Abstract
Dendritic cells (DC) are essential in immunity due to their role in activating T cells thereby promoting anti-tumorigenic responses. Tumor cells, however, hijack the immune system causing T cell exhaustion and DC dysfunction. Tumor-induced T cell exhaustion may be reversed through immune checkpoint blockade (ICB), however, this treatment fails to show clinical benefit in many patients. While ICB serves to reverse T cell exhaustion, DCs are still necessary to prime, activate and direct the T cells to target tumor cells. In this review we will provide a brief overview of DC function, describe mechanisms by which DC functions are disrupted by the tumor microenvironment and highlight recent developments in DC cancer vaccines.
Keywords: Dendritic Cells, Tumor Microenvironment, Immune-suppression, Cancer, Immunotherapy, Vaccines
EMERGENCE OF DC BASED VACCINES
Dendritic