Abstract
Neural and temperamental mechanisms through which a genetic risk marker in the γ-amino butyric acid α2 receptor subunit (GABRA2) impacts adolescent functioning were investigated. Participants (N = 80; 29 female) completed an emotional word task during functional magnetic resonance imaging. Behavioral control, negative emotionality, and resiliency temperament constructs were assessed. Externalizing and internalizing problems were the outcomes. Those with the GABRA2 minor allele had reduced activation to positive words in the angular gyrus, middle temporal gyrus, and cerebellum, and to negative words in frontal, parietal, and occipital cortices. Reduced activation in the angular gyrus predicted greater negative emotionality, and in turn, elevated externalizing problems. Reduced activation in the inferior parietal cortex predicted greater resiliency, and in turn, low externalizing problems.
Keywords: functional magnetic resonance imaging (fMRI), genes, temperament, externalizing, mediation
Research examining genetic effects on psychopathology has proliferated. Genetic variants act as catalysts for a cascade of behaviors that typically precede psychopathology. An example are polymorphisms in the γ-amino butyric acid (GABA) system through the gene encoding the γ-amino butyric acid α2 receptor subunit (GABRA2). GABA is a major inhibitory neurotransmitter and acts to reduce neuronal excitability across the central nervous system (Edenberg et al., 2004). Replicated associations support the role of the GABRA2 minor (G) allele on problem behavior in adulthood, including alcohol and drug dependence (e.g., Covault, Gelernter, Hesselbrock, Nellissery, & Kranzler, 2004; Edenberg et al., 2004). Prior work has also supported the role of GABRA2 polymorphisms as risk factors for problem behavior in adolescence, including conduct disorder (Dick et al., 2006; Trucco Villafuerte, Heitzeg, Burmeister, & Zucker, 2014). Moreover, one study demonstrated that rule breaking mediated the association between GABRA2 polymorphisms and later substance use during late adolescence (Trucco et al., 2014). This work highlights the importance of testing prospective mechanistic pathways of genetic risk. Yet, this represents only the first step toward elucidating mechanisms underlying genetic effects on maladjustment. Given support for neurobiological differences in risk for addiction (Kalivas and Volkow, 2005), alterations in brain activation may represent pathways of GABRA2 risk to adolescent problem behavior. The aim of the current study was to determine whether individual differences in neural response to emotional stimuli using functional magnetic resonance imaging (fMRI) and childhood temperament mediate the relation between GABRA2 variants and the development of externalizing and internalizing problems in adolescence.
This study targets a specific polymorphism to more precisely characterize the mechanistic structure of problem behavior. Although its functional significance is still unclear, GABRA2 polymorphisms are associated with neural phenotypes, including electroencephalographic activity in adults (Edenberg et al., 2004), activation in the ventral tegmental area and medial frontal cortex in response to alcohol cues in adults (Kareken et al., 2010), and activation of the insular cortex (Villafuerte et al., 2012) and nucleus accumbens (Heitzeg et al., 2014) during reward anticipation in children and adults. GABRA2 also impacts alcohol use via personality differences in adulthood (Villafuerte, Strumba, Stoltenberg, Zucker & Burmeister, 2013). Similar genetic and neurobiological factors may be relevant to phenotypes more germane to adolescence, including temperament and externalizing behavior. To our knowledge, the current study is the first to examine the neurobiological underpinnings of emotion and temperamental factors as mediators between GABRA2 and externalizing and internalizing symptoms.
Recent developmental cascade models detail how psychopathology originates from an interplay of genetic and physiological factors (Dodge, Malone, Lansford, Miller, Pettit, & Bates, 2009). Behavioral genetic models demonstrate that temperament and externalizing behavior are not only genetically influenced, but share a high degree of genetic overlap (Saudino, 2005). Moreover, cascade models posit a sequential progression from biological risk to progressively riskier and more problematic behaviors over time. Namely, biological risk may first be expressed as difficult temperament in childhood, which then progresses to adolescent behavior problems and to more problematic behaviors in adulthood, such as illicit drug use (Dodge et al., 2009). Given that temperament is viewed as an inherited set of motor, emotional, and attentional processes, it is not surprising that childhood temperament predicts adolescent externalizing behavior (Frick and Morris 2004). High negative emotionality (e.g., anger, irritability) predicts externalizing and internalizing behavior (Eisenberg et al., 2001). Low levels of resiliency, which reflect a reduced ability to adaptively respond to novel environments, and low levels of behavioral control, which reflect a reduced ability to appropriately contain impulses, also predict externalizing and internalizing symptoms (Hofer, Eisenberg, & Reiser, 2010). It is important to note that the temperamental construct of resiliency is distinct from the construct of resilience to adversity that is often used in the maltreatment/trauma literature (Cichetti, 2010). Although it is likely that temperamental resiliency may increase the likelihood of overcoming severe adversity, it is likely only one of many factors that impact resilience following a traumatic event (Wong et al., 2006).
Since temperament is believed to stem directly from individual differences in genetic makeup and physiological factors, it follows that childhood temperament is not only more proximal to the expression of genetic risk compared to problem behavior and psychopathology, but temperament, especially resiliency and behavioral control, are likely to mediate the relationship between genetic and physiological factors and later externalizing and internalizing symptomatology. For example, one study demonstrated that even though the effects between genetic risk factors and externalizing behavior were relatively weak, the association between genetic risk factors and temperament (i.e., behavioral control and resiliency) were more robust, as was the association between temperament and later externalizing behavior (Trucco, Hicks, Villafuerte, Nigg, Burmeister, & Zucker, 2016). Similarly, other studies have demonstrated that although the effect size of the association between genetic risk variants and problem behavior is small (e.g., Haberstick, Smolen & Hewitt, 2006; Dick et al., 2006; Trucco et al., 2016), the effect size between temperament and problem behavior is in the moderate to large range (e.g., Burgess, Marshall, Rubin, & Fox, 2003; Oldehinkel, Kartman, de Winter, Veenstra, 2004). Thus, temperament is likely to have a relatively stronger impact on behavior problems compared to specific genetic risk factors. Examining the role of temperament and neurobiological differences as potential mediators is likely to extend prior research by providing a more fine-grained understanding of a mechanistic pathway from GABRA2 to problem behavior in adolescence.
