Abstract
Ovarian cancer is one of the leading causes of death related to the female reproductive system in western countries. Adverse side effects and resistance to platinum based chemotherapy have become the major obstacles for ovarian cancer treatment. Natural products have gained great attention in cancer treatment in recent years. Chinese bayberry leaves flavonoids (BLF) containing rich content of myricitrin (myricetin 3-O-rhamnoside) and a part of quercetrin (quercetin 3-rhamnoside) inhibited the growth of an ovarian cancer cell line A2780/CP70. Such inhibitory effects might be due to the induction of apoptosis and G1 cell cycle arrest. BLF treatment increased the expression of cleaved caspase-3 and -7 and induced apoptosis via a Erk-dependent caspase-9 activation intrinsic apoptotic pathway by up-regulating the pro-apoptotic proteins (Bad and Bax) and down-regulating the anti-apoptotic proteins (Bcl-xL and Bcl-2), which were also in consistency with the results from Hoechst 33342 staining and flow cytometry analysis. Furthermore, by reducing the expression of cyclin D1 and CDK4 and p-Erk, BLF elevated the distribution of G1 phase in cell cycle and thus caused G1 cell cycle arrest. Overall, these results indicated that BLPs could be a valuable resource of natural compound for ovarian cancer treatment.
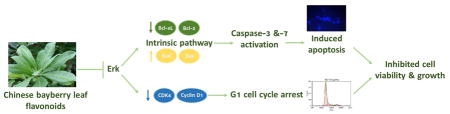
Graphical Abstract
1. Introduction
Ovarian cancer as one of the most lethal gynecological cancers in the female reproductive system affects roughly one in 75 women in the United States [1]. Although the first-line therapy might benefit about 80% of patients with ovarian cancer, 75% of those patients still experience tumor recurrence, which causes a huge concern towards the treatment of ovarian cancer patients [2]. Cisplatin is one of the most widely used chemotherapy drug for treating ovarian cancer. However, taking cisplatin treatment might bring some side effects to patients and tumor recurrence is still unavoidable due the development of resistance. Treatment failure and death in more than 90% of patients with metastatic disease are thought to be caused by drug resistance [3]. Therefore, it is urgent to seek for new agents for cancer treatment and bring less side effects to patients. Natural products from plants have drawn great attention in the fight against cancer in recent years. Extensive studies have shown that flavonoids derived from plants exhibited anti-cancer properties via inducing apoptosis, causing cell cycle arrest and inhibiting angiogenesis etc. based on different in vivo and in vitro models [4].
By mechanism, there are two types of resistance – the nononcogenic resistance and oncogenic resistance. The former one is due to prevention of drug-target interaction while the latter one is due to alterations in the regulation of apoptosis and cell cycle. In oncogenic resistance, a drug interacts with its targets, however, some downstream pathways related to apoptosis are blocked, and thus oncogenic resistance is also considered as apoptosis avoidance resistance [5]. Apoptosis also known as a process of programmed cell death plays a crucial role in maintaining cell homeostasis. Avoidance of apoptosis is a hallmark of cancer [6]. Therefore, inducing apoptosis to overcome oncogenic resistance is one of the potential therapeutics for cancer patients [3, 7]. Furthermore, cell cycle events, which involve four different phases (G0, G1, S and G2) strictly take place in cells and lead to cell division and duplication of its DNA. Defected cell cycle events result in uncontrolled cell proliferation, which is considered as one of the hallmarks of cancer. Oncogenic processes exhibit their greatest effects by targeting G1 phase progression [8]. During the G1 phase, cells can be regulated by mitogens, antiproliferative cytokines and other extracellular signals by either advancing towards another division or withdrawing from the cycle into a resting state (G0) [9]. Cyclin-dependent protein kinases (CDKs) and D-type cyclins have been reported to control the G1 cell cycle progression by forming the holoenzyme complexes. Therefore, the G1 cell cycle checkpoint is considered as the molecular target for cancer treatment by focusing on the CDKs and D type cyclins complex.
Chinese bayberry (Myrica rubra Sieb. et Zucc.) has been cultivated in Southern China for more than 2000 years and is popular among local people. However, leaves from bayberry trees are always abandoned after harvest, which causes huge ecological waste and awaits further utilization and development. Flavonoids from Chinese bayberry leaves (BLF) contain rich content of myricitrin and a part of quercetrin as its major components and exhibited strong anti-oxidant property based on the chemical and cellular assays from a previous study from our group [10]. Antioxidant activity of natural phytochemicals is related to other bioactivities, such as anti-cancer and antiproliferative activities [11]. Previous studies have shown that myricitrin, quercetrin and some other flavonols with similar structures such as myricetin and quercetin exhibited potent anti-cancer properties by inducing apoptosis and G1 cell cycle arrest via different pathways [12, 13]. Although many studies have focused on the anti-cancer properties of flavonoids based on different cancer cell models, however, no efforts have been made to clarify the effects of BLF on ovarian cancer cells. Thus, the present study aims to demonstrate the inhibitory effects of BLF on the growth of an ovarian cancer cell line A2780/CP70 in terms of its regulation on apoptosis and cell cycle arrest. Our results showed that BLF induced apoptosis in A2780/CP70 cells by targeting the intrinsic apoptotic proteins and caused G1 cell cycle arrest via the Erk pathway.
