Abstract
Background & Aims
Individuals with cystic fibrosis are at increased risk of colorectal cancer (CRC) compared to the general population, and risk is higher among those who received an organ transplant. We performed a cost-effectiveness analysis to determine optimal CRC screening strategies for patients with cystic fibrosis.
Methods
We adjusted the existing MISCAN-Colon microsimulation model to reflect increased CRC risk and lower life-expectancy in patients with cystic fibrosis. Modeling was performed separately for individuals who never received an organ transplant and patients who had received an organ transplant. We modeled 76 colonoscopy screening strategies that varied the age range and screening interval. The optimal screening strategy was determined based on a willingness to pay threshold of $100,000 per life-year gained. Sensitivity and supplementary analyses were performed, including fecal immunochemical test (FIT) as an alternative test, earlier ages of transplantation, and increased rates of colonoscopy complications, to assess if optimal screening strategies would change.
Results
Colonoscopy every 5 years, starting at an age of 40 years, was the optimal colonoscopy strategy for patients with cystic fibrosis who never received an organ transplant; this strategy prevented 79% of deaths from CRC. Among patients with cystic fibrosis who had received an organ transplant, optimal colonoscopy screening should start at an age of 30 or 35 years, depending on the patient’s age at time of transplantation. Annual FIT screening was predicted to be cost-effective for patients with cystic fibrosis. However, the level of accuracy of the FIT in population is not clear.
Conclusions
Using a MISCAN-Colon microsimulation model, we found screening of patients with cystic fibrosis for CRC to be cost effective. Due to the higher risk in these patients for CRC, screening should start at an earlier age with a shorter screening interval. The findings of this study (especially those on FIT screening) may be limited by restricted evidence available for patients with cystic fibrosis.
Keywords: Colonoscopy screening, microsimulation modeling, screening ages, decision analysis, cystic fibrosis, colorectal cancer screening
Introduction
Cystic fibrosis is the most common, life shortening, autosomal recessive genetic disease among Caucasians.1 Approximately 35,000 children and adults have cystic fibrosis in the United States (US), with worldwide prevalence estimated in more than 70,000 individuals.2, 3 Cystic fibrosis is caused by a mutation in the cystic fibrosis transmembrane conductance regulator gene. Cystic fibrosis impacts multiple organ systems, including respiratory and gastrointestinal.4 Due to advances in disease management, detection, and therapy, survival has increased in individuals with cystic fibrosis. The median predicted survival age increased from 33.3 to 41.7 years between 2000 and 2015 and currently more than half of individuals with cystic fibrosis are aged 18 or older.4 However, with improved survival, individuals with cystic fibrosis increasingly become at risk for other diseases that typically occur at older ages, especially those involving the gastrointestinal tract.5
Gastrointestinal malignancies are an emerging health problem among individuals with cystic fibrosis. Several studies have shown an increased risk of digestive tract cancers and an increased early incidence and progression of adenomatous colorectal polyps to colorectal cancer (CRC).5–8 Screening for CRC is a well-established intervention that has been shown to reduce the burden of CRC in the general population.9–17 Screening generally starts at the age of 50 for the average risk population, with those at higher risk (such as those with family history of CRC (first-degree relatives, FDR) or Lynch Syndrome) commencing at an earlier age.18 Although those with cystic fibrosis fall into the latter category (their CRC risk exceeds that of those with FDR), their lower life-expectancy may lead to a different trade-off between the benefits and harms of CRC screening. At present, there are no specific recommendations for screening and surveillance for this population.
We performed a decision analysis for the Cystic Fibrosis Foundation and Cystic Fibrosis CRC Screening Task Force (CFCRCSTF),19 to explore the benefits, harms, and costs of CRC screening in the CF population and determine the most appropriate CRC screening strategy using a modeling approach.
Materials and Methods
We used the Microsimulation Screening Analysis-Colon (MISCAN-Colon) model (Erasmus University Medical Center, Rotterdam, The Netherlands) to assess the effectiveness and costs of screening for CRC among individuals with cystic fibrosis. This model is part of the Cancer Intervention and Surveillance Modeling Network (CISNET).20
MISCAN-Colon model description
MISCAN-Colon is a well-established stochastic microsimulation model for CRC. The structure, underlying assumptions, and calibration of this model have been described in previous studies and in the model appendix.20, 21 Briefly, MISCAN-Colon simulates the life histories of many individuals from birth to death (first without screening and subsequently with screening). As each simulated individual ages, zero, one, or more than one adenomas may develop. These adenomas can progress in size and may develop into (preclinical) cancer. Survival after cancer diagnosis depends on age, stage and the localization of the cancer at diagnosis.22 The introduction of screening may alter the simulated life histories: detection and removal of adenomas may prevent some cancer cases or may detect others at an earlier stage (favorable survival). MISCAN-Colon quantifies the effectiveness and the costs of screening by comparing all the life histories with screening with the corresponding life histories without screening.
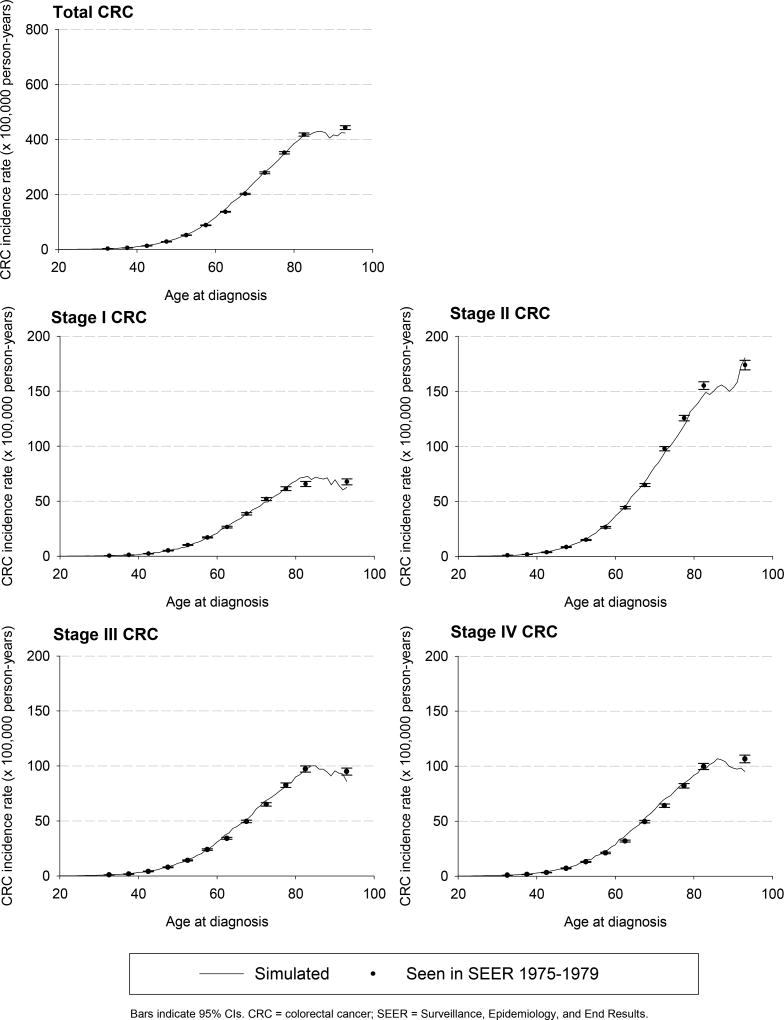
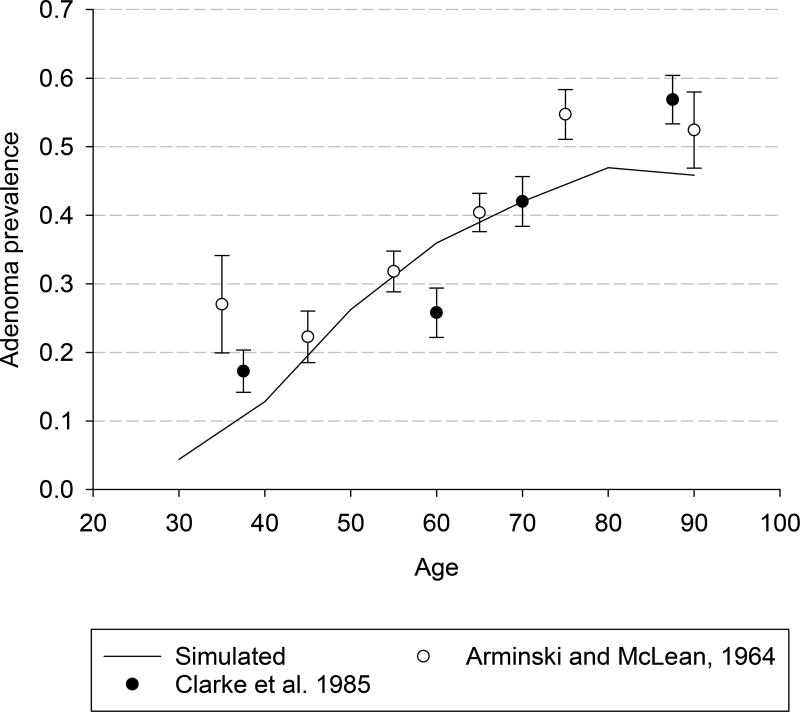
MISCAN-Colon was first calibrated to age-, stage-, and localization-specific incidence of CRC as seen in the US general population in the SEER (Surveillance, Epidemiology, and End Results) program before the introduction of the screening (years between 1975 and 1979, Appendix Figure 1)23 and the age-specific prevalence distribution of adenomas seen in autopsy studies (Appendix Figure 2).24–33 Adenoma dwell time and the preclinical duration of CRC were calibrated to the outcomes of the randomized clinical trials (RCTs) evaluating screening using guaiac fecal occult blood tests and sigmoidoscopy.9–12, 14, 34
Adaptions of the MISCAN-Colon model to the cystic fibrosis population
The MISCAN-Colon model was adjusted to reflect the increased CRC risk and the elevated all-cause mortality in individuals with cystic fibrosis. Modeling was performed separately for individuals who never received a transplant and those who were post-transplant to account for differences in CRC risk and survival between these two groups (non-transplant vs. transplant patients). We assumed that the higher CRC risk in both groups was caused by a more frequent adenoma onset (increased probability of adenoma occurrence across all ages) which would result in more CRC.
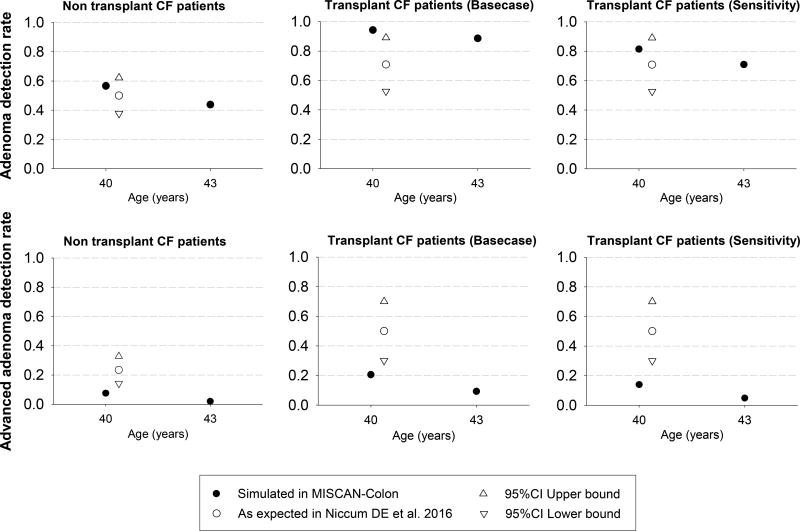
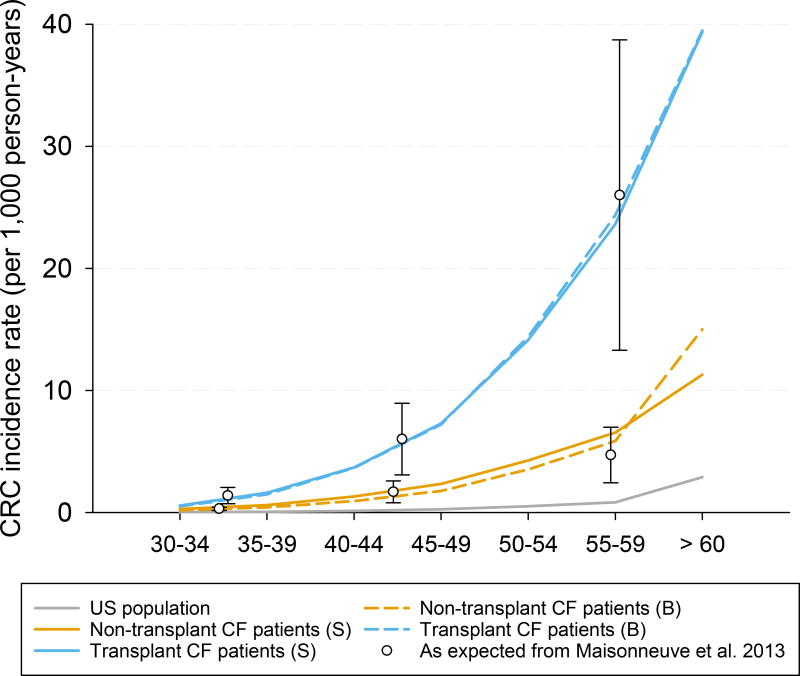
For individuals with cystic fibrosis who have not had a transplant, the parameters of the model were adjusted to replicate the 7-fold higher CRC risk observed in a 20-year study of 48,188 individuals with cystic fibrosis included in the Cystic Fibrosis Foundation Patient Registry (CFFPR) (Figure 1).6 Adenoma and advanced adenoma (i.e., large adenoma ≥ 10 mm) detection rates at two different screening rounds were computed and compared with the adenoma detection rates observed in an observational study of people with cystic fibrosis undergoing colonoscopy screening (Appendix Figure 3).8 The model was also adjusted to reflect the overall mortality of individuals with cystic fibrosis in 2015.4
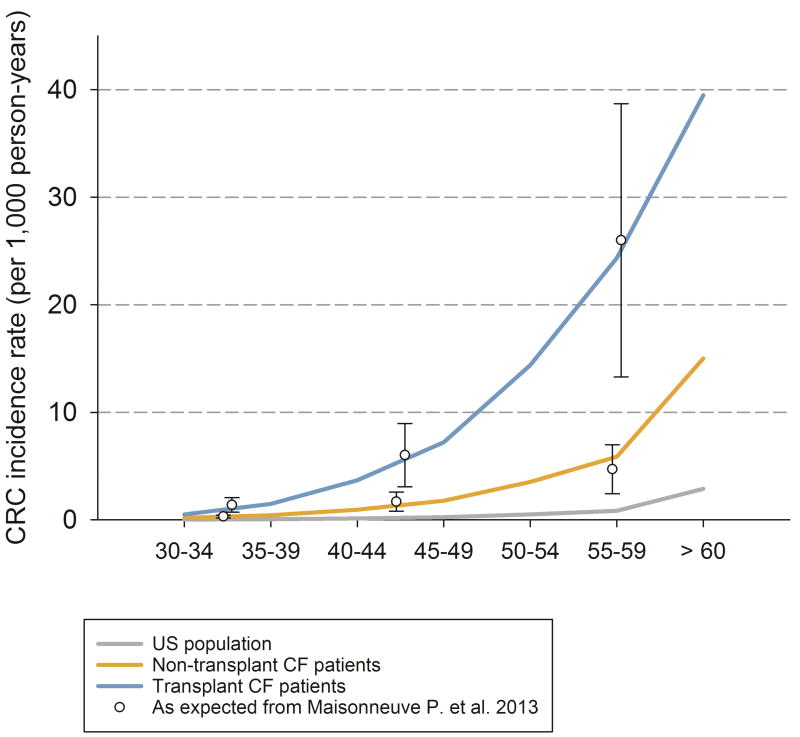
Figure 1.
