Abstract
Glutamatergic afferents from the cerebral cortex are the main excitatory drive of striatal projection neurons. The metabotropic glutamate receptor 4 (mGluR4) presynaptically modulates transmission at corticostriatal synapses, and is considered as a potent drug target for Parkinson’s disease and other brain disorders. To better characterize the anatomical substrate that underlies the functional effects of mGluR4 in the striatum, we undertook electron microscopic localization studies of mGluR4 expression in the mouse striatum. Our data demonstrate that more than 80% mGluR4-immunoreactive structures are accounted for by unmyelinated axons and axon terminals, and that almost 50% putative glutamatergic terminals (i.e. forming asymmetric synapses) express mGluR4 in the mouse striatum. Using vGluT1 as a presynaptic marker of glutamatergic corticostriatal boutons, our findings indicate: 1) all striatal mGluR4-positive terminals co-express vGluT1 immunoreactivity, 2) 44.3% total striatal glutamatergic terminals co-express vGluT1 and mGluR4, and 3) mGluR4 is expressed in 73.4% of total striatal vGluT1-positive terminals. To determine if mGluR4 terminals target preferentially direct versus indirect pathway neurons, mGluR4 immunostaining was combined with D1 receptor immunoreactivity. These data showed that around 30% mGluR4-imunoreactive glutamatergic terminals target D1 receptor-positive spines (i.e. direct pathway neurons), while almost 70% formed synapses with D1 receptor-negative spines. Thus, these immuno-electron microscopic studies indicate that pre-synaptic mGluR4 in striatal glutamatergic terminals is expressed almost exclusively in cortical boutons to subserve regulatory influences upon a large contingent of corticostriatal terminals that preferentially target the “indirect” pathway striatal projection neurons in mice.
Keywords: metabotropic, glutamate, cortex, striatum, vesicular glutamate transporter, dopamine receptor
INTRODUCTION
The cerebral cortex is the main source of glutamatergic inputs to the striatum. Because of its importance in regulating striatal outflow, a tight control of corticostriatal synapses is essential for normal basal ganglia function. Various pre- and post-synaptic mechanisms involving dopaminergic, GABAergic and glutamatergic receptors have been characterized as important regulators of corticostriatal transmission and synaptic plasticity. Among those, are different subtypes of pre- and post-synaptic metabotropic glutamate receptors (mGluRs) (Smith et al., 2001, Gubellini et al., 2004, Conn et al., 2005, Kuwajima et al., 2007, Bogenpohl et al., 2013, Gregory et al., 2013). The mGluR4, a member of the group III mGluR family, has received significant attention because of its powerful presynaptic regulation of corticostriatal transmission, and its relevance as potential target for antiparkinsonian therapy (Beurrier et al., 2009, Cuomo et al., 2009, Johnson et al., 2009, Jones et al., 2012, Le Poul et al., 2012, Bennouar et al., 2013). However, it is not clear if the expression of mGluR4 is a common characteristic of all cortical terminals or only a feature shared by a subset of corticostriatal afferents.
Thus, one of the goals of this study was to assess the proportion of striatal glutamatergic terminals that co-express mGluR4 and the vesicular glutamate transporter 1 (vGluT1), a marker of cortical terminals (Fremeau et al., 2004, Lacey et al., 2005, Raju et al., 2008, Smith et al., 2014), in the mouse striatum. Knowing that the main targets of cortical glutamatergic terminals in the striatum are the two main populations of striatal output neurons (so-called direct and indirect pathway neurons), and that activation of either pathway induces opposite effects upon basal ganglia outflow and consequent motor behavior (Kravitz et al., 2010, Kravitz et al., 2012, Freeze et al., 2013), a second goal of this study was to determine if cortical mGluR4-positive terminals preferentially target a specific population of striatal projection neurons.
MATERIALS and METHODS
Animal and tissue preparation
Five adult male Swiss colony-derived FVB mice (Charles River Laboratories) were deeply anesthetized with a cocktail of Ketamine (60–100mg/kg) and domitor (0.1mg/kg), and transcardially perfused with cold Ringer’s solution, followed by fixative consisting of 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer (0.1M; pH 7.4). All animal procedures were approved by the animal care and use committee of Emory University and conform to the U.S. National Institutes of Health guidelines.
After perfusion, the brains were removed from the skull and post-fixed in 4% paraformaldehyde for 5–24 hours. Following post-fixation, brains were cut with a vibrating microtome into 60μm-thick coronal sections that were stored in phosphate-buffered saline (PBS) at 4°C until further processing. Selected sections at the level of the striatum were put in a 1.0% sodium borohydride solution for 20 minutes and thereafter rinsed in PBS before being processed for immunocytochemistry.
Antibodies
MGluR4
The rabbit polyclonal anti-mGluR4a antibody (Zymed/Invitrogen, 1:200, Catalog #513100, Lot #60103066A2) used in this study was raised against a synthetic peptide derived from the C-terminal 200 amino acids of the rat mGluR4 protein specific for the mGluR4a splice variant. Studies by the manufacturer confirmed the reactivity on Western blots (Mr=93,000–110,000) using rat brain cell lysate. Tissue from mGluR4 knockout mice is completely devoid of immunostaining when probed with this antibody (Smith et al., unpublished data).
