery into physiological PDAs due to the different stages and regions of cage deployment and delivery. Local maxima and minima in the curves are due to the unique recovery shapes of the multi-cage design expanding at different diameters.
Figure 8.
Radial force and wall tension analysis. A: Wall tension generated by Amplatz® canine ductal occluder (ACDO), τACDO, thin-walled nitinol foam cage prototype (NFCTn), τTn, and thick-walled nitinol foam cage prototype (NFCTk), τTk. Wall tension caused by the ACDO was approximately twice that of the NFCTk and three times that of the NFCTn. B.1–B.3: Contact pressure/strut force of the ACDO, NFCTn, NFCTk respectively (PSACDO, sTn, and sTk). C.1–C.3: Blockwise pressure/force and wall tension of the ACDO, NFCTn, NFCTk respectively (PBACDO, bTn, and bTk). B.2 and C.2 show a front view of Blockwise with an NFC deployed within the bare bore and within the vessel mimic, respectively. The starred points indicate the diameter at which the device and vessel mimic reached stress equilibrium, and the point at which maximum vessel distention had occurred. Strut/Blockwise force and contact/Blockwise pressure between the NFCs and ACDO is not comparable due to differences in device geometry, however, wall tension developed due to device expansion can be compared. Three NFCTn and NFCTk and one ACDO were tested at least three times each at each Blockwise bore diameter.
The plots in Figure 8C.1–C.3 display the compressive pressure/force applied by the Blockwise blades (PBACDO, bTn, bTk) that prevented device expansion within the vessel mimic and the wall tension (τACDO, τTn, τTk) generated within the vessel wall due to device expansion. At smaller bore diameters, the Blockwise applies a compressive force, preventing device cage expansion, and the wall tension is low at smaller bore diameters as the Blockwise is taking most of the load. However, as the bore diameter increases, the strength of the vessel wall matches the radial force imparted by the device cage, creating a stress equilibrium between the vessel mimic and device (Figure 8C.1–C.3, starred region). At this point, maximum vessel distension has occurred and the Blockwise compressive force approaches zero. While it is difficult to compare strut force and contact pressure between the NFCs and ACDO due to differences in geometry, wall tension developed due to device expansion can be compared. Figure 8A shows the wall tension of all three devices overlaid for comparison. Table 2 shows the wall tension and vessel distension, the difference between the bore diameter at stress equilibrium and the original vessel OD. The ACDO generated 0.031 ± 0.001 N/mm of wall tension compared to 0.011 ± 0.002 and 0.016 ± 0.003 N/mm generated by the NFCTn and NFCTk, respectively. The distension of both the NFCTn and NFCTk prototypes and ACDO was approximately 1, 1.5, and 2.5 mm, respectively. While the large amount of vessel distention in all devices could lead to clinical concerns such as vessel perforation, there has not been clinical evidence of such incidence due to oversizing in stenting (Duerig et al., 2000) or PDA procedures in literature. While there have not been reported incidences of vessel perforation using the ACDO, in our experience, clinicians have expressed concerns on the amount of over sizing in the ampulla in some cases. However, animal studies have shown that stents can protrude as much as 2 mm further the adventitia and remain covered with connective tissue (Duerig et al., 2000). Additionally, the estimated wall tension of the canine aorta just prior to rupture was calculated as 0.91 N/mm (Cohen et al., 1972; Moriwaki et al., 2011). Both the prototypes and the commercial device generated wall tensions that were an order of magnitude lower than this value. Overall, the lower vessel distension (1.67× lower) and wall tension (2× lower) of the NFC prototypes indicate that implantations of this device should not result in significant vessel trauma or ampulla rupture.
Table 2.