Several studies have investigated biological correlates of temperament and personality. Adolescents with conduct disorder (CD) demonstrated reduced activation in response to negatively valenced pictures in the right anterior cingulate and left amygdala compared to controls (Sterzer, Stadler, Krebs, Kleinschmidt & Poustka, 2005); higher novelty seeking scores were associated with lower anterior cingulate activity in a follow-up study using a similar task (Stadler, Sterzer, Schmeck, Krebs, Kleinschmidt, & Poutska, 2007). More recent work suggests that hypoactivation in response to negatively valenced pictures (e.g., fearful faces) in the amygdala may be more characterstic of a subset of youth with a disruptive behavioral disorder and elevated callous-unemotional traits, compared to youth with attention deficit hyperactivity disorder (ADHD), and matched controls (Jones, Laurens, Herba, Barker, & Viding, 2009; Marsh et al., 2008). One study with adults demonstrated that increased brain activation to positively valenced pictures in both cortical (e.g., inferior temporal gyrus) and subcortical (e.g., putamen) regions were associated with extraversion, while increased brain activation to negative pictures in left frontal and temporal cortical regions were associated with neuroticism (Canli, Zhao, Desmond, Kang, Gross, & Gabrieli, 2001). Extraversion and novelty seeking are closely linked to low behavioral control, while neuroticism is associated with emotional reactivity such as negative emotionality and low resiliency (Eisenberg et al., 2001). Thus, it is likely that difficult temperament and the progression to problem behavior may be due in part to abnormalities in the processing of affective information. Namely, a deficiency in experiencing negative emotions, as demonstrated by decreased activation to negatively valenced stimuli, may lead to a lack of empathy or guilt (Herpertz & Sass, 2000), which is more characteristic of youth with difficult temperament. Prior work has also extended this work to examine possible genetic factors that are associated with emotion processing and subsequent differences in personality. For example, several studies have examined how a specific genetic risk factor related to alcohol dependence (corticotropin-releasing hormone receptor 1) was associated with differences in brain activation during an emotionally valenced word task using late adolescent and adult samples (Glaser et al., 2014; Hsu et al., 2012). Furthermore, individual differences in brain activation to emotionally valenced stimuli went on to impact levels of neuroticism and negative emotionality. Accordingly, it is possible that differences in brain activation to positively and negatively valenced words may represent a mechanism through which GABRA2 impacts temperament during earlier developmental periods.
Current Study
The current study focused on youth participating in the Michigan Longitudinal Study (MLS), which oversamples families of men charged with drunk driving who met criteria for an alcohol use disorder (AUD). Thus, this sample represents youth at high-risk for behavioral problems (see Park & Schepp, 2015). First, we examined GABRA2-related neurobiological differences in emotional reactivity to both positively and negatively valenced words in children (mean age 10.78). We chose emotional words over other emotional stimuli (e.g., faces, pictures) as words necessitate more cognitive processing effort, resulting in subtler influences of genetic risk consistent with prior work (Hsu et al., 2012). We hypothesized that minor (G) allele carriers would demonstrate reduced activation to negatively valenced words, consistent with work supporting GABA as an inhibitory neurotransmitter and prior work on adolescents with CD (Dick et al., 2006; Sterzer et al., 2005; Trucco et al., 2014). We further hypothesized that decreased activation to negative words among children with the minor allele would predict difficult temperament, which in turn would predict externalizing and internalizing problems in adolescence. We examined externalizing and internalizing symptomatology as outcomes given prior work supporting an association between GABRA2 and rule-breaking behavior (Trucco et al., 2014) as well as anxiety (Enoch, Schwartz, Albaugh, Virkkunen & Goldman, 2006). We did not limit our analyses to, or make specific hypotheses regarding, particular brain regions given the paucity of imaging studies on GABRA2 in childhood that focus on emotional reactivity. We also did not make hypotheses regarding responses to positively valenced words as few studies have examined this in youth. Accordingly, the current study represents a preliminary investigation of potential neurobiological mechanisms underlying the development of adolescent externalizing and internalizing problems.
Method
Participants
Participants were youth (N = 80; 29 female) recruited from the MLS to participate in an fMRI study (see Table 1). The MLS is an ongoing prospective study investigating the development of substance use disorders (SUD) among a high-risk sample of families. Namely, the MLS follows families from three different substance use risk categories: 1) families with fathers convicted of drunk driving meeting criteria for an alcohol use disorder (AUD; high risk), 2) a control sample of families from the same neighborhoods as the high risk families where neither parent had a history of SUD (low risk), and 3) community-identified men with an AUD diagnosis and their families (moderate risk) who were identified during community canvassing used to acquire the control families. See Zucker and colleagues (1996) for a full description of the MLS.
Table 1.
Demographic and Psychometric Variables
| AA | AG | GG | Total Sample | Test | |
|---|---|---|---|---|---|
| N | 30 | 39 | 11 | 80 | |
|
| |||||
| Demographic Data | |||||
| Sex (count: M/F) | 17/13 | 24/15 | 10/1 | 51/29 | p = .113ˆ |
| Race and Ethnicity (%) | p = .179ˆ | ||||
| Caucasian | 17.5 | 35.0 | 11.2 | 63.8 | |
| African American | 10.0 | 5.0 | 1.2 | 16.2 | |
| Biracial | 5.0 | 6.2 | 0.0 | 11.2 | |
| Hispanic | 5.0 | 2.5 | 1.2 | 8.8 | |
|
| |||||
| Parental AUD (count: FH+/FH−) | 26/4 | 31/8 | 10/1 | 67/13 | p = .641ˆ |
|
| |||||
| Age at fMRI Scan (mean [SD]) | 10.49 (1.50) |
11.02 (1.27) |
10.68 (1.14) |
10.78 (1.35) | F(2,77) = 1.36, p = .263 |
|
| |||||
| Lifetime Diagnosis (count)* | |||||
| ADHD, any type | 6 | 4 | 0 | 10 | p = .225ˆ |
| Generalized Anxiety Disorder | 1 | 0 | 0 | 1 | p = .520ˆ |
| Major Depression Disorder | 0 | 0 | 0 | 0 | – |
| Oppositional Defiant Disorder | 4 | 4 | 0 | 8 | p = .681ˆ |
| Conduct Disorder | 0 | 1 | 0 | 1 | p = 1.000ˆ |
Note. Race and ethnicity sample totals do not match the sum of the percentages given for each subgroup due to rounding. G refers to the GABRA2 minor allele and A refers to the GABRA2 major allele. AUD = alcohol use disorder; FH+ = alcohol use disorder in one or both parents; FH- = alcohol use disorder in neither parent; ADHD = attention deficit hyperactivity disorder;
Fisher’s two-sided exact test;
7 participants missing DSM-IV diagnoses.
For the current study, participants were excluded if they had any neurological, acute, uncorrected, or chronic medical illness; current Axis I disorder (not including anxiety, CD, ADHD, or SUD); current or recent (within 6 months) treatment with centrally active medications; MRI contraindications (e.g., metal implants, claustrophobia); IQ less than 70; or history of psychosis in first-degree relatives. Participants taking ADHD medication were asked to abstain at least 48 hours before the scan.
Procedure
Participants and multiple other reporters (e.g., teachers, research interviewers) completed assessments following initial recruitment into the MLS (Wave 1, ages 3 to 5) with subsequent assessments occurring every 3 years. Beginning between ages 7 and 12, children were also scanned every 1–2 years. Informed consent from parents and teachers was obtained, as was youth assent from participants.