2. Results
2.1 Effects of BLF and cisplatin on A2780/CP70 ovarian cancer cell viability
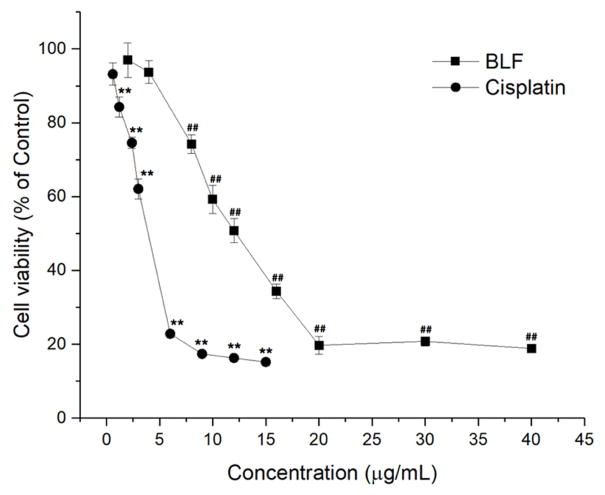
CellTiter 96 Aqueous One Solution Cell Proliferation assay was performed to investigate the effects of BLF and cisplatin on the viability of A2780/CP70 ovarian cancer cells. Figure 1 shows that both BLF and cisplatin dose-dependently inhibited the viability of A2780/CP70 ovarian cancer cells (p < 0.01). The cell viability rate decreased from 93.73 ± 3.08% to 59.22 ± 3.79% after treating with BLF from 2 μg/mL to 10 μg/mL. The IC50 of BLF and cisplatin cell viability curve were 10.57 μg/mL and 3.45 μg/mL, respectively. Although the ability to inhibit the cell viability of A2780/CP70 cells of cisplatin was stronger than that of BLF, BLF still had strong inhibitory effects on A2780/CP70 cells. The IC50 of BLF was lower than that of some other natural products, such as theaflavin-3,3′-digallate (IC50 was more than 17.9 μg/mL on OVCAR-3 cells) [14] and galangin (IC50 was more than 11 μg/mL on A2780/CP70 cells) [15].
Figure 1.
BLF and cisplatin inhibited the viability of A2780/CP70 cells in a dose dependent manner. (**) p < 0.01, compared with the control of cisplatin. (##) p < 0.01, compared with the control of BLF. Cells treated with culture medium containing 0.01% DMSO was used as the control.
2.2 BLF induced apoptosis in A2780/CP70 ovarian cancer cells
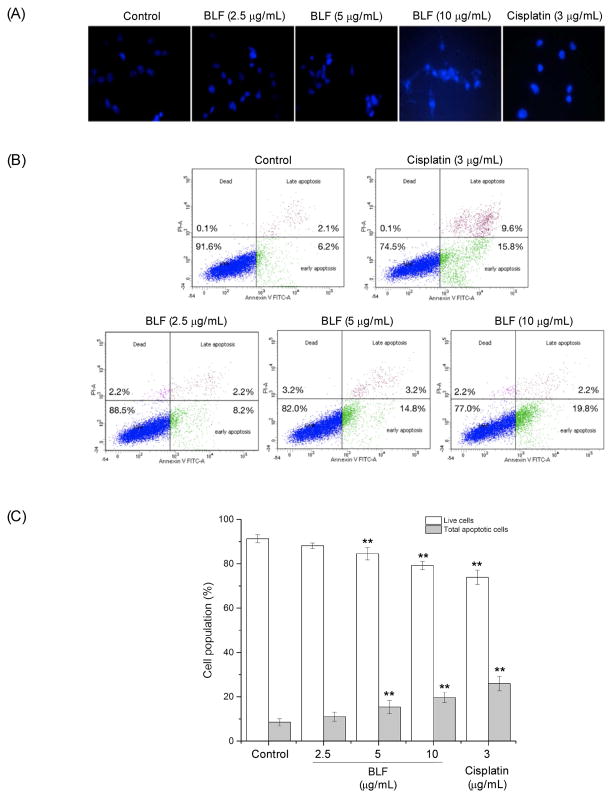
To determine whether BLF inhibited the viability of A2780/CP70 cells by inducing apoptosis, cells were analyzed by the Hoechst 33342 DNA staining and flow cytometry. Figure 2A shows the Hoechst 33342 stained cells under a fluorescence microscope. After treating with BLF or cisplatin, cells showed more condensed and fragmented nuclei and were much brighter than the control group, which indicated that BLF or cisplatin treatment induced more apoptotic cells than the control group. Such results were further verified by the flow cytometry assay. Figure 2B and C show that BLF significantly decreased the percentage of live cells and increased the percentage of apoptotic cells in a dose-dependent manner (p < 0.01). The total apoptotic cells increased from 8.5 ± 1.6% (BLF at 2.5 μg/mL) to 19.7 ± 2.3% (BLF at 10 μg/mL), which was comparable to that of cisplatin at 3 μg/mL. These results indicated that the inhibitory effect of BLF on A2780/CP70 cell viability might be due to inducing apoptosis.
Figure 2.
BLF induced apoptosis in A2780/CP70 cells. (A) Hoechst 33342 staining of A2780/CP70 cells detected by a fluorescent microscope after treating with BLF (2.5, 5 and 10 μg/mL) or cisplatin (3 μg/mL) for 24 h. Highly condensed or fragmented nuclei represent apoptotic cells. (B) Flow cytometry analysis of A2780/CP70 cells. Cell were treated with BLF (2.5, 5 and 10 μg/mL) or cisplatin (3 μg/mL) for 24 h, then stained with Annexin V-FITC and PI solution and analyzed with flow cytometry. (C) Percentage of live and total apoptotic cells after treatment with BLF (2.5, 5 and 10 μg/mL) or cisplatin (3 μg/mL). Results are representative of three independent experiments and are expressed as mean ± SD. (**) p < 0.01, compared with the control. Cells treated with culture medium containing 0.01% DMSO was used as the control.