VGluT1
Rabbit anti-VGluT1 antibodies were used as markers of corticostriatal (vGluT1-positive) glutamatergic projections (Lacey et al., 2005, Raju and Smith, 2005, Fujiyama et al., 2006, Raju et al., 2008). The commercially available Rabbit anti-VGluT1 antibodies (MaB Technologies, Cat # VGT1-3, Lot # GA062B, 1:5000) were raised against a peptide from the COOH terminus of the rat vesicular glutamate transporter 1 (rvGluT1), corresponding to amino acids 543–560 (cATHSTVQPPRPPPPVRDY). (Raju et al., 2006). Specificity studies from our laboratory and others using immunoblotting of brain lysate revealed single bands at ~60 kDa. Preadsorbtion of primary antibody with synthetic peptide (0.2– 0.4 μg/ml) overnight at 4°C abolished immunoreactivity, whereas preadsorbtion with a similar, but non-identical, peptide preserved immunoreactivity (Montana et al., 2004, Raju and Smith, 2005). Immunohistochemical experiments on striatal tissue using the VGluT1 antibody in control tissue and tissue preadsorbed with vGluT1, but not vGluT2 peptide further confirmed the specificity of the antibody (Raju et al., 2006).
D1R
Spines containing the D1 receptor were identified using the Sigma Aldrich monoclonal rat anti-D1 dopamine receptor antibody (Catalog #D187-250uL; clone 1-1-F11 s.E6; immunoglobulin purified). According to the information provided by the manufacturer, the antibody was produced by immunizing rats with a 97 amino acid synthetic peptide corresponding to the C-terminus of the human D1 dopamine receptor. The D187 antibody is specific for human (DRD1 Gene ID 1812), monkey, and rat (Drd1a Gene ID 24316) D1 dopamine receptors. The antibody is supplied as a solution in 20 mM sodium phosphate, pH 7.2, containing 150 mM sodium chloride and 0.02% sodium azide. The antiserum mainly labeled striatal spines and some dendrites. Tests for the specificity of this antibody have been published in previous studies (Levey et al., 1993, Hersch et al., 1995). The D1 staining pattern seen in our study with this antibody corresponds to the pattern described using this and other D1 antisera in previous (Levey et al., 1993, Smiley et al., 1994, Hersch et al., 1995, Yung et al., 1995, Smith and Villalba, 2008).
MGluR4 Immunoperoxidase Light and Electron Microscopy Labeling
After sodium borohydride treatment, selected coronal tissue sections were incubated for 1 hour at room temperature (RT) in PBS containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.3% Triton X-100, followed by the primary antibody solution (1:200 dilution) containing 1% NGS, 1% BSA, and 0.3% Triton X-100 in PBS for 24 hours at RT. After three rinses in PBS, sections were incubated in secondary biotinylated goat anti-rabbit IgGs at a concentration of 1:200 (Vector Laboratories, Burlingame, CA) for 90 minutes. The sections were rinsed again in PBS and then incubated for another 90 minutes with the avidin-biotin peroxidase complex (ABC) at a dilution of 1:100 (Vector Laboratories). Finally, the sections were washed twice in PBS and once in Tris buffer (50 mM; pH 7.6) and transferred to a solution containing 0.025% 3,3′ -diaminobenzidine tetrahydrochloride (DAB; Sigma), 10 mM imidazole, and 0.005% hydrogen peroxide in Tris buffer for 10 minutes producing a diffusible reaction product to identify the immunoreactivity for the receptors. Sections were rinsed in PBS, mounted onto gelatin-coated slides, dehydrated, and then coverslipped with Permount. Immunostained sections were then scanned with a high resolution.
The incubation procedures to localize mGluR4 at the electron microscopic level were as follows: After sodium borohydride treatment, selected tissue sections were transferred to a cryoprotectant solution (PB, 0.05M, pH 7.4, containing 25% sucrose and 10% glycerol) for 20 minutes and then frozen in the –80°C freezer for 20 minutes. The tissue was returned to the cryoprotectant solution and then, via a decreasing gradient of cryoprotectant solutions (100%, 70%, 50%, 30%) the tissue was returned to PBS. Brain tissue sections were incubated for 1 hour at RT in PBS containing 10% NGS, 1% BSA, followed by a 48 hour incubation at 4 degrees centigrade with primary antibodies diluted in a solution of normal serum (1%), 1% BSA and PBS. The rest of the protocol was the same as for the LM procedure. Following the DAB reaction, the sections were transferred to PB (0.1 M, pH 7.4) for 10 min and exposed to 1% osmium tetroxide diluted in PB for 20 min. They were then rinsed with PB and dehydrated in an increasing gradient of ethanol (50%, 70%, 90%, 100%). Uranyl acetate (1%) was added to the 70% alcohol to increase contrast at the electron microscope. The sections were then treated with propylene oxide before being embedded in epoxy resin (Durcupan, ACM; Fluka, Buchs, Switzerland) for 12 hours, mounted on microscope slides, and placed in a 60°C oven for 48 hours. Blocks of tissue were cut into 60 nm ultrathin sections with an ultramicrotome (Ultracut T2; Leica, Nussloch, Germany) and serially collected on single-slot Piloform-coated copper grids. The sections were stained with lead citrate for 5 min (Reynolds, 1963) and examined with a Zeiss EM-10C electron microscope (Thornwood, NY).
Analysis of mGluR4-immunostained material
To analyze the overall pattern of synaptic connection of mGluR4-positive elements, one block of striatal tissue from each animal was prepared and cut in ultrathin sections. In the electron microscope, sections were scanned for the presence of immunoperoxidase-labeled structures recognized by their electron-dense reaction product. For each animal, 50 photographs of randomly selected immunoreactive elements collected from superficial sections of the blocks were digitized at 20,000×. This resulted in a total surface of 7,200 μm2 of striatal tissue to be examined for each mGluR experiment. Immunoreactive elements were categorized as presynaptic (axons, terminals) or postsynaptic (dendrites, spines) neuronal structures on the basis of ultrastructural features. The relative abundance of mGluR4-positive neuronal elements in the striatum was calculated by dividing the number of labeled elements over the total surface area of striatum examined. To obtain the percent of labeled elements in a specific category, the number of labeled elements in this group was divided by the number of total number of identified elements of that type.