Vessel mimic distension for each device type: Amplatz® canine ductal occluder (ACDO), thin-walled nitinol foam cage prototype (NFCTn), and thick-walled nitinol foam cage prototype (NFCTk). Vessel distension is defined as the difference between the bore diameter at equilibrium and the unloaded vessel mimic outer diameter. The ACDO generated the highest wall tension and vessel distension.
| Device Type | Wall Tension at Stress Equilibrium (N/mm) |
Approximate Vessel Distension at Stress Equilibrium (mm) |
|---|---|---|
| ACDO | 0.031 ± 0.001 | 2.5 |
| NFCTn | 0.011 ± 0.002 | 1 |
| NFCTk | 0.016 ± 0.003 | 1.5 |
It should be noted that the radial force values measured include contributions of both the proximal and distal cages, representing a worst-case deployment wherein the entire device was deployed into a straight tubular, Type III (geometry not shown) PDA (Miller et al., 2006). In a standard deployment, only the proximal cage and waist apply outward forces to the ductus. The distal cage resides along the pulmonary artery wall and therefore should not exert an outward force on the vessel. However, it was not possible to replicate this due to the straight, cylindrical geometry of the Blockwise, meaning the radial force may be slightly overestimated for all devices including the ACDO.
3.3 Device Migration in Simplified in vitro Models
Migration was measured as movement of the distal tip of the device relative to the deployed position for stability evaluation. Qualitative inspection of the images captured of the devices deployed into the 3 and 5 mm MDD models (Figures 9 and 10 respectively) do not show motion of either the NFCTn or ACDO frames. However, measurements of distal tip motion displayed minimal movement of both the NFCTn and ACDO. The device migration in the 3 mm MDD models (2× oversized MDD) was lower or comparable to the devices deployed into the 5 mm MDD (1.2×) sized models, with values shown in Table 3. The axial migration of the NFCTn prototype is negligible, when compared to that of the ACDO, except for within the IIA 3mm at 300 mmHg. The greatest migration occurred at a pressure gradient of 300 mmHg in the simplified IIA 3 mm and WA 3 mm models for the NFCTn and within the IIA 3×4 mm and WA 3 mm for the ACDO. Zero migration indicates that either there was no motion seen, or that the distal tip retracted towards the minimal ductal diameter. The retraction occurs in response to the ampulla widening and allowing the proximal and waist portion of the cage to more fully recover to their unconstrained shape.
Figure 9.
Dislocation study using 3 mm minimal ductal diameter (MDD) simplified models. Thin-walled nitinol foam cage prototypes (NFCTn) deployed in all 3 mm MDD patent ductus arteriosus morphologies and subjected to increasing pressures (top to bottom). Simplified schematic of each model shown in the bottom row. Baseline images were recorded at 100 mmHg, immediately post deployment. All other images are shown after five minutes at the noted pressure. Minor motion of the distal tip (pulmonary artery side) was present in all models, and was comparable to that seen by the Amplatz® canine ductal occluder. Proximal cage collapse was apparent at a pressure gradient of 200 mmHg and above.
Figure 10.
Dislocation study using 5 mm minimal ductal diameter (MDD) simplified models. Thin-walled nitinol foam cage prototypes (NFCTn) deployed in all 5 mm MDD patent ductus arteriosus morphologies and subjected to increasing pressures (top to bottom). Simplified schematic of each model shown in the bottom row. Baseline images were recorded at 100 mmHg, immediately post deployment. All other images are shown after five minutes at the noted pressure. Negligible motion of the distal tip was present, comparable to that of the Amplatz® canine ductal occluder. Proximal cage compression was not as apparent as the larger MDD afforded less resistance to flow.
Table 3.
Thin-walled nitinol foam cage prototype (NFCTn) and Amplatz® canine ductal occluder (ACDO) migration in simplified, flexible patent ductus arteriosus models. Migration seen by the NFCTn was comparable to that of the ACDO in both the 3 mm and 5 mm minimal ductal diameter models at physiological and elevated pressures. (ACDO, n=3, except WA 3mm 300 mmHg, n=2. NFCTn, n= 9, except IIA 3×4mm 300 mmHg, n=8. NFCTn IIA 3×4mm 100, 200, 300 mmHg: images from one trial unmeasured due to displacement of the scale).