Measures
fMRI Task
Emotion processing was assessed using a passive word task (Glaser et al., 2014; Heitzeg, Nigg, Yau, Zubieta, & Zucker, 2008). On average, participants were 10.78 (± 1.35) years old when they completed the fMRI for the current study. Words were selected from the Affective Norms for English Words (Bradley and Lang, 1999), which provides valence and arousal norms on separate scales of 1 to 9, with 1 indicating negative valence and low arousal, respectively, and 9 indicating positive valence and high arousal, respectively. Thirty-six words were selected for each condition: negative (valence < 3, arousal > 5; e.g., ugly, mad), neutral (4.5 < valence < 5.5, arousal > 2; e.g., bench, talk), and positive (valence > 7, arousal > 5; e.g., happy, fun).
Words were presented in a block design. Each block had 6 trials (i.e., single word presentations). Each trial lasted 4 seconds: 3 seconds of stimulus-on and 1 second of stimulus-off (during which a fixation mark appeared on the screen). For each trial, participants pressed a button if they understood the word. This was done to encourage passive viewing rather than affective evaluation. Following each block, participants were told to relax and look at the blank screen for 18 seconds. There were 3 runs, each comprising 6 blocks — 2 blocks of each condition — counterbalanced using a Latin Squares design, for a total of 6 blocks (36 words) per condition. The task lasted 12 minutes and 36 seconds. Following the fMRI scan, participants completed a questionnaire in which they were asked to rate the emotional valence of each word on a 9-point scale.
Temperament
The California Child Q-sort (CCQ; Block and Block, 1980) consists of 100 cards with statements describing behavior and personality characteristics of children. Following the day long assessment protocol with each child, the Master’s level research interviewer sorted the cards into nine categories ranging from “least descriptive” to “most descriptive” in order to describe the child’s behavior and personality. Items we selected for analyses were those that pertained to resiliency, negative emotionality, and behavioral control based on the work of Eisenberg and colleagues (2001). The resiliency subscale was comprised of 23 descriptors (e.g., recoups after stress), the negative emotionality subscale was comprised of 11 descriptors (e.g., cries easily), and the behavioral control subscale was comprised of 14 descriptors (e.g., physically cautious). The internal validity for these subscales were adequate in the current sample (αs = 0.70 – 0.88). On average, participants were 11.62 (± 1.76) years old when administered the CCQ for the current study.
Externaling and internalizing symptoms
Externalizing symptoms were assessed using T-scores from the aggressive (e.g., bullying) and delinquency subscales (e.g., steals) of the Teacher Report Form (TRF; Achenbach, 1991). Internalizing symptoms were assessed using T-scores from the withdrawn depressed (e.g., unhappy), anxious depressed (e.g., nervous), and somatic complaints (e.g., headaches) subscales (αs = 0.86 – 0.92). On average, participants were 13.27 (± 0.88) years old when they were rated for the current study.
Control variables
Parent’s lifetime AUD status (0 = neither parent had AUD, 1 = one or both parents had AUD in their lifetime), child’s biological sex, and age at brain scan were also included as covariates.
Genotyping
DNA was genotyped using the Illumina Addiction biology SNP array using the Illumina GoldenGate platform (Hodgkinson et al., 2008). GABRA2 SNP rs279858 (exon 5, K132K) was examined given prior work demonstrating associations between the minor allele and problem behavior and maladaptive personality (Trucco et al., 2014; Villafuerte et al., 2013). All MLS participants (n=1139) were also genotyped for 150 ancestry informative markers (Hodgkinson et al., 2008), and ethnic factor scores were calculated using principal component analysis in SAS 9.3 as in prior work (Glaser et al., 2014). The four scores explaining the highest variance (~96%) were examined to control for population stratification.
fMRI Data Acquisition
Participants were scanned on a 3.0T GE Signa scanner (GE Healthcare) using a T2*-weighted single-shot combined spiral in out sequence (repetition time [TR] = 2000 ms; echo time [TE] = 30 ms; flip angle = 90°; field of view [FOV] = 200 mm; 64 × 64 matrix; in plane resolution = 3.12 × 3.12 mm; slice thickness = 4 mm; Glover & Law, 2001). A high-resolution anatomical T1-weighted scan was obtained (TR = 25 ms; minimum TE; FOV = 25 cm; 256 × 256 matrix; slice thickness = 1.4 mm). To minimize motion, foam padding was used around the head.
Analysis Plan
Since the sample included siblings, multilevel linear modeling assessed potential clustering within families. The amount of variance accounted for by family clustering on study variables was negligible (p values > .08). Thus, given the complexity of the analyses, multilevel linear modeling was not considered further. Missing data patterns were assessed using Little’s MCAR test in SPSS 20: (χ2 = 133.27[117], p = .14). This suggests that missingness did not have a strong impact on findings. Nevertheless, full information maximum likelihood (FIML) estimator in Mplus was implemented.
fMRI data preprocessing
Functional images were reconstructed using an iterative algorithm (Fessler, Lee, Olafsson, Shi & Noll, 2005). Runs exceeding 3 mm translation or 3° rotation in any direction were removed. For the remaining data, participant head motion was corrected using FSL 5.0.2.2 (Analysis Group, FMRIB, Oxford, United Kingdom; Jenkinson, Bannister, Brady, & Smith, 2002). Slice timing corrections, normalization, and smoothing were conducted in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). Functional images were spatially normalized to the Montreal Neurological Institute (MNI) template and smoothed with a 6 mm full-width half-maximum Gaussian spatial smoothing kernel to improve signal-to-noise ratio and account for differences in anatomy. Low frequency noise was removed with a high-pass filter (128 seconds).
Individual subject statistical maps
Analyses were completed using a general linear model. Negative, neutral, and positive words were modeled separately (i.e., each four second trial in its respective block) with the canonical hemodynamic response function. Six motion parameters and white matter signal intensity were modeled as nuisance regressors to remove residual motion artifacts and capture non-task-related noise, respectively. Two contrasts of interest were modeled to isolate affective processing and control for nonspecific lexical and visual processing: positive words versus neutral words (POS) and negative words versus neutral words (NEG).
fMRI group analyses
First, we identified brain regions associated with GABRA2 during the processing of emotional words. Correlational analyses were conducted in SPM8 to detect differences in the hemodynamic response to emotional words related to the number of G alleles (i.e., AA = 0, AG = 1, GG = 2). This additive approach makes the fewest assumptions and is consistent with prior work (e.g., Heitzeg et al., 2014). Type I error was controlled at α = .05 by establishing the statistical significance threshold at p < .001, uncorrected for multiple comparisons, with a 35 voxel extent, determined using simulation results generated by AlphaSim in AFNI (Cox, 1996). Average beta weights from clusters significantly related to GABRA2 were extracted using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002).