CRC incidence expected in individuals with cystic fibrosis according to Maisonneuve P. et al. 2013 and CRC incidence simulated in Microsimulation Screening Analysis-Colon model without screening in the US general population, non-transplant, and transplant cystic fibrosis patients assuming a higher CRC risk through a more frequent adenoma onset (base case analysis). Note: Bars indicate 95% confidence intervals; CRC = colorectal cancer; CF = Cystic Fibrosis.
In all analyses for cystic fibrosis transplant patients, we assumed the same adenoma risk as the non-transplant cystic fibrosis population until organ transplantation. We assumed a more frequent onset of adenomas immediately after organ transplant. A 30-fold increase in CRC risk was based on the US cohort study by Maisonneuve et al6 (Figure 1). Simulated adenoma and advanced adenoma detection rates were computed and are reported in Appendix Figure 3. In addition to a higher CRC risk, we also assumed that transplanted individuals with cystic fibrosis had a higher risk of dying of CRC once diagnosed. The increased CRC death-specific risk was modeled as a hazard ratio of 2 based on the excess risk of CRC death using the model provided by Rutter CM et al.22 Life-expectancy post transplantation was based on life tables for individuals with cystic fibrosis after lung transplantation. Lung transplants constitute 90% of transplantations in individuals with cystic fibrosis.4 Our model reflected the International Society for Heart and Lung Transplantation’s data which shows that, for individuals with cystic fibrosis, post-transplant survival is related to time since the transplant and not age.35 We simulated this entire population with transplant at the age of 30 years (the median age of transplant) and assessed earlier ages of transplantation in sensitivity analyses to assess if the optimal screening strategies would change.
Screening strategies simulated
For both groups (transplant and non-transplant individuals with cystic fibrosis), a cohort of 10 million individuals, aged 30 years in 2017, was simulated with the adjusted MISCAN-Colon model under 76 different colonoscopy screening strategies (a total of 152 different screening strategies). The strategies differed with respect to i) screening interval (3, 5 or 10 years for colonoscopy; ii) age to start (30, 35, 40, 45, 50); and iii) age to end screening (55, 60, 65, 70, 75 years). Furthermore, an additional cohort of 10 million individuals aged 30 years in 2017 without cystic fibrosis was simulated to enable a comparison of outcomes between the cystic fibrosis population and the US general population under the recommended US CRC screening guidelines (colonoscopy starting at age 50 repeated every 10 years).
In addition, given that colonoscopy might be very demanding for individuals with cystic fibrosis, we explored the fecal immunochemical test (FIT) as a possible and hypothetically adequate alternative in this population. As such, we performed a specific supplementary analysis including also annual FIT screening (25 screening strategies).
Screening assumptions
Test characteristics and complication rates for each screening test were based on studies in the general population (Appendix Table 1),36–40 as specific information for the cystic fibrosis population are not available.
Modeling FIT screening strategies, we assumed that patients with a positive FIT result were referred for a diagnostic colonoscopy (positive threshold: 100 ng/ml buffer, equals to 20 µg/g feces).37 Individuals with adenomas detected and removed during a screening or diagnostic colonoscopy were assumed to enter colonoscopy surveillance according to the current general population guidelines,18 except for colonoscopy screening strategies with 3 year screening interval where a more intensive colonoscopy surveillance interval was introduced in line with the screening interval: every 3 years. We assumed 100% adherence to screening, diagnostic and surveillance tests.
Because it is reasonable to consider that the performance of CRC screening in cystic fibrosis population may be different with regards to colonoscopy complications, adverse events related to a more intensive bowel preparation, and the efficacy of FIT, we address these aspects in specific sensitivity analyses to assess if the optimal screening strategies would be affected.
CRC screening costs and outcomes
The cost-effectiveness analyses were carried out from a societal perspective. The costs of screening tests were based on the 2014 Medicare payment rates including co-payments (Appendix Table 2). Complication costs were obtained from a cost analysis study of cases hospitalized after endoscopy in 2007.41 Patient time costs were added to both.42 The cost of life years (LYs) with CRC care were based on the SEER-Medicare linked data analysis and included co-payments and patient time costs.43 All costs were adjusted to 2015 using the annual average Consumer Price Indexes provided by US Bureau of Labor Statistics.44 For each simulated cohort, we computed the effectiveness (i.e., CRC cases prevented, CRC deaths prevented, and LYs gained) and costs of the screening. LYs gained (LYG) from screening and costs were discounted by applying the conventional 3% annual discount rate.
Cost-effectiveness analyses
We determined the cost-effectiveness of each screening strategy and compared these results to no screening. Subsequently, we performed an incremental cost-effectiveness analysis to determine the optimal screening strategy. To do this we: i) ranked all the screening strategies by increasing costs; ii) excluded all the screening strategies that were more costly and less effective than other strategies (“strongly dominated strategies”); iii) deleted the screening strategies that were less costly and less effective than another but provided an additional LY at higher incremental costs (“weakly dominated strategies”); iv) calculated for all remaining strategies (“efficient strategies”, or strategies on the “efficient frontier”) the incremental cost-effectiveness ratio (ICER) as the ratio between additional costs and additional clinical benefits (in this case LY gained) of a specific screening strategy compared to the previous less expensive strategy (i.e., strategy with costs lower and closest to the strategy of interest); and v) selected the optimal strategy assuming a willingness to pay threshold of $100,000 per LYG.
Sensitivity analyses
We conducted multiple sensitivity analyses to test the robustness of the model results under a variety of different assumptions. These assumptions included: i) lowering colonoscopy test sensitivity for small and medium size adenomas (0.65 and 0.80 respectively); ii) a more proximal CRC location (50% of CRC in the right colon); iii) increasing colonoscopy complication rates two-fold; iv) increasing the risk of cardiovascular complications associated with colonoscopy (5- and 10-fold increased risk, including respiratory arrest); v) lowering FIT specificity (0.90); vi) a worst case for FIT considering a lower specificity (0.75) and sensitivity (i.e. 36% reduced) in cystic fibrosis population (different FIT performances); vii) biennial screening intervals for FIT; viii) lowering adherence to the screening test (80%); ix) more intensive colonoscopy surveillance (3 years) for all the screening strategies; and x) increasing costs due to increased patient time (Appendix Table 2).
Additionally, among the non-transplant people with cystic fibrosis, we analyzed the impact of: i) a higher CRC risk (10-fold increased risk compared to general population); ii) a higher CRC risk (7-fold) due to a shorter adenoma dwell time (94% reduced, extremely fast adenoma progression) instead of a more frequent adenoma onset (Appendix Figure 4); and iii) a higher all-cause mortality in older ages (≥ 45 years).45 For the individuals with cystic fibrosis who have had a transplant, we investigated the impact of: i) differential age of transplant (20 and 25 years-old in 2017); ii) additional colonoscopy screening strategies (starting at age 32, every 5 years); iii) increased CRC risk (45-fold increased risk) with a more proximal CRC location (50% of CRC in the right colon); iv) utilization of the same age-specific mortality rate observed among non-transplant individuals with cystic fibrosis after age 50 years; and v) higher CRC risk due to a combination of shorter adenoma dwell time (50% reduced) and higher adenoma onset (16-fold increased risk calibrated to replicate the increased CRC incidence among these individuals, Appendix Figure 4).
Results
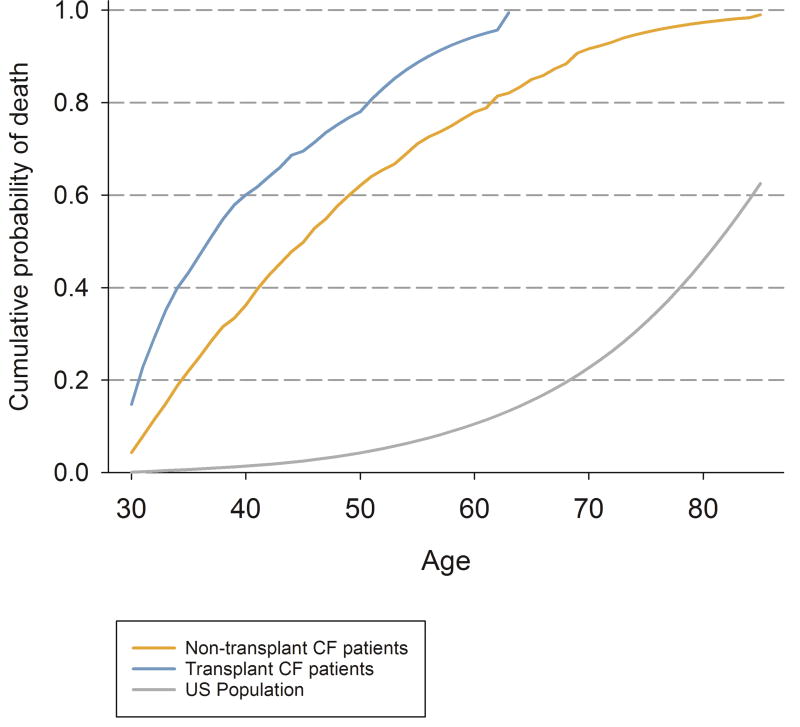
Without screening, the model predicted 19.1 CRC deaths per 1,000 30-year old individuals with cystic fibrosis who have not had a transplant. Among those who had a transplant, 22.3 CRC deaths per 1,000 individuals were predicted to die from CRC (Table 1). The recommended US CRC screening strategy was estimated to prevent more than 73% of the CRC deaths among the US general population, 66% of CRC deaths among individuals with cystic fibrosis, and 39% of individuals with cystic fibrosis post-transplant. However, only 22% of individuals who received a transplant and 36% of those who did not were predicted to survive in the model until age 50, thereby meeting the age requirement to participate in this screening strategy (Figure 2).
Table 1.
Number of colorectal cancer (CRC) deaths predicted, prevented, and screening life-years gained estimated with Microsimulation Screening Analysis-Colon model without screening and with recommended screening scenarios for the US general population, for transplant and non-transplant Cystic Fibrosis patients.
| Screening strategies | CRC deaths Predicteda |
CRC deaths Preventeda |
Reduction in CRC Mortality (%) |
LYGa,b |
|---|---|---|---|---|
|
| ||||
| US general population: | ||||
| Without screening | 27.8 | - | - | - |
| Colonoscopy, Ages 50–75 (10) | 7.4 | 20.4 | 73.4 | 56.0 |
| No transplant CF patients: | ||||
| Without screening | 19.1 | - | - | - |
| Colonoscopy, Ages 50–75 (10) | 6.5 | 12.6 | 66.0 | 30.3 |
| Transplant CF patients: | ||||
| Without screening | 22.3 | - | - | - |
| Colonoscopy, Ages 50–75 (10) | 13.6 | 8.7 | 39.0 | 14.5 |
CRC = Colorectal cancer; LYG, Life years gained compared with no screening; (n) = screening interval; CF = Cystic fibrosis.
These values were computed per 1,000 30 years-old US individuals in 2017, 1,000 30 years-old no transplant CF patients in 2017, and 1,000 30 years-old transplant CF patients (with organ transplant at age 30) in 2017 for, respectively, US general population, no transplant and transplant CF patients;
LYG from screening were discounted (3%).
Figure 2.
Cumulative Risk (%) of death for all causes simulated with Microsimulation Screening Analysis-Colon model for US general population, transplant, and non-transplant Cystic Fibrosis patients without screening. Note: CF = Cystic Fibrosis.
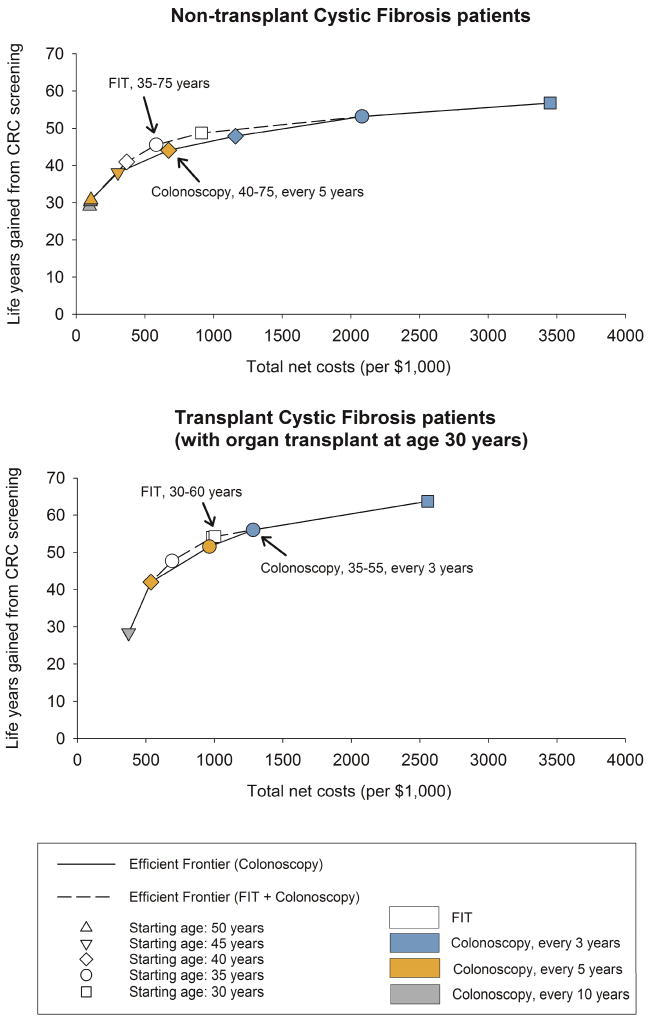
The costs and benefits of all simulated screening strategies for transplant and non-transplant individuals with cystic fibrosis were investigated (Appendix Tables 3–6) and strategy-specific efficient frontiers are reported in Figure 3. Among the efficient colonoscopy screening strategies, LYG from screening varied from 29 to 57 (per 1,000 individuals age 30 years) for non-transplant and from 28 to 64 for transplant cystic fibrosis patients. Higher benefits were associated with colonoscopy screening every 3 years from age 30 to 75, while the lower values for LYG for individuals with cystic fibrosis with and without organ transplant were observed, respectively, screening with once-lifetime colonoscopy at age 50 and 10-yearly colonoscopy from age 45 to 55.
Figure 3.