2.3 BLF regulated key proteins in apoptotic pathways
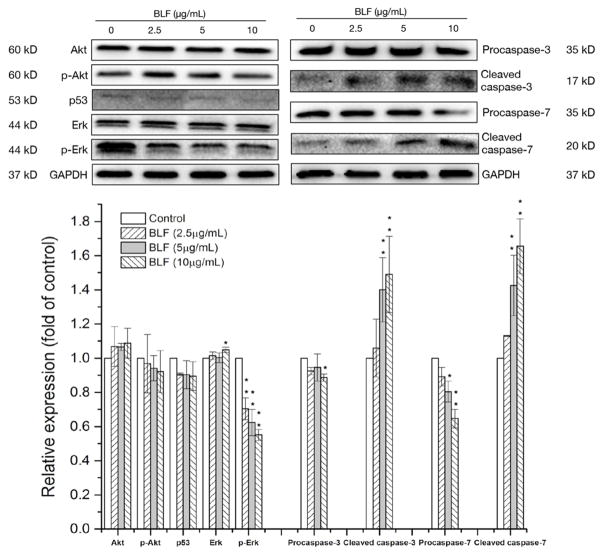
Various signaling pathways involve in the regulation of apoptosis, such as Akt, p53 and Erk pathways[16]. Thus, key proteins regulating cellular apoptosis were investigated in the present study. Figure 3 shows that BLF did not show obvious effects on p-Akt and p53, however, significantly reduced the expression of p-Erk in a dose dependent manner (p < 0.01). As a result, both of procaspase-3 and procaspase-7 decreased, while the expression of cleaved caspase-3 and cleaved caspase-7 significantly increased in a dose-dependent relationship (p < 0.01). Caspase-3 and caspase-7 are the key contributors to apoptosis and can be activated through proteolytic processing. BLF at 10 μg/mL increased the expression of cleaved caspase-3 and cleaved caspase-7 for about 1.47 ± 0.14 and 1.62 ± 0.16 fold of control, which suggests that BLF could induce apoptosis in A2780/CP70 cells via the Erk pathway.
Figure 3.
BLF regulated key proteins in apoptotic pathways in A2780/CP70 cells. Cells were treated with BLF (2.5, 5 and 10 μg/mL) for 24 h. Akt, p-Akt, p53, p-Erk, Erk, procaspase-3, cleaved caspase-3, procaspase-7, cleaved caspase-7 and GAPDH protein expression were detected by Western blot analysis and quantified by Image J software. Some blots presented were cropped and some full-length blots were presented in Supplementary Information file. Results are representative of three independent experiments and are expressed as mean ± SD. (*) p < 0.05 and (**) p < 0.01, compared with the control. Cells treated with culture medium containing 0.01% DMSO was used as the control.
2.4 Effects of BLF on intrinsic and extrinsic apoptotic pathways
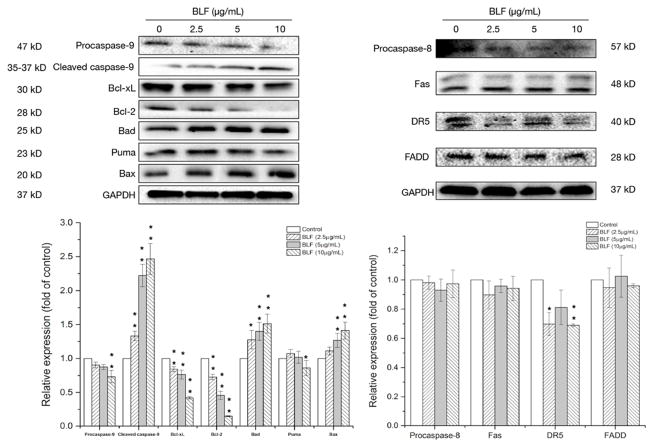
Induction of apoptosis can be classified into two main pathways, which are the intrinsic (the mitochondrial) pathway and extrinsic (the death receptor) pathway [16]. To clarify whether the intrinsic or extrinsic pathways involved in the BLF-induced apoptosis or not, key proteins in both pathways were determined by western blot assay. In the intrinsic apoptotic pathway, both of the pro-apoptotic Bcl-2 family (Bad, Puma and Bax) and the anti-apoptotic Bcl-2 family (Bcl-xL and Bcl-2) are vital for the regulation of apoptosis. Figure 4 shows that BLF treatment dose-dependently reduced the expression of Bcl-xL and Bcl-2 and increased the expression of Bad and Bax, however, did not show obvious effects on the expression of Puma. Activation of the pro-apoptotic proteins (Bad and Bax) and inhibition of the anti-apoptotic proteins (Bcl-xL and Bcl-2) by BLF resulted in the elevation of cleaved caspase-9, which is an important member of the caspase family and can be activated by proteolytic processing. Cleaved caspase-9 further interact with other caspase members, such as caspase-3 and caspase-7 to initiate apoptosis [17]. The expression of cleaved caspase-9 of BLF at 10 μg/mL was 2.45 ± 0.06 fold of control. However, BLF did not show obvious effects on the key proteins in extrinsic apoptotic pathway, including procaspase-8, Fas and FADD. Taken together, BLF mainly targeted the intrinsic apoptotic pathway to activate caspase-9 and further induced apoptosis in A2780/CP70 cells.
Figure 4.