| NFCTn Axial Migration (mm) | ACDO Axial Migration (mm) | |||||
|---|---|---|---|---|---|---|
| 100 mmHg | 200 mmHg | 300 mmHg | 100 mmHg | 200 mmHg | 300 mmHg | |
|
|
||||||
| IIA 3mm | 0.0 ± 0.1 | 0.1 ± 0.1 | 0.5 ± 0.3 | 0.0 ± 0.1 | 0.1 ± 0.4 | 0.1 ± 0.3 |
| IIA 3×4mm | 0.0 ± 0.2 | 0.1 ± 0.3 | 0.2 ± 0.4 | 0.0 ± 0.1 | 0.1 ±0.2 | 0.4 ± 0.1 |
| IIB 3mm | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| WA 3mm | 0.0 ± 0.1 | 0.2 ± 0.2 | 0.6 ± 0.2 | 0.0 ± 0.2 | 0.3 ± 0.2 | 0.6 ± 0.3 |
| IIA 5mm | 0.1 ± 0.1 | 0.0 ± 0.3 | n/a | 0.1 ± 0.2 | 0.0 ± 0.2 | n/a |
| IIB 5mm | 0.0 ± 0.2 | 0.0 ± 0.3 | n/a | 0.1 ± 0.1 | 0.1 ± 0.2 | n/a |
| WA 5mm | 0.1 ± 0.1 | 0.2 ± 0.2 | n/a | 0.0 ± 0.1 | 0.1 ± 0.2 | n/a |
Average migration within each model was analyzed across pressure gradients and device type. Significant differences in migration only occurred within the IIA 3mm and WA 3mm models. Within the IIA 3mm and WA 3mm models, the NFCTn exhibited statistically significant higher migration at 300 mmHg compared to the migration seen at both 100 and 200 mmHg, and the ACDO exhibited significantly higher migration at 300 mmHg compared to 100 mmHg in the WA 3mm model. Additionally, the migration of the ACDO was significantly lower than that of the NFCTn when subjected to 300 mmHg within the IIA 3mm model.
Device migration occurred in response to flow passing through and compressing the foam into the distal cage, elongating the cage, but not dislodging the frame. Since device migration was measured as the axial motion of the distal cage, the foam compression could exaggerate the migration measurements such that cage elongation was measured and not actual dislocation of the prototype through the anatomy.
A secondary result of the foam compression was partial collapse of the proximal cage. The drag imparted on the foam that caused elongation of the distal cage also pulled the attached proximal nub toward the MDD. Proximal cage collapse was most pronounced in the 3 mm IIA and IIB models (Figure 9). However, the compression may not be as pronounced when implanted in vivo as flow across the device will be negligible following foam embolization. Standard delivery practices of the ACDO indicate that the device should not be released from the delivery cable until only a minor persistent flow through the ductus is present or it is completely occluded (Nguyenba and Tobias, 2007), thereby minimizing compression and migration due to flow.
It is promising that the NFCTn prototypes, even with an oversize factor of only 1.2×, remained stable at up to twice the physiological pressure, despite clinical recommendations for devices to have a waist 2× oversized relative to the MDD. Additionally, two-dimensional angiography of the PDA does not always result in an accurate MDD measurement and therefore it is important that a safety factor is incorporated into the device sizing. Whereas, the simplified models exhibited a rigid MDD, the flexible nature of the canine PDA may allow the struts in the waist to more fully expand, allowing the device to more closely return to its programmed shape. Greater expansion of the waist will result in frame conformance similar to that seen in the oversized MDD models, reducing the prototype’s protrusion into the pulmonary artery and minimizing the effects of foam compression. While the flexible, thin-walled PDMS models did not allow for extensive MDD dilation, minimal NFC migration in the oversized MDD’s demonstrated that the device could withstand a vessel configuration which may occur after deployment.
Overall, the minimal measured migration of the NFCTn prototypes is comparable to that of the ACDO in all models tested at physiological and elevated pressures. The test shows that there is a minimal risk of NFC device migration following implantation.
3.4 Device Deployment and Retraction in Physiological in vitro Models
3.4.1 Device Deployment and Retraction Characteristics
The NFCTk and NFCTn prototypes and ACDO were successfully delivered to both IIA and IIB physiological models. The angle at which the models were deployed as well as the rigid nature of the model did not allow the proximal cages of any NFC devices or the ACDO to return and hold their preformed shape and conform to the ampulla taper as expected in the elastic PDA vessel of a dog. Figure 11, below shows the deployment sequence of an NFCTk deployed into the IIB Model.
Figure 11.