Efficient frontiers with efficient screening strategies for non-transplant cystic fibrosis and transplant cystic fibrosis patients. Total costs and life-years gained from screening were discounted (3% discounting rate) and 100% adherence was assumed for screening, diagnostic and surveillance test. Optimal screening strategies are labelled and indicated by arrows.
For non-transplant individuals with cystic fibrosis, when only colonoscopy was considered as a screening test, the optimal colonoscopy strategy was one screen every 5 years from 40 to 75 years with an ICER of $84,000 per LY gained (Table 2). This strategy predicted 25 CRC cases and 4 CRC deaths to occur equating to a reduction of 52% in CRC incidence and 79% for CRC mortality (Table 2). Among transplanted cystic fibrosis patients, colonoscopy screening repeated every 3 years between age 35 and 55 was optimal. It prevented 82% of CRC mortality (ICER of $71,000 per LY gained), compared with no screening (Table 3).
Table 2.
Efficient screening strategies among non-transplant Cystic Fibrosis patients according to screening tests used.
| Outcomes per 1,000 non-transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (3% discounted)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening tests
|
Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb CRC incidencec |
(%) CRC mortalityc |
ICER (*$1,000) |
||
| FIT | COLs | |||||||||||||
|
| ||||||||||||||
| Colonoscopy strategies (main analysis)
| ||||||||||||||
| No screening | 0 | 0 | 0 | 23 | 0 | 52 | 19 | 134 | 0 | 1,918 | 0 | 0 | 0 | - |
| COL 50–55 y, 10 y | 0 | 214 | 334 | 558 | 3 | 32 | 7 | 127 | 29 | 2,016 | 97 | 38 | 62 | 3 |
| COL 50–60 y, 10 y | 0 | 225 | 345 | 579 | 3 | 31 | 7 | 127 | 30 | 2,021 | 103 | 40 | 66 | 4 |
| COL 50–60 y, 5 y | 0 | 234 | 354 | 597 | 3 | 31 | 6 | 126 | 31 | 2,025 | 107 | 41 | 67 | 9 |
| COL 50–70 y, 5 y | 0 | 235 | 354 | 598 | 3 | 31 | 6 | 126 | 31 | 2,026 | 107 | 41 | 67 | 14 |
| COL 45–75 y, 5 y | 0 | 394 | 531 | 931 | 3 | 27 | 5 | 117 | 38 | 2,222 | 303 | 48 | 74 | 27 |
| COL 40–70 y, 5 y | 0 | 689 | 724 | 1,417 | 4 | 25 | 4 | 109 | 44 | 2,591 | 673 | 52 | 79 | 62 |
| COL 40–75 y, 5 y | 0 | 689 | 724 | 1,417 | 4 | 25 | 4 | 109 | 44 | 2,591 | 673 | 52 | 79 | 84 |
| COL 40–75 y, 3 y | 0 | 793 | 1,301 | 2,097 | 5 | 20 | 3 | 93 | 48 | 3,078 | 1,159 | 63 | 84 | 128 |
| COL 35–75 y, 3 y | 0 | 1,482 | 1,700 | 3,185 | 5 | 18 | 2 | 84 | 53 | 4,000 | 2,082 | 67 | 88 | 174 |
| COL 30–75 y, 3 y | 0 | 2,671 | 2,062 | 4,734 | 6 | 17 | 2 | 77 | 57 | 5,370 | 3,451 | 68 | 90 | 383 |
|
| ||||||||||||||
| All screening strategies (supplementary analysis)
| ||||||||||||||
| No screening | 0 | 0 | 0 | 23 | 0 | 52 | 19 | 134 | 0 | 1,918 | 0 | 0 | 0 | - |
| COL 50–55 y, 10 y | 0 | 214 | 334 | 558 | 3 | 32 | 7 | 127 | 29 | 2,016 | 97 | 38 | 62 | 3 |
| COL 50–60 y, 10 y | 0 | 225 | 345 | 579 | 3 | 31 | 7 | 127 | 30 | 2,021 | 103 | 40 | 66 | 4 |
| COL 50–60 y, 5 y | 0 | 234 | 354 | 597 | 3 | 31 | 6 | 126 | 31 | 2,025 | 107 | 41 | 67 | 9 |
| COL 50–70 y, 5 y | 0 | 235 | 354 | 598 | 3 | 31 | 6 | 126 | 31 | 2,026 | 107 | 41 | 67 | 14 |
| FIT 40–75 y | 4,125 | 0 | 300 | 519 | 2 | 38 | 5 | 164 | 41 | 2,286 | 368 | 28 | 75 | 25 |
| FIT 35–75 y | 6,772 | 0 | 367 | 675 | 2 | 36 | 4 | 163 | 46 | 2,501 | 583 | 31 | 78 | 47 |
| FIT 30–75 y | 10,783 | 0 | 427 | 872 | 2 | 36 | 4 | 163 | 49 | 2,830 | 912 | 32 | 80 | 103 |
| COL 35–75 y, 3 y | 0 | 1,482 | 1,700 | 3,185 | 5 | 18 | 2 | 84 | 53 | 4,000 | 2,082 | 67 | 88 | 263 |
| COL 30–75 y, 3 y | 0 | 2,671 | 2,062 | 4,734 | 6 | 17 | 2 | 77 | 57 | 5,370 | 3,451 | 68 | 90 | 383 |
COL indicates colonoscopy; CRC, colorectal cancer; FIT = Fecal Immunochemical Test; LY, Life-years; LYG, LY gained compared with no screening; ICER, Incremental cost-effectiveness ratio (Costs/LYs gained).
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Bold rows indicate optimal screening strategies.
Table 3.
Efficient screening strategies among Transplant Cystic Fibrosis patients according to screening tests used.
| Outcomes per 1,000 transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (with organ transplant at age 30, 3% discounted)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening test
|
Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb CRC incidencec |
(%) CRC mortalityc |
ICER (*$1,000) |
||
| FIT | COLs | |||||||||||||
|
| ||||||||||||||
| Colonoscopy strategies (main analysis)
| ||||||||||||||
| No screening | 0 | 0 | 0 | 30 | 0 | 52 | 22 | 115 | 0 | 2,065 | 0 | 0 | 0 | - |
| COL 45–55 y, 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2,438 | 374 | 25 | 57 | 1 |
| COL 45–55 y, 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2,439 | 374 | 25 | 58 | 7 |
| COL 40–55 y, 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2,601 | 536 | 36 | 70 | 12 |
| COL 35–55 y, 5 y | 0 | 607 | 838 | 1,451 | 3 | 31 | 5 | 122 | 52 | 3,028 | 963 | 41 | 77 | 45 |
| COL 35–55 y, 3 y | 0 | 642 | 1,265 | 1,912 | 4 | 26 | 4 | 110 | 56 | 3,347 | 1,282 | 49 | 82 | 71 |
| COL 30–55 y, 3 y | 0 | 1,511 | 1,826 | 3,340 | 5 | 25 | 3 | 99 | 64 | 4,622 | 2,558 | 53 | 87 | 166 |
|
| ||||||||||||||
| All screening strategies (supplementary analysis)
| ||||||||||||||
| No screening | 0 | 0 | 0 | 30 | 0 | 52 | 22 | 115 | 0 | 2,065 | 0 | 0 | 0 | - |
| COL 45–55 y, 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2,438 | 374 | 25 | 57 | 1 |
| COL 45–55 y, 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2,439 | 374 | 25 | 58 | 7 |
| COL 40–55 y, 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2,601 | 536 | 36 | 70 | 12 |
| FIT 35–55 y | 3,419 | 0 | 377 | 620 | 2 | 42 | 6 | 175 | 48 | 2,756 | 691 | 19 | 72 | 27 |
| FIT 30–55 y | 6,702 | 0 | 460 | 811 | 2 | 41 | 5 | 177 | 54 | 3,050 | 985 | 21 | 76 | 47 |
| FIT 30–60 y | 6,722 | 0 | 463 | 816 | 2 | 41 | 5 | 178 | 54 | 3,068 | 1,003 | 20 | 77 | 86 |
| COL 35–55 y, 3 y | 0 | 642 | 1,265 | 1,912 | 4 | 26 | 4 | 110 | 56 | 3,347 | 1,282 | 49 | 82 | 156 |
| COL 30–55 y, 3 y | 0 | 1,511 | 1,826 | 3,340 | 5 | 25 | 3 | 99 | 64 | 4,622 | 2,558 | 53 | 87 | 166 |
COL indicates colonoscopy; CRC, colorectal cancer; FIT = Fecal Immunochemical Test; LY, Life-years; LYG, LY gained compared with no screening; ICER, Incremental cost-effectiveness ratio (Costs/LYs gained).
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Bold rows indicate optimal screening strategies.
When both FIT and colonoscopy screening strategies were jointly modeled (supplementary analysis), the optimal screening strategy was annual FIT between age 35 and 75 with an ICER of $47,000 per LY gained (Table 2) for non-transplant individuals with cystic fibrosis. When compared to no screening, it could prevent 31% of CRC cases and 78% of the CRC deaths (16 CRC cases and 15 deaths per 1,000). FIT was also cost-effective for cystic fibrosis individuals who had undergone organ transplant with annual FIT between ages 30 and 60 achieving a reduction in CRC incidence of 20% and mortality of 77% with an ICER of $86,000 per LY gained (Table 3).
Sensitivity analyses
For many of the sensitivity analyses, the optimal screening strategy remained the same as the base case (Table 4). For non-transplant individuals with cystic fibrosis, the optimal age to stop colonoscopy screening was sensitive to our assumptions for higher all-cause mortality in older ages (55 years) or increased risk of cardiovascular complications (70 years). A colonoscopy screening interval of every 3 years was more optimal when adenoma dwell time was reduced and CRC risk was increased with more proximal adenoma location. Higher costs for colonoscopy (more time required for patients to be prepared for colonoscopy and to recover from its complications) resulted in a later age to start screening (45 years). When all strategies were investigated (supplementary analysis), FIT start age was earlier (30 years) when adenoma dwell time was shortened and CRC risk was increased. A reduction in specificity and sensitivity of FIT increased the age of starting screening to 40 years. FIT screening should stop at age 60 when higher overall mortality was assumed among individuals with cystic fibrosis in older ages. FIT was not cost-effective when a biennial interval was considered.
Table 4.
The optimal screening strategies in base case and sensitivity analyses for transplant and non-transplant Cystic Fibrosis individuals.
| Assumptions for the sensitivity analyses |
Non-transplant CF patients |
Transplant CF patients |
||
|---|---|---|---|---|
| Colonoscopy (main analysis) |
All tests (supplementary analysis) |
Colonoscopy (main analysis) |
All tests (supplementary analysis) |
|
| Base case | COL 40–75 (5) | FIT 35–75 | COL 35–55 (3) | FIT 30–60 |
| Worst-case sensitivity for colonoscopy test | B | B | B | B |
| More proximal adenoma location | B | B | B | B |
| Higher rates of colonoscopy complications | B | B | B | B |
| Higher rates of cardiovascular complications (5-fold increased) | COL 40–70 (5) | B | B | B |
| Higher rates of cardiovascular complications (10-fold increased) | COL 40–70 (5) | B | B | B |
| Worst-case specificity for FIT (0.90) | B | B | B | B |
| Worst-case for specificity (0.75) and sensitivity (36% reduced) for FIT | B | FIT 40–75 | B | COL 35–55 (3) |
| Biennial screening intervals for FIT | B | COL 40–75 (5) | B | COL 35–55 (3) |
| Lower adherence for screening tests | B | B | B | B |
| Intensive surveillance | B | B | B | B |
| Higher patient time costs | COL 45–75 (5) | B | COL 35–55 (5) | FIT 30–55 |
| Only for no transplant CF patients | ||||
| Increased CRC risk with more proximal adenoma location (10-fold increased risk) | COL 40–75 (3) | FIT 30–75 | - | - |
| Shorter adenoma dwelling time (94% reduced) | COL 40–70 (3) | FIT 30–75 | - | - |
| Higher overall mortality in older ages (≥ 45 years) | COL 40–55 (5) | FIT 35–60 | - | - |
| Only for transplant CF patients | ||||
| Organ transplant at: | ||||
| Age 20 | - | - | COL 30–55 (10) | FIT 25–55 |
| Age 25 | - | - | COL 30–55 (5) | FIT 25–55 |
| Additional colonoscopy screening strategy (every 5 years) starting at age 32 for transplant patients (organ transplant at age 30) | - | - | B | B |
| Increased CRC risk with more proximal adenoma location (45-fold increased risk) | - | - | B | COL 35–55 (3) |
| Age-specific overall mortality rates of non-transplant CF after 50 years | - | - | COL 35–60 (3) | COL 35–60 (3) |
| Shorter adenoma dwelling time (50% reduced) with adjusted CRC risk (16-fold increased) | - | - | B | COL 35–55 (3) |
B = Optimal strategy is the same of the base case; COL = Colonoscopy; FIT = Fecal Immunochemical Test; (n) = screening interval; CF = Cystic fibrosis.
Among transplant cystic fibrosis patients, less intense colonoscopy screening (every 5 years) was optimal when higher patient time costs were considered. For individuals with cystic fibrosis who had an organ transplant before age 30, colonoscopy screening was optimal from 30 years. However, optimal screening interval varied according to the age at organ transplant: every 10 years up to age 55 for those with transplantation at age 20; and every 5 years up to age 55 for those that had a transplant at age 25. When we assumed that older individuals with cystic fibrosis who had an organ transplant (≥ 50 years) had the same overall mortality as the non-transplant, the age to stop screening increased to 60 years of age. Considering all screening strategies (supplementary analysis), FIT screening was not considered cost-effective when there was an increased CRC risk (45-fold), a shorter adenoma dwell time, biennial FIT, lower FIT sensitivity and specificity, and when the same age-specific mortality of non-transplant cystic fibrosis individuals (for those older than 50 years) were assumed for transplant cystic fibrosis patients. Optimal screening strategies among these individuals also varied according the age of organ transplant: FIT screening should start at age 25 when individuals with cystic fibrosis underwent a transplantation at age 20 or 25 years.
Discussion
Recent studies have highlighted the necessity of tailored CRC screening for individuals with cystic fibrosis, reporting that these individuals have an increased risk of CRC compared to the average population.5–8 Using an established micro-simulation model, adjusted for the characteristics of cystic fibrosis populations, we found that the recommended US CRC screening strategy for the general population was not optimal for individuals with cystic fibrosis. A greater reduction in CRC mortality could be achieved if screening started before age 50 in both individuals who have and have not received an organ transplantation. Colonoscopy every five years starting at age 40 in individuals with cystic fibrosis who have not received a transplant was shown, in our study, to significantly improve LYG and CRC mortality at an acceptable cost (ICER of $84,000 per LY gained). Our cost-effectiveness analysis suggests, for cystic fibrosis patients who underwent organ transplantation, more intensive colonoscopy screening starting at ages 30 (transplant at age 20 or 25) or 35 (transplant at age 30), through to age 55. The optimal screening interval varied according to age at organ transplant and patient time costs. The model also suggested that screening with FIT could be more cost-effective than colonoscopy (supplementary analysis), but specific evidence of its performance in the cystic fibrosis population is required before considering this screening modality.