MGluR4/vGluT1 co-localization
Because the mGluR4 and vGluT1 antibodies are both generated in rabbits, co-localization studies on the same section using two different markers was not possible. Thus, we used the double immunoperoxidase cocktail experiment approach (Hersch et al., 1995, Lei et al., 2004, Bordelon et al., 2005, Mitrano and Smith, 2007) to determine the extent of vGluT1/mGluR4 co-localization in the mouse striatum.
In brief, series of three sections at the level of the dorsal striatum from each animal were incubated with the following antibodies: vGluT1, mGluR4 or vGluT1 + mGluR4, and localized using the ABC immunoperoxidase method described above. Blocks from corresponding regions of the striatum were taken from each section and cut in ultrathin sections. At the electron microscopic level, 50 micrographs of randomly selected immunoreactive elements from the surface of each block were digitized at 20,000×. This resulted in a total surface of 7,200μm2 of striatal tissue to be examined for each experiment.
From this material, the following information was collected:
Percent total striatal glutamatergic terminals that co-express mGluR4/vGluT1: (% striatal terminals forming asymmetric synapses labeled with vGluT1 antibodies alone + % terminals forming asymmetric synapses labeled with mGluR4 antibodies alone) ‒ % terminals forming asymmetric synapses labeled mGluR4+vGluT1 antibodies cocktail [see (Hersch et al., 1995, Lei et al., 2004, Mitrano and Smith, 2007) for further details].
Percent total vGluT1 terminals that co-express mGluR4: (% striatal terminals forming asymmetric synapses labeled with mGluR4+vGluT1 antibodies cocktail / % striatal terminals forming asymmetric synapses labeled with vGluT1 antibodies alone) X 100.
Percent total mGluR4 terminals that co-express vGluT1: (% striatal terminals forming asymmetric synapses labeled with mGluR4+vGluT1 antibodies cocktail / % striatal terminals forming asymmetric synapses labeled with mGluR4 antibodies alone) X 100.
MGluR4/D1R double immunostaining
To study the relationships between mGluR4-positive terminals and D1-positive or D1-negative neurons, double immunoperoxidase or immunogold/immunoperoxidase methods were used. The tissue processing for the double immunoperoxidase approach was the same as described above for the mGluR4 labeling, except that a cocktail of D1R/mGluR4 antibodies were used. After completion of the primary antibodies reaction, each antigen was localized separately using specific secondary antibodies and the ABC method as described above.
In sections processed to localize mGluR4 with the pre-embedding immunogold method and D1R with immunoperoxidase, the following procedure was used. After cryoprotectant, sections were preincubated for 1hr in a solution containing 10% NGS and 10% NHS in PBS-BSA (0.005% BSA, 0.05% Tween 20, and 0.001% gelatin in PBS) before being transferred to a 1% NHS and 1% NGS in PBS-BSA solution containing the rabbit anti-mGluR4 and rat anti-D1R antibodies for 24 hr at room temperature. Next, the tissue was rinsed in PBS-BSA and incubated for 2 hr in the secondary 1.4 nm gold-conjugated goat anti-rabbit IgGs (Nanogold; Nanoprobes, Stonybrook, NY) at a concentration of 1:100 and a secondary biotinylated horse anti-rat IgGs (Vector Laboratories, Burlingame, CA) at a concentration of 1:200 in 1% NGS and 1% NHS in PBS-BSA. The sections were then rinsed with PBS followed by 2% Acetate Buffer rinses. Tissue then underwent silver intensification of gold particles for 5–10 min using the HQ silver kit (Nanoprobes). After the intensification, the tissue was rinsed in Acetate buffer and the sections were put in a solution containing 1:100 avidin-biotin-peroxidase complex (Vector). The tissue was washed in PBS and then rinsed with TRIS buffer before being transferred into a solution containing 0.01% imidazole, 0.0005% hydrogen peroxide and 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma, St. Louis, MO) for 10 minutes. The remainder of the procedure, involving dehydration, osmification and embedding was the same as that described above for the immunoperoxidase material.
Analysis of mGluR4/D1 double-immunostained material
To determine the relationships between mGluR4-positive terminals and D1-containing elements, double immunostained striatal tissue from each animal was scanned for the presence of mGluR4-positive terminals. When such boutons were found, their postsynaptic targets were identified and categorized as immunoreactive or not for D1. The proportion of mGluR4-positive terminals in contact with D1-positive or D1-negative elements was then calculated for each animal using either of the double labeling methods and statistically compared with t-Test. To ensure that the sensitivity of the D1 antibodies was the same across all double immunoreactions, the relative percentage of D1-positive vs D1-negative spines was calculated for each block of tissue analyzed in these studies. To do so, a total of 30 electron micrographs were randomly taken at 20,000X from the surface of sections. The percentages of labeled and unlabeled spines were then calculated from these images and averaged across blocks of tissue from the different animals.
Control experiments
Control incubations for all experiments included omission of either primary antibodies in turn to test for nonspecific secondary antibody binding.
RESULTS
Striatal MGluR4 Immunoreactivity
At the light microscopic level, the mGluR4 striatal neuropil displayed a homogenous pattern similar to that described in previous studies (Kinzie et al., 1995, Ohishi et al., 1995b, Bradley et al., 1996, Corti et al., 1998, Kosinski et al., 1999, Simonyi et al., 2000, Corti et al., 2002, Kuramoto et al., 2007). Other basal ganglia nuclei, like the globus pallidus also displayed strong mGluR4 immunoreactivity (Fig. 1).
Figure 1.
