Abstract
Background
Sleep Disturbances (SDs) are a symptom common to mental health disorders (MHD) and substance use disorders (SUD). We aimed to identify the value of SD as a predictor for subsequent treatment of illicit drug and alcohol use disorders (SUDs) in primary care and relative to the predictive value of mental health disorders (MHDs).
Methods
We used electronic health records data from ambulatory primary care in a safety net Boston area healthcare system from 2013-2015 (n=83,920). SUD (separated into illicit drug use disorder and alcohol use disorder) and MHD were identified through ICD-9 codes and medical record documentation. We estimated Cox proportional hazard models to examine the risk of SUD across four comparison groups (SD only, SD and MHD, MHD only, and neither SD nor MHD).
Results
Compared to patients with no sleep or MHD, patients with SD had a greater risk for subsequent SUD treatment. Approximately one-fifth of patients with SD were treated for an illicit drug use disorder and approximately 12% were treated for alcohol use disorder. Risk for SUD treatment, estimated at over 30% by the end of the study, was greatest for patients with a MHD, either alone or comorbid with SD. Risk was greater for older patients and men, and lower for minority patients.
Conclusions
SD and MHD, individually and comorbid, significantly predict subsequent treatment of illicit drug and alcohol use disorder in primary care. Screening and evaluation for SD should be a routine practice in primary care to help with identifying SUD risk.
Keywords: sleep disturbance, substance use disorder, adequate care
1. Introduction
Sleep disturbances (SD) include inability to sleep (insomnia), excessive sleepiness (hypersomnia), daytime sleepiness, abnormal movement or behavior during sleep, or inability to fall asleep at the appropriate time (Harvey & Asarnow, 2014). Sleep disturbances are considered as symptoms of other diagnoses, such as psychiatric disorders including major depressive disorder, bipolar disorder, and autism (Baker & Richdale, 2015; Cohen & Sharkey, 2017) or medical illness, such as diabetes, obesity, and cardiovascular disease (Kamath, Prpich, & Jillani, 2015). SDs are a symptom of virtually all mental health disorders (MHD) and substance use disorders (SUD), most commonly insomnia or hypersomnia (Arnedt, Conroy, & Brower, 2012; Chokroverty, 2000; Krystal, Thakur, & Roth, 2008; Vandrey, Babson, Herrmann, & Bonn-Miller, 2014). Studies examining the effects of alcohol, cocaine, cannabis, opiates, and other addictive substances on sleep clearly indicate they are associated with, and contribute to, disordered sleep (Garcia & Salloum, 2015). Although SD is most often a symptom of another illness or syndrome, primary disorders such as obstructive sleep apnea, chronic insomnia, and restless leg syndrome are commonly first diagnosed in primary care settings rather than in specialty behavioral health care (Bailes et al., 2016). If SDs are indicators of risk for SUDs, screening for and identifying SD and related distress and morbidity in primary care could be helpful in providing early identification of risk for drug and alcohol use problems.
SD in childhood is associated with the early onset of alcohol use disorders (AUD) in adolescence (Hasler, Kirisci, & Clark, 2016; Wong, Brower, Fitzgerald, & Zucker, 2004; Wong, Brower, & Zucker, 2009). One way this has been interpreted is that subjects with SD may have the tendency to self-medicate with alcohol and tranquilizers to promote sleep, or abuse stimulants to stay awake during the day (Aira, Hartikainen, & Sulkava, 2008). Other theories suggest that SD might be part of a prodromal predisposition of an individual who is at risk for SUD (Wong et al., 2004) or that poor sleep exacerbates existing MHDs such as depression or ADHD, which in turn leads to substance use risk (Morse, MacMaster, Kodad, & Robledo, 2014). However, the predictive value of SD as an indicator of risk for SUDs in adults is less understood. If SDs have predictive value for identifying adult individuals in primary care who may be at risk for later needing treatment for SUDs, this would provide an important opportunity to address that risk early. SDs that are not clearly linked to a behavioral health disorder are usually identified and treated in primary care settings rather than in specialty behavioral health services. The potential benefit of screening for SDs in primary care is especially important given the movement towards behavioral health treatment integration in primary care (Stoop, Nefs, Pommer, Pop, & Pouwer, 2015) which can include the implementation of brief interventions for sleep (Chung, Lee, Yeung, Chan, Chung, & Lin, 2017).