Deployment sequence of thick-walled nitinol foam cage prototype (NFCTk) in physiological IIB model delivered by a veterinary cardiologist under fluoroscopic guidance. A: Sheath deployed across the minimal ductal diameter. The compressed NFCTk can be seen within the sheath. B: Distal cage deployed. C: Distal cage pulled against main pulmonary artery (MPA) ostium. The black arrows indicate the location of the distal cage. D: Proximal cage deployed and device released from delivery wire.
Retraction was successful in all device types (NFCTn, NFCTk, ACDO) tested. For each device, the sheath was positioned against the proximal struts and pulling on the delivery cable. For the NFCTn and NFCTk prototypes, retraction was attempted after the foam had expanded (~2–5 mins). The force required to retract the NFCTk was comparable to that of the ACDO.
While the pressure drop across the ductus was set to 120 mmHg, and the flow rate on the pump was constant for the entire test, the pressure gauges were not positioned immediately proximal and distal to the model ductus. Therefore, the actual pressure drop across the PDA may have presented lower than measured. However, the goal of this experiment was not to assess stability, as evaluated in Device Stability Testing (see Section 2.4), but rather to obtain feedback on deployment characteristics and potential future improvements, discussed below.
3.4.1.1 Veterinary Cardiologist Feedback on Thin-Walled NFC Device Characteristics
The NFCTn was successfully deployed, retracted, and repositioned, and passed a light push/pull test performed by a boarded veterinary cardiologist (Gordon SG) experienced in catheter based PDA closure. However, there were some concerns regarding the tactile feedback during delivery as well as device visibility under fluoroscopy. First, the NFCTn was more difficult to identify under fluoroscopy compared to the NFCTk and the ACDO (Figure 12). This lack of strut opacity forced the cardiologist to rely mostly on the radiopaque markers located on the proximal and distal tips during placement. Secondly, during deployment of the distal cages, cardiologists typically feel and visualize the distal disk of the ACDO as it is advanced out of the sheath and abruptly expands to its preformed shape. In comparison to both the ACDO and NFCTk prototype, the NFCTn exhibited minimal feedback to the cardiologist in this regard. Additionally, the distal cage of the NFCTn was easily deformed and did not provide a noticeable change in resistance to the cardiologist during gentle retraction to indicate that the distal cage had engaged the pulmonary side of the ductal ostium. Lastly, the proximal cage did not return to its programmed cup shape following delivery in the PDA ampulla, and following delivery of the proximal cage, it was relatively easy to push the entire device through the ductus into the pulmonary artery. It was noted that the lack of stability noted with a gentle push test in combination with the lack of tactile feedback during delivery/deployment may make the NFCTn prototype sub-optimum in comparison to the ACDO. However, it is believed that these issues are related to the thin struts and increasing their thickness and respective stiffness, could improve these qualities.
Figure 12.
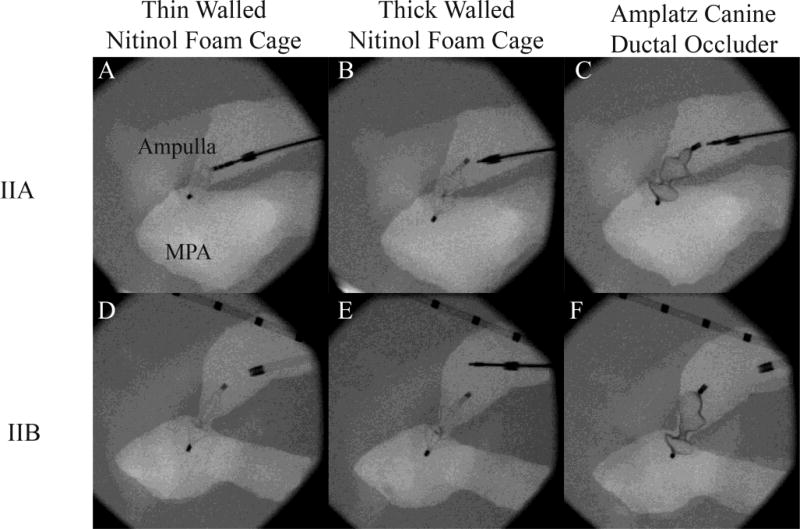
Devices deployed into physiological patent ductus arteriosus models by a veterinary cardiologist under fluoroscopic guidance. A–C: Thin-walled nitinol foam cage prototype (NFCTn) (still attached to delivery cable), thick-walled nitinol foam cage prototype (NFCTk), and Amplatz® canine ductal occluder (ACDO) (left to right) deployed into the IIA physiological model. D–F: NFCTn, NFCTk, and ACDO (left to right) fully deployed into the IIB physiological model. All devices were successfully delivered, with the NFCTk and ACDO having a similar feel and deployment characteristics.