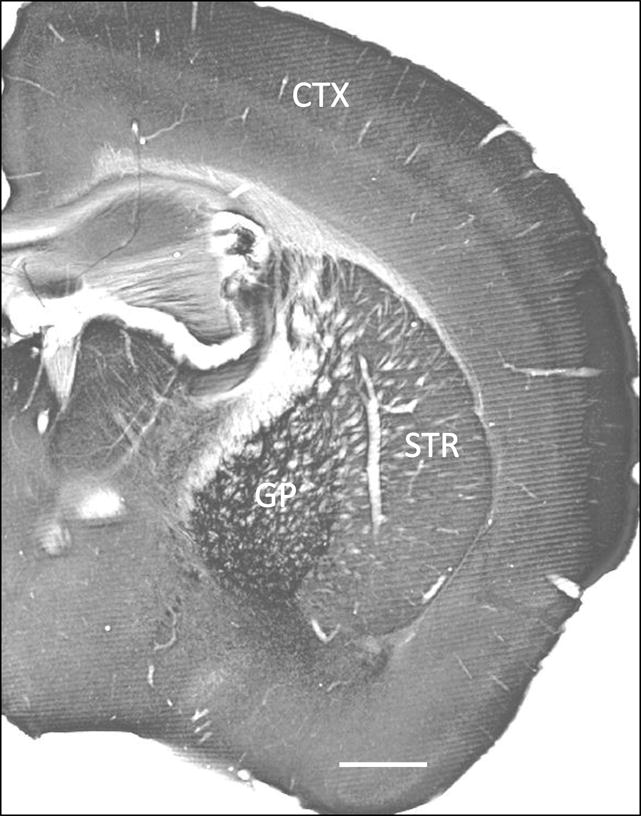
mGluR4 immunoreactivity in wild type mouse brain tissue: cortex (CTX), striatum (STR), and globus pallidus (GP). Scale bar: 1 mm.
At the electron microscopic level, pre-synaptic mGluR4 labeling was found to be aggregated in the pre-synaptic grids of putative glutamatergic terminals (Fig. 2A–B; 3A–D); a typical pattern of group III mGluR immunoreactivity throughout the CNS (Shigemoto et al., 1997, Ferraguti et al., 2005). A quantitative analysis of labeled elements randomly collected from our material revealed that 86.7% ±1.4 mGluR4 immunoreactivity was localized in presynaptic elements, including unmyelinated axons and putative glutamatergic terminals (Fig. 2E. A total of 47.0% ±3.0 of all terminals forming asymmetric synapses in the striatum were immunolabeled for mGluR4 and contacted dendritic spines (Fig. 2F). While the majority of mGluR4 labeling was found in presynaptic elements, a small amount of labeling was found post synaptically in dendrites and spines (Fig. 2A,B,E). Our quantitative analysis revealed that 12.6% ±1.7 of spine heads in the striatum were immunoreactive for mGluR4 (Fig. 2E)
Figure 2.
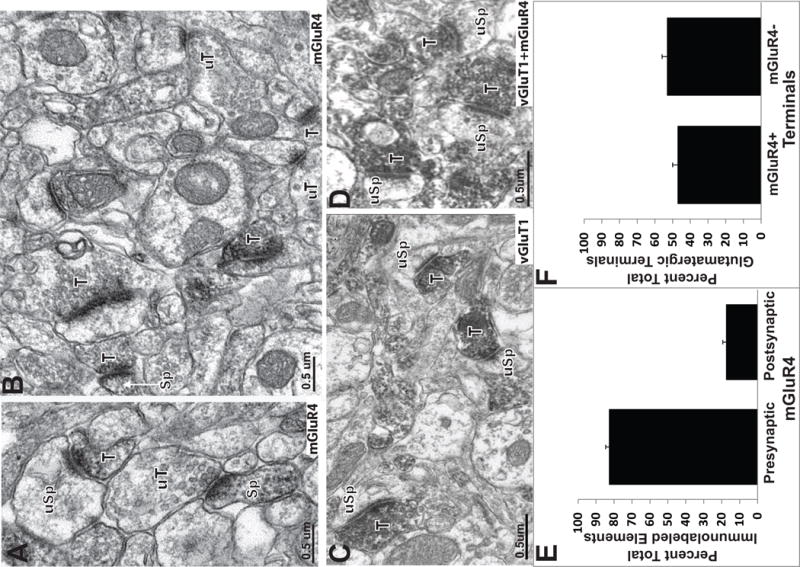
MGluR4/vGluT1 co-localization in the striatum. (A-B) Electron micrographs of mGluR4-positive terminals (T) and spines (Sp). Immunoreactive boutons frequently contact unlabelled dendritic spines (uSp). Unlabeled terminals in the neuropil are also indicated (uT). (C) vGluT1-immunoreactive terminals in contact with unlabeled dendritic spines. (D) Immunoreactive terminals (T) in contact with unlabeled spines (uSp) in striatal tissue incubated with a cocktail of vGluT1 and mGluR4 antibodies. (E) Histograms showing the relative percentages of presynaptic (ie terminals and axons) vs postsynaptic (spines, dendrites) mGluR4-positive neuronal elements in the striatum. (F) Histogram showing the relative percentage of total striatal glutamatergic terminals immunoreactive or not for mGluR4.
Figure 3.