Despite the lower life-expectancy reported in cystic fibrosis population, the model suggests – especially for those who have not undergone an organ transplantation – that screening should be repeated until age 75 years. Few individuals with cystic fibrosis currently reach this age, but once they survive to a certain age (i.e. 65–70) their excess risk of dying compared to the general population becomes smaller and a death from CRC becomes more likely. Thus, screening is effective until age 75. However, the model was adjusted to reflect data on individuals with cystic fibrosis provided by the CFFPR which contains only a very small number of individuals at older ages. Moreover, a previous study has shown that some death dates were missing in the CFFPR, especially for individuals with cystic fibrosis older than 45 years, when compared with national vital statistics.45 Therefore, the model results on the age to stop screening could be less robust than those obtained on the age to start screening. A specific sensitivity analysis, carried out assuming a higher overall mortality in cystic fibrosis long-term survivors as reported by Nick et al. in Colorado,45 confirmed this hypothesis (Table 4). This potentially incomplete ascertainment of outcomes may also affect estimates for CRC incidence. In that case, we would have underestimated the risk of CRC and the optimal colonoscopy screening strategy would be even more intensive than the base case: colonoscopy screening should start at age 40 and repeated every 3 years.
At the same time, our model suggests to screen individuals with cystic fibrosis who have had an organ transplant up to age 55. This difference is mainly related to the higher CRC risk seen in cystic fibrosis individuals after transplantation. Performing our analysis on transplant cystic fibrosis individuals (assuming transplant at age 30 years), the model predicted that all these patients developed one or more adenomas before age 55 and, therefore, entered colonoscopy surveillance rather than attending subsequent screening rounds. As a result, outcomes of similar strategies with different ages to stop screening, above age 60, were the same (Appendix Tables 5–6). Although individuals with cystic fibrosis had a more frequent adenoma onset after organ transplant, the increase in CRC incidence was not as immediate, potentially due to the lag-time in the progression between adenoma and CRC.46 This was shown in our analysis for starting screening age in transplant cystic fibrosis patients that underwent organ transplant at age 30.
Specific screening recommendations already exist for several groups of individuals at higher risk of CRC: individuals with family history of CRC (FDR) are recommended to undergo colonoscopy every 5–10 years, starting at age 40.47 Individuals with Lynch syndrome should undergo colonoscopy every 1–2 years starting at age 20–25 years.48 CRC risk in cystic fibrosis population falls somewhere between the risk of these different groups, with the risk in transplant patients (30-fold increase compared to general population)6 being higher than Lynch syndrome patients.49 This indicates that individuals with cystic fibrosis should potentially have similar recommendations as these other high risk groups. However, it is also necessary to consider the different life expectancy of individuals with cystic fibrosis compared to individuals in other high risk groups as this may influence the balance between the harms and benefits of screening. This effect may be seen in Table 1. Although patients with cystic fibrosis have an up to 30-fold increased CRC risk compared to average US individuals, CRC deaths predicted among them were less than reported for the US general population (19.1 and 22.3 versus 27.8 per 1,000) due to their more elevated other cause mortality (70% of the deaths in cystic fibrosis individuals are related to cardiorespiratory causes)4. While early diagnosis may prevent a CRC death, screening may result in an over-diagnosis due to cystic fibrosis-related competing causes of death and can incur in additional costs from screening and treatment. Thus, CRC screening guidelines for the other high risk group cannot be simply generalized to individuals with cystic fibrosis. This may explain why, unlike for individuals with Lynch syndrome, more intensive screening strategies were not found to be cost-effective for the cystic fibrosis population.
Several studies have recently highlighted the necessity of tailored CRC screening for the cystic fibrosis population5–8 and, to our knowledge, this is the first study to assess the cost-effectiveness of CRC screening in these individuals. The results of this formal decision analysis, which was requested by the Cystic Fibrosis Foundation and CFCRCSTF to inform the cystic fibrosis CRC screening consensus recommendations19 have provided important suggestions for clinicians, researchers, and policy makers who were tasked with developing an appropriate CRC screening policy for people with cystic fibrosis in the US. However, the findings of this study should be interpreted with caution considering the following limitations. First, we did not model the natural history of CRC separately for men and women. Epidemiological studies among cystic fibrosis patients report gender differences: women experience a lower risk of developing CRC6 and lower life-expectancy50 than men. Considering these differences, a less intensive CRC screening strategy could be optimal for women with cystic fibrosis. However, there is little data on CRC incidence and mortality in these patients and even less is stratified by gender, meaning this differentiation is not yet feasible. Second, our analysis was not stratified for pulmonary function (an important clinical indicator of the health of individuals with cystic fibrosis). Although Niccum and colleagues only considered cystic fibrosis patients with predicted FEV1 ≥ 40% eligible to CRC screening,8 the available data for individuals with cystic fibrosis did not permit this additional model stratification. The most recent Cystic Fibrosis Patient Registry Annual Report showed that up to 75% of individuals with cystic fibrosis aged 40 years had a predicted FEV1 ≥ 40%.4 If screening was limited to this subset of individuals, the balance between harms and benefits of screening in individuals with cystic fibrosis would become more favorable.
Furthermore, we assumed that adenomas in persons with cystic fibrosis could arise following the same localization-specific distribution observed in autopsy studies for the general population24–33 and with the same increased risk – 7-fold compare to general population – in both the colon and rectum. Although Maisonneuve and colleagues reported that CRC cases were mainly located in the colon of individuals with cystic fibrosis (26 out 28 cases),6 a direct calibration of the adenoma localization-specific onset distribution was not possible as limited data is currently available. To address this, we performed a sensitivity analysis to assess the effects of assuming a different localization-specific distribution for adenoma onset in people with cystic fibrosis and screening strategy outcomes were not sensitive to this assumption (Table 4).
Several factors may cause the higher risk of CRC in the cystic fibrosis population, but information about the rationale of this increased risk remains unclear. We assumed that the higher risk of CRC shown in the cystic fibrosis population was due to a more frequent adenoma onset. This assumption was validated for non-transplant patients, but not for individuals with cystic fibrosis who had an organ transplant (Appendix Table 3). A shorter adenoma dwell time may also play a role in the progression from adenoma to CRC. To investigate this, we performed a specific sensitivity analysis assuming a shorter dwell time (50% reduced, faster adenoma progression) and more elevated adenoma onset (16-fold increased risk) for transplant cystic fibrosis patients. The results of this sensitivity analysis were validated with adenoma detection rates observed in an observational study of cystic fibrosis patients undergoing colonoscopy screening (Appendix Table 3).8 However, this analysis revealed that our cost-effectiveness outcomes were not sensitive to this assumption. Our model does not explicitly describe adenoma histology and that may explain the lower simulated rates of colonoscopy detected advanced adenomas (Appendix Figure 3).
In our study, assumptions on colonoscopy performance, complications, polypectomy safety, costs (including sedation costs), and adverse events of bowel preparation were informed by data from the general population and the Medicare population,40 because specific empirical data for the cystic fibrosis population were not available. For colonoscopy performance, this assumption seems reasonable, as model-predicted adenoma detection rates were close to observed (Appendix Figure 3). However, it may be reasonable to assume that risk of complications and/or inadequate bowel preparation is higher in people with cystic fibrosis compared to the general population. Also, the more intensive and extended bowel preparation regimens for individuals with cystic fibrosis and additional colonoscopy investigations because of inadequate bowel preparation could lead to a further increase in adverse events. To address this concern, we performed specific sensitivity analyses on colonoscopy performance and rate of complications (especially for cardiovascular adverse events, including respiratory arrest, Appendix Table 7 and 8). Results of these analyses showed that the optimal screening starting ages and intervals were not sensitive to changes in these assumptions (Table 4).
The feasibility of colonoscopy in individuals with cystic fibrosis and its capacity to early detect CRC and adenomas in these individuals was suggested by the findings of a small observational study conducted in Minnesota.8 Moreover, colonoscopy is the screening test of choice for higher risk groups.47, 48 We therefore focused our main analysis and interpretation of our results on this screening modality. However, given the potential burden of colonoscopy and colonoscopy preparation to the cystic fibrosis patient, we thought it was pertinent to also consider FIT as a possible and hypothetically adequate alternative. As such, we performed a specific supplementary analysis including annual FIT screening. We found that this screening modality was cost-effective and optimal among individuals with cystic fibrosis. However, because information on FIT characteristics in this population is lacking, the analysis was performed using FIT characteristics from the general population.37 In individuals with cystic fibrosis, the presence of blood in feces could be related to several gastrointestinal disorders,51 which could affect the effectiveness and cost-effectiveness of FIT screening in cystic fibrosis population. Sensitivity analyses revealed that our results on cost-effectiveness of FIT depend on screening intensity and the test characteristics as assumed in this analysis, especially for post-transplant cystic fibrosis patients. Hence, before considering FIT as the preferred screening modality, FIT performance must be tested in the cystic fibrosis population to better explore its effectiveness in early detection of CRC and adenomas among this population. If future studies confirm that FIT in individuals with cystic fibrosis performs as well as or better than we assumed in our sensitivity analyses, FIT may be considered an attractive screening option for this population. In the meantime, FIT could be considered for those not willing to undergo colonoscopy.
Despite its limitations, this study has important clinical and policy implications. This study indicates that there is benefit to earlier CRC screening in the cystic fibrosis population and can be done at acceptable costs. The findings of this analysis support clinicians, researchers, and policy makers who aim to define a tailored CRC screening for individuals with cystic fibrosis in the US. Meanwhile, outcomes of screening in individuals with cystic fibrosis should be closely monitored to accumulate evidence on the performance and safety of CRC screening in these individuals.
Acknowledgments
Funding: This study was funded by the Cystic Fibrosis Foundation (CFF), the Cancer Intervention and Surveillance Modeling Network consortium (CISNET, grant U01CA199335), and Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (Ann G. Zauber, PhD; P30 CA008748).
Appendix
Appendix Figure 1.
Colorectal cancer incidence seen before the introduction of screening versus incidence simulated by Microsimulation Screening Analysis-Colon model.
Appendix Figure 2.
Adenoma prevalence seen in selected autopsy studies versus prevalence simulated by Microsimulation Screening Analysis-Colon model. Observed results are shown only for the 2 largest studies on which the model has been calibrated. The model has additionally been calibrated to 8 other autopsy studies. Bars indicate 95% CIs.
Appendix Figure 3.
Adenoma and advanced adenoma detection rate simulated with Microsimulation Screening Analysis-Colon (MISCAN-Colon model) and observed in a colonoscopy observational study among Cystic Fibrosis patients.
Appendix Figure 4.
CRC incidence expected in CF individuals according to Maisonneuve P. et al. 2013 and CRC incidence simulated in Microsimulation Screening Analysis-Colon model without screening in US general population, non-transplant, and transplant CF patients assuming higher CRC risk through a combination of a more frequent adenoma onset and a faster adenoma progression (sensitivity analysis).
Note: Bars indicate 95% CIs; CRC = colorectal cancer; CF = Cystic Fibrosis; B = Base case analysis; and S = Sensitivity analysis.
MISCAN-Colon model description (Model appendix)
General Model Structure
MISCAN-Colon is a stochastic microsimulation model for the CRC useful to explain and predict trends in CRC incidence and mortality rates and to assess the effects and costs of primary prevention and screening for CRC.17
The model simulates the life history of each person at individual level, rather than as proportions of a cohort. For that reason, the model allows the time dependence between future and past state transitions. However, in contrast to most traditional Markov models, MISCAN-Colon does not use yearly transition probabilities but it generates durations in states. This solution increases the model flexibility and the computational performance. In addition, the model simulates sequences of events by drawing from distribution of probability or durations, rather than using fixed values. Hence, the results of the model are subject to random variation.
MISCAN-Colon consists of 3 modules: a demography module, natural history module, and screening module.
The Demography Module
MISCAN-Colon model draws a date of birth and a date of no-CRC death for each individual simulated, using birth and life tables (representative of the population under consideration). The model restricts the maximum age a person can achieve to 100 years.
The Natural History Module
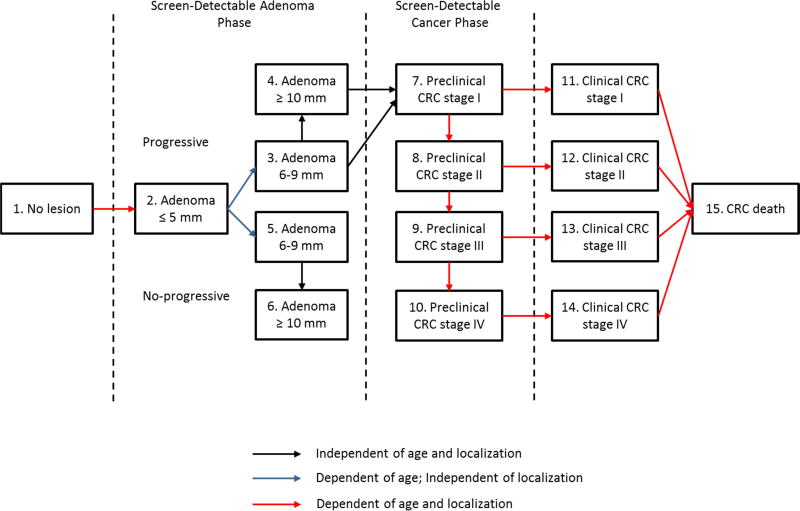
As each simulated person ages, 1 or more adenomas may develop (Appendix Figure 5). These adenomas ca be either progressive or no-progressive and both can grow in size from small (<5 mm) to medium (6–9 mm) and then to large (> 10 mm). Only progressive adenomas can develop into preclinical cancer, which may progress through stage I to IV. However, during each stage, CRC may be diagnosis because of symptoms. After CRC clinical diagnosis, survival time is simulated using age-, stage-, and localization-specific survival estimates for clinically diagnosed CRC based on a study published by Rutter and colleagues.19 For synchronous CRCs, the survival is based on the most advance cancer. The date of death for CRC patients is the earliest simulate date of death (due to CRC or another cause).
The probability of adenoma onset differs among the individuals and it depends on the person’s age and risk index. For that reason, most persons do not develop adenomas and some others develop many. The distribution of adenoma over the colon and rectum was assumed equals to the distribution of cancer cases seen in SEER before the introduction of screening.38 The personal risk index and the age-specific onset of adenomas were calibrated to adenoma prevalence data obtained in several autopsy studies (Appendix Figure 2).21–30, 38 Furthermore, the age-specific probability of adenoma progressivity and the age-, localization--specific transition between preclinical and clinical cancer stages were calibrated to SEER data on age-, stage- and localization-specific incidence of CRC in pre-screening years (i.e., 1975–1979, Appendix Figure 1).38 The average duration of the preclinical cancer stages were calibrated according to data obtained from randomized, controlled trials (RCTs) evaluating screening using guaiac fecal occult blood tests.10, 11, 14 The average duration between the adenoma onset and the progression into preclinical cancer (adenoma dwell time) was calibrated to the data on interval cancer seen in a sigmoidoscopy screening RCT.9 Furthermore, we assumed: an equal overall dwell time for adenoma developing into cancer from medium (30% of all CRCs) and from large size adenomas (70% of all CRCs); exponential distribution for all duration in the adenoma and preclinical cancer phases; perfect correlation for the durations within adenoma and preclinical cancer (quicker growing from small adenoma and medium-sized adenoma, quicker developing into preclinical CRC); absence of correlation between durations in the adenoma phase and duration in the preclinical cancer phase.