3.4.1.2 Veterinary Cardiologist Feedback on Thick-Walled NFC Device Characteristics
The NFCTk prototype was successfully deployed, retracted, repositioned, and passed a strong push/pull test in which the cardiologist applied much more force than what is typically used in normal ACDO deliveries. The increased strut wall thickness for NFCTk (0.06 mm) compared to the NFCTn (0.038 mm,) greatly improved the overall feel and visibility of the device under fluoroscopy (Figures 12). The NFCTk strut thickness did offer more resistance than the NFCTn while advancing through the catheter, but it was still noted to be less than the ACDO, and was not an issue. The distal cage of the NFCTk exhibited characteristic tactile feedback when the distal cage was delivered and offered noticeably higher resistance compared to the NFCTn prototype when retracted against the pulmonic ostium of the ductus, indicating to the cardiologist that it was in proper position and the proximal cage could be unsheathed. Like the NFCTk, the proximal cage of the NFCTk did not return to its programmed cupped shape. It was noted that this prototype felt similar to the ACDO as it was unsheathed and the cardiologist received appropriate tactile feedback to allow appropriate cage delivery and positioning. In summary, the increased tactile feedback and greater stiffness provided by the NFCTk prototype increases its ease of delivery compared to the NFCTn device.
3.4.1.3 Veterinary Cardiologist Feedback on ACDO Characteristics
The ACDO was successfully deployed, retracted, and repositioned in the models. It offered the most resistance while advancing through the sheath, even compared to the NFCTk. The proximal cage of the ACDO did not return to its preformed shape and looked nearly identical to that of the NFCTk. This indicated that all devices (ACDO and NFC) were either oversized relative to the ampulla of the model, or more likely, the rigid model did not allow for ampulla distention and expansion, preventing return to their cupped geometries. In the future, flexible PDA models using the physiological anatomies should be created to more accurately mimic the deployment characteristics.
5. Conclusions
While the ACDO is effective at treating canine PDA, it’s large delivery system profile can be limiting when treating smaller dogs with narrow vasculature. The superelasticity of the dense nitinol mesh of the ACDO is used to provide both stability and occlusion, whereas in the NFC, the superelasticity of the nitinol is used to provide stability and an SMP foam is used for occlusion. The reduction in material volumes in the NFC designs has multiple benefits, including improvement in ease of delivery by reducing both the force required to advance the device through the catheter and having an equivalent force required to retract the device into the sheath, and potentially reducing the cost of production.
The NFC prototypes conformed to all design criteria. An interesting aspect of this design is the scalable nature of the prototype. Like the previous version of the prototype, the frame is scalable in that by changing the length of the laser cut struts, the diameter of the cages can be increased or decreased. As the delivery profile of the NFC is determined by the OD of the nitinol tube and compressed SMP foam, we predict that larger waist diameter NFCs can be delivered into smaller catheters and sheaths compared to comparable waist ACDOs. Additionally, by reducing the OD of the nitinol tubing used to create the frame, NFCs with a waist of 4 mm and smaller may be able to be delivered through a 4 Fr catheter (~1 mm ID). Figure 13 shows conceptual 4 mm and 8 mm waist NFC devices alongside the tested 6 mm waist NFC prototype.
Figure 13.
Nitinol foam cage (NFC) device sizes compared to 6 mm Amplatz® canine ductal occluder (ACDO). Left to right: 6 mm ACDO, 4mm NFC (0.889 mm (0.035”) OD × 0.787 mm (0.031”) ID tubing), 6mm NFC (1.12 mm OD × 1 mm ID tubing), 8mm NFC (1.12 mm OD × 1 mm ID tubing).