BLF regulated key proteins in intrinsic and extrinsic apoptotic pathways in A2780/CP70 cells. Cells were treated with BLF (2.5, 5 and 10 μg/mL) for 24 h. Procaspase-9, cleaved caspase-9, Bcl-xL, Bcl-2, Bad, Puma, Bax, procaspase-8, Fas, DR5, FADD and GAPDH protein expression were detected by Western blot analysis and quantified by Image J software. Some blots presented were cropped and some full-length blots were presented in Supplementary Information file. Results are representative of three independent experiments and are expressed as mean ± SD. (*) p < 0.05 and (**) p < 0.01, compared with the control. Cells treated with culture medium containing 0.01% DMSO was used as the control.
2.5 BLF induced G1 cell cycle arrest in A2780/CP70 ovarian cancer cells
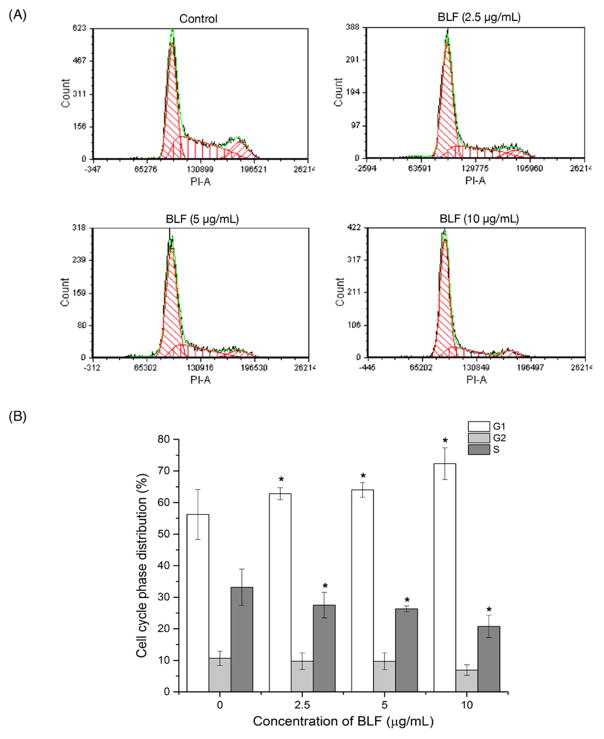
Other than inducing apoptosis, we further investigated whether BLF treatment caused any cell cycle-related events to inhibit the growth of A2780/CP70 cells, thus, the cell cycle phase distribution of cells treated with BLF (2.5, 5, 10 μg/mL) was analyzed by flow cytometry after propidium iodide staining. Figure 5A shows that the distribution of G1 significantly increased after treating with BLF (p < 0.05). BLF at 10 μg/mL increased 28.6% of G1 cell cycle distribution compared with that of the control (Figure 5B). However, the distribution of S phase slightly decreased and treatment with BLF did not show obvious effects on G2 phase. The flow cytometry analysis indicated that BLF induced G1 cell cycle arrest in A2780/CP70 cells.
Figure 5.
BLF induced G1 cell cycle arrest in A2780/CP70 cells. (A and B) BLF induced G1 cell cycle arrest by increasing the distribution of G1 phase. Cell were treated with BLF (2.5, 5 and 10 μg/mL) for 24 h, fixed in 70% and stained with propidium iodide. DNA contents were determined by flow cytometry. Cells treated with culture medium containing 0.01% DMSO was used as the control. Results are representative of three independent experiments and are expressed as mean ± SD. (*) p < 0.05.
2.6 BLF regulated cell cycle G1-related proteins
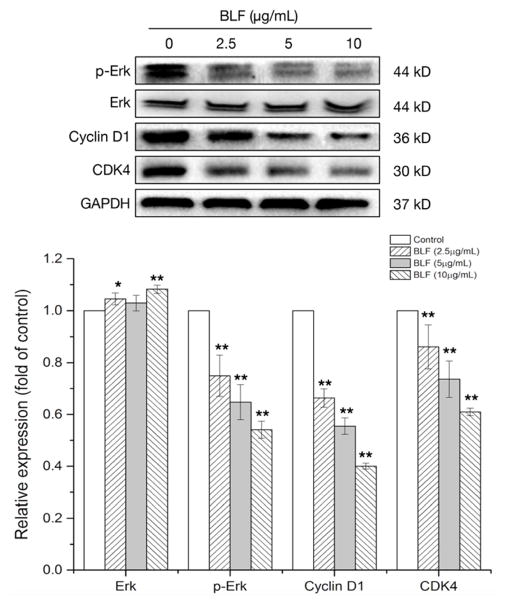
Based on the results from the flow cytometry analysis, key proteins related to the regulation of G1 cell cycle were determined by western blot analysis after BLF treatment. Erk has been reported to control G1 cell cycle phase via regulating the cyclin-CDK4/6 complex [18]. Figure 6 shows that BLF reduced the expression of p-Erk and thus inactivated the Erk. Furthermore, BLF at 10 μg/mL reduced the expression of cyclin D1 and CDK4 for about 60.0% and 39.2% respectively compared with those of the control. These results indicated that the BLF-induced G1 cell cycle arrest might be attributed to the downregulation of Erk and cyclin D1/CDK4 complex.
Figure 6.
BLF regulated cell cycle G1-related proteins. Cells were treated with BLF (2.5, 5 and 10 μg/mL) for 24 h. Erk, p-Erk, Cyclin D1, CDK4 and GAPDH protein expression were detected by Western blot analysis and quantified by Image J software. Some blots presented were cropped and some full-length blots were presented in Supplementary Information file. Results are representative of three independent experiments and are expressed as mean ± SD. (*) p < 0.05 and (**) p < 0.01, compared with the control. Cells treated with culture medium containing 0.01% DMSO was used as the control.