The Screening Module
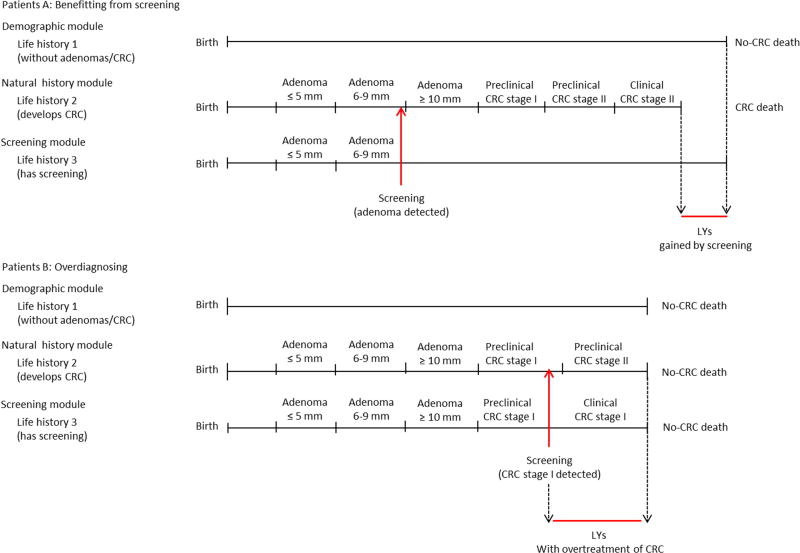
Screening will modify some of the simulated life histories: Some cancer cases will be prevented by the detection and removal of adenomas or by detection in an earlier stage (favourable survival). As seen in RCTs on guaiac fecal occult blood testing, the stage-specific survival of screen-detected CRC was more favourable compared to clinically detected CRC, even after the lead-time bias correction.12 Hence, we assigned those screen-detected cancer cases - that without screening would have been clinically detected in the same stage – a survival corresponding to a cancer that is 1 stage less progressive. The only exceptions were screen-detected stage IV cancer cases: we assigned the survival of a clinically diagnosed stage IV cancer. Furthermore, together with the positive effects of screening, we also modelled over-diagnosis, overtreatment, and colonoscopy-related complications.36
Integrating Modules
For each person simulated, a date of birth and a date of no-CRC death (a lifetime history without adenoma or CRC) are generated from the demography module. In patient A in Appendix Figure 6, the natural history module generates an adenoma. This adenoma progress into preclinical cancer (diagnosed as stage II CRC due to symptoms) and results in CRC death before non-CRC death would have occurred. However, in the screening module, a screening examination is introduced: the adenoma is detected; removed; and the CRC death prevented. The positive effect of the screening intervention is indicated by the green arrow and represents the increased life years gained for this patients and due to screening. Another example is the patient B. He develops an adenoma and it would never have been diagnosed in a no screening scenario. However, during the screening examination, CRC is screen-detected in stage I and - for this patient - screening results in over diagnosis and overtreatment of CRC (no LYs gained, but only additional LYs with CRC care).
Results for US general population (included in this study)
According to the MISCAN-Colon model, up to 73% of CRC deaths may be avoided by introducing CRC screening in US general population (Table 1). While this result may appear elevated considering the findings of several RCTs,9, 15–17 it is in accordance with assumptions made in our analysis. We investigated the impact of screening in the entire colorectum with 100% adherence to screening (in each screening round), and surveillance tests. The RCTs mainly investigated the effect of screening on the left colon (once-only flexible sigmoidoscopy (FS)), reporting a 22–31% reduction in CRC mortality with a compliance ranging from 58% to 71%.9, 15, 17 Schoen et al. reported a 50% reduction in distal CRC mortality in those invited to FS (54% of adherence in those invited to repeat screening every 5 or 3 years).16 Furthermore, the MISCAN-Colon model is calibrated and validated against data from UK FS screening trial.34
Appendix Figure 5.
The general model structure of MISCAN-Colon model.
Appendix Figure 6.
Integrating modules with two examples.
Appendix Table 1.
Test Characteristics of Colonoscopy and Fecal Immunochemical Tests (FIT)
| Tests
|
||
|---|---|---|
| Test Characteristic | Colonoscopya | FITb |
|
| ||
| Specificity, % | 0.86c | 0.964 |
| Sensitivity, % | ||
| Small adenomas (≤5mm) | 0.75 | 0.076d |
| Medium adenomas (6-9 mm) | 0.85 | 0.076d |
| Large adenomas (≥10 mm) | 0.95 | 0.238e |
| CRCs that would not have been clinically detected in their current stage | 0.95 | 0.625f |
| CRCs that would have been clinically detected in their current stage | 0.95 | 0.886f |
| Reach | 95% reaches the cecum; the reach of the remaining 5% is distributed uniformly over colon and rectum | Whole colon and rectum |
| Complication rate | Increases exponentially with ageg | 0 |
| Mortality rate | 0.0000191h | 0 |
The sensitivity of colonoscopy for the detection of adenomas and CRC within the reach of the endoscope was obtained from a systematic review on miss rates seen in tandem colonoscopy studies33;
FIT characteristics were based on a large US based study comparing multi-targeted Stool DNA with FIT in a screening setting34;
Specificity for colonoscopy is therefore based on an adenoma prevalence study of patients undergoing screening colonoscopy36;
Sensitivity for non-advanced adenomas (not reported separately for medium adenomas);
Sensitivity for advanced adenomas (not reported for large adenomas);
These estimates were found by calibrating our model outcomes to the per-person sensitivities given in the multi-targeted Stool DNA with FIT 34;
Age-specific risks for complications of colonoscopy requiring a hospital admission or emergency department visit were obtained from a study by Warren et al37;
Appendix Table 2.
Costs associated with colorectal cancer screening in the base case and cost sensitivity analysis.
| Costs, $a | Higher costs for colonoscopy, $ (Sensitivity analysis)e |
|
|---|---|---|
|
| ||
| Per FIT | 40 | - |
| Per colonoscopy | ||
| Without polypectomy/biopsy | 880 | 1,400 |
| With polypectomy/biopsy | 1,200 | 1,700 |
| Per complication of colonoscopy | ||
| Seriousb GI complications | 8,100 | 11,200 |
| Otherc GI complications | 6,200 | 7,600 |
| Cardiovascular complicationsd | 6,700 | 8,500 |
| Per LY with CRC care | ||
| Initial care | ||
| Stage I CRC | 36,900 | - |
| Stage II CRC | 49,500 | - |
| Stage III CRC | 60,100 | - |
| Stage IV CRC | 78,200 | - |
| Continuing care | ||
| Stage I CRC | 3,100 | - |
| Stage II CRC | 2,900 | - |
| Stage III CRC | 4,100 | - |
| Stage IV CRC | 12,300 | - |
| Terminal Care, ending in CRC death | ||
| Stage I CRC | 64,200 | - |
| Stage II CRC | 63,900 | - |
| Stage III CRC | 67,400 | - |
| Stage IV CRC | 88,900 | - |
| Terminal Care, ending in other-cause death | ||
| Stage I CRC | 19,400 | - |
| Stage II CRC | 17,400 | - |
| Stage III CRC | 21,600 | - |
| Stage IV CRC | 50,200 | - |
GI = Gastro intestinal; FIT = Fecal immunochemical test.
Costs are presented in 2015 U.S. dollars and include co-payments and patient time costs (i.e., the opportunity costs of spending time on screening or being treated for a complication or CRC) but do not include travel costs, costs of lost productivity, and unrelated health care and non–health care costs in added years of life. We assumed that the value of patient time was equal to the median wage rate in 2014: $17.01/h. Cost values were estimated for the year 2014. We assumed that FITs, colonoscopies, and complications used up 1, 8, and 16 h of patient time, respectively. Patient time costs were already included in the estimates for the costs of LYs with CRC care obtained from a study by Yabroff et al40; All costs were adjusted for the year 2015 using the annual average Consumer Price Indexes provided by US Bureau of Labor Statistics41;
Serious GI complications included perforations, gastrointestinal bleeding, or transfusions;
Other GI complications included paralytic ileus, nausea and vomiting, dehydration, or abdominal pain;
Cardiovascular complications included myocardial infarction or angina, arrhythmias, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, or shock;
We assumed that colonoscopies, and complications used up 40 and 190 h of patient time, respectively.
Appendix Table 3.
Outcomes with colonoscopy screening strategies that vary by the ages to begin and end screening among non-transplant Cystic Fibrosis patients.
| Outcomes per 1,000 non-transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (3% discounted)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening tests
|
Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb CRC incidencec |
(%) CRC mortalityc |
Efficient strategy |
||
| FIT | COLs | |||||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 23 | 0 | 52 | 19 | 134 | 0 | 1918503 | 0 | 0 | 0 | Dominated |
| COL 50–55 y | ||||||||||||||
| 3 y | 0 | 234 | 566 | 808 | 4 | 28 | 6 | 119 | 32 | 2148408 | 229905 | 47 | 70 | Dominated |
| 5 y | 0 | 231 | 352 | 591 | 3 | 31 | 6 | 126 | 31 | 2023494 | 104991 | 41 | 66 | Dominated |
| 10 y | 0 | 214 | 334 | 558 | 3 | 32 | 7 | 127 | 29 | 2015966 | 97463 | 38 | 62 | Efficient |
| COL 50–60 y | ||||||||||||||
| 3 y | 0 | 242 | 575 | 825 | 4 | 27 | 6 | 119 | 33 | 2155878 | 237376 | 48 | 71 | Dominated |
| 5 y | 0 | 234 | 354 | 597 | 3 | 31 | 6 | 126 | 31 | 2025210 | 106707 | 41 | 67 | Efficient |
| 10 y | 0 | 225 | 345 | 579 | 3 | 31 | 7 | 127 | 30 | 2021207 | 102704 | 40 | 66 | Efficient |
| COL 50–65 y | ||||||||||||||
| 3 y | 0 | 244 | 576 | 827 | 4 | 27 | 6 | 119 | 33 | 2157230 | 238727 | 48 | 71 | Dominated |
| 5 y | 0 | 235 | 354 | 598 | 3 | 31 | 6 | 126 | 31 | 2025651 | 107148 | 41 | 67 | Dominated |
| 10 y | 0 | 225 | 345 | 579 | 3 | 31 | 7 | 127 | 30 | 2021207 | 102704 | 40 | 66 | Dominated |
| COL 50–70 y | ||||||||||||||
| 3 y | 0 | 244 | 576 | 828 | 4 | 27 | 6 | 119 | 33 | 2157394 | 238892 | 48 | 71 | Dominated |
| 5 y | 0 | 235 | 354 | 598 | 3 | 31 | 6 | 126 | 31 | 2025716 | 107213 | 41 | 67 | Efficient |
| 10 y | 0 | 226 | 346 | 580 | 3 | 31 | 6 | 127 | 30 | 2021651 | 103148 | 40 | 66 | Dominated |
| COL 50–75 y | ||||||||||||||
| 3 y | 0 | 244 | 576 | 828 | 4 | 27 | 6 | 119 | 33 | 2157431 | 238928 | 48 | 71 | Dominated |
| 5 y | 0 | 235 | 354 | 598 | 3 | 31 | 6 | 126 | 31 | 2025729 | 107227 | 41 | 67 | Dominated |
| 10 y | 0 | 226 | 346 | 580 | 3 | 31 | 6 | 127 | 30 | 2021651 | 103148 | 40 | 66 | Dominated |
| COL 45–55 y | ||||||||||||||
| 3 y | 0 | 419 | 896 | 1320 | 4 | 23 | 4 | 105 | 41 | 2475197 | 556694 | 56 | 78 | Dominated |
| 5 y | 0 | 390 | 528 | 925 | 3 | 28 | 5 | 117 | 38 | 2219433 | 300930 | 47 | 73 | Dominated |
| 10 y | 0 | 361 | 505 | 873 | 3 | 28 | 5 | 119 | 37 | 2195135 | 276632 | 46 | 72 | Dominated |
| COL 45–60 y | ||||||||||||||