Based on the supporting stability and deployment results for the NFC devices, we plan to pursue the NFCTk design in future studies due to the cardiologist’s positive feedback regarding its performance and familiar feel. The increased strut thickness should not negatively impact device stability or increase migration, and may improve these aspects by further resisting compression. Strut integrity of the NFCTk devices will need to be evaluated in a future study using improved fabrication techniques, as the current excimer laser cutting method results in rough cut strut edges, which could reduce fatigue life. With these changes to the strut thickness, and the positive results from the presented studies, including acute strut integrity, lower generated wall tension, minimal device migration, and positive veterinary cardiologist feedback, it is believed that the NFC could be used to treat canine PDA.
Patent ductus arteriosus occlusion prototype compared to commercial device
Prototype exhibited acute integrity: one million cycles
Prototype generated lower wall tension in mock vessel wall
Prototype exhibited stability at physiological and elevated pressures in vitro
Veterinary cardiologist feedback: similar deployment qualities to commercial device
Acknowledgments
This work was supported by the NIH National Institute of Neurological Disorders and Stroke Grant U01-NS089692.
Abbreviations
- PDA
Patent ductus arteriosus
- ADO
Amplatzer™ Duct Occluder
- AVP
Amplatzer™ Vascular Plug
- ACDO
Amplatz® Canine Ductal Occluder
- NFC
Nitinol foam cage
- SMP
Shape memory polymer
- Tg
Glass Transition Temperature
- MDD
Minimal ductal diameter
- MPA
Main pulmonary artery
- NFCTn
Thin-walled nitinol foam cage
- NFCTk
Thick-walled nitinol foam cage
- NFCTkEP
Electropolished thick-walled nitinol foam cage
- WA
Wide ampulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achen SE, Miller MW, Gordon SG, Saunders AB, Roland RM, Drourr LT. Transarterial ductal occlusion with the Amplatzer vascular plug in 31 dogs. J. Vet. Intern. Med. 2008;22:1348–1352. doi: 10.1111/j.1939-1676.2008.0185.x. [DOI] [PubMed] [Google Scholar]
- Birchard SJ, Bonagura JD, Fingland RB. Results of ligation of patent ductus-arteriosus in dogs - 201 cases (1969–1988) J. Am. Vet. Med. Assoc. 1990;196:2011–2013. [PubMed] [Google Scholar]
- Buchanan JW. Patent Ductus Arteriousus Morphology, Pathogenesis, Types and Treatment. J. Vet. Cardiol. 2001;3:7–16. doi: 10.1016/S1760-2734(06)70010-8. [DOI] [PubMed] [Google Scholar]
- Bureau SP, Monnet E, Orton EC. Evaluation of survival rate and prognostic indicators for surgical treatment of left-to-right patent ductus arteriosus in dogs: 52 cases (1995–2003) J. Am. Vet. Med. Assoc. 2005;227:1794–1799. doi: 10.2460/javma.2005.227.1794. [DOI] [PubMed] [Google Scholar]
- Campbell FE, Thomas WP, Miller SJ, Berger D, Kittleson MD. Immediate and late outcomes of transarterial coil occlusion of patent ductus arteriosus in dogs. J. Vet. Intern. Med. 2006;20:83–96. doi: 10.1892/0891-6640(2006)20[83:ialoot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cohen J, Litwin SB, Aaron A, Fine S. The rupture force and tensile strength of canine aortic tissue. J. Surg. Res. 1972;13:321–333. doi: 10.1016/0022-4804(72)90083-2. [DOI] [PubMed] [Google Scholar]
- Duerig TW, Tolomeo DE, Wholey M. An overview of superelastic stent design. Minimally Invasive Therapy & Allied Technologies. 2000;9:235–246. doi: 10.1080/13645700009169654. [DOI] [PubMed] [Google Scholar]
- Eyster GE, Eyster JT, Cords GB, Johnston J. Patent ductus arteriosus in the dog: Characteristics of occurrence and results of surgery in one hundred consecutive cases. J. Am. Vet. Med. Assoc. 1976;168:435–438. [PubMed] [Google Scholar]