SDs can have implications for both new onset and relapse SUDs. Adults in recovery from alcohol and illicit drug use may continue to have sleep problems long after achieving abstinence, and persistent SD may be predictive of SUD relapse (Babson, Boden, Harris, Stickle, & Bonn-Miller, 2013; Brower, Krentzman, & Robinson, 2011; Kaplan, McQuaid, Primich, & Rosenlicht, 2014). Studies suggest the monitoring of adults who use alcohol to fall asleep (alcohol as a hypnotic), as this use may be representative of how sleep problems evolve into SUD (Kolla et al., 2015). SD may pre-date and potentially be a predictor for SUD in at least a subset of primary care patients. Both SD and SUD are often under-identified and untreated (Bartlett, Marshall, Williams, & Grunstein, 2008; Teplin, Raz, Daiter, Varenbut, & Tyrrell, 2006), but SD may be even more likely to be missed than SUD. One study found that while questions about smoking and alcohol use were asked in 100% of routine visits, at least one question about sleep health (How is your sleep?) was asked in only 40% of visits (Sorscher, 2008).
In this paper, we report on a study evaluating sleep disturbances (SD) as potential predictors of later SUD diagnosis and treatment in an adult primary care sample within a large safety-net hospital that offers care for indigent and uninsured patients. Our objectives are to understand the predictive value of SD associated with substance use problems in adult populations, and whether identifying SD could be helpful for screening and intervening early in the risk for SUD, and identify which group of patients would most benefit from screening. We hypothesize that persons with sleep disturbance are more likely to be subsequently diagnosed and treated for a SUD as compared to individuals without sleep disturbance, regardless of whether SD is comorbid with a MHD.
2. Methods
This study is part of the International Latino Research Partnership (ILRP; NIDA R01DA034952 and NIMH R01MH100155). Data for this study consisted of electronic health records (EHR) data from January 1, 2013 - September 1, 2015. We collected EHR data from primary care sites associated with a large Boston area safety net and academic community health care system serving over 140,000 patients in primary care, specialty care, emergency services, hospital care, maternity care, and behavioral health. The system serves vulnerable populations and engages a community psychiatry and public health mission in its practice and medical training. The patient population is multicultural, and approximately 40% of patients speak a language other than English, most commonly Spanish, Portuguese, and Haitian Creole. The study was approved by the institutional review boards of the participating institutions.
We limited the current study sample to four distinct adult populations: patients with no sleep disorder and no mental health disorder; patients with a diagnosis of a sleep disorder (SD) but no mental health disorder, patients with a diagnosis of sleep and mental health disorder, and patients with mental health disorder only. Patients in these groups “enter” the data on the day of their first visit for treatment of the SD and are followed until September 1, 2015. The fourth group was the lower risk comparison group and included patients that never received a diagnosis for SD or mental health disorder during the 32 months of data collection. These patients “enter” the data at the time of their first medical or behavioral health care visit, and are followed until September 1, 2015. The EHR sample was comprised of 60.06% female and 39.94% male patients; and diverse across race/ethnicity (46.06% White, 16.44% Black, 28.84% Latino, 7.67% Asian).
Because of our specific interest in the progression from SD and MHD to SUD (separated into alcohol abuse and illicit drug use disorder), we excluded from this sample patients with prior treatment for SUD that preceded treatment for SD. To ensure that patients in the sample were regular patients at the health care system under study, we excluded individuals if they had less than three primary care visits between 2013 and 2015. Other than age (patients less than 18 years of age not included), no other exclusion criteria were used.
We identified SD in the EHR through treatment linked to the International Classification of Diseases, Ninth Revision (ICD-9) codes (780.5X unspecified sleep disturbance or 327.3X Circadian rhythm sleep disorders). We identified mental health disorder through service use linked to the behavioral health International Classification of Diseases, Ninth Revision (ICD-9) codes 291, 292, and 295-314, with the exception of the substance use disorder ICD-9 codes described below. This includes a range of psychiatric disorders including unipolar and bipolar affective disorders, anxiety disorders, Attention Deficit Hyperactivity Disorder among other common psychiatric diagnoses.
We examined two dependent variables (follow-up treatment for illicit drug use disorder, and alcohol use disorder), using the following ICD-9 codes: illicit drug dependence (304.XX, 648.3X, 655.5X, 760.72, 73, 75, 779.5, 965.0X), illicit drug abuse (292.XX; 305.20-23, 30-33, 40-43, 50-53, 60-63, 70-73, 80-83, 90-93), alcohol dependence (303.9X; 357.5; 425.5; 535.3; 571.0, 1, 2, 3; V11.3), and alcohol abuse (291.XX; 303.00-03; 305.00-03; V79.1; 790.3). Given the established associations between age, sex, race/ethnicity and rates of substance use (Compton, Thomas, Stinson, & Grant, 2007), we adjusted for these variables in multivariate Cox proportional hazard models, an analytic approach increasingly used to identify risk factors for developing alcohol and substance disorders (Gowin, Sloan, Stangl, Vatsalya, & Ramchandani, 2017). Sex was categorized as female or male, race/ethnicity as non-Latino White, non-Latino Black, non-Latino Asian, and Latino, and age was entered into the regression models as a continuous variable. We included three physical health conditions prevalent in primary care and associated with risk for SD, MHD and SUDs (Young, Skatrud, & Peppard, 2004): HIV (ICD-9 codes of 042.XX), cardiovascular disease (ICD-9 codes of 429.2X, 401.XX, or 402.XX), and diabetes (ICD-9 code of 250.XX) as control variables. The variables indicating diagnosis of these illnesses are not mutually exclusive, ie, patients with multiple disorders can be coded as a “1” for multiple conditions. Benzodiazepine prescriptions and pain medications are also adjusted for given their significant association with SUD and mental health disorder (Darke & Hall 1995; SAMHSA, 2012) and their potential for confounding the relationship between sleep and MH disorder and SUD.