| 3 y | 0 | 424 | 901 | 1331 | 4 | 23 | 4 | 105 | 41 | 2479908 | 561406 | 56 | 79 | Dominated |
| 5 y | 0 | 393 | 530 | 930 | 3 | 27 | 5 | 117 | 38 | 2221064 | 302561 | 48 | 74 | Dominated |
| 10 y | 0 | 361 | 505 | 873 | 3 | 28 | 5 | 119 | 37 | 2195135 | 276632 | 46 | 72 | Dominated |
| COL 45–65 y | ||||||||||||||
| 3 y | 0 | 425 | 902 | 1332 | 4 | 23 | 4 | 105 | 41 | 2480593 | 562090 | 56 | 79 | Dominated |
| 5 y | 0 | 394 | 531 | 931 | 3 | 27 | 5 | 117 | 38 | 2221496 | 302994 | 48 | 74 | Dominated |
| 10 y | 0 | 364 | 507 | 877 | 3 | 28 | 5 | 119 | 37 | 2197099 | 278597 | 46 | 73 | Dominated |
| COL 45–70 y | ||||||||||||||
| 3 y | 0 | 426 | 902 | 1333 | 4 | 23 | 4 | 105 | 41 | 2480935 | 562432 | 57 | 79 | Dominated |
| 5 y | 0 | 394 | 531 | 931 | 3 | 27 | 5 | 117 | 38 | 2221588 | 303085 | 48 | 74 | Dominated |
| 10 y | 0 | 364 | 507 | 877 | 3 | 28 | 5 | 119 | 37 | 2197099 | 278597 | 46 | 73 | Dominated |
| COL 45–75 y | ||||||||||||||
| 3 y | 0 | 426 | 902 | 1333 | 4 | 23 | 4 | 105 | 41 | 2480965 | 562462 | 57 | 79 | Dominated |
| 5 y | 0 | 394 | 531 | 931 | 3 | 27 | 5 | 117 | 38 | 2221593 | 303090 | 48 | 74 | Efficient |
| 10 y | 0 | 364 | 507 | 877 | 3 | 28 | 5 | 119 | 37 | 2197180 | 278677 | 46 | 73 | Dominated |
| COL 40–55 y | ||||||||||||||
| 3 y | 0 | 788 | 1297 | 2088 | 5 | 20 | 3 | 93 | 48 | 3073485 | 1154982 | 62 | 84 | Dominated |
| 5 y | 0 | 685 | 721 | 1411 | 4 | 25 | 4 | 109 | 44 | 2589046 | 670543 | 52 | 78 | Dominated |
| 10 y | 0 | 584 | 654 | 1243 | 4 | 27 | 5 | 113 | 41 | 2486943 | 568440 | 48 | 73 | Dominated |
| COL 40–60 y | ||||||||||||||
| 3 y | 0 | 791 | 1300 | 2094 | 5 | 20 | 3 | 93 | 48 | 3075938 | 1157436 | 63 | 84 | Dominated |
| 5 y | 0 | 688 | 723 | 1416 | 4 | 25 | 4 | 109 | 44 | 2590515 | 672012 | 52 | 79 | Dominated |
| 10 y | 0 | 592 | 663 | 1261 | 4 | 27 | 5 | 114 | 42 | 2491257 | 572754 | 49 | 76 | Dominated |
| COL 40–65 y | ||||||||||||||
| 3 y | 0 | 793 | 1301 | 2097 | 5 | 20 | 3 | 93 | 48 | 3077629 | 1159127 | 63 | 84 | Dominated |
| 5 y | 0 | 689 | 724 | 1417 | 4 | 25 | 4 | 109 | 44 | 2590952 | 672449 | 52 | 79 | Dominated |
| 10 y | 0 | 592 | 663 | 1261 | 4 | 27 | 5 | 114 | 42 | 2491257 | 572754 | 49 | 76 | Dominated |
| COL 40–70 y | ||||||||||||||
| 3 y | 0 | 793 | 1301 | 2097 | 5 | 20 | 3 | 93 | 48 | 3077867 | 1159364 | 63 | 84 | Dominated |
| 5 y | 0 | 689 | 724 | 1417 | 4 | 25 | 4 | 109 | 44 | 2591030 | 672527 | 52 | 79 | Efficient |
| 10 y | 0 | 593 | 663 | 1261 | 4 | 27 | 4 | 114 | 42 | 2491646 | 573143 | 49 | 77 | Dominated |
| COL 40–75 y | ||||||||||||||
| 3 y | 0 | 793 | 1301 | 2097 | 5 | 20 | 3 | 93 | 48 | 3077874 | 1159371 | 63 | 84 | Efficient |
| 5 y | 0 | 689 | 724 | 1417 | 4 | 25 | 4 | 109 | 44 | 2591048 | 672546 | 52 | 79 | Optimal |
| 10 y | 0 | 593 | 663 | 1261 | 4 | 27 | 4 | 114 | 42 | 2491646 | 573143 | 49 | 77 | Dominated |
| COL 35–55 y | ||||||||||||||
| 3 y | 0 | 1473 | 1691 | 3167 | 5 | 18 | 2 | 85 | 53 | 3992038 | 2073535 | 66 | 87 | Dominated |
| 5 y | 0 | 1194 | 907 | 2105 | 4 | 24 | 4 | 104 | 48 | 3174604 | 1256101 | 54 | 81 | Dominated |
| 10 y | 0 | 939 | 802 | 1745 | 4 | 26 | 4 | 110 | 45 | 2900743 | 982240 | 51 | 78 | Dominated |
| COL 35–60 y | ||||||||||||||
| 3 y | 0 | 1481 | 1699 | 3182 | 5 | 18 | 2 | 84 | 53 | 3998695 | 2080192 | 66 | 88 | Dominated |
| 5 y | 0 | 1197 | 909 | 2110 | 4 | 24 | 4 | 104 | 48 | 3175886 | 1257384 | 55 | 81 | Dominated |
| 10 y | 0 | 939 | 802 | 1745 | 4 | 26 | 4 | 110 | 45 | 2900743 | 982240 | 51 | 78 | Dominated |
| COL 35–65 y | ||||||||||||||
| 3 y | 0 | 1482 | 1700 | 3185 | 5 | 18 | 2 | 84 | 53 | 4000132 | 2081629 | 67 | 88 | Dominated |
| 5 y | 0 | 1198 | 909 | 2111 | 4 | 24 | 4 | 104 | 48 | 3176328 | 1257825 | 55 | 82 | Dominated |
| 10 y | 0 | 941 | 803 | 1749 | 4 | 25 | 4 | 110 | 45 | 2902724 | 984222 | 51 | 79 | Dominated |
| COL 35–70 y | ||||||||||||||
| 3 y | 0 | 1482 | 1700 | 3185 | 5 | 18 | 2 | 84 | 53 | 4000269 | 2081766 | 67 | 88 | Dominated |
| 5 y | 0 | 1198 | 909 | 2111 | 4 | 24 | 4 | 104 | 48 | 3176413 | 1257910 | 55 | 82 | Dominated |
| 10 y | 0 | 941 | 803 | 1749 | 4 | 25 | 4 | 110 | 45 | 2902724 | 984222 | 51 | 79 | Dominated |
| COL 35–75 y | ||||||||||||||
| 3 y | 0 | 1482 | 1700 | 3185 | 5 | 18 | 2 | 84 | 53 | 4000326 | 2081823 | 67 | 88 | Efficient |
| 5 y | 0 | 1198 | 909 | 2111 | 4 | 24 | 4 | 104 | 48 | 3176422 | 1257919 | 55 | 82 | Dominated |
| 10 y | 0 | 942 | 803 | 1749 | 4 | 25 | 4 | 110 | 45 | 2902790 | 984287 | 51 | 79 | Dominated |
| COL 30–55 y | ||||||||||||||
| 3 y | 0 | 2664 | 2056 | 4722 | 6 | 17 | 2 | 78 | 56 | 5363829 | 3445326 | 68 | 89 | Dominated |
| 5 y | 0 | 2019 | 1081 | 3103 | 4 | 23 | 3 | 99 | 51 | 4054185 | 2135683 | 56 | 83 | Dominated |
| 10 y | 0 | 1469 | 930 | 2404 | 4 | 26 | 4 | 107 | 47 | 3490661 | 1572158 | 51 | 77 | Dominated |
| COL 30–60 y | ||||||||||||||
| 3 y | 0 | 2670 | 2061 | 4733 | 6 | 17 | 2 | 77 | 57 | 5368594 | 3450091 | 68 | 90 | Dominated |
| 5 y | 0 | 2022 | 1083 | 3108 | 4 | 23 | 3 | 99 | 52 | 4055530 | 2137027 | 56 | 83 | Dominated |
| 10 y | 0 | 1478 | 939 | 2421 | 4 | 25 | 4 | 107 | 48 | 3494835 | 1576333 | 53 | 80 | Dominated |
| COL 30–65 y | ||||||||||||||
| 3 y | 0 | 2670 | 2062 | 4734 | 6 | 17 | 2 | 77 | 57 | 5369313 | 3450810 | 68 | 90 | Dominated |
| 5 y | 0 | 2022 | 1084 | 3109 | 4 | 23 | 3 | 99 | 52 | 4056006 | 2137503 | 56 | 83 | Dominated |
| 10 y | 0 | 1478 | 939 | 2421 | 4 | 25 | 4 | 107 | 48 | 3494835 | 1576333 | 53 | 80 | Dominated |
| COL 30–70 y | ||||||||||||||
| 3 y | 0 | 2671 | 2062 | 4734 | 6 | 17 | 2 | 77 | 57 | 5369646 | 3451143 | 68 | 90 | Dominated |
| 5 y | 0 | 2022 | 1084 | 3109 | 4 | 23 | 3 | 99 | 52 | 4056097 | 2137594 | 56 | 83 | Dominated |
| 10 y | 0 | 1479 | 939 | 2422 | 4 | 25 | 4 | 107 | 48 | 3495178 | 1576675 | 53 | 80 | Dominated |
| COL 30–75 y | ||||||||||||||
| 3 y | 0 | 2671 | 2062 | 4734 | 6 | 17 | 2 | 77 | 57 | 5369680 | 3451178 | 68 | 90 | Efficient |
| 5 y | 0 | 2022 | 1084 | 3109 | 4 | 23 | 3 | 99 | 52 | 4056118 | 2137616 | 56 | 83 | Dominated |
| 10 y | 0 | 1479 | 939 | 2422 | 4 | 25 | 4 | 107 | 48 | 3495178 | 1576675 | 53 | 80 | Dominated |
COL indicates colonoscopy; CRC, colorectal cancer; LY, Life-years; LYG, LY gained compared with no screening; Grey row indicates optimal screening strategy.
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Appendix Table 4.
Outcomes with FIT screening strategies that vary by the ages to begin and end screening among non-transplant Cystic Fibrosis patients.
| Outcomes per 1,000 non-transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (3% discounted)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening tests
|
Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb CRC incidencec |
(%) CRC mortalityc |
Efficient strategy |
||
| FIT | COLs | |||||||||||||
|
| ||||||||||||||
| No screening | 0 | 23 | 0 | 23 | 0 | 52 | 19 | 134 | 0 | 1918503 | 0 | 0 | 0 | Dominated |
| FIT 50–55 y | 864 | 0 | 120 | 210 | 1 | 45 | 12 | 153 | 19 | 2038443 | 119940 | 13 | 37 | Dominated |
| FIT 50–60 y | 1164 | 0 | 148 | 255 | 1 | 43 | 9 | 158 | 25 | 2052169 | 133666 | 18 | 50 | Dominated |
| FIT 50–65 y | 1300 | 0 | 158 | 273 | 2 | 42 | 8 | 161 | 27 | 2063754 | 145251 | 20 | 58 | Dominated |
| FIT 50–70 y | 1353 | 0 | 161 | 279 | 2 | 42 | 7 | 163 | 28 | 2072133 | 153630 | 20 | 61 | Dominated |
| FIT 50–75 y | 1369 | 0 | 162 | 281 | 2 | 42 | 7 | 163 | 28 | 2074725 | 156222 | 20 | 62 | Dominated |
| FIT 45–55 y | 1959 | 0 | 194 | 329 | 2 | 42 | 10 | 155 | 28 | 2115102 | 196599 | 19 | 48 | Dominated |
| FIT 45–60 y | 2228 | 0 | 216 | 366 | 2 | 40 | 8 | 159 | 32 | 2126869 | 208366 | 23 | 59 | Dominated |
| FIT 45–65 y | 2352 | 0 | 226 | 382 | 2 | 40 | 7 | 162 | 34 | 2137804 | 219302 | 25 | 65 | Dominated |
| FIT 45–70 y | 2400 | 0 | 228 | 388 | 2 | 39 | 6 | 163 | 35 | 2145442 | 226939 | 25 | 69 | Dominated |
| FIT 45–75 y | 2416 | 0 | 229 | 389 | 2 | 39 | 6 | 164 | 35 | 2148080 | 229577 | 25 | 70 | Dominated |
| FIT 40–55 y | 3696 | 0 | 268 | 463 | 2 | 40 | 9 | 155 | 34 | 2252661 | 334158 | 23 | 54 | Dominated |
| FIT 40–60 y | 3948 | 0 | 288 | 497 | 2 | 39 | 7 | 159 | 38 | 2266287 | 347784 | 27 | 64 | Dominated |
| FIT 40–65 y | 4064 | 0 | 296 | 512 | 2 | 38 | 6 | 162 | 40 | 2276322 | 357819 | 28 | 70 | Dominated |
| FIT 40–70 y | 4110 | 0 | 299 | 517 | 2 | 38 | 5 | 163 | 41 | 2283302 | 364799 | 28 | 73 | Dominated |
| FIT 40–75 y | 4125 | 0 | 300 | 519 | 2 | 38 | 5 | 164 | 41 | 2286016 | 367513 | 28 | 75 | Efficient |
| FIT 35–55 y | 6360 | 0 | 336 | 622 | 2 | 39 | 8 | 155 | 39 | 2469942 | 551440 | 26 | 58 | Dominated |
| FIT 35–60 y | 6602 | 0 | 356 | 654 | 2 | 37 | 6 | 159 | 43 | 2482622 | 564119 | 29 | 68 | Dominated |
| FIT 35–65 y | 6714 | 0 | 364 | 668 | 2 | 36 | 5 | 162 | 45 | 2492455 | 573952 | 30 | 74 | Dominated |
| FIT 35–70 y | 6758 | 0 | 366 | 674 | 2 | 36 | 4 | 163 | 45 | 2498628 | 580125 | 31 | 77 | Dominated |
| FIT 35-75 y | 6772 | 0 | 367 | 675 | 2 | 36 | 4 | 163 | 46 | 2501004 | 582501 | 31 | 78 | Optimal |
| FIT 30–55 y | 10379 | 0 | 397 | 821 | 2 | 38 | 8 | 155 | 42 | 2800037 | 881534 | 27 | 61 | Dominated |
| FIT 30–60 y | 10616 | 0 | 416 | 852 | 2 | 36 | 6 | 159 | 46 | 2812063 | 893560 | 30 | 70 | Dominated |
| FIT 30–65 y | 10726 | 0 | 424 | 866 | 2 | 36 | 5 | 161 | 48 | 2821545 | 903042 | 32 | 76 | Dominated |
| FIT 30–70 y | 10769 | 0 | 427 | 871 | 2 | 36 | 4 | 162 | 49 | 2828060 | 909557 | 32 | 78 | Dominated |
| FIT 30–75 y | 10783 | 0 | 427 | 872 | 2 | 36 | 4 | 163 | 49 | 2830319 | 911816 | 32 | 80 | Efficient |
COL indicates colonoscopy; CRC, colorectal cancer; LY, Life-years; LYG, LY gained compared with no screening; FIT , Fecal immunochemical test; Grey row indicates optimal screening strategy.
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Appendix Table 5.
Outcomes with colonoscopy screening strategies that vary by the ages to begin and end screening among transplant Cystic Fibrosis patients.