- Franks JN. Patent ductus arteriosus. In: Harari J, editor. Small animal surgery secrets. second. Hanley & Belfus; Philadelphia, PA: 2004. pp. 120–124. [Google Scholar]
- Glaus TM, Berger F, Ammann FW, Kiowski W, Ohlert S, Boller M, Kastner S, Reusch CE, Sisson D. Closure of large patent ductus arteriosus with a self-expanding duct occluder in two dogs. J. Small. Anim. Pract. 2002;43:547–550. doi: 10.1111/j.1748-5827.2002.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Goodrich KR, Kyles AE, Kass PH, Campbell F. Retrospective comparison of surgical ligation and transarterial catheter occlusion for treatment of patent ductus arteriosus in two hundred and four dogs (1993–2003) Vet. Surg. 2007;36:43–49. doi: 10.1111/j.1532-950X.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- Gordon SG, Miller MW. Transarterial coil embolization for canine patent ductus arteriosus occlusion. Clin. Tech. Small. An. P. 2005;20:196–202. doi: 10.1053/j.ctsap.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Gordon SG, Saunders AB, Achen SE, Roland RM, Drourr LT, Hariu C, Miller MW. Transarterial ductal occlusion using the Amplatz® canine duct occluder in 40 dogs. J. Vet. Cardiol. 2010;12:85–92. doi: 10.1016/j.jvc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hogan DF, Green HW, Sanders RA. Transcatheter closure of patent ductus arteriosus in a dog with a peripheral vascular occlusion device. J. Vet. Cardiol. 2006;8:139–143. doi: 10.1016/j.jvc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Jackson WF, Henderson RA. Ligature placement in closure of patent ductus arteriosus. J. Am. Anim. Hosp. Assoc. 1979;15:55–58. [Google Scholar]
- Lendlein A, Kelch S. Shape-memory polymers. Angew. Chem. Int. Edit. 2002;41:2034–2057. [PubMed] [Google Scholar]
- MacWilliams J. Optimization of nitinol shape setting through post laser cutting processing. In: Venugopalan R, Wu M, editors. Medical device materials III: Proceedings from the Materials & Processes for Medical Devices Conference 2005; ASM International; Materials Park, OH. 2006. pp. 69–72. [Google Scholar]
- Maitland DJ, Small W, Ortega JM, Buckley PR, Rodriguez J, Hartman J, Wilson TS. Prototype laser-activated shape memory polymer foam device for embolic treatment of aneurysms. J. Biomed. Opt. 2007;12:030504. doi: 10.1117/1.2743983. [DOI] [PubMed] [Google Scholar]
- Miller MW, Gordon SG, Saunders AB, Arsenault WG, Meurs KM, Lehmkuhl LB, Bonagura JD, Fox PR. Angiographic classification of patent ductus arteriosus morphology in the dog. J. Vet. Cardiol. 2006;8:109–114. doi: 10.1016/j.jvc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Moriwaki T, Oie T, Takamizawa K, Murayama Y, Fukuda T, Omata S, Kanda K, Nakayama Y. Variations in local elastic modulus along the length of the aorta as observed by use of a scanning haptic microscope (SHM) J. Artif. Organs. 2011;14:276–283. doi: 10.1007/s10047-011-0596-2. [DOI] [PubMed] [Google Scholar]
- Nguyenba TP, Tobias AH. The Amplatz canine duct occluder: A novel device for patent ductus arteriosus occlusion. J. Vet. Cardiol. 2007;9:109–117. doi: 10.1016/j.jvc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Nguyenba TP, Tobias AH. Minimally invasive per-catheter patent ductus arteriosus occlusion in dogs using a prototype duct occluder. J. Vet. Intern. Med. 2008;22:129–134. doi: 10.1111/j.1939-1676.2007.0009.x. [DOI] [PubMed] [Google Scholar]
- Paprottka KJ, Paprottka PM, Reiser MF, Waggershauser T. Comparative study of the corrosion behavior of peripheral stents in an accelerated corrosion model: experimental in vitro study of 28 metallic vascular endoprostheses. Diagn. Interv. Radiol. 2015;21:403–409. doi: 10.5152/dir.2015.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DF. Epidemiologic and genetic studies of congenital heart disease in the dog. Circ. Res. 1968;23:171–202. doi: 10.1161/01.res.23.2.171. [DOI] [PubMed] [Google Scholar]