Mediation
Next, we tested whether brain activity and temperament (resiliency, negative emotionality, and behavioral control) mediated the effect of GABRA2 on maladjustment. Multiple mediator path models were examined in MPlus 7.11. The first step estimated a path model that included GABRA2, brain areas that differed across GABRA2 genotypes, and temperament. To limit convergence issues, only covariates that were correlated with study variables were included. Paths were estimated for each exogenous variable (i.e., GABRA2, covariates) to each brain area of interest, and from GABRA2 and each brain area of interest to temperament. Covariances were included between all exogenous variables, between brain areas, and between each temperament dimension. Modification indexes (>5) were examined to determine whether additional paths should be included to improve model fit.
The second step involved dropping non-significant paths and adding paths from each remaining brain area and temperament dimension on externalizing and internalizing problems. A covariance was included between externalizing and internalizing. Separate path models were estimated for POS and NEG associations. Bias-corrected confidence intervals (BCCIs) using a bootstrapping sample (n = 5000) were used to assess mediated effects.
Results
Brain regions that were identified as differing across number of G alleles were not correlated with maladjustment, with one exception. Activation in the parahippocampal gyrus was negatively correlated with externalizing behavior (r = −0.38, p = 0.005).
Post-Scanning Questionnaire
A mixed-design 3 (word type) × 3 (GABRA2 group) analysis of variance (ANOVA) was performed to test for differences on emotional valence ratings (with Greenhouse-Geisser correction). There was no main effect of GABRA2 (F2,76 = 2.12, p = 0.127) and no interaction (F2.66,101.15 = 0.25, p = 0.841). Consistent with previous work (Heitzeg et al., 2008), there was a significant main effect of word type in the expected direction (positive > neutral > negative; F1.33,101.15 = 349.19, p < .001).
Brain Imaging
POS contrast
In a whole brain search, GABRA2 minor allele frequency was negatively correlated with blood oxygen-level dependent (BOLD) signal in three regions (see Table 2): left angular gyrus (Brodmann area [BA] 39; Figure 1A), right middle temporal gyrus (BA 37), and right cerebellum (declive). There were no positive correlations between GABRA2 and activation to positive words.
Table 2.
Brain Imaging Results
| Cluster Size | BA/Hemi | MNI Coordinates | t-value | |||
|---|---|---|---|---|---|---|
| Label | x | y | z | |||
| Positive Words vs. Neutral Words (POS) | ||||||
|
| ||||||
| Angular Gyrus (−) | 36 | 39/L | −36 | −60 | 26 | 3.96*** |
| Middle Temporal Gyrus (−) | 81 | 37/R | 44 | −66 | 4 | 4.30*** |
| Cerebellum (−) | 50 | –/R | 48 | −64 | −24 | 4.18*** |
|
| ||||||
| Negative Words vs. Neutral Words (NEG) | ||||||
|
| ||||||
| Orbitofrontal Cortex (−) | 101 | 11/L | −10 | 24 | −24 | 3.81*** |
| Calcarine Sulcus (−) | 74 | 31/L | −28 | −66 | 10 | 3.92*** |
| Precuneus (−) | 389 | 39/R | 28 | −60 | 24 | 4.51*** |
| Superior Frontal Gyrus (−) | 159 | 6/R | 32 | 14 | 56 | 4.41*** |
| Inferior Parietal Cortex (−) | 88 | 40/R | 38 | −48 | 44 | 3.74*** |
| Cuneus (−) | 78 | 7/R | 6 | −78 | 38 | 3.85*** |
| Parahippocampal Gyrus (+) | 57 | 36/L | −22 | −4 | −34 | 4.26*** |
Note. Brain regions correlated with number of G alleles. Negative (−) or positive (+) correlation, where AA = 0, AG = 1, and GG = 2. All clusters are significant at p < .001 (uncorrected) with a 35 voxel extent, corresponding to a corrected-level threshold of p < .05. Cluster size indicates number of voxels; BA, Brodmann area; Hemi, hemisphere; MNI, Montreal Neurological Institute; L, left; R, right;
p < .001
Figure 1.
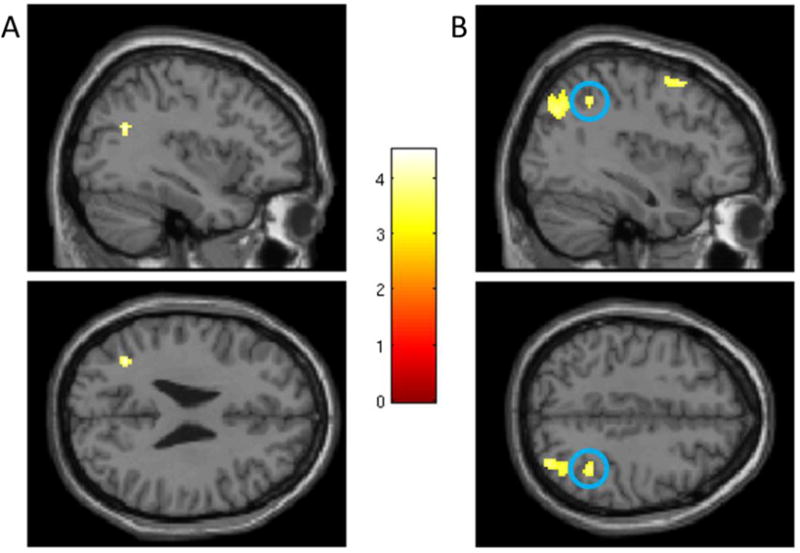
(A) Left angular gyrus activation (centered at x = −36, y = −60, z = 26; top: sagittal view, bottom: axial view) during POS condition. (B) Right inferior parietal cortex activation (circled; centered at x = 38, y = −48, z = 44) during NEG condition. The color bar represents t-values. Coordinates are in MNI space.
NEG contrast
In a whole brain search, GABRA2 minor allele frequency was negatively correlated with BOLD signal in six regions (see Table 2): left orbitofrontal cortex (BA 11), left calcarine sulcus/posterior cingulate (“calcarine sulcus”; BA 31), right precuneus (BA 39), right superior middle frontal gyrus (“superior frontal gyrus”; BA 6), right inferior parietal cortex (BA 40; Figure 1B), and right cuneus/precuneus (“cuneus”; BA 7). The left parahippocampal gyrus (BA 36) was positively correlated with GABRA2. See Table 3 for significant task effects for the POS and NEG contrasts, irrespective of genotype.
Table 3.