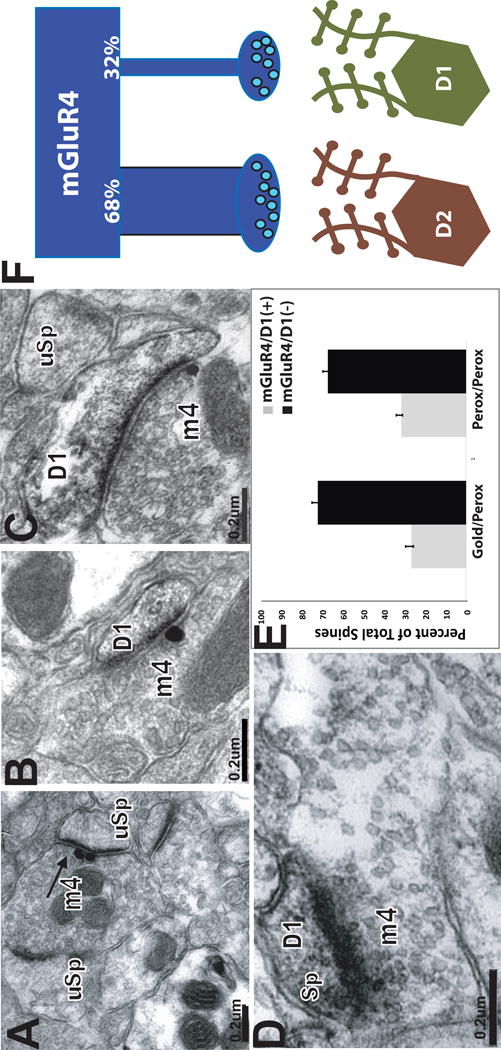
Targeting of D1-positive and D1-negative spines by mGluR4-labeled terminals. In panels A-C, mGluR4 is localized with gold, D1 is labeled with peroxidase. In panel D both mGuR4 and D1 are localized with immunoperoxidase. (A-D) mGluR4– positive terminal forming asymmetric synapses with D1-negative (A) or D1-positive (B-D) dendritic spines. (E) Histogram showing the relative percentages of mGluR4-immunoreactive terminals forming asymmetric synapses with D1-positive (gray bars) or D1-negative (black bars) spines in striatal tissue in which D1R immunostaining was localized with pre-embedding immunogold (left two bars) or pre-embedding immunoperoxidase (right two bars). (F) Schematic of differential innervation of direct and indirect striatal output pathways by mGluR4-positive terminals. MGluR4-positive terminals target preferentially indirect pathway neurons in the mouse striatum.
MGluR4 expression in corticostriatal glutamatergic terminals
The extent of mGluR4 expression in corticostriatal terminals was determined by means of the double immunoperoxidase cocktail approach using mGluR4 and vGluT1 antibodies. In singly stained tissue, the pattern of labeling for the mGluR4 and vGluT1 was distinctly different, the vGluT1 labeling was dense and filled the entire terminal, whereas the mGluR4 immunoreactivity was confined to the pre-synaptic active zones of labeled terminals, as shown in Figure 2A–D. In line with recent quantitative data from our laboratory and others (Raju and Smith, 2005, Raju et al., 2008), the vGluT1 antibodies labeled 64.0% ±4.7 of total terminals forming asymmetric synapses, while 47.1% +/− 3.0 of putative glutamatergic terminals displayed mGluR4 immunoreactivity. The cocktail experiments yielded to 66.7% ±3.8 of labeled terminals forming asymmetric synapses when the striatal tissue was co-immunostained for vGluT1 and mGluR4. Thus, based on these data, algebric calculations described in the Methods sectionrevealed that 44.3% +/− 3.2 putative striatal glutamatergic terminals (ie forming asymmetric synapses) co-express vGluT1 and mGluR4 in the mouse striatum. Of the total striatal vGluT1-positive striatal terminals, 73.4% +/− 5.3 co-express mGluR4. Finally, our findings also demonstrate that 100% mGluR4-containing terminals represent a subset of vGluT1-positive corticostriatal boutons (see Table 1).
Table 1.
Quantitative estimates and synaptic relationships of MGluR4-positive glutamatergic terminals in the mouse striatum
| % total mGluR4+ glutamatergic terminals vs total striatal glutamatergic terminals | 47.1% +/−3.0 |
| % mGluR4/vGluT1+ terminals vs total striatal glutamatergic terminals | 44.3% +/−3.2 |
| % total vGluT1+ terminals that express mGluR4 | 73.4% +/−5.3 |
| % total mGluR4+ terminals that express vGluT1 | 100% |
| % mGluR4+ terminals in contact with D1+ spines | 32.2%+/−1.5 |
| % mGluR4+ terminals in contact with D1-spines | 67.8%+/−1.5 |
MGluR4-positive terminals in contact with direct and indirect pathway neurons
To determine the prevalence of direct synaptic contacts between mGluR4-positive glutamatergic terminals and direct or indirect output pathway neurons, we combined the D1 receptor immunostaining, as marker of the direct pathway neurons, with the mGluR4 localization. The D1 receptor antibody labeled dendrites and spines. In line with previous findings from rat studies (Hersch et al., 1995, Lei et al., 2004), D1-labeled spines accounted for 51.2% of all spines in the mouse striatum, suggesting that the remaining D1-negative spines most likely represent D2 spines of indirect pathway neurons.
Thus, from tissue that contained both immunoperoxidase (used to label D1) and immunogold (used to label mGluR4) (Fig. 3A–C), spines contacted by a total of 142 gold-labeled mGluR4 terminals was categorized as immunoreactive or not for D1. This first series of studies revealed that 27.3%±1.9 of mGluR4-positive terminals contacted D1-positive spines (Fig. 3E). To further confirm these observations, we employed a double immunoperoxidase technique which resulted in peroxidase deposit in both mGluR4-positive elements and D1 receptor-immunoreactive structures (Fig. 2E). Because the immunoreactivity for either antigen is almost completely separate (postsynaptic D1 and presynaptic mGluRs), and that very few terminals in the striatum display D1 immunoreactivity, we assumed that the terminal labeling in this material was indicative of mGluR4 immunoreactivity, while postsynaptic labeling was mainly associated with D1 receptor labeling. The double immunoperoxidase method resulted in similar results as those obtained with the immunoperoxidase/immunogold double labeling method. From this material, 32.2%± 1.5 mGluR4-immunoreactive terminals formed synapses with D1-positive spines (Fig. 3E; Table 1).