2.1. Analytical Methods
First, we conducted chi-square analyses to compare the probability of receiving treatment for SUD, split into illicit drug use disorder and alcohol use disorder across the four groups of interest, as well as differences between the groups in the covariates described above.
Next, we estimated Kaplan-Meier survival function curves to account for SD/MHD group differences in the amount of exposure time (ie, patients “enter” the data at different time periods during 32 months of data collection and therefore have different time windows of risk of being diagnosed with SUD), and the right-censoring of patients (to account for the probability that treatment for a SUD diagnosis could occur after September 1, 2015) (Hosmer & Lemeshow, 2008). These curves graphically demonstrate the difference in time to treatment for a SUD diagnosis. Separate curves were created for each of the four groups, plotting at day-long intervals the proportion of the number of cases with SUD over the number of cases without SUD remaining in each time period. The hazard rates associated with these curves were calculated and statistically compared using the nonparametric log-rank test of equality (Mantel, 1966).
We then estimated Cox proportional hazard models to examine the risk of SUD, comparing hazard rates across our four groups (SD only, SD and MHD, MHD only, and no SD or MHD), adjusting for the covariates described above. We considered all individuals that had not yet received treatment for SUD to be right-censored at the end of our data (September 1, 2015). We assessed the Cox proportional hazards assumption using tests of Schoenfeld (1982) residuals and by visual estimation of log-log plots of the estimated survival curves. We used Wald tests (Korn & Graubard, 1990) to test significance in group hazard rate differences. All analyses were conducted using Stata 14 (StataCorp, 2014).
3. Results
Table 1 identifies differences between the four groups; 12.31% were treated for illicit drug use disorder over the study time period and 7.04% were treated for alcohol use disorder. Illicit drug use was further broken down to show that 7.33% were treated for cocaine, a third of a percent (0.33%) were treated for marijuana use, and 6.79% were treated for illicit prescription drug use. Chi-square results indicated that those with co-morbid sleep and mental health disorders were most likely to have alcohol or illicit drug use disorder treatment. The breakdown of type of sleep disorder within those diagnosed with sleep disorder was 6.9% circadian rhythm sleep disorder; 21.4% sleep disturbance; 1.3% sleep apnea; 63.5% insomnia, unspecified; 7.3% hypersomnia; and 0.9% disruption of 24-hour sleep wake cycle (the total is greater than 100% because several patients had multiple diagnoses).
Table 1.
Descriptive statistics (in percent unless otherwise noted) for total sample and four comparison groups, with chi-square tests for differences between the four groups of patients (N=83,920).
| Total Sample | With no sleep or mental health disorder | Sleep disorder only | With Comorbid Sleep Disorder and Mental Health Disorder | Mental Health Disorder only | p-value for tests between 4 groups | |
|---|---|---|---|---|---|---|
| Number of primary care visits over data collection period | 13.50 | 11.58 | 19.11 | 27.51 | 16.39 | .0001 |
| Alcohol Use Disorder = Yes | 7.04 | 5.16 | 10.72 | 15.39 | 10.68 | .0001 |
| Illicit Drug Use Disorder = Yes | 12.31 | 9.30 | 14.78 | 24.68 | 18.66 | .0001 |
| Cocaine Use Disorder = Yes | 7.33 | 6.49 | 11.64 | 13.42 | 8.40 | .0001 |
| Marijuana Use Disorder = Yes | 0.33 | 0.17 | 0.17 | 0.92 | 0.69 | .0001 |
| Prescription Drug Abuse = Yes | 6.79 | 4.14 | 7.12 | 17.16 | 12.62 | .0001 |
| Race/Ethnicity | ||||||
| White | 46.06 | 41.94 | 42.63 | 58.12 | 56.06 | .0001 |
| Black | 16.44 | 18.16 | 18.59 | 11.37 | 12.20 | .0001 |
| Latino | 28.84 | 29.56 | 30.70 | 26.53 | 26.99 | .0001 |
| Asian | 7.67 | 9.23 | 7.50 | 3.38 | 4.00 | .0001 |
| Female | 60.06 | 58.49 | 46.27 | 58.92 | 65.93 | .0001 |
| Male | 39.94 | 41.51 | 53.73 | 41.08 | 34.07 | .0001 |
| Diabetes = 1 | 28.08 | 25.51 | 44.05 | 46.66 | 31.05 | .0001 |
| Cardio = 1 | 5.11 | 4.25 | 9.25 | 12.14 | 6.11 | .0001 |
| HIV = 1 | 9.11 | 8.02 | 9.88 | 14.07 | 11.38 | .0001 |
| Pain Medication | 34.48 | 31.00 | 42.34 | 53.62 | 40.67 | .0001 |
Figures 1 and 2 present Kaplan-Meier survival curves for illicit drug use disorder and alcohol use treatment, respectively. These curves represent the different survival functions, or the different hazard over time of receiving treatment for illicit drug use disorder or alcohol use disorder by sleep/mental health group. In both cases, SD alone significantly predicted both alcohol and illicit drug use disorder, and the group with comorbid sleep and mental health disorder had the greatest hazard of treatment for subsequent alcohol use disorder (approximately 15% of the sample by the end of the study period) and illicit drug use disorder (approximately 30% of the sample by the end of the study period). Mental health disorder alone was associated with a greater risk for illicit drug use compared to sleep disturbance alone, however, risk patterns for alcohol use disorder were similar for sleep disturbance and mental health disorder.