Task Effects
| Cluster Size | BA/Hemi | MNI Coordinates | t-value | |||
|---|---|---|---|---|---|---|
| Label | x | y | z | |||
| Positive Words vs. Neutral Words (POS) | ||||||
|
| ||||||
| Medial Frontal Gyrus | 402 | 10/L | −2 | 60 | 14 | 4.33 |
| Medial Frontal Gyrus | 336 | 10/L | −4 | 54 | −6 | 4.11 |
| Superior Frontal Gyrus | 39 | 8/L | −20 | 36 | 46 | 3.56 |
| Anterior Cingulate | 35 | 32/L | −6 | 20 | −8 | 3.53 |
|
| ||||||
| Negative Words vs. Neutral Words (NEG) | ||||||
|
| ||||||
| Inferior Frontal Gyrus | 1496 | 47/L | −44 | 28 | −4 | 6.57 |
| Inferior Frontal Gyrus | 924 | 47R | 46 | 20 | −12 | 5.79 |
| Middle Frontal Gyrus | 58 | 9/R | 40 | 16 | 28 | 3.63 |
| Medial Frontal Gyrus | 2040 | 9/L | −4 | 54 | 42 | 5.94 |
| Medial Frontal Gyrus | 118 | 11/L | −4 | 52 | −12 | 4.46 |
| Anterior Cingulate | 151 | 32/L | −10 | 32 | −6 | 4.36 |
| Superior Temporal Gyrus | 43 | 22/R | 50 | −22 | −8 | 3.82 |
| Middle Temporal Gyrus/Insula | 675 | 21/L | −52 | −30 | −4 | 5.07 |
| Parahippocampal Gyrus | 38 | 28/R | 14 | −10 | −16 | 3.94 |
| Inferior Occipital Gyrus | 178 | 18/R | 34 | −94 | −10 | 4.62 |
| Inferior Occipital Gyrus | 195 | 19/L | −42 | −76 | −12 | 4.33 |
| Thalamus | 394 | –/B | 0 | −10 | 6 | 4.37 |
| Thalamus | 174 | –/L | −6 | −30 | −4 | 4.48 |
One peak voxel is reported per cluster. All clusters were significant at p < .001 (uncorrected) with a 35 voxel extent, corresponding to a corrected-level threshold of p < .05. Cluster size indicates number of voxels.
BA, Brodmann area; Hemi, hemisphere; MNI, Montreal Neurological Institute; L, left; R, right; B, bilateral
Mediation Models
Boys had lower activation in the angular gyrus (r = −0.23, p < .05) and middle temporal gyrus (r = −0.22, p < .05), one ethnic factor was negatively correlated with GABRA2 (r = −0.24, p < .05), and parent AUD status was associated with lower behavioral control (r = −0.25, p < .05). Accordingly, sex, ethnicity, and parental AUD were included as covariates.
POS contrast
In the first step, we tested three sets of associations: 1) genotype on brain activation (angular gyrus, middle temporal gyrus, and cerebellum activation); 2) genotype on temperament; and 3) brain activation on temperament. Adolescents with the GG genotype had blunted activation in the angular gyrus (path estimate = −0.40, p < .001), middle temporal gyrus (path estimate = −0.40, p < .001), and cerebellum (path estimate = −0.45, p < .001). Activation in the angular gyrus predicted lower negative emotionality (path estimate = −0.31, p < .05) and greater behavioral control (path estimate = 0.37, p < .01).
In the second step, we trimmed non-significant brain areas and resiliency, and added externalizing and internalizing behavior (see Figure 2A). The effect of GABRA2 on angular gyrus activation remained significant, as was the effect of angular gyrus activation on negative emotionality and behavioral control. The indirect effect of GABRA2 on negative emotionality (0.20, 95% BCCI = 0.06 – 0.43) and behavioral control (−0.15, 95% BCCI = −0.34 – −0.04) via angular gyrus activation were significant. Approximately 34.5% of the total effect of GABRA2 on negative emotionality operated through angular gyrus activation, while 42.0% of the total effect of GABRA2 on behavioral control operated through angular gyrus activation. In turn, negative emotionality predicted greater externalizing behavior. The indirect effect of angular gyrus activation on externalizing behavior via negative emotionality (−1.54, 95% BCCI = −4.17 – −0.22) was significant. Approximately 65.7% of the total effect of angular gyrus activation on externalizing behavior operated through negative emotionality. Accordingly, there was evidence for multiple mediation in the association between GABRA2 and externalizing behavior through angular gyrus activation during POS and negative emotionality.
Figure 2.
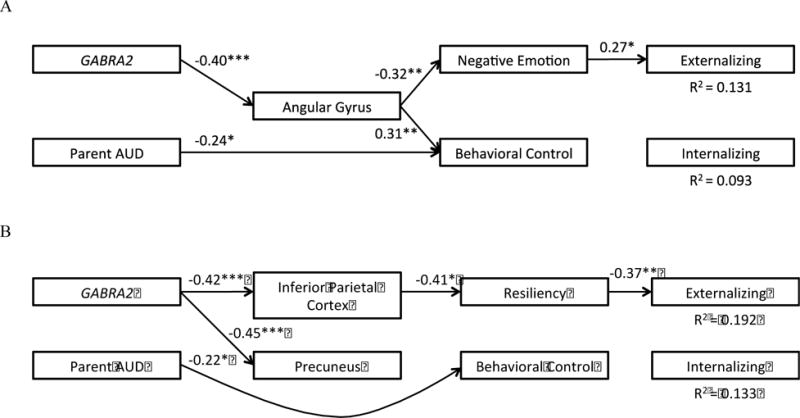
Path models testing whether brain activity and temperament mediate the effects of GABRA2 on maladjustment. (A) Standardized path coefficients in POS condition. Model fit: χ2 = 11.46 (10), p = .32, RMSEA = 0.04, CFI = 0.96, TLI = 0.89. (B) Standardized path coefficients in NEG condition. Model fit: χ2 = 5.11 (5), p = .40, RMSEA = 0.02, CFI = 1.00, TLI = 0.99. Only significant paths are represented (* = p < .05, ** = p < .01, *** = p < .001). Biological sex and ethnicity are not depicted.
NEG contrast
In the first step, we tested three sets of associations: 1) genotype on brain activation (orbitofrontal cortex, calcarine sulcus, precuneus, superior frontal gyrus, inferior parietal cortex, cuneus, and parahippocampal gyrus activation); 2) genotype on temperament; and 3) brain activation on temperament. GABRA2 negatively predicted brain activation in the orbitofrontal cortex, calcarine sulcus, precuneus, superior frontal gyrus, inferior parietal cortex, and cuneus (all ps < .001), but positively predicted brain activation in the parahippocampal gyrus (0.42, p < .001). Activation in the inferior parietal cortex predicted lower resiliency (−0.57, p < .05), while precuneus activation predicted lower behavioral control (−0.56, p < .05).
In the second step, we trimmed non-significant brain areas and negative emotionality, and added externalizing and internalizing behavior (see Figure 2B). The effect of GABRA2 on inferior frontal gyrus activation and precuneus activation remained significant. The effect of inferior parietal cortex activation on resiliency remained significant; however, the effect of precuneus activation on behavioral control was no longer significant. The indirect effect of GABRA2 on resiliency via inferior parietal cortex activation (0.18, 95% BCCI = 0.04 – 0.39) was significant. Approximately 73.7% of the total effect of GABRA2 on resiliency operated through inferior parietal cortex activation. In turn, resiliency predicted lower externalizing behavior. The indirect effect of inferior parietal cortex activation on externalizing behavior via resiliency (2.00, 95% BCCI = 0.30 – 5.41) was significant. Approximately 32.1% of the total effect of inferior parietal cortex activation on externalizing behavior operated through resiliency. Accordingly, there was evidence for multiple mediation in the association between GABRA2 and externalizing behavior through inferior parietal cortex activation during NEG and resiliency.