| Outcomes per 1,000 transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (with organ transplant at age 30, 3% discounted) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Screening tests | Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb (%) | Efficient strategy |
|||
|
| ||||||||||||||
| FIT | COLs | CRC incidencec |
CRC mortalityc |
|||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 30 | 0 | 52 | 22 | 115 | 0 | 2064654 | 0 | 0 | 0 | Dominated |
| COL 50–55 y | ||||||||||||||
| 3 y | 0 | 125 | 173 | 314 | 2 | 48 | 13 | 143 | 15 | 2437339 | 372685 | 8 | 40 | Dominated |
| 5 y | 0 | 125 | 152 | 293 | 1 | 48 | 14 | 143 | 14 | 2420864 | 356209 | 8 | 39 | Dominated |
| 10 y | 0 | 124 | 151 | 293 | 1 | 48 | 14 | 143 | 14 | 2419742 | 355087 | 8 | 39 | Dominated |
| COL 50–60 y | ||||||||||||||
| 3 y | 0 | 125 | 173 | 314 | 2 | 48 | 13 | 143 | 15 | 2437341 | 372687 | 8 | 40 | Dominated |
| 5 y | 0 | 125 | 152 | 293 | 1 | 48 | 14 | 143 | 14 | 2420865 | 356211 | 8 | 39 | Dominated |
| 10 y | 0 | 124 | 151 | 293 | 1 | 48 | 14 | 143 | 14 | 2420644 | 355990 | 8 | 39 | Dominated |
| COL 50–65 y | ||||||||||||||
| 3 y | 0 | 125 | 173 | 314 | 2 | 48 | 13 | 143 | 15 | 2437341 | 372687 | 8 | 40 | Dominated |
| 5 y | 0 | 125 | 152 | 293 | 1 | 48 | 14 | 143 | 14 | 2420865 | 356211 | 8 | 39 | Dominated |
| 10 y | 0 | 124 | 151 | 293 | 1 | 48 | 14 | 143 | 14 | 2420644 | 355990 | 8 | 39 | Dominated |
| COL 50–70 y | ||||||||||||||
| 3 y | 0 | 125 | 173 | 314 | 2 | 48 | 13 | 143 | 15 | 2437341 | 372687 | 8 | 40 | Dominated |
| 5 y | 0 | 125 | 152 | 293 | 1 | 48 | 14 | 143 | 14 | 2420865 | 356211 | 8 | 39 | Dominated |
| 10 y | 0 | 124 | 151 | 293 | 1 | 48 | 14 | 143 | 14 | 2420644 | 355990 | 8 | 39 | Dominated |
| COL 50–75 y | ||||||||||||||
| 3 y | 0 | 125 | 173 | 314 | 2 | 48 | 13 | 143 | 15 | 2437341 | 372687 | 8 | 40 | Dominated |
| 5 y | 0 | 125 | 152 | 293 | 1 | 48 | 14 | 143 | 14 | 2420865 | 356211 | 8 | 39 | Dominated |
| 10 y | 0 | 124 | 151 | 293 | 1 | 48 | 14 | 143 | 14 | 2420644 | 355990 | 8 | 39 | Dominated |
| COL 45–55 y | ||||||||||||||
| 3 y | 0 | 200 | 416 | 628 | 3 | 38 | 9 | 137 | 29 | 2481276 | 416622 | 27 | 59 | Dominated |
| 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2438899 | 374244 | 25 | 58 | Efficient |
| 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2438362 | 373707 | 25 | 57 | Efficient |
| COL 45–60 y | ||||||||||||||
| 3 y | 0 | 200 | 416 | 628 | 3 | 38 | 9 | 137 | 29 | 2481280 | 416625 | 27 | 59 | Dominated |
| 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2438902 | 374247 | 25 | 58 | Dominated |
| 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2438362 | 373707 | 25 | 57 | Dominated |
| COL 45–65 y | ||||||||||||||
| 3 y | 0 | 200 | 416 | 628 | 3 | 38 | 9 | 137 | 29 | 2481280 | 416625 | 27 | 59 | Dominated |
| 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2438902 | 374247 | 25 | 58 | Dominated |
| 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2438362 | 373707 | 25 | 57 | Dominated |
| COL 45–70 y | ||||||||||||||
| 3 y | 0 | 200 | 416 | 628 | 3 | 38 | 9 | 137 | 29 | 2481280 | 416625 | 27 | 59 | Dominated |
| 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2438902 | 374247 | 25 | 58 | Dominated |
| 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2438362 | 373707 | 25 | 57 | Dominated |
| COL 45–75 y | ||||||||||||||
| 3 y | 0 | 200 | 416 | 628 | 3 | 38 | 9 | 137 | 29 | 2481280 | 416625 | 27 | 59 | Dominated |
| 5 y | 0 | 200 | 343 | 554 | 2 | 39 | 9 | 139 | 28 | 2438902 | 374247 | 25 | 58 | Dominated |
| 10 y | 0 | 199 | 342 | 553 | 2 | 39 | 9 | 139 | 28 | 2438362 | 373707 | 25 | 57 | Dominated |
| COL 40–55 y | ||||||||||||||
| 3 y | 0 | 328 | 774 | 1109 | 3 | 30 | 6 | 123 | 44 | 2707578 | 642923 | 42 | 74 | Dominated |
| 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2600975 | 536321 | 36 | 70 | Efficient |
| 10 y | 0 | 320 | 582 | 909 | 3 | 34 | 7 | 129 | 42 | 2597514 | 532860 | 35 | 70 | Dominated |
| COL 40–60 y | ||||||||||||||
| 3 y | 0 | 328 | 774 | 1109 | 3 | 30 | 6 | 123 | 44 | 2707578 | 642924 | 42 | 74 | Dominated |
| 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2600975 | 536321 | 36 | 70 | Dominated |
| 10 y | 0 | 320 | 582 | 909 | 3 | 34 | 7 | 129 | 42 | 2597584 | 532929 | 35 | 70 | Dominated |
| COL 40–65 y | ||||||||||||||
| 3 y | 0 | 328 | 774 | 1109 | 3 | 30 | 6 | 123 | 44 | 2707578 | 642924 | 42 | 74 | Dominated |
| 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2600975 | 536321 | 36 | 70 | Dominated |
| 10 y | 0 | 320 | 582 | 909 | 3 | 34 | 7 | 129 | 42 | 2597584 | 532929 | 35 | 70 | Dominated |
| COL 40–70 y | ||||||||||||||
| 3 y | 0 | 328 | 774 | 1109 | 3 | 30 | 6 | 123 | 44 | 2707578 | 642924 | 42 | 74 | Dominated |
| 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2600975 | 536321 | 36 | 70 | Dominated |
| 10 y | 0 | 320 | 582 | 909 | 3 | 34 | 7 | 129 | 42 | 2597584 | 532929 | 35 | 70 | Dominated |
| COL 40–75 y | ||||||||||||||
| 3 y | 0 | 328 | 774 | 1109 | 3 | 30 | 6 | 123 | 44 | 2707578 | 642924 | 42 | 74 | Dominated |
| 5 y | 0 | 324 | 591 | 923 | 3 | 34 | 7 | 129 | 42 | 2600975 | 536321 | 36 | 70 | Dominated |
| 10 y | 0 | 320 | 582 | 909 | 3 | 34 | 7 | 129 | 42 | 2597584 | 532929 | 35 | 70 | Dominated |
| COL 35–55 y | ||||||||||||||
| 3 y | 0 | 642 | 1265 | 1912 | 4 | 26 | 4 | 110 | 56 | 3346546 | 1281892 | 49 | 82 | Optimal |
| 5 y | 0 | 607 | 838 | 1451 | 3 | 31 | 5 | 122 | 52 | 3028100 | 963446 | 41 | 77 | Efficient |
| 10 y | 0 | 571 | 788 | 1364 | 3 | 32 | 6 | 125 | 49 | 2980739 | 916084 | 39 | 75 | Dominated |
| COL 35–60 y | ||||||||||||||
| 3 y | 0 | 642 | 1265 | 1912 | 4 | 26 | 4 | 110 | 56 | 3346548 | 1281894 | 49 | 82 | Dominated |
| 5 y | 0 | 607 | 838 | 1451 | 3 | 31 | 5 | 122 | 52 | 3028100 | 963446 | 41 | 77 | Dominated |
| 10 y | 0 | 571 | 788 | 1364 | 3 | 32 | 6 | 125 | 49 | 2980739 | 916084 | 39 | 75 | Dominated |
| COL 35–65 y | ||||||||||||||
| 3 y | 0 | 642 | 1265 | 1912 | 4 | 26 | 4 | 110 | 56 | 3346548 | 1281894 | 49 | 82 | Dominated |
| 5 y | 0 | 607 | 838 | 1451 | 3 | 31 | 5 | 122 | 52 | 3028100 | 963446 | 41 | 77 | Dominated |
| 10 y | 0 | 571 | 788 | 1364 | 3 | 32 | 6 | 125 | 49 | 2980739 | 916084 | 39 | 75 | Dominated |
| COL 35–70 y | ||||||||||||||
| 3 y | 0 | 642 | 1265 | 1912 | 4 | 26 | 4 | 110 | 56 | 3346548 | 1281894 | 49 | 82 | Dominated |
| 5 y | 0 | 607 | 838 | 1451 | 3 | 31 | 5 | 122 | 52 | 3028100 | 963446 | 41 | 77 | Dominated |
| 10 y | 0 | 571 | 788 | 1364 | 3 | 32 | 6 | 125 | 49 | 2980739 | 916084 | 39 | 75 | Dominated |
| COL 35–75 y | ||||||||||||||
| 3 y | 0 | 642 | 1265 | 1912 | 4 | 26 | 4 | 110 | 56 | 3346548 | 1281894 | 49 | 82 | Dominated |
| 5 y | 0 | 607 | 838 | 1451 | 3 | 31 | 5 | 122 | 52 | 3028100 | 963446 | 41 | 77 | Dominated |
| 10 y | 0 | 571 | 788 | 1364 | 3 | 32 | 6 | 125 | 49 | 2980739 | 916084 | 39 | 75 | Dominated |
| COL 30–55 y | ||||||||||||||
| 3 y | 0 | 1511 | 1826 | 3340 | 5 | 25 | 3 | 99 | 64 | 4622190 | 2557535 | 53 | 87 | Efficient |
| 5 y | 0 | 1316 | 1080 | 2400 | 4 | 29 | 4 | 117 | 57 | 3888961 | 1824306 | 43 | 80 | Dominated |
| 10 y | 0 | 1134 | 971 | 2110 | 4 | 31 | 5 | 121 | 54 | 3656737 | 1592083 | 41 | 77 | Dominated |
| COL 30–60 y | ||||||||||||||
| 3 y | 0 | 1511 | 1826 | 3340 | 5 | 25 | 3 | 99 | 64 | 4622193 | 2557539 | 53 | 87 | Dominated |
| 5 y | 0 | 1316 | 1080 | 2400 | 4 | 29 | 4 | 117 | 57 | 3888964 | 1824309 | 43 | 80 | Dominated |
| 10 y | 0 | 1134 | 971 | 2110 | 4 | 31 | 5 | 121 | 54 | 3656827 | 1592172 | 41 | 78 | Dominated |
| COL 30–65 y | ||||||||||||||
| 3 y | 0 | 1511 | 1826 | 3340 | 5 | 25 | 3 | 99 | 64 | 4622193 | 2557539 | 53 | 87 | Dominated |
| 5 y | 0 | 1316 | 1080 | 2400 | 4 | 29 | 4 | 117 | 57 | 3888964 | 1824309 | 43 | 80 | Dominated |
| 10 y | 0 | 1134 | 971 | 2110 | 4 | 31 | 5 | 121 | 54 | 3656827 | 1592172 | 41 | 78 | Dominated |
| COL 30–70 y | ||||||||||||||
| 3 y | 0 | 1511 | 1826 | 3340 | 5 | 25 | 3 | 99 | 64 | 4622193 | 2557539 | 53 | 87 | Dominated |
| 5 y | 0 | 1316 | 1080 | 2400 | 4 | 29 | 4 | 117 | 57 | 3888964 | 1824309 | 43 | 80 | Dominated |
| 10 y | 0 | 1134 | 971 | 2110 | 4 | 31 | 5 | 121 | 54 | 3656827 | 1592172 | 41 | 78 | Dominated |
| COL 30–75 y | ||||||||||||||
| 3 y | 0 | 1511 | 1826 | 3340 | 5 | 25 | 3 | 99 | 64 | 4622193 | 2557539 | 53 | 87 | Dominated |
| 5 y | 0 | 1316 | 1080 | 2400 | 4 | 29 | 4 | 117 | 57 | 3888964 | 1824309 | 43 | 80 | Dominated |
| 10 y | 0 | 1134 | 971 | 2110 | 4 | 31 | 5 | 121 | 54 | 3656827 | 1592172 | 41 | 78 | Dominated |
COL indicates colonoscopy; CRC, colorectal cancer; LY, Life-years; LYG, LY gained compared with no screening; Grey row indicates optimal screening strategy.
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Appendix Table 6.
Outcomes with FIT screening strategies that vary by the ages to begin and end screening among transplant Cystic Fibrosis patients.
| Outcomes per 1,000 transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (with organ transplant at age 30, 3% discounted) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Screening tests | Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb (%) | Efficient strategy |
|||
|
| ||||||||||||||
| FIT | COLs | CRC incidencec |
CRC mortalityc |
|||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 30 | 0 | 52 | 22 | 115 | 0 | 2064654 | 0 | 0 | 0 | Dominated |
| FIT 50–55 y | 327 | 0 | 88 | 189 | 1 | 54 | 15 | 153 | 13 | 2467521 | 402866 | −3 | 35 | Dominated |
| FIT 50–60 y | 360 | 0 | 92 | 198 | 1 | 54 | 14 | 155 | 13 | 2496400 | 431746 | −4 | 36 | Dominated |
| FIT 50–65 y | 364 | 0 | 92 | 199 | 1 | 54 | 14 | 156 | 13 | 2501575 | 436920 | −4 | 37 | Dominated |
| FIT 50–70 y | 364 | 0 | 92 | 199 | 1 | 54 | 14 | 156 | 13 | 2501575 | 436920 | −4 | 37 | Dominated |
| FIT 50–75 y | 364 | 0 | 92 | 199 | 1 | 54 | 14 | 156 | 13 | 2501575 | 436920 | −4 | 37 | Dominated |
| FIT 45–55 y | 813 | 0 | 181 | 322 | 1 | 48 | 11 | 165 | 25 | 2517648 | 452994 | 8 | 53 | Dominated |
| FIT 45–60 y | 840 | 0 | 184 | 329 | 1 | 48 | 10 | 167 | 26 | 2541004 | 476350 | 7 | 54 | Dominated |
| FIT 45–65 y | 843 | 0 | 184 | 329 | 1 | 49 | 10 | 167 | 26 | 2545324 | 480670 | 7 | 54 | Dominated |
| FIT 45–70 y | 843 | 0 | 184 | 329 | 1 | 49 | 10 | 167 | 26 | 2545324 | 480670 | 7 | 54 | Dominated |
| FIT 45–75 y | 843 | 0 | 184 | 329 | 1 | 49 | 10 | 167 | 26 | 2545324 | 480670 | 7 | 54 | Dominated |
| FIT 40–55 y | 1722 | 0 | 283 | 466 | 2 | 44 | 8 | 172 | 38 | 2589414 | 524759 | 15 | 65 | Dominated |
| FIT 40–60 y | 1745 | 0 | 286 | 472 | 2 | 45 | 8 | 173 | 38 | 2610117 | 545463 | 14 | 66 | Dominated |
| FIT 40–65 y | 1748 | 0 | 286 | 473 | 2 | 45 | 8 | 173 | 38 | 2614004 | 549350 | 14 | 66 | Dominated |
| FIT 40–70 y | 1748 | 0 | 286 | 473 | 2 | 45 | 8 | 173 | 38 | 2614004 | 549350 | 14 | 66 | Dominated |
| FIT 40–75 y | 1748 | 0 | 286 | 473 | 2 | 45 | 8 | 173 | 38 | 2614004 | 549350 | 14 | 66 | Dominated |
| FIT 35–55 y | 3419 | 0 | 377 | 620 | 2 | 42 | 6 | 175 | 48 | 2755648 | 690993 | 19 | 72 | Efficient |
| FIT 35–60 y | 3440 | 0 | 380 | 625 | 2 | 42 | 6 | 177 | 48 | 2774500 | 709845 | 18 | 73 | Dominated |
| FIT 35–65 y | 3443 | 0 | 380 | 626 | 2 | 43 | 6 | 177 | 48 | 2778104 | 713449 | 18 | 73 | Dominated |
| FIT 35–70 y | 3443 | 0 | 380 | 626 | 2 | 43 | 6 | 177 | 48 | 2778104 | 713449 | 18 | 73 | Dominated |
| FIT 35–75 y | 3443 | 0 | 380 | 626 | 2 | 43 | 6 | 177 | 48 | 2778104 | 713449 | 18 | 73 | Dominated |
| FIT 30–55 y | 6702 | 0 | 460 | 811 | 2 | 41 | 5 | 177 | 54 | 3049935 | 985281 | 21 | 76 | Efficient |
| FIT 30–60 y | 6722 | 0 | 463 | 816 | 2 | 41 | 5 | 178 | 54 | 3067680 | 1003026 | 20 | 77 | Optimal |
| FIT 30–65 y | 6725 | 0 | 463 | 816 | 2 | 42 | 5 | 178 | 54 | 3071052 | 1006398 | 20 | 77 | Dominated |
| FIT 30–70 y | 6725 | 0 | 463 | 816 | 2 | 42 | 5 | 178 | 54 | 3071052 | 1006398 | 20 | 77 | Dominated |
| FIT 30–75 y | 6725 | 0 | 463 | 816 | 2 | 42 | 5 | 178 | 54 | 3071052 | 1006398 | 20 | 77 | Dominated |
COL indicates colonoscopy; CRC, colorectal cancer; LY, Life-years; LYG, LY gained compared with no screening; FIT, Fecal immunochemical test; Grey row indicates optimal screening strategy.