Fig. 1.
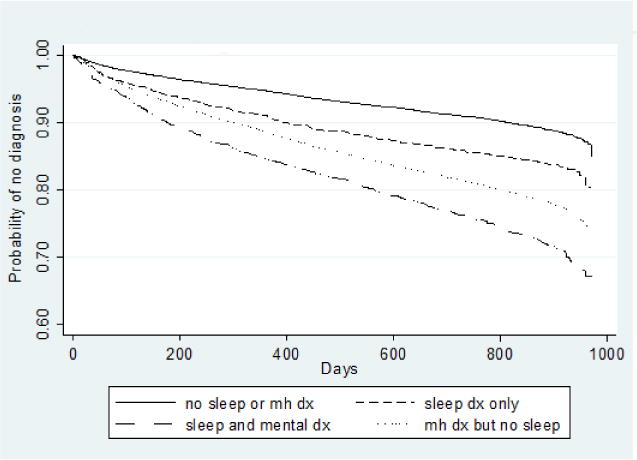
Kaplan-Meier survival curves for time to diagnosis of illicit drug abuse or dependence. dx = diagnosis.
Fig. 2.
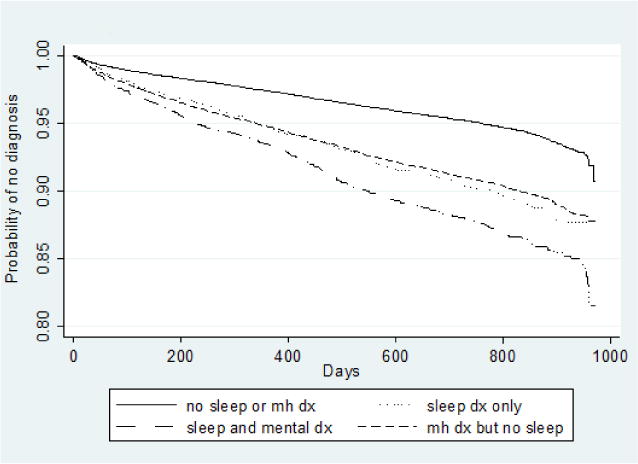
Kaplan-Meier survival curves for time to diagnosis of alcohol abuse or dependence. dx = diagnosis.
After covariate adjustment, Cox proportional hazard model results show that SD was associated with a greater overall risk of treatment for alcohol or illicit drug use disorder diagnosis as compared to those without sleep or mental health disorder, after adjustment for race, gender, age, and other covariates (Tables 2 and 3). The results were similar in direction and significance for alcohol use disorder, and were similar in direction but not significance for illicit drug use disorder. In addition, the greater hazard rates for those with both MHD and SD compared to those with no mental health or SD persist in Cox proportional hazard models of alcohol use disorder and illicit drug use disorder.
Table 2.
Hazard ratios from survival analysis of time to diagnosis of illicit drug use disorder
| VARIABLES | (1) Full Sample |
(2) Excluding those with a prior illicit drug use disorder |
|---|---|---|
| No Sleep, No Mental Health DX (referent) | ||
| Sleep DX only | 1.294*** (0.0762) |
1.101 (0.0700) |
| Sleep DX + Mental Health DX | 2.115*** (0.0962) |
1.899*** (0.0904) |
| Mental Health DX only | 1.889*** (0.0470) |
1.887*** (0.0470) |
| Age | 1.003*** (0.000676) |
1.003*** (0.000679) |
| Female | 0.811*** (0.0180) |
0.810*** (0.0182) |
| White (reference) | ||
| Black | 0.896*** (0.0280) |
0.897*** (0.0282) |
| Asian | 0.753*** (0.0373) |
0.755*** (0.0375) |
| Latino | 0.769*** (0.0207) |
0.767*** (0.0208) |
| Cardio DX | 1.029 (0.104) |
1.025 (0.104) |
| HIV DX | 1.791*** (0.109) |
1.793*** (0.109) |
| Diabetes DX | 2.947*** (0.0753) |
2.943*** (0.0758) |
| Any pain medication | 1.468*** (0.0335) |
1.472*** (0.0338) |
| Any benzodiazepine | 1.326*** (0.0352) |
1.330*** (0.0355) |
Hazard ratios represent the change in the expected log of the hazard ratio relative to a one unit change in the covariate of interest, adjusting for all other predictors
Standard errors of the hazard ratios are reported in parentheses.