Discussion
This study examined potential neurobiological and endophenotypic mechanisms through which GABRA2 variants impact externalizing and internalizing symptoms. The minor (G) allele was associated with decreased neural activation in the angular gyrus, middle temporal gyrus, and cerebellum to positively valenced words and decreased neural activation in the orbitofrontal cortex, calcarine sulcus, precuneus, superior frontal gyrus, inferior parietal cortex, and cuneus to negatively valenced words. There was evidence for multiple mediation in the association between GABRA2 and externalizing behavior through angular gyrus activation to positively valenced words and negative emotionality. There was also evidence for multiple mediation in the association between GABRA2 and externalizing behavior through inferior parietal cortex activation to negatively-valenced words and resiliency.
Prior studies demonstrate that emotional word tasks activate brain regions spanning the angular gyrus (BA 39) and middle temporal gyrus (posterior; BA 21) in adult samples (Hsu et al., 2012; Kuchinke et al., 2005). Moreover, word versus non-word recognition takes place largely in the left angular gyrus (BA 39), as well as the left dorsal prefrontal cortex in the superior (BA 6, 8, 9) and middle (BA 6, 8) frontal gyri, left posterior cingulate gyrus and precuneus (BA 23, 29–31, 7) and the junction of the left posterior middle temporal and inferior temporal gyri (BA 21, 37; Binder et al., 2003). The work of Hsu and colleagues (2012) extends this work by examining whether adults carrying a specific genetic risk factor (corticotropin-releasing hormone receptor 1) exhibit differences in brain activation to negative words and whether personality traits were associated with brain activity depending on genotype. Findings indicate that differences in activation in the middle temporal gyrus were associated with neuroticism (a construct that is highly correlated with negative emotionality and low resiliency) depending on genotype (Hsu et al., 2012). Overall, these findings are consistent with the brain regions that demonstrated differences across GABRA2 genotypes in the current study, as well as a relationship between differences in brain activity and negative emotionality and resiliency in this adolescent sample.
Several theories can help explain how neural hypoactivation to emotionally charged experiences relates to later externalizing behavior. One theory is the stimulation-seeking theory. Stimulation-seeking theory posits that low arousal in youth exhibiting externalizing behavior, especially in youth with CD, causes an unpleasant internal state (Frick and Morris, 2004). These individuals seek out external stimulation from the environment to increase their arousal to an optimal level. According to Heller and Nitschke’s (1997) valence (pleasant vs. unpleasant) and arousal (low vs. high) dichotomy, the processing of arousal is mediated primarily by the temporoparietal cortex; this is supported by functional neuroimaging, lesion, and event-related potential electroencephalography studies (e.g., Dolcos and Cabeza, 2002). These youth may be more likely to seek out external stimulation from the environment, regardless of whether it is positive or negative, to increase their arousal to an optimal level.
Moreover, a robust biological marker of externalizing is autonomic underarousal as indexed by low electrodermal arousal and autonomic hyporesponsiveness to emotional stimuli. Adolescents diagnosed with CD demonstrated lower electrodermal response to pictures independent of valence (Herpertz et al., 2005) and abnormal right dorsal anterior cingulate cortex deactivation when viewing negative pictures compared to controls (Sterzer et al., 2005). Given replicated associations between GABRA2 and externalizing behavior and CD (Dick et al., 2006; Trucco et al., 2014), it is not surprising that lower emotional arousal characterizes those with the G allele in the current study. It is also not surprising that brain regions that demonstrated differences in activation across genotype in the current study were consistent with prior work examining differences in brain activation to negatively valenced stimuli among youth with CD. It is possible that G allele carriers experience low arousal as evidenced by decreased neural activation in temporoparietal regions to both positively and negatively valenced words. In turn, this low level of arousal among G allele carriers may contribute to the development of externalizing behavior over time. Engagement in externalizing behavior may be one way in which G allele carriers try to increase their arousal to an optimal level. Consistent with cascade models, G allele carriers may need to engage in progressively riskier behaviors over time, such as illicit drug use in adulthood, to maintain an optimal level of arousal. These findings may provide a greater understanding of neurobiological factors associated with the GABRA2 minor allele and provide support for the linkage of the externalizing pathway to substance use in late adolescence found in prior studies (Trucco et al., 2014). Given that GABRA2 and emotional hypoarousal are associated primarily with externalizing behavior, this may also explain weak effects on internalizing behavior. It is important to note that the current study did not include a direct measure of arousal. Accordingly, future work is necessary to confirm this interpretation.
Another theory that can help us understand how hypoactivation to emotionally valenced stimuli can contribute to later externalizing behavior is the emotion dysregulation theory. The theory suggests that impairment in emotion regulation can impact later problem behavior (Cappadocia, Desrocher, Pepler, & Schroeder, 2009). That is, youth diagnosed with CD often experience affect-evoked suppression of neural activity, which likely interferes with cognitive functions that are integral to the regulation of emotional behaviors and recognition of emotionally valenced stimuli, such as facial expressions (Blair, Colledge, Murray, & Mitchell, 2001; Sterzer et al., 2005). When viewing emotionally valenced pictures, males with severe CD demonstrated less activation in the amygdala and the anterior cingulate cortex compared to controls (Sterzer et al., 2005). A study using the emotional word paradigm demonstrated that adolescents and young adults at risk for developing alcohol dependence had decreased ventral striatal and extended amygdala activation compared to resilient youth, which was further associated with more externalizing problems (Heitzeg et al., 2008). Although specific brain regions in the current study differ from prior work, the association between blunted activation to emotionally valenced words and behavior problems is consistent. There are several factors that could account for differences in specific activation regions identified. First, emotion circuitry is developing from childhood into adulthood (e.g., Blakemore & Choudhury, 2006); thus, the current findings may be specific to emotion circuitry in late childhood. Second, the hypotheses tested and analytical approaches differed across studies, with previous work (Sterzer et al., 2005; Heitzeg et al., 2008) comparing brain activation based on groups that differed in behavior problems, and the current work focusing on GABRA2 variation. It is also important to note that the regions identified in this study have been associated with other processes beyond emotion dysregulation and arousal. For example, the angular gyrus is integral to semantic retrieval (Price, 2000), attentional processing, and encoding salient events in the environment (see Seghier, 2013). Therefore, other processes may be involved that were not directly examined.