DISCUSSION
The findings of this study demonstrate that mGluR4 is expressed in a subset of vGluT1-positive cortical glutamatergic neurons that target preferentially D1-negative “indirect pathway” striatal projection neurons in mice. These results support electrophysiological studies showing that mGluR4 regulates glutamatergic transmission at corticostriatal synapses, but also provide evidence suggesting that this mGluR4-mediated modulation of cortical projections may preferentially affect indirect over direct striatofugal neurons in mice.
Ultrastructural Localization of mGluR4
Despite previous qualitative description of presynaptic mGluR4 immunoreactivity in the rat and monkey striatum (Kinzie et al., 1995, Ohishi et al., 1995b, Bradley et al., 1996, Saugstad et al., 1997, Corti et al., 1998, Kosinski et al., 1999, Simonyi et al., 2000, Corti et al., 2002, Kuramoto et al., 2007), no study provided a quantitative assessment of mGluR4 localization in the striatum. At the ultrastructural level, various studies suggested a presynatic localization of mGluR4 in the active zones of some glutamatergic terminals, although the exact source of these boutons was not characterized (Bradley et al., 1999, Kosinski et al., 1999, Corti et al., 2002). Our data are in line with these previous observations, but further demonstrate that mGluR4 is expressed exclusively in a subset of cortical glutamatergic terminals in mice.
Although not directly assessed in this study, our findings suggest that pre-synaptic regulation of glutamatergic transmission may affect cortical, but not thalamic afferents, in the mouse striatum. A small number of studies demonstrated mGluR4 and mGluR7 mRNA expression in the rodent thalamus (Tanabe et al., 1993, Ohishi et al., 1995a, Saugstad et al., 1997, Corti et al., 1998, Kinoshita et al., 1998), but direct anatomical and functional evidence for pre-synaptic expression of group III mGluRs in thalamostriatal terminals remains to be demonstrated.
MGluR4 immunoreactivity was also found in a small population of postsynaptic elements in the mouse striatum. A few previously published reports described postsynaptic group III mGluRs localization in the retina on amacrine cells and, occasionally, on hippocampal pyramidal cell dendrites, (Brandstatter et al., 1996, Bradley et al., 1999) but the postsynaptic role of this receptor is unknown.
MGluR4/vGluT1 Co-localization
Our double immunolabeling data revealed that about 75% of striatal vGluT1-positive terminals co-express mGluR4, indicating that mGluR4 is an important regulator of corticostriatal transmission, but that a subset of cortical terminals may lack this pre-synaptic regulatory mechanism. Whether these negative data are due to technical limitations or genuine lack of mGluR4 cannot be completely ascertained, but one may consider the possibility that some of these mGluR4-negative cortical terminals may express other pre-synaptic mGluR subtypes, particularly mGluR7 or mGluR2 which, in fact, are both strongly expressed in the dorsal striatum (Kinoshita et al., 1998; Testa et al., 1998; Smith et al., 2001). The possibility that these different group III and group II mGluRs co-exist at the level of single cortical terminals should also be considered. Considering the strikingly different sensitivity of mGluR4 and mGluR7 receptor subtype to glutamate (Conn and Pin, 1997, De Blasi et al., 2001, Niswender and Conn, 2010), their possible co-expression at the active zones of glutamatergic synapses may provide a wide range of regulatory mechanisms by which group III mGluRs could influence glutamatergic transmission in the striatum. Future studies are needed to further assess the extent of co-expression of group III and group II mGluRs at the level of single glutamatergic corticostriatal terminals.
Our assessment of the extent of mGluR4/vGluT1 co-localization is based on data obtained from antibody cocktail experiments in which both antigens are localized with the immunoperoxidase method. This approach was successfully applied by our group and others to assess co-localization of other receptor subtypes in the striatum and cerebral cortex (Hersch et al., 1995, Lei et al., 2004, Bordelon et al., 2005, Mitrano and Smith, 2007). Because both the vGluT1 and mGluR4 antibodies were generated in rabbits, these antibodies could not be used in traditional double labeling experiments that localize each antigen with different markers. The benefit of the double immunoperoxidase method used here is that the sensitivity of the marker used to localize each antigen (i.e. peroxidase) is the same for the different antibodies, thereby reducing the likelihood of false negative data due to the reduced sensitivity and poor tissue penetration of gold-conjugated antibodies, commonly used in double immuno EM studies. However, an important technical issue to consider is that the quantitative assessment of the total number of labeled elements in each experiment is based on the relative abundance of labeled and unlabeled structures encountered in the tissue under analysis, thereby assuming that unlabeled elements are genuinely devoid of significant immunoreactivity for the antigen under study. To make sure this is the case, we sampled tissue taken only from the most superficial sections of the blocks where antibodies have full access to their antigens. Taking into consideration the little inter-individual variability between animals used in the same group, we believe that our sample strategy was accurate and, most importantly, consistent across experiments.