Statistically significant at p<0.001 level
p<0.01
p<0.05
Table 3.
Hazard ratios from survival analysis of time to diagnosis of alcohol use disorder
| VARIABLES | (1) Full Sample |
(2) Excluding those with a prior alcohol use disorder |
|---|---|---|
| No Sleep, No Mental Health DX (referent) | ||
| Sleep DX only | 1.493*** (0.106) |
1.296*** (0.0764) |
| Sleep DX + Mental Health DX | 1.692*** (0.106) |
2.113*** (0.0964) |
| Mental Health DX only | 1.586*** (0.0550) |
1.889*** (0.0470) |
| Age | 1.010*** (0.000920) |
1.003*** (0.000676) |
| Female | 0.531*** (0.0161) |
0.811*** (0.0181) |
| White (reference) | ||
| Black | 0.730*** (0.0349) |
0.895*** (0.0280) |
| Asian | 0.714*** (0.0517) |
0.753*** (0.0373) |
| Latino | 1.011 (0.0355) |
0.770*** (0.0207) |
| Cardio DX | 1.459*** (0.171) |
1.030 (0.104) |
| HIV DX | 1.290** (0.128) |
1.792*** (0.109) |
| Diabetes DX | 1.470*** (0.0589) |
2.949*** (0.0754) |
| Any pain medication | 1.963*** (0.0621) |
1.468*** (0.0335) |
| Any benzodiazepine | 1.494*** (0.0532) |
1.325*** (0.0352) |
Hazard ratios represent the change in the expected log of the hazard ratio relative to a one unit change in the covariate of interest, adjusting for all other predictors.
Standard errors of the hazard ratios are reported in parentheses.
Statistically significant at p<0.001 level
p<0.01
p<0.05
4. Discussion
Results of this study highlight important considerations in the identification, treatment, and potential risk for alcohol and illicit drug use disorders in patients with SD, and comorbid SD and MHD, and as compared to having MHD alone. Our hypothesis that persons with various sleep disturbances are more likely to be later diagnosed and treated for SUD was supported by study findings for alcohol disorders, but only partially supported in findings for illicit drug use disorders.
Sleep complaints are highly prevalent in primary care populations (Foley, Ancoli-Israel, Britz, & Walsh, 2004) and research has consistently shown that patients with the highest risk for SD are those with pain, limited activity, and poor overall physical and mental health (Dinges et al., 1999; Mundt, Eisenschenk, & Robinson, 2017). Because SD are associated with significant health impacts (Drager, McEvoy, Barbe, Lorenzi-Filho, & Redline, 2017; Lillis et al., 2017), screening positive for symptoms of SD should prompt further diagnostic evaluation (Teplin et al., 2006). A meta-analysis found that SD can even be associated with suicidal ideation, a relationship that did not show risk moderation by depression being present (Pigeon, Pinquart, & Conner, 2012). Although we tried to include individuals whose SD pre-dated the initiation of documented diagnosis and treatment for drug or illicit drug use disorder, it is likely that some of the individuals in the sample had prior substance use, and that in those cases our observations reflected SD predicting alcohol/illicit drug use disorder relapse. However, screening may be helpful regardless of whether SD predicts initial incidence or relapse.
4.1. Limitations
A limitation of our study is the use of the hospital electronic medical records, which rely on clinician comprehensiveness and accuracy regarding the identification and coding of SD, SUD and MHD. Because of variability in clinicians’ attention to sleep symptoms and use of screening tools, under-identification and under-diagnosis of SD in the sample is likely. This study did not examine the possible role that psychiatric factors might play in mediating between sleep related problems and SUD. In addition, our SD variable represents a variety of sleep problems including obstructive sleep apnea, insomnia, and restless leg syndrome and is not representative of a homogenous group. The sample that receives care in this safety net hospital is more vulnerable than the general population, with higher representation of women and Latinos. This may lead to an underestimate of SUD, which is more prevalent for White individuals, and may highlight mental health disorders more prevalent in women and vulnerable populations (Compton et al., 2007). This study should be replicated using data from populations in less adverse circumstances. Our data also cannot capture those individuals who have SD and need but never seek substance use treatment, our primary outcome.