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Appendix Table 7.
Efficient colonoscopy screening strategies among non-transplant Cystic Fibrosis patients (assuming 5-fold and 10-fold increased rates of cardiovascular complications).
| Outcomes per 1,000 non-transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (3% discounted) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Screening tests | Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb (%) | ICER (*$1,000) |
|||
|
| ||||||||||||||
| FIT | COLs | CRC incidencec |
CRC mortalityc |
|||||||||||
|
| ||||||||||||||
| Colonoscopy strategies (5-fold increased rates of cardiovascular complications) | ||||||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 23 | 0 | 52 | 19 | 134 | 0 | 1919770 | 0 | 0 | 0 | - |
| COL 50–55 y, 10 y | 0 | 214 | 334 | 558 | 5 | 32 | 7 | 127 | 29 | 2034143 | 114374 | 38 | 62 | 4 |
| COL 50–60 y, 10 y | 0 | 225 | 345 | 579 | 6 | 31 | 7 | 127 | 30 | 2040522 | 120753 | 40 | 66 | 5 |
| COL 50–55 y, 5 y | 0 | 231 | 352 | 591 | 6 | 31 | 7 | 126 | 30 | 2042926 | 123156 | 41 | 66 | 10 |
| COL 50–60 y, 5 y | 0 | 234 | 354 | 597 | 6 | 31 | 6 | 126 | 31 | 2044880 | 125111 | 41 | 66 | 10 |
| COL 50–70 y, 5 y | 0 | 235 | 354 | 598 | 6 | 31 | 6 | 126 | 31 | 2045449 | 125679 | 41 | 67 | 16 |
| COL 45–75 y, 5 y | 0 | 394 | 531 | 931 | 7 | 27 | 5 | 117 | 38 | 2244517 | 324748 | 48 | 74 | 28 |
| COL 40–70 y, 5 y | 0 | 689 | 724 | 1417 | 8 | 25 | 4 | 109 | 44 | 2616434 | 696665 | 52 | 78 | 64 |
| COL 40–75 y, 5 y | 0 | 689 | 724 | 1417 | 8 | 25 | 4 | 109 | 44 | 2616456 | 696686 | 52 | 78 | 101 |
| COL 40–75 y, 3 y | 0 | 793 | 1301 | 2097 | 10 | 20 | 3 | 93 | 47 | 3110299 | 1190530 | 63 | 84 | 137 |
| COL 35–75 y, 3 y | 0 | 1482 | 1700 | 3185 | 11 | 18 | 2 | 84 | 52 | 4035494 | 2115724 | 67 | 87 | 191 |
| COL 30–75 y, 3 y | 0 | 2671 | 2062 | 4734 | 11 | 17 | 2 | 77 | 55 | 5405835 | 3486065 | 68 | 89 | 453 |
|
| ||||||||||||||
| Colonoscopy strategies (10-fold increased rates of cardiovascular complications) | ||||||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 23 | 1 | 52 | 19 | 134 | 0 | 1921353 | 0 | 0 | 0 | - |
| COL 50–55 y, 10 y | 0 | 214 | 334 | 558 | 9 | 32 | 7 | 127 | 29 | 2056855 | 135502 | 38 | 62 | 5 |
| COL 50–60 y, 10 y | 0 | 225 | 345 | 579 | 9 | 31 | 7 | 127 | 30 | 2064656 | 143303 | 40 | 65 | 7 |
| COL 50–55 y, 5 y | 0 | 231 | 352 | 591 | 9 | 31 | 7 | 126 | 30 | 2067209 | 145856 | 41 | 66 | 10 |
| COL 50–60 y, 5 y | 0 | 234 | 354 | 597 | 10 | 31 | 6 | 126 | 30 | 2069459 | 148106 | 41 | 66 | 11 |
| COL 50–70 y, 5 y | 0 | 235 | 354 | 598 | 10 | 31 | 6 | 126 | 30 | 2070110 | 148756 | 41 | 66 | 19 |
| COL 45–75 y, 5 y | 0 | 394 | 531 | 931 | 11 | 27 | 5 | 117 | 37 | 2273139 | 351786 | 48 | 73 | 29 |
| COL 40–70 y, 5 y | 0 | 689 | 724 | 1417 | 13 | 25 | 4 | 109 | 43 | 2648184 | 726831 | 52 | 78 | 68 |
| COL 40–75 y, 3 y | 0 | 793 | 1301 | 2097 | 16 | 20 | 3 | 93 | 46 | 3150810 | 1229457 | 63 | 83 | 151 |
| COL 35–75 y, 5 y | 0 | 1198 | 909 | 2110 | 13 | 24 | 4 | 103 | 47 | 3237207 | 1315854 | 55 | 80 | 209 |
| COL 35–75 y, 3 y | 0 | 1482 | 1700 | 3184 | 17 | 18 | 3 | 84 | 51 | 4079400 | 2158046 | 67 | 86 | 217 |
| COL 30–75 y, 3 y | 0 | 2670 | 2061 | 4733 | 18 | 17 | 2 | 77 | 53 | 5450861 | 3529508 | 68 | 87 | 636 |
COL indicates colonoscopy; CRC, colorectal cancer; FIT = Fecal Immunochemical Test; LY, Life-years; LYG, LY gained compared with no screening; ICER, Incremental cost-effectiveness ratio (Costs/LYs gained).
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Bold rows indicate optimal screening strategies.
Appendix Table 8.
Efficient colonoscopy screening strategies among transplant Cystic Fibrosis patients (assuming 5-fold and 10-fold increased rates of cardiovascular complications).
| Outcomes per 1,000 transplant cystic fibrosis individuals free of diagnosed cancer at age 30 years in 2017 (with organ transplant at age 30, 3% discounted) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Screening tests | Surveillance COLs |
Total COLs |
Compli -cations |
CRC Casesc |
CRC deatha,c |
LY with CRC |
LYGb | Total costs (*$1,000) |
Net costs (*$1,000) |
Reductionsb (%) | ICER (*$1,000) |
|||
|
| ||||||||||||||
| FIT | COLs | CRC incidencec |
CRC mortalityc |
|||||||||||
|
| ||||||||||||||
| Colonoscopy strategies (5-fold increased rates of cardiovascular complications) | ||||||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 30 | 0 | 52 | 22 | 115 | 0 | 2065435 | 0 | 0 | 0 | - |
| COL 45–55 y, 10 y | 0 | 199 | 342 | 553 | 4 | 39 | 10 | 139 | 28 | 2452010 | 386576 | 25 | 57 | 2 |
| COL 45–55 y, 5 y | 0 | 200 | 343 | 554 | 4 | 39 | 9 | 139 | 28 | 2452589 | 387154 | 25 | 57 | 8 |
| COL 40–55 y, 5 y | 0 | 324 | 591 | 923 | 6 | 34 | 7 | 129 | 42 | 2618834 | 553399 | 36 | 70 | 12 |
| COL 35–55 y, 5 y | 0 | 607 | 838 | 1451 | 7 | 31 | 5 | 122 | 51 | 3049189 | 983754 | 41 | 77 | 46 |
| COL 35–55 y, 3 y | 0 | 642 | 1265 | 1912 | 8 | 26 | 4 | 110 | 56 | 3373257 | 1307822 | 49 | 82 | 75 |
| COL 30–55 y, 3 y | 0 | 1511 | 1825 | 3339 | 10 | 25 | 3 | 99 | 63 | 4652949 | 2587514 | 53 | 86 | 177 |
|
| ||||||||||||||
| Colonoscopy strategies (10-fold increased rates of cardiovascular complications) | ||||||||||||||
|
| ||||||||||||||
| No screening | 0 | 0 | 0 | 30 | 0 | 52 | 22 | 115 | 0 | 2066410 | 0 | 0 | 0 | - |
| COL 45–55 y, 10 y | 0 | 199 | 341 | 553 | 7 | 39 | 10 | 139 | 28 | 2469059 | 402648 | 25 | 57 | 3 |
| COL 45–55 y, 5 y | 0 | 200 | 342 | 554 | 7 | 39 | 10 | 139 | 28 | 2469686 | 403276 | 25 | 57 | 10 |
| COL 40–55 y, 5 y | 0 | 324 | 591 | 923 | 9 | 34 | 7 | 129 | 42 | 2641176 | 574766 | 36 | 70 | 14 |
| COL 35–55 y, 5 y | 0 | 607 | 838 | 1451 | 11 | 31 | 5 | 122 | 51 | 3075548 | 1009138 | 41 | 76 | 49 |
| COL 35–55 y, 3 y | 0 | 642 | 1265 | 1911 | 13 | 26 | 4 | 110 | 55 | 3406590 | 1340180 | 49 | 81 | 85 |
| COL 30–55 y, 3 y | 0 | 1511 | 1825 | 3339 | 16 | 25 | 3 | 99 | 62 | 4691373 | 2624962 | 53 | 85 | 194 |
COL indicates colonoscopy; CRC, colorectal cancer; FIT = Fecal Immunochemical Test; LY, Life-years; LYG, LY gained compared with no screening; ICER, Incremental cost-effectiveness ratio (Costs/LYs gained).
Including deaths from complications of screening;
compared with no screening;
CRC cases and CRC death were not discounted.
Bold rows indicate optimal screening strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests: All authors disclose no conflicts of interest.
Contributors AG and IL-V were responsible for study coordination: AG, AGZ, and IL-V were responsible for the study design and first version of the manuscript. AG, AGZ, IL-V, and DRC drafted the final manuscript. All authors gave critical revisions on intellectual content of the manuscript and approved the final manuscript.
References
- 1.Parkins MD, Parkins VM, Rendall JC, et al. Changing epidemiology and clinical issues arising in an ageing cystic fibrosis population. Ther Adv Respir Dis. 2011;5:105–19. doi: 10.1177/1753465810386051. [DOI] [PubMed] [Google Scholar]
- 2.Farrell PM. The prevalence of cystic fibrosis in the European Union. J Cyst Fibros. 2008;7:450–3. doi: 10.1016/j.jcf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13:1173–9. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation Patient Registry 2015 Annual Data Report. Bethesda, Maryland: Available on: https://www.cff.org/Our-Research/CF-Patient-Registry/2015-Patient-Registry-Annual-Data-Report.pdf 2016. Cystic Fibrosis Foundation. [Google Scholar]
- 5.Billings JL, Dunitz JM, McAllister S, et al. Early colon screening of adult patients with cystic fibrosis reveals high incidence of adenomatous colon polyps. J Clin Gastroenterol. 2014;48:e85–8. doi: 10.1097/MCG.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 6.Maisonneuve P, Marshall BC, Knapp EA, et al. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105:122–9. doi: 10.1093/jnci/djs481. [DOI] [PubMed] [Google Scholar]
- 7.Meyer KC, Francois ML, Thomas HK, et al. Colon cancer in lung transplant recipients with CF: increased risk and results of screening. J Cyst Fibros. 2011;10:366–9. doi: 10.1016/j.jcf.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Niccum DE, Billings JL, Dunitz JM, et al. Colonoscopic screening shows increased early incidence and progression of adenomas in cystic fibrosis. J Cyst Fibros. 2016;15:548–53. doi: 10.1016/j.jcf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 10.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult22 blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50:29–32. doi: 10.1136/gut.50.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, et al. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115:2410–9. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 15.Holme Ø, Løberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: A randomized clinical trial. JAMA. 2014;312:606–615. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: Follow-up findings of the italian randomized controlled trial - SCORE. J Natl Cancer Inst. 2011;103:1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Hadjiliadis D, Khoruts A, Zauber AG, Hempstead SE, Maisonneuve P, Lowenfels AB for the CF Colorectal Cancer Screening Taskforce. Cystic Fibrosis Colorectal Cancer Screening Consensus Recommendations. Work in progress 2017 [Google Scholar]
- 20.Vogelaar I, van Balegooijen M, Zauber AG, et al. Model profiler of the MISCAN-Colon miscosimulation model for colorectal cancer. Deparment of Public Health; Erasmus, MC: 2004. available from: http://cisnet.flexkb.net/mp/pub/cisnet_colorectal_sloankettering_profile.pdf. [Google Scholar]
- 21.van Hees F, Habbema JD, Meester RG, et al. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis. Ann Intern Med. 2014;160:750–9. doi: 10.7326/M13-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutter CM, Johnson EA, Feuer EJ, et al. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst. 2013;105:1806–13. doi: 10.1093/jnci/djt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence— SEER 9 Regs Limited Use, Nov 2002 Sub (1973–2000) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; Released April 2003, based on the November 2002 submission. [Google Scholar]
- 24.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7:249–61. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 25.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61:1472–6. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–6. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36:179–86. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 28.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508–14. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24:799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 30.LJ B. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4:277–82. [Google Scholar]
- 31.Rickert RR, Auerbach O, Garfinkel L, et al. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847–57. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49:819–25. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835–42. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. Jama. 2016;315:2595–609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belli EV, Landolfo K, Keller C, et al. Lung cancer following lung transplant: single institution 10 year experience. Lung Cancer. 2013;81:451–4. doi: 10.1016/j.lungcan.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 36.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 37.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal40 cancer screening. N Engl J Med. 2014;370:1287–97. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 38.Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 39.Schroy PC, 3rd, Coe A, Chen CA, et al. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159:13–20. doi: 10.7326/0003-4819-159-1-201307020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–57. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 41.Leffler DA, Kheraj R, Garud S, et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch Intern Med. 2010;170:1752–7. doi: 10.1001/archinternmed.2010.373. [DOI] [PubMed] [Google Scholar]
- 42.Bureau of Labor Statistics. Current Employment Statistics (National) Accessed at www.bls.gov/web/empsit/ceseesummary.htm, on 16 August 2016.
- 43.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 44.The United States Department of Labor. Bureau of Labor Statistics. Consumer Price Index. Available from: http://www.bls.gov/cpi/tables.htm.
- 45.Nick JA, Chacon CS, Brayshaw SJ, et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2010;182:614–26. doi: 10.1164/rccm.201001-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morson B. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 48.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. Jama. 2006;296:1507–17. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 49.Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. Jama. 2011;305:2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 50.MacKenzie T, Gifford AH, Sabadosa KA, et al. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161:233–41. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly T, Buxbaum J. Gastrointestinal Manifestations of Cystic Fibrosis. Dig Dis Sci. 2015;60:1903–13. doi: 10.1007/s10620-015-3546-7. [DOI] [PubMed] [Google Scholar]