3. Discussion
Ovarian cancer is one of the most lethal gynecological cancers among women in the world. Patients who experienced platinum based chemotherapy might acquire some side effects and resistance to treatment drugs, which is the major impediment for ovarian cancer treatment. Natural products have gained huge attention in cancer treatment in recent years and flavonoids have been reported to exhibit anti-cancer properties through various signaling pathways based on many different in vivo and in vitro studies [4]. In the present study, we found that BLF strongly inhibited the cell viability of ovarian cancer cells A2780/CP70 (Figure 1) and afterwards demonstrated that the BLF-induced inhibitory effects on cell viability might be due to the induction of apoptosis and G1 cell cycle arrest.
Apoptosis is a process of programmed cell death that occurs in multicellular organisms and plays an important role in maintaining regular functions and activities of cells. One possible mechanism that tumor cells gain resistance to chemotherapy drugs might be due to the resistance to apoptosis [5]. Based on the results of the Hoechst 33342 staining and the flow cytometry assay, our data indicated that BLF induced apoptosis in A2780/CP70 cells by showing much brighter and more condensed nuclei within cells (Figure 2A) and significantly higher percentage of apoptotic cells (Figure 2B & C). Many pathways are involved in the regulation of apoptosis, such as Akt, p53 and Erk pathways. Akt can be activated by phosphorylation and further stimulates cell survival by inhibiting apoptosis [19]. Activation of Erk is necessary for cell survival and proliferation and it can be activated by phosphorylation in response to a diverse range of stimuli, such as mitogens, growth factors and cytokines. However, p53 as a tumor suppressor protein can induce DNA repair, apoptosis and cell cycle arrest [20]. Among these key proteins, BLF did not show obvious effects on the expression of Akt and p53, however, significantly reduced the expression of p-Erk in A2780/CP70 cells, which resulted in the activation of caspase-3 and caspase-7 (Figure 3). Both of caspase-3 and caspase-7 belong to the cysteine-aspartic acid protease (caspase) family and act as crucial executioner proteins of apoptosis. Activation of caspase-3 requires proteolysis from its inactive proenzymes at conserved aspartic residues to produce two subunits, and therefore form the active enzyme. During apoptosis, caspase-7 is activated via proteolytic processing by upstream caspases at Asp23, Asp 198 and Asp 206 to produce mature and active subunits [17]. By mainly targeting Erk, BLF treatment activated caspase-3 and caspase-7 by increasing the expression of cleaved caspase-3 and cleaved caspase-7 and thus induced apoptosis in A2780/CP70 cells. Such results were also in accordance with some previous studies that flavonoids, such as myricitrin, myricetin, quercetrin and quercetin caused apoptosis in different cancer cell models via the Erk pathway [12, 21].
Once apoptosis begins, it inevitably leads to cell death, thus, the initiation of apoptosis is strictly regulated by activation mechanisms [16]. Two well-known mechanisms contributed to apoptosis are the intrinsic (the mitochondrial) apoptotic pathway and extrinsic (the death receptor) apoptotic pathway. The intrinsic apoptotic pathway is mediated by mitochondrial outer membrane permeabilization, which leads to the release of cytochrome c into cytoplasm, and cytochrome c then forms apoptosome and initiates the activation of the caspase cascade via caspase-9 to result in apoptosis [22]. In the extrinsic apoptotic pathway, tumor necrosis factor-related apoptosis-inducing ligands bind with their respective death receptors, such as DR4/DR5 or Fas, and then interacts with Fas-associated death domain protein (FADD), which functions as an important adaptor in coupling death signaling from membrane receptors to caspase-8 and further activates the caspase-3 [23]. Activation of either intrinsic or extrinsic apoptotic pathway leads to the activation and cleavage of procaspase-3, -6 or -7 and afterwards results in the progress of apoptosis [24]. In the present study, key proteins in both intrinsic and extrinsic pathways were determined by western blot assays after the BLF treatment. The expression of the proteins in the extrinsic apoptotic pathway, such as procaspase-8, Fas and FADD did not show obvious change after BLF treatment. On the other hand, BLF significantly regulated key proteins in the intrinsic apoptotic pathway. Bad as a member of the Bcl-2 family is a pro-apoptotic protein that promotes cell death by displacing Bax from binding to anti-apoptotic proteins, such as Bcl-2 and Bcl-xL [25]. Bax forms oligomers and translocates from cytosol to the mitochondrial membrane to interact with pore proteins and thus increases the permeability of the membrane, which results in the release of cytochrome c from mitochondria and activation of caspase-9 and other caspases required for apoptosis [26]. On the other hand, Bcl-xL can heterodimerize with the apoptotic proteins or form some mitochondrial outer membrane pores that are non-permeable to pro-apoptotic molecules and thus prevent apoptosis [27]. Bcl-2 is localized to the outer membrane of mitochondria, where it plays a vital role in promoting cell survival and inhibiting the activity of pro-apoptotic proteins [25]. BLF treatment dose-dependently activated the pro-apoptotic proteins (Bad and Bax) and inhibited the activities of anti-apoptotic proteins (Bcl-xL and Bcl-2) (Figure 4) and therefore induced apoptosis by mainly focusing on the intrinsic apoptotic pathway.