Both the stimulation-seeking and the emotion dysregulation theory support temperament as a mechanism through which neurobiological underpinnings impact later functioning. Autonomic underarousal was associated with greater impulsivity and lower effortful control (Fowles, Kochanska, & Murray, 2000), which is consistent with our findings that reduced angular gyrus activation predicted lower levels of behavioral control during POS. Prior work also suggests that hypofunction in the right temporoparietal cortex in depressed individuals may signal reduced arousal to positively valenced stimuli and low positive emotionality (Kovacs and Lopez-Duran, 2010). Our findings indicate that reduced angular gyrus activation predicted greater negative emotionality during POS. Similarly, deactivation in response to emotionally valenced pictures may represent deficits in processing emotional cues from the environment that lead to engagement in externalizing behavior (Stadler et al., 2007; Sterzer et al., 2005), especially among a subgroup of youth with conduct problems and elevated callous-unemotional traits (Jones et al., 2009; Marsh et al., 2008). Youth high in externalizing were characterized as emotionally undercontrolled (i.e., high on negative emotionality, low on resilience) compared to youth with internalizing problems and healthy controls (Eisenberg et al., 2001).
There may be a potential tradeoff to reduced activation to emotional stimuli among carriers of the G allele. When exposed to negative stimuli, reduced activation seems protective. Reduced activation to negative words predicted greater resiliency, which then predicted low externalizing behavior. Yet, reduced activation may interfere with experiencing pleasure. Reduced activation to positive words predicted greater negative emotionality, which then predicted high externalizing behavior. Reduced activation to emotionally valenced stimuli among those carrying the G allele may promote adaptive or maladaptive outcomes depending on the context. This is consistent with work supporting GABRA2 as a plasticity factor (e.g., Trucco, Villafuerte, Burmeister, & Zucker, in press; Trucco, Villafuerte, Heitzeg, Burmeister, & Zucker, 2016). That is, GABRA2 variants may increase susceptibility to maladaptive and supportive contexts alike. Involvement in delinquent behavior such as stealing may provide the stimulation needed to obtain optimal arousal for some youth. Similarly, prosocial behavior such as athletics may increase arousal to an optimal level. These adolescents may be susceptible to social contexts that promote risk-taking behaviors such as affiliation with deviant peers, as well as social contexts that promote more adaptive functioning such as prosocial peers. Thus, traditional conceptualizations of GABRA2 variants as purely risk factors may be imprecise as these youth may also demonstrate more adaptive outcomes depending on the context. Although social contexts were not assessed in this study, it may be that exposure to emotional words had a similar effect on youth as environmental exposures. Future work examining these associations across social contexts is necessary.
Limitations to the current study should be noted. Our sample was comprised mostly of boys and enriched with families with AUD. Our findings may not generalize to females or healthy community-based samples. Also, there is likely a more complex structure underlying problem behavior, with the included variables explaining a small amount of variance. Future studies should examine other pathways to youth maladjustment, especially those relevant to internalizing. Although the distribution of GABRA2 genotypes is consistent with prior work, a small portion of G homozygotes were examined given our sample size. Future work should include social contexts as moderators given strong evidence for gene × environment interactions. Given these limitations, study findings should be considered preliminary and caution is warranted when drawing inferences given that a replication sample was not included.
Clinical Implications.
Our findings indicate that a key mechanism through which GABRA2 variants impact later problem behavior is via brain activation to emotionally valenced stimuli and temperament. Yet, this pathway is not straightforward. Blunted activation to emotional stimuli may pose a risk in some contexts, but may be protective in other contexts among those carrying the minor GABRA2 allele. Not only does this demonstrate that genes are not necessarily deterministic, it suggests that important nuances in pathways to adolescent functioning can help guide individualized approaches to treatment. For example, if blunted activation to emotional stimuli demonstrated among G-allele carriers is a result of autonomic underaousal, then these adolescents may benefit from interventions focused on increasing arousal levels through biofeedback techniques, especially towards positively-valenced stimuli. Given that the efficacy of biofeedback for use with adolescents has not been widely tested (Raine, 1996), it may be more useful as an addition to multimodal treatment packaging for externalizing behavior than as a standalone technique. For example, the Fast Track Prevention Program (Conduct Problems Prevention Research Group, 1992) is a multicomponent intervention program that targets social-cognitive deficits related to emotion regulation among other key risk factors (i.e., parent behavior management, peer relations, academic skills and classroom behavior). Similarly, the Making Choices: Social Problem Solving Skills for Children (MC) program may be beneficial for these youth since there is a focus on strengthening emotional, social, and cognitive skills associated with building relationships (Fraser et al., 2005). Yet, given the current state of knowledge regarding the genetic architecture underlying adolescent behavior problems, genetic screenings are likely to provide minimal gain in the effectiveness of interventions with possible costs. For example, genetic screenings that connect a genetic disposition to externalizing behavior could lead to stigmatization and discrimination (Chhangur, Weeland, Matthys, & Overbeek, 2015). Although these proposed approaches may be especially beneficial for youth carrying the minor GABRA2 allele, these findings should be replicated with larger and more diverse samples before firm recommendations for individualized treatment based on genetic makeup can be offered.
Acknowledgments
This research was supported by the National Institutes of Health [K08AA023290 to E.M.T., R01DA027261 to M.M.H & R.A.Z., R01AA12217 to R.A.Z. & M.M.H, R37/R01AA07065 to R.A.Z. & M.M.H., T32DA07267 to Margaret Gnegy, T32DA07268 to John Traynor, T32AA07477 to Fred Blow]; and the University of Michigan [UL1TR000433 to L.M.C.]. We thank families that participated in the Michigan Longitudinal Study.
Footnotes
The authors declare no conflicts of interest.