4.2. Clinical Implications
The treatment of SD should include monitoring for the development of alcohol and illicit drug use towards early identification, non-narcotic medication treatment, and non-pharmacological interventions for substance use problems (Arnedt, Conroy, Armitage, & Brower, 2011; Babson, Feldner, & Badour, 2010; Britton et al., 2010). Providers in mental health and primary care should consider routine screening and identification of SD so that treatment can be initiated effectively and early (Teplin et al., 2006). Using brief screening tools for mental health disorders (eg, PHQ-9; Kroenke, Spitzer, & Williams, 2002) can help in identifying sleep problems which are symptoms of a mental health disorders such as depression (ie., questions about insomnia and hypersomnia which are part of the depression disorder symptom criteria). Conversely, patients with SD should be thoroughly evaluated for mental health disorders. Symptoms suggestive of SD are common, but are not routinely and specifically screened for in the primary care setting. Validated questionnaires and screening tools that have been developed for identifying shift work related SD can efficiently identify patients at risk for common SD in this setting (Barger et al., 2012). A study in Australia found that a subjective screening measure of sleep difficulty was helpful and feasible in identifying individuals in a community pharmacy setting with likelihood for obstructive sleep apnea, insomnia, and/or restless leg syndrome, the most common sleep disturbances encountered in primary care settings (Fuller et al., 2014). Other available screening measures include the Cleveland Sleep Habits questionnaire (CSHQ; Mustafa, Erokwu, Ebose, & Strohl, 2005), Berlin questionnaire (Chung et al., 2008), and Epworth Sleepiness Scale (ESS; Johns, 1991). Objective and mobile markers of sleep exist through rest/activity cycle-measuring wrist band devices (Dijk, 2016), and these can supplement other more subjective measures including self-reported sleep latency, duration of sleep, and restfulness. Patients who screen positive for SD and present with subjective complaints regarding their sleep, may warrant further questioning about whether they are self-medicating, or have a history of SUD. In our sample, individuals prescribed benzodiazepines or pain medication and/or who had medical comorbidity also showed increased risk of needing treatment for AUD and SUD. The Michigan Alcohol Screening Test (MAST; Selzer, 1971) and the Drug Abuse Screening Test (DAST; Skinner, 1982) or Alcohol Use Identification Test (AUDIT; Reinert & Allen, 2002) can identify alcohol and drug problems among patients presenting with SD, both alone or comorbid with mental health problems (Boschloo et al., 2010). In addition, interventions that address SD among patients with and without comorbid mental health problems are needed (Conroy & Arnedt, 2014; Kaplan et al., 2014), especially those that can be delivered or initiated in primary care, and that can address behavioral, medical, and psychiatric comorbidity. In primary care, clinical encounters, a limited sleep history should be conducted followed by documentation of any identified SD, a brief intervention such as sleep hygiene counseling, a referral to a sleep evaluation if initial brief interventions are not helpful, and a referral to a psychiatry clinic for any significant comorbid diagnosis or symptoms.
Screening, Brief Intervention, and Referral to Treatment (SBIRT) is an evidenced-based practice used to identify, reduce, and prevent problematic use, abuse, and dependence on alcohol and drugs (Aldridge, Linford, & Bray, 2017). SBIRT can be easily used in primary care settings and enables healthcare professionals to systematically screen and assist patients who may not be seeking help for a SUD, but whose drinking or drug use may cause or complicate their ability to successfully handle health, work, or family issues. In a landmark report, the Institute of Medicine specifically cited the SBIRT model as a promising practice for SUD treatment (Institute of Medicine, 2001). SDs are not usually specifically screened as part of SBIRT practice, although screening for SD could identify an important potential indicator that a patient is at risk for subsequent SUD in primary care. This SBIRT approach could offer patients a comprehensive response to a significant health problem, while promoting healthy sleep, and the prevention of alcohol and substance use problems. Our study sheds light on the issue of SD as an indicator of risk of SUD. Future research is needed to more clearly understand the relationship between SD, SUD, and MHD to continue to better inform prevention and intervention efforts.
Supplementary Material
Highlights.
Patients with sleep disturbances have a higher risk for SUD treatment
Patients with a mental health disorder had the greatest risk for SUD treatment
Screening for sleep disturbances may help identify SUD risk in primary care
Acknowledgments
Research reported in this publication was supported by the National Institute On Drug Abuse (NIDA) of the National Institutes of Health under Award Number R01DA034952 and the National Institute of Mental Health (NIMH) under Award number R01MH100155. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: None.