Many cancer cells exhibit defective cell-cycle checkpoints, which leads to uncontrolled proliferation and growth [28]. Cells undergo four stages (G0, G1, G2 and S phases) to divide and duplicate its DNA. During G1 phase, cells synthesize mRNA and proteins in preparation for the following steps leading to mitosis. Many proteins are required for the progression through the G1 phase of cell cycle, such as cyclin D1 and CDKs. Cyclin D1 can be synthesized quickly and accumulates in the nucleus, however, it will be degraded when cells enter the S phase. Cyclin D1 as a regulatory subunit of CDK4/6 can assemble with CDK4/6 into holoenzyme complexes to regulate the G1/S phase transition and the entry into the S phase [8]. Thus, many investigations focused on G1 cell cycle checkpoint by targeting the cyclin D1-CDK4 complex as one of the anti-cancer therapeutics. Erk pathway not only participates in the regulation of apoptosis, but also controls G1 cell cycle phase. Erk has been reported to regulate cyclin D1 transcriptional induction via Fos family member proteins and also control the assembly of cyclin-CDK complex via the CDK translocation [18]. Results from the flow cytometry analysis indicated that the G1 cell cycle phase distribution significantly increased after BLF treatment compared with the control (Figure 5). Western blot assay further demonstrated that the BLF-induced G1 arrest might be associated with Erk and cyclin D1-CDK4 complex by exhibiting significantly reduced expression of p-Erk, cyclin D1 and CDK4 after BLF treatment (Figure 6). These results were also in agreement with some previous studies that flavonoids such as quercetin and myricetin induced G1 cell cycle arrest via the inhibition of D type cyclins and CDKs in different cancer cell models, including ovarian cancer cells [13, 29], [30].
Flavonoids are widespread in many different types of plants or plant products and frequently occur as glycosides. For instance, myricitrin and quercetrin which can be extracted from the fruits, leaves and bark of Chinese bayberry trees and other plants, are the 3-O-rhamnoside form of myricetin and quercetin respectively [31]. Myricitrin and quercetrin are converted to their aglycone forms through intestinal microflora [32] and their aglycones (myricetin and quercetin) were reported to induce apoptosis on different cancer cell models by affecting the intrinsic apoptotic proteins via the Erk pathway [33]. Furthermore, some functional groups of flavonoids, such as 2,3 double bond, 3-OH group and 4-oxo on the C ring were reported to affect the function of mitochondria membrane by decreasing the fluidity via inhibiting the respiratory chain of mitochondria or causing uncoupling, which might partially be attributed to apoptosis [34]. Containing rich myricitrin and a part of quercetrin and other flavonoids, BLF showed strong capacities in the induction of apoptosis and G1 cell cycle arrest via the Erk pathway. Except for the major effects brought by myricitrin, its synergistic effects with other flavonoids might also played a role as well. Many previous studies showed that combination of two or more flavonoids exerted stronger inhibition on cancer growth due to the synergistic and additive effects and such benefit might be due to the complex mixture of phytochemicals present in whole foods [35, 36]. With myricitrin as its major component and a part of quercetrin and other flavonoids, BLF owned the potential to be a valuable source of natural products to benefit ovarian cancer patients given the fact that myricitrin has been listed as “generally recognized as safe” by the US [32]. However, the dosage and in vivo toxicity of BLF awaits further investigation in the future.
4. Conclusion
In the present study, we demonstrated that BLF had strong inhibitory effects on the growth of an ovarian cancer cell line A2780/CP70 by inducing apoptosis and G1 cell cycle arrest via the Erk pathway. BLF reduced the expression of p-Erk and then activated the caspase cascade by elevating the expression of cleaved caspase-3, -7 and -9. BLF treatment significantly up-regulated pro-apoptotic proteins and down-regulated anti-apoptotic proteins in intrinsic apoptotic pathway to induce apoptosis rather than the extrinsic apoptotic pathway. Furthermore, by downregulating the expression of p-Erk, cyclin D1 and CDK4, BLF induced G1 cell cycle arrest in A2780/CP70 cells, which was also in accordance with the flow cytometry analysis. Taken together, BLF showed potent anti-cancer property by inducing apoptosis via a Erk-dependent caspase-9 activation intrinsic apoptotic pathway and inducing G1 cell cycle arrest in A2780/CP70 cells. BLF owns the potential to be developed as a value source of dietary compounds for ovarian cancer treatment to promote public health.
5. Materials and methods
5.1 Materials and reagents
Propidium iodide, dimethyl sulfoxide were purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA). Antibodies against Akt, phospho-Akt, p53, procaspase-3, cleaved caspase-3, procaspase-7, cleaved caspase-7, Procaspase-9, cleaved caspase-9, Bcl-xL, Bcl-2, Puma, procaspase-8, Fas, DR5, FADD, Cyclin D1 and CDK4 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against Erk, phospho-Erk, Bad, Bax and GAPDH were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA).
5.2 Flavonoids from Chinese bayberry leaves
BLF used in the present study was obtained from our previous study [10]. The content of total flavonoids, myricitrin and quercetrin of BLF are 920.78 ± 18.88 mg/g DW, 184.36 ± 5.96 mg/g DW and 127.05 ± 0.42 μg/g DW, respectively. Detailed information about the preparation and identification of BLF was presented in Supplementary Materials. BLF was presented as yellow powder and was kept in −80 °C refrigerator for long term storage. BLF was firstly dissolved in dimethyl sulfoxide (DMSO) at 100 mg/mL to prepare a stock solution, which was diluted with culture medium to treat cells at 2.5, 5 and 10 μg/mL (DMSO concentration was 0.0025%, 0.005% and 0.01%, respectively). Cells treated with culture medium containing 0.01% DMSO was used as the control. Cisplatin was firstly dissolved in PBS at 1.5 mg/mL to prepare a stock solution, which was then diluted with culture medium to treat cells at 3 μg/mL for the Hoechst 33342 staining assay and flow cytometry analysis.