There is strong evidence that group III mGluRs play an important modulatory role on glutamatergic transmission in slices of rodent striatum (Pisani et al., 1997, Gubellini et al., 2004, 2014; Beurrier et al., 2009). Group III mGluR agonist, L-SOP (10 μM) significantly reduced the EPSP amplitude at the corticostriatal synapse in a reversible manner without modifying the postsynaptic sensitivity to glutamate (Pisani et al., 1997). L-AP4, another group III agonist reversibly and dose-dependently decreased the amplitude of the corticostriatal EPSP (Calabresi et al., 1993, 1996, Pisani et al., 1997). In a more recent study using specific mGluR4-related compounds, direct evidence for mGluR4-mediated presynaptic regulation of corticostriatal transmission was demonstrated (Beurrier et al., 2009). Future studies using drugs specific for mGluR4 or mGluR7 could help better understand the functional significance of the strong expression level of these two pre-synaptic mGluRs in corticostriatal terminals.
MGluR4-positive glutamatergic terminals vs direct and indirect striatal projection neurons
Although previous studies have confirmed the existence of mGluR4 in striatal glutamatergic terminals, it was not clear from these studies if the mGluR4-positive boutons preferentially target direct or indirect striatal output neurons. This issue was directly addressed in the present study. Because specific D2 receptor antibodies from a non-rabbit species were not available, rat anti-D1 receptor antibodies were used to identify the spines of the direct MSNs, while unlabeled spines were considered as belonging to indirect pathway neurons [see also (Hersch et al., 1995, Yung et al., 1995, Day et al., 1996, Lei et al., 2004, Deng et al., 2006)]. This assumption was supported by quantitative data showing that ~ 50% spines in the blocks of tissue used in our double-immunolabeling experiments displayed D1 immunoreactivity. Thus, using this approach, we found that almost 70% mGluR4-positive terminals were in contact with D1-negative spines, suggesting that indirect pathway neurons are preferentially targeted by corticostriatal terminals that express mGluR4. It is unlikely that this was due to a low sensitivity of the D1 labeling method because, as expected, almost half spines displayed D1 immunoreactivity. The possibility that this was due to the low penetration of gold-conjugated antibodies in the tissue is also unlikely, because the peroxidase was used to localize D1, while the gold labeling identified mGluR4-containing terminals. Furthermore, the fact that similar results were found when either gold- or peroxidase-conjugated antibodies were used to label mGluR4 terminals also rule out this possibility. Thus, studies using specific mGluR4 allosteric modulators in striatal slices of mice with GFP-labeled D1 or D2 dopamine receptors are warranted to assess the functional significance of these findings.
MGluR4-A Potential Target for Parkinson’s disease therapy
Recent data have provided strong evidence for the potential antiparkinsonian effects of group III mGluR agonists and allosteric modulators in acute rodent models of parkinsonism. MGluR4 can selectively modulate striatopallidal transmission, raising the interesting possibility that activation of mGluR4 could decrease the excessive inhibition of the GP that has been postulated to occur in Parkinson’s disease (Marino et al., 2003, Valenti et al., 2003). Consistent with this, intracerebroventricular, intrapallidal or systemic injections of group III mGluR agonists or mGluR4 allosteric potentiators produces therapeutic benefit in both acute and chronic rodent models of Parkinson’s disease (Valenti et al., 2003, Gubellini et al., 2004, Lopez et al., 2007, Jones et al., 2012, Bennouar et al., 2013). In light of findings presented in this study and previous electrophysiological data from slices of rat striatum (Beurrier et al., 2009; Gubellini et al., 2014), we believe that group III mGluRs in corticostriatal glutamatergic terminals represent another potential target through which mGluR4 potentiators could mediate their antiparkinsonian effects. Furthermore, the fact that activation of mGluR4 in corticostriatal afferents may affect preferentially “overactive” indirect pathway neurons, adds another benefit to this therapeutic approach. Thus, these findings, combined with the development of specific mGluR4-related drugs that display good bioavailability and pharmacokinetic properties, set the stage for promising mGluR4-mediated therapies in PD and related basal ganglia disorders.
Acknowledgments
This work was supported by grants from the National Institute of Health to YS (R01 NS037423; R01NS037948) and the Yerkes National Primate Center NIH base grant (P51-OD011132). Thanks are due to Susan Jenkins and Jean-Francois Pare for technical assistance.
References
- Bennouar KE, Uberti MA, Melon C, Bacolod MD, Jimenez HN, Cajina M, Kerkerian-Le Goff L, Doller D, Gubellini P. Synergy between L-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: implications for Parkinson’s disease treatment and dyskinesia. Neuropharmacology. 2013;66:158–169. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Bogenpohl J, Galvan A, Hu X, Wichmann T, Smith Y. Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultrastructural localization and electrophysiological effects of activation in the striatopallidal complex. Neuropharmacology. 2013;66:242–252. doi: 10.1016/j.neuropharm.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordelon JR, Smith Y, Nairn AC, Colbran RJ, Greengard P, Muly EC. Differential localization of protein phosphatase-1alpha, beta and gamma1 isoforms in primate prefrontal cortex. Cerebral cortex. 2005;15:1928–1937. doi: 10.1093/cercor/bhi070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. The Journal of comparative neurology. 1999;407:33–46. [PubMed] [Google Scholar]
- Brandstatter JH, Koulen P, Kuhn R, van der Putten H, Wassle H. Compartmental localization of a metabotropic glutamate receptor (mGluR7): two different active sites at a retinal synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4749–4756. doi: 10.1523/JNEUROSCI.16-15-04749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Heterogeneity of metabotropic glutamate receptors in the striatum: electrophysiological evidence. The European journal of neuroscience. 1993;5:1370–1377. doi: 10.1111/j.1460-9568.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends in neurosciences. 1996;19:19–22. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nature reviews Neuroscience. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annual review of pharmacology and toxicology. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Corti C, Restituito S, Rimland JM, Brabet I, Corsi M, Pin JP, Ferraguti F. Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. The European journal of neuroscience. 1998;10:3629–3641. doi: 10.1046/j.1460-9568.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- Cuomo D, Martella G, Barabino E, Platania P, Vita D, Madeo G, Selvam C, Goudet C, Oueslati N, Pin JP, Acher F, Pisani A, Beurrier C, Melon C, Kerkerian-Le Goff L, Gubellini P. Metabotropic glutamate receptor subtype 4 selectively modulates both glutamate and GABA transmission in the striatum: implications for Parkinson’s disease treatment. Journal of neurochemistry. 2009;109:1096–1105. doi: 10.1111/j.1471-4159.2009.06036.x. [DOI] [PubMed] [Google Scholar]
- Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hien TT, White NJ. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219–223. doi: 10.1016/s0140-6736(96)09096-4. [DOI] [PubMed] [Google Scholar]
- De Blasi A, Conn PJ, Pin J, Nicoletti F. Molecular determinants of metabotropic glutamate receptor signaling. Trends in pharmacological sciences. 2001;22:114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. Journal of chemical neuroanatomy. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JD, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:10520–10536. doi: 10.1523/JNEUROSCI.2547-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Unzai T, Nakamura K, Nomura S, Kaneko T. Difference in organization of corticostriatal and thalamostriatal synapses between patch and matrix compartments of rat neostriatum. The European journal of neuroscience. 2006;24:2813–2824. doi: 10.1111/j.1460-9568.2006.05177.x. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Niswender CM. Pharmacology of metabotropic glutamate receptor allosteric modulators: structural basis and therapeutic potential for CNS disorders. Progress in molecular biology and translational science. 2013;115:61–121. doi: 10.1016/B978-0-12-394587-7.00002-6. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Progress in neurobiology. 2004;74:271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Melon C, Dale E, Doller D, Kerkerian-Le Goff L. Distinct effects of mGluR4 receptor positive allosteric modulators at corticostriatal vs. striatopallidal synapses may differentially contribute tothier antiparkinsonian action. Neuropharmacology. 2014;85:166–177. doi: 10.1016/j.neuropharm.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS & neurological disorders drug targets. 2009;8:475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson’s disease. The Journal of pharmacology and experimental therapeutics. 2012;340:404–421. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. The Journal of comparative neurology. 1998;393:332–352. [PubMed] [Google Scholar]
- Kinzie JM, Saugstad JA, Westbrook GL, Segerson TP. Distribution of metabotropic glutamate receptor 7 messenger RNA in the developing and adult rat brain. Neuroscience. 1995;69:167–176. doi: 10.1016/0306-4522(95)00244-d. [DOI] [PubMed] [Google Scholar]
- Kosinski CM, Risso Bradley S, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Jr, Young AB, Standaert DG. Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. The Journal of comparative neurology. 1999;415:266–284. [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto E, Fujiyama F, Unzai T, Nakamura K, Hioki H, Furuta T, Shigemoto R, Ferraguti F, Kaneko T. Metabotropic glutamate receptor 4-immunopositive terminals of medium-sized spiny neurons selectively form synapses with cholinergic interneurons in the rat neostriatum. The Journal of comparative neurology. 2007;500:908–922. doi: 10.1002/cne.21216. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6249–6260. doi: 10.1523/JNEUROSCI.3819-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey CJ, Pothecary CA, Salt TE. Modulation of retino-collicular transmission by Group III metabotropic glutamate receptors at different ages during development. Neuropharmacology. 2005;49(Suppl 1):26–34. doi: 10.1016/j.neuropharm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Bolea C, Girard F, Poli S, Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, Hodge LM, Smith KM, DiLella AG, Liverton N, Hess F, Browne SE, Reynolds IJ. A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson’s disease. The Journal of pharmacology and experimental therapeutics. 2012;343:167–177. doi: 10.1124/jpet.112.196063. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Valenti O, O’Brien JA, Williams DL, Jr, Conn PJ. Modulation of inhibitory transmission in the rat globus pallidus by activation of mGluR4. Annals of the New York Academy of Sciences. 2003;1003:435–437. doi: 10.1196/annals.1300.045. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. The Journal of comparative neurology. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual review of pharmacology and toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. The Journal of comparative neurology. 1995a;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Nomura S, Ding YQ, Shigemoto R, Wada E, Kinoshita A, Li JL, Neki A, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neuroscience letters. 1995b;202:85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36:845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. The European journal of neuroscience. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. The Journal of comparative neurology. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Smith Y. Differential Localization of Vesicular Glutamate Transporters 1 and 2 in the Rat Striatum. In: Bolam JP, et al., editors. The Basal Ganglia VIII Advances in Behavioral Biology. Vol. 56. USA: Springer US; 2005. pp. 601–610. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of cell biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad JA, Kinzie JM, Shinohara MM, Segerson TP, Westbrook GL. Cloning and expression of rat metabotropic glutamate receptor 8 reveals a distinct pharmacological profile. Molecular pharmacology. 1997;51:119–125. doi: 10.1124/mol.51.1.119. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Miller LA, Sun GY. Region-specific decline in the expression of metabotropic glutamate receptor 7 mRNA in rat brain during aging. Brain research Molecular brain research. 2000;82:101–106. doi: 10.1016/s0169-328x(00)00189-3. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Charara A, Paquet M, Kieval JZ, Pare JF, Hanson JE, Hubert GW, Kuwajima M, Levey AI. Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. Journal of chemical neuroanatomy. 2001;22:13–42. doi: 10.1016/s0891-0618(01)00098-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T, Bolam JP. The thalamostriatal system in normal and diseased states. Frontiers in systems neuroscience. 2014;8:5. doi: 10.3389/fnsys.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Movement disorders: official journal of the Movement Disorder Society. 2008;23(Suppl 3):S534–547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Freiberg IK, Weiss SW, Standaert DS. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]