References
- Aira M, Hartikainen S, Sulkava R. Drinking alcohol for medicinal purposes by people aged over 75: A community-based interview study. Family Practice. 2008;25(6):445–449. doi: 10.1093/fampra/cmn065. [DOI] [PubMed] [Google Scholar]
- Aldridge A, Linford R, Bray J. Substance use outcomes of patients served by a large US implementation of Screening, Brief Intervention and Referral to Treatment (SBIRT) Addiction. 2017;112(Suppl 2):43–53. doi: 10.1111/add.13651. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: A randomized controlled pilot trial. Behavior Research and Therapy. 2011;49(4):227–233. doi: 10.1016/j.brat.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Sleep and substance use disorders. In: Morin CM, Espie CA, Morin CM, Espie CA, editors. The Oxford Handbook of Sleep and Sleep Disorders. New York, NY, US: Oxford University Press; 2012. pp. 526–554. [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, Bonn-Miller MO. Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. Journal of Substance Abuse Treatment. 2013;44(4):438–443. doi: 10.1016/j.jsat.2012.08.224. [DOI] [PubMed] [Google Scholar]
- Babson KA, Feldner MT, Badour CL. Cognitive behavioral therapy for sleep disorders. Psychiatric Clinics of North America. 2010;33(3):629–640. doi: 10.1016/j.psc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Bailes S, Rizzo D, Baltzan M, Grad R, Pavilanis A, Creti L, Libman E. Manifestations of insomnia in sleep apnea: Implications for screening and treatment. Behavioral Sleep Medicine. 2016;14(4):429–441. doi: 10.1080/15402002.2015.1017098. [DOI] [PubMed] [Google Scholar]
- Baker EK, Richdale AL. Sleep patterns in adults with a diagnosis of high-functioning autism spectrum disorder. Sleep: Journal of Sleep and Sleep Disorders Research. 2015;38(11):1765–1774. doi: 10.5665/sleep.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger LK, Ogeil RP, Drake CL, O’Brien CS, Ng KT, Rajaratnam SM. Validation of a questionnaire to screen for shift work disorder. Sleep. 2012;35(12):1693–1703. doi: 10.5665/sleep.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DJ, Marshall NS, Williams A, Grunstein RR. Predictors of primary medical care consultation for sleep disorders. Sleep Medicine. 2008;9(8):857–864. doi: 10.1016/j.sleep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Smit JH, van den Brink W, Veltman DJ, Beekman ATF, Penninx BWJH. The performance of the Alcohol Use Disorder Identification Test (AUDIT) in detecting alcohol abuse and dependence in a population of depressed or anxious persons. Journal of Affective Disorders. 2010;126(3):441–446. doi: 10.1016/j.jad.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Britton WB, Bootzin RR, Cousins JC, Hasler BP, Peck T, Shapiro SL. The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: A treatment-development study. Substance Abuse. 2010;31(2):86–97. doi: 10.1080/08897071003641297. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Krentzman A, Robinson EAR. Persistent insomnia, abstinence, and moderate drinking in alcohol ‐ dependent individuals. The American Journal on Addictions. 2011;20(5):435–440. doi: 10.1111/j.1521-0391.2011.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokroverty S. Diagnosis and treatment of sleep disorders caused by co-morbid disease. Neurology. 2000;54(5, Suppl 1):S8–S24. [PubMed] [Google Scholar]
- Chung KF, Lee CT, Yeung WF, Chan MS, Chung EW, Lin WL. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2017 doi: 10.1093/fampra/cmx122. [DOI] [PubMed] [Google Scholar]
- Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Shapiro CM. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- Cohen ZL, Sharkey KM. Insomnia in psychiatric disorders. In: Attarian HP, Attarian HP, editors. Clinical Handbook of Insomnia. Totowa, NJ, US: Humana Press; 2017. pp. 267–281. [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Conroy DA, Arnedt JT. Sleep and substance use disorders: an update. Current Psychiatry Reports. 2014;16(10):487. doi: 10.1007/s11920-014-0487-3. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W. Levels and correlates of polydrug use among heroin users and regular amphetamine users. Drug Alcohol Depend. 1995;39(3):231–235. doi: 10.1016/0376-8716(95)01171-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ. Biomarkers, old and new: For sleep, sleepiness, circadian phase and much more. Journal of Sleep Research. 2016;25(3):255–256. doi: 10.1111/jsr.12435. [DOI] [PubMed] [Google Scholar]
- Dinges D, Ball E, Fredrickson P, Kiley J, Kryger M, Richardson G, National Center on Sleep Disorders Research Working Group Recognizing problem sleepiness in your patients. American Family Physician. 1999;59(4):937–944. [PubMed] [Google Scholar]
- Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep Apnea and Cardiovascular Disease: Lessons From Recent Trials and Need for Team Science. Circulation. 2017;136(19):1840–1850. doi: 10.1161/circulationaha.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of Psychosomatic Research. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Fuller JM, Wong KK, Grunstein R, Krass I, Patel J, Saini B. A comparison of screening methods for sleep disorders in Australian community pharmacies: a randomized controlled trial. PLoS One. 2014;9(6):e101003. doi: 10.1371/journal.pone.0101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AN, Salloum IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review. The American Journal on Addictions. 2015 doi: 10.1111/ajad.12291. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. Am J Psychiatry. 2017;174(11):1094–1101. doi: 10.1176/appi.ajp.2017.16101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Asarnow LD. Insomnia. In: Hofmann SG, Dozois DJA, Rief W, Smits JAJ, Hofmann SG, Dozois DJA, Rief W, Smits JAJ, editors. The Wiley Handbook of Cognitive Behavioral Therapy. 1-3. Wiley-Blackwell; 2014. pp. 541–565. [Google Scholar]
- Hasler BP, Kirisci L, Clark DB. Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and other drug involvement. J Stud Alcohol Drugs. 2016;77(4):649–655. doi: 10.15288/jsad.2016.77.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd. New York: Wiley; 2008. [Google Scholar]
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kamath J, Prpich G, Jillani S. Sleep disturbances in patients with medical conditions. Psychiatric Clinics of North America. 2015;38(4):825–841. doi: 10.1016/j.psc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Kaplan KA, McQuaid J, Primich C, Rosenlicht N. An evidence-based review of insomnia treatment in early recovery. Journal of Addiction Medicine. 2014;8(6):389–394. doi: 10.1097/ADM.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Kolla BP, Schneekloth T, Mansukhani MP, Biernacka JM, Hall-Flavin D, Karpyak V, Frye MA. The association between sleep disturbances and alcohol relapse: A 12-month observational cohort study. The American Journal on Addictions. 2015;24(4):362–367. doi: 10.1111/ajad.12199. [DOI] [PubMed] [Google Scholar]
- Korn E, Graubard B. Simultaneous testing of regression coefficients with complex survey data: Use of Bonferroni t statistics. American Statistician. 1990;44(4):270–276. [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine. 2002;64(2):258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Thakur M, Roth T. Sleep disturbance in psychiatric disorders: Effects on function and quality of life in mood disorders, alcoholism, and schizophrenia. Annals of Clinical Psychiatry. 2008;20(1):39–46. doi: 10.1080/10401230701844661. [DOI] [PubMed] [Google Scholar]
- Lillis TA, Gerhart J, Bouchard LC, Cvengros J, O’Mahony S, Kopkash K, Burns J. Sleep disturbance mediates the association of post-traumatic stress disorder symptoms and pain in patients with cancer. Am J Hosp Palliat Care. 2017 doi: 10.1177/1049909117739299. 1049909117739299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50(3):163–170. [PubMed] [Google Scholar]
- Morse SA, MacMaster SA, Kodad V, Robledo K. The impact of a sleep hygiene intervention on residents of a private residential facility for individuals with co-occurring mental health and substance use disorders: Results of a pilot study. Journal of Addictions Nursing. 2014;25(4):204–208. doi: 10.1097/JAN.0000000000000050. [DOI] [PubMed] [Google Scholar]
- Mundt JM, Eisenschenk S, Robinson ME. An examination of pain’s relationship to sleep fragmentation and disordered breathing across common sleep disorders. Pain Med. 2017 doi: 10.1093/pm/pnx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep Breath. 2005;9(2):57–63. doi: 10.1007/s11325-005-0016-z. [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): A review of recent research. Alcoholism: Clinical and Experimental Research. 2002;26(2):272–279. doi: 10.1097/00000374-200202000-00016. [DOI] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. The American Journal of Psychiatry. 1971;127(12):1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Sorscher AJ. How is your sleep: a neglected topic for health care screening. The Journal of the American Board of Family Medicine. 2008;21(2):141–148. doi: 10.3122/jabfm.2008.02.070167. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software Release 14.0. College Station, TX: Stata Corporation; 2014. [Google Scholar]
- Stoop CH, Nefs G, Pommer AM, Pop VJ, Pouwer F. Effectiveness of a stepped care intervention for anxiety and depression in people with diabetes, asthma or COPD in primary care: A randomized controlled trial. J Affect Disord. 2015;184:269–276. doi: 10.1016/j.jad.2015.05.063. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration(SAMHSA), Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. 2012 Retrieved from Rockville, MD: https://www.samhsa.gov/data/sites/default/files/DAWN096/DAWN096/SR096EDHighlights2010.pdf. [PubMed]
- Teplin D, Raz B, Daiter J, Varenbut M, Tyrrell M. Screening for substance use patterns among patients referred for a variety of sleep complaints. The American Journal of Drug and Alcohol Abuse. 2006;32(1):111–120. doi: 10.1080/00952990500328695. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Babson KA, Herrmann ES, Bonn-Miller MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. International Review of Psychiatry. 2014;26(2):237–247. doi: 10.3109/09540261.2014.901300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clinical and Experimental Research. 2004;28(4):578–587. doi: 10.1097/01.ALC.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Zucker RA. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Medicine. 2009;10(7):787–796. doi: 10.1016/j.sleep.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