5.3 Cell culture and reagents
Human ovarian cancer cell line A2780/CP70 was kindly provided by Dr. Bing-Hua Jiang from Department of Microbiology, Immunology, and Cell Biology, West Virginia University, Morgantown, WV, USA. Cells were cultured in RPMI 1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% US-qualified fetal bovine serum (Invitrogen, Grand Island, NY, USA) at 37 °C with 5% CO2.
5.4 Cell viability assay
A2780/CP70 cells were seeded into 96-well plates at a density of 2 × 104 per well in medium with 10% FBS at 37 °C with 5% CO2 and were allowed to attach the bottom overnight and then treated with BLPs at different concentrations for 24 h. Cell viability was determined by using CellTiter 96 Aqueous One Solution Cell Proliferation assay (Promega, Madison, WI, USA) based on the manufacturer’s instructions. The results were expressed as a percentage compared to control cells (vehicle treatment).
5.5 Hoechst 33342 staining for apoptosis assessment
A2780/CP70 cells were seeded into 96-well plates at a density of 1 × 105 per well in medium with 10% FBS at 37 °C with 5% CO2 and were allowed to attach the bottom overnight. Afterwards, cells were treated with cisplatin (3 μg/mL) or BLF (2.5, 5 and 10 μg/mL) for 24 h. Cells were stained with 10 μg/mL Hoechst 33342 (Sigma, St. Louis, MO, USA) in PBS for 10 min at 37 °C in the dark. Afterwards, cells were assessed under a fluorescence microscope (ZEISS) and data were collected from three independent experiments.
5.6 Flow cytometry analysis for apoptosis
The apoptotic cells were detected by an Alexa Fluor 488 Annexin V/Dead Cell Apoptosis Kit (Invitrogen). Cells were harvested and centrifuged for 10 min at 1500 rpm after treating with cisplatin (3 μg/mL) or BLF (2.5, 5 and 10 μg/mL) for 24 h. Afterwards, cells were washed with PBS twice and suspended in binding buffer with Alexa Fluor 488 Annexin V and propidium iodide (PI) in the dark at room temperature for 15 min. The stained cells were analyzed by a flow cytometry system (BD Biosciences, San Jose, CA, USA) within 1 h with the emission wavelength at 530 nm and excitation wavelength at 488nm.
5.7 Flow cytometry analysis for cell cycle
A2780/CP70 cells were treated with cisplatin (3 μg/mL) or BLF (2.5, 5 and 10 μg/mL) for 24 h and were harvested by digestion with trypsin and centrifugation (1500 rpm for 15 min). The cell pellets were suspended with 70% ethanol at −20 °C overnight. Afterwards, cells were washed with PBS twice and incubated with RNase (180 μg/mL) for 30 min at 37 °C, and followed by incubation with PI solution (final concentration 50 μg/mL) for 30 min in the dark. Cells were analyzed by flow cytometry system (BD Biosciences) and results were analyzed by FCS software (De Novo Software, CA, USA).
5.8 Western blot
A2780/CP70 cells were treated with BLF (2.5, 5 and 10 μg/mL) for 24 h and were harvested with M-PER Mammalian Protein Extraction Reagent and Halt Protease and Phosphatase Inhibitor (Thermo Fisher Scientific, Waltham, MA, USA). Total protein concentrations were determined by the BCA protein kit (Thermo Fisher Scientific). Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride filters membrane (GE Healthcare, Chicago, IL, USA). Afterwards, membranes were blocked with 5% of nonfat milk with TBST for 1 h and then were incubated with the first antibody over-night at 4 °C and appropriate secondary antibodies conjugated with horseradish peroxidase. Antibodies against Akt, phospho-Akt, p53, procaspase-3, cleaved caspase-3, procaspase-7, cleaved caspase-7, Procaspase-9, cleaved caspase-9, Bcl-xL, Bcl-2, Bad, Puma, Bax, procaspase-8, Fas, DR5, FAD, CDK4 and cyclin D1 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against GAPDH, Erk, phospho-Erk and GAPDH were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Bands were detected by the ECL Western blot detection reagents (Thermo Fisher Scientific) and exposed to a Mini-Protean 3 System (Bio-Rad, Atlanta, GA, USA).
5.9 Statistical analysis
Results are presented as the mean ± standard deviation (SD) for at least three replicates for each sample. Statistical analyses were performed using the SPSS program, version 17.0 (SPSS Inc., 2009). Data were analysed by ANOVA and significant differences were set at p < 0.05 and p < 0.01.
Supplementary Material
HIGHLIGHTS.
BLF inhibited cell viability of cisplatin-resistant ovarian cancer A2780/CP70 cells
Flow cytometry analysis showed that BLF induced apoptosis in A2780/CP70 cells
BLF activated caspase-3 and caspase-7 to induce apoptosis in A2780/CP70 cells
BLF induced apoptosis via the Erk-induced intrinsic apoptotic pathway
BLF induced G1 cell cycle arrest in A2780/CP70 cells
Acknowledgments
The authors thank Dr. Kathy Brundage from the Flow Cytometry Core at the West Virginia University for providing technical help on apoptosis analysis. This research was supported by NIH grants P20RR016477 from the National Center for Research Resources and P20GM103434 from the National Institute for General Medical Sciences (NIGMS) awarded to the West Virginia IDeA Network of Biomedical Research Excellence. This research was supported by Grant Number P20GM104932 from NIGMS, a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH. This study was also supported by COBRE grant GM102488/RR032138, ARIA S10 grant RR020866, FORTESSA S10 grant OD016165 and INBRE grant GM103434. This research was also supported by the National Natural Science Foundation of China (C200501) and the National Key Research and Development Program (2016YFD0400805).