References
- Achenbach TM. Manual for the Youth Self-Report for ages 11–18. Burlington: University of Vermonth Department of Psychiatry; 1991. [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15(3):372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29:491–498. doi: 10.1023/A:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Block JH, Block J. The role of ego-control and ego-resiliency in the organization of behavior. In: Collins WA, editor. Development of cognition, affect, and social relations: The Minnesota Symposia on Child Psychology. Vol. 13. Hillsdale: Erlbaum; 1980. pp. 39–101. [Google Scholar]
- Bradley MM, Lang PJ. Affective Norms for English Words (ANEW) Gainsville: NIMH Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Paper presented at: 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan: 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Burgess KB, Marshall PJ, Rubin KH, Fox NA. Infant attachment and temperament as predictors of subsequent externalizing behaviors are cardiac physiology. Journal of Child Psychology and Psychiatry. 2003;44:819–831. doi: 10.1111/1469-7610.00167. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JDE. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037//0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Cappadocia MC, Desrocher M, Pepler D, Schroeder JH. Contextualizing the neurobiology of conduct disoreder in an emotion dysregulation framework. Clinical Psychology. 2009;29:506–518. doi: 10.1016/j.cpr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Chhangur RR, Weeland J, Matthys W, Overbeek G. Gene by environment research to prevent externalizing behavior: Ethical questions raised from a public healthcare perspective. Public Health Ethics. 2015;8:295–304. doi: 10.1093/phe/phv024. [DOI] [Google Scholar]
- Conduct Problems Prevention Research Group. A developmental and clinical model for the prevention of conduct disorders: The FAST Track Program. Development and Psychopathology. 1992;4:509–527. [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol depedence. American Journal of Medical Genetics B: Neuropsychiatry Genetics. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE. A dynamic cascade model of the development of substance-use onset. Monographs of the Society for Research in Child Development. 2009;74:1–134. doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Cabeza R. Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:252–263. doi: 10.3758/CABN.2.3.252. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Begleiter H. Variations in GABRA 2, encoding the α2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. American Journal of Meidcal Genetics Part B: Neuropsychiatric Genetics. 2006;141:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J, Lee S, Olafsson V, Shi H, Noll D. Toeplitz-based iterative image reconstruction for MRI with correction for magnetic field inhomogeneity. IEEE Transactions on Signal Processing. 2005;53:3393–3402. doi: 10.1109/TSP.2005.853152. [DOI] [Google Scholar]
- Fowles DC, Kochanska G, Murray K. Electrodermal activity and temperament in preschool children. Psychophysiology. 2000;37:777–787. [PubMed] [Google Scholar]
- Fraser MW, Galinsky MJ, Smokowski PR, Day SH, Terzian MA, Rose RA, Guo S. Social information-precessing skills training to promote social competence and prevent aggressive behavior in the the third grades. Journal of Consulting and Clinical Psychology. 736:1045–1055. doi: 10.1037/t10619-000. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Morris S. Temperament and developmental pathways to conduct problems. Journal of Clinical Child and Adolescent Psychology. 2004;33:54–68. doi: 10.1207/S15374424JCCP3301_6. [DOI] [PubMed] [Google Scholar]
- Glaser Y, Zubieta JK, Hsu DT, Villafuerte S, Mickey BJ, Trucco EM, Heitzeg MM. Right ventrolateral prefrontal cortex mediates the effect of the corticotropin-releasing hormone receptor 1 (CRHR1) gene variation on negative emotionality and alcohol use. Journal of Neuroscience. 2014;34:4099–4107. doi: 10.1523/JNEUROSCI.3672-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;24:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Smolen A, Hewitt JK. Family-based association test of the 5HTTLPR and aggressive behavior in a general population sample of children. Biological Psychiatry. 2006;59:836–843. doi: 10.1016/j.biopsych.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: Differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism, Clinical and Experimental Research. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Villafuerte S, Weiland BJ, Enoch M, Burmeister M, Zubieta J, Zucker RA. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39:3077–3086. doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. Regional brain activity in emotion: A framework for understanding cognition in depression. Cognition & Emotion. 1997;11:637–661. doi: 10.1080/026999397379845a. [DOI] [Google Scholar]
- Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. American Journal of Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen P, Heinz E, Lobos EA, Goldman D. Addictions biology: Haplotype-based analysis for 130 candidate genes on a single array. Alcohol and Alcoholism. 2008;42:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer C, Eisenberg N, Reiser M. The role of socialization, effortful control, and ego-resiliency in French adolescents’ social functioning. Journal of Research on Adolescence. 2010;20:555–582. doi: 10.1111/j.1532-7795.2010.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Mickey BJ, Langenecker SA, Heitzeg MM, Love TM, Wang H, Kennedy SE, Peciña M, Shafir T, Hodgkinson CA, Enoch MA, Goldman D, Zubieta JK. Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene influences fMRI signal responses during emotional stimulus processing. Journal of Neuroscience. 2012;32(9):3253–3260. doi: 10.1523/JNEUROSCI.5533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivation to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/foc.5.2.foc208. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherhill L, Dzemidzic M, Bragulat W, Cox C, Talavage T, O’Connor SJ, Foroud T. A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcoholism: Clinical and Experimental Research. 2010;34:2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Lopez-Duran N. Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: Implications for prevention. Journal of Child Psychology and Psychiatry. 2010;51:472–496. doi: 10.1111/j.1469-7610.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo MLH, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. NeuroImage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Blair RJR. Reduced anygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, de Winter AF, Veenstra R. Temperament profiles associated with internalizing and externalizing problems in preadolescence. Development & Psychopathology. 2004;16:421–440. doi: 10.1017/S0954579404044591. [DOI] [PubMed] [Google Scholar]
- Park S, Schepp KG. A systematic review of research on children of alcoholics: Their inherent resilience and vulnerability. Journal of Child and Family Studies. 2015;25(5):1222–1231. doi: 10.1007/s10826-014-9930-7. [DOI] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior biosocial perspectives and treatment implications. Annals of the New York Academy of Sciences. 1996;794(1):46–59. doi: 10.1111/j.1749-6632.1996.tb32508.x. [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. Journal of Developmental and Behavioral Pediatrics. 2005;26:214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus multiple functions and multiple subdivisions. The Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: Association with temperament traits. Journal of Psychiatric Reearch. 2007;41:410–417. doi: 10.1016/j.jpsychires.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Trucco EM, Hicks BM, Villafuerte S, Nigg JT, Burmeister M, Zucker RA. Temperament and externalizing behavior as mediators of genetic risk on adolescent substance use. Journal of Abnormal Psychology. 2016;125(4):565–575. doi: 10.1037/abn0000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA. Rule breaking mediates the developmental association between GABRA2 and adolescent substance abuse. Journal of Child Psychology and Psychiatry. 2014;55:1372–1379. doi: 10.1111/jcpp.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Burmeister M, Zucker RA. Beyond risk: Prospective effects of GABA Receptor Subunit Alpha-2 (GABRA2) × Positive Peer Involvement on adolescent behavior. Development and Psychopathology. doi: 10.1017/S0954579416000419. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA. Susceptibility effects of GABA receptor subunit alpha-2 (GABRA2) variants and parental monitoring on externalizing behavior trajectories: Risk and protection conveyed by the minor allele. Development and Psychopathology. 2016;28:15–26. doi: 10.1017/S0954579415000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Yao W-YW, Majczenko K, Zubieta J-K, Zucker RA, Burmeister M. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Molecular Psychiatry. 2012;17:511–519. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Strumba V, Stoltenberg SF, Zucker RA, Burmeister M. Impulsiveness mediates the association between GABRA2 SNPs and lifetime alcohol problems. Genes, Brain, and Behavior. 2013;12:525–531. doi: 10.1111/gbb.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K. Behavioral control and resiliency in the onset of alcohol and illict drug use: A prospective study from preschool to adolescence. Child Development. 2006;77:1016–1033. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerlad HE, Bingham CR, Sanford K. Other evidence for at least two alcoholisms II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology. 1996;8:831–848. doi: 10.1017/S0954579400007458. [DOI] [Google Scholar]