- Rodriguez JN, Clubb FJ, Wilson TS, Miller MW, Fossum TW, Hartman J, Tuzun E, Singhal P, Maitland DJ. In vivo response to an implanted shape memory polyurethane foam in a porcine aneurysm model. J. Biomed. Mater. Res. A. 2014a;102:1231–1242. doi: 10.1002/jbm.a.34782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JN, Miller MW, Boyle A, Horn J, Yang CK, Wilson TS, Ortega JM, Small W, Nash L, Skoog H, Maitland DJ. Reticulation of low density shape memory polymer foam with an in vivo demonstration of vascular occlusion. J. Mech. Behav. Biomed. 2014b;40:102–114. doi: 10.1016/j.jmbbm.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AB, Achen SE, Gordon SG, Miller MW. Utility of transesophageal echocardiography for transcatheter occlusion of patent ductus arteriosus in dogs: Influence on the decision-making process. J. Vet. Intern. Med. 2010;24:1407–1413. doi: 10.1111/j.1939-1676.2010.0587.x. [DOI] [PubMed] [Google Scholar]
- Schuessler A, Strohel M. Status and trends of nitinol micromachining techniques. In: Pelton AR, Duerig T, editors. SMST-2003: Proceedings of the International Conference on Shape Memory and Superelastic Technologies; SMST Society Inc; Menlo Park, CA. 2004. pp. 135–141. [Google Scholar]
- Singh MK, Kittleson MD, Kass PH, Griffiths LG. Occlusion devices and approaches in canine patent ductus arteriosus: Comparison of outcomes. J. Vet. Intern. Med. 2012;26:85–92. doi: 10.1111/j.1939-1676.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- Sisson D. Use of a self-expanding occluding stent for nonsurgical closure of patent ductus arteriosus in dogs. J. Am. Vet. Med. Assoc. 2003;223:999–1005. doi: 10.2460/javma.2003.223.999. [DOI] [PubMed] [Google Scholar]
- Small IVW, Singhal P, Wilson TS, Maitland DJ. Biomedical applications of thermally activated shape memory polymers. J. Mater. Chem. 2010;20:3356–3366. doi: 10.1039/B923717H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BJ, Luis-Fuentes V, Darke PGG. Comparison of the incidence of residual shunting between two surgical techniques used for ligation of patent ductus arteriosus in the dog. Vet. Surg. 2003;32:231–237. doi: 10.1053/jvet.2003.50025. [DOI] [PubMed] [Google Scholar]
- Stauthammer CD, Olson J, Leeder D, Hohnadel K, Hanson M, Tobias AH. Patent ductus arteriosus occlsuion in small dogs utilizing a low profile Amplatz canine ductal occluder prototype. J. Vet. Cardiol. 2015;17:203–209. doi: 10.1016/j.jvc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. European Radiology. 2004;14:292–301. doi: 10.1007/s00330-003-2022-5. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Soda A, Saida Y, Sugihara K, Takashima K, Shibazaki A, Yamane Y. Evaluation of the efficacy and safety of coil occlusion for patent ductus arteriosus in dogs. J. Vet. Med. Sci. 2007;69:857–859. doi: 10.1292/jvms.69.857. [DOI] [PubMed] [Google Scholar]
- Tobias AH, Stauthammer CD. Minimally invasive per-catheter occlusion and dilation procedures for congenital cardiovascular abnormalities in dogs. Vet. Clin. N. Am-Small. 2010;40:581–603. doi: 10.1016/j.cvsm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Van Israel N, French AT, Dukes-McEwan J, Corcoran BM. Review of left-to-right shunting patent ductus arteriosus and short term outcome in 98 dogs. J. Small. Anim. Pract. 2002;43:395–400. doi: 10.1111/j.1748-5827.2002.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Wierzbicki MA, Bryant J, Miller MW, Keller B, Maitland DJ. Mechanical and in vitro evaluation of an experimental canine patent ductus arteriosus occlusion device. J. Mech. Behav. Biomed. 2016;59:156–167. doi: 10.1016/j.jmbbm.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]