Footnotes
Author Contributions
Y.Z. designed research, carried out the experiments, and prepared the manuscript. C.Y.W. and Y.C.C. participated in the design of the study. Y.C.C., G.O.R., X.Q.Y. and S.G.C. conceived and coordinated the project, and prepared the manuscript. All authors read and approved the final manuscript.
Competing financial interests
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart B, Wild CP. World Cancer Report. 2014. [Google Scholar]
- 2.Wang Y, Compton C, Rankin GO, Cutler SJ, Rojanasakul Y, Tu Y, Chen YC. 3-Hydroxyterphenyllin, a natural fungal metabolite, induces apoptosis and S phase arrest in human ovarian carcinoma cells. International Journal of Oncology. 2017;50(4):1392–1402. doi: 10.3892/ijo.2017.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nature Reviews Cancer. 2003;3(7):502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 4.Ravishankar D, Rajora AK, Greco F, Osborn HM. Flavonoids as prospective compounds for anti-cancer therapy. The International Journal of Biochemistry & Cell Biology. 2013;45(12):2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Blagosklonny MV. Prospective strategies to enforce selectively cell death in cancer cells. Oncogene. 2004;23(16):2967–2975. doi: 10.1038/sj.onc.1207520. [DOI] [PubMed] [Google Scholar]
- 6.Blagosklonny MV. Carcinogenesis, cancer therapy and chemoprevention. Cell Death and Differentiation. 2005;12(6):592–602. doi: 10.1038/sj.cdd.4401610. [DOI] [PubMed] [Google Scholar]
- 7.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 8.Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 9.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Chen S, Wei C, Gong H, Li L, Ye X. Chemical and Cellular Assays Combined with In Vitro Digestion to Determine the Antioxidant Activity of Flavonoids from Chinese Bayberry (Myrica rubra Sieb. et Zucc) Leaves. PLoS One. 2016;11(12):e0167484. doi: 10.1371/journal.pone.0167484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural and Food Chemistry. 2007;55(22):8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 12.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. The Journal of Nutritional Biochemistry. 2007;18(7):427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Han A, Chen E, Singh RK, Chichester CO, Moore RG, Singh AP, Vorsa N. The cranberry flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. International Journal of Oncology. 2015;46(5):1924–1934. doi: 10.3892/ijo.2015.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y, Rankin GO, Tu Y, Chen YC. Theaflavin-3, 3′-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. International Journal of Oncology. 2016;48(1):281–292. doi: 10.3892/ijo.2015.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Chen AY, Rojanasakul Y, Ye X, Rankin GO, Chen YC. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. Journal of Functional Foods. 2015;15:464–475. doi: 10.1016/j.jff.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang H-G, Reed JC, Nicholson DW, Alnemri ES. Ordering the cytochrome c–initiated caspase cascade: hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9–dependent manner. The Journal of Cell Biology. 1999;144(2):281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochimica et Biophysica Acta. 2007;1773(8):1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88(4):435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 20.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 21.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Letters. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 23.Scaffidi C, Volkland J, Blomberg I, Hoffmann I, Krammer PH, Peter ME. Phosphorylation of FADD/MORT1 at Serine 194 and Association with a 70-kDa Cell Cycle-Regulated Protein Kinase. The Journal of Immunology. 2000;164(3):1236–1242. doi: 10.4049/jimmunol.164.3.1236. [DOI] [PubMed] [Google Scholar]
- 24.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology. 2013;14(1):32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 26.Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proceedings of the National Academy of Sciences. 1998;95(25):14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. The Journal of Biological Chemistry. 2001;276(22):19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapuetic targets in cancer. Cell Proliferation. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zang W, Wang T, Wang Y, Li M, Xuan X, Ma Y, Du Y, Liu K, Dong Z, Zhao G. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumor Biology. 2014;35(12):12583–12592. doi: 10.1007/s13277-014-2579-4. [DOI] [PubMed] [Google Scholar]
- 30.Moon S-K, Cho G-O, Jung S-Y, Gal S-W, Kwon TK, Lee Y-C, Madamanchi NR, Kim C-H. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochemical and Biophysical Research Communications. 2003;301(4):1069–1078. doi: 10.1016/s0006-291x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 31.Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food and Chemical Toxicology. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 32.Hobbs CA, Swartz C, Maronpot R, Davis J, Recio L, Koyanagi M, Hayashi SM. Genotoxicity evaluation of the flavonoid, myricitrin, and its aglycone, myricetin. Food and Chemical Toxicology. 2015;83:283–292. doi: 10.1016/j.fct.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) The Journal of Nutrition. 2006;136(11):2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 34.Dorta DJ, Pigoso AA, Mingatto FE, Rodrigues T, Prado IM, Helena AF, Uyemura SA, Santos AC, Curti C. The interaction of flavonoids with mitochondria: effects on energetic processes. Chemico-biological Interactions. 2005;152(2–3):67–78. doi: 10.1016/j.cbi.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combiantions of phytochemicals. Amerian Journal of Clinical Nutrition. 2003;78(3):517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 36.Campbell JK, King JL, Harmston M, Lila MA, Erdman JW. Synergistic Effects of Flavonoids on Cell Proliferation in Hepa-1c1c7 and LNCaP Cancer Cell Lines. Journal of Food Science. 2006;71(4):S358–S363. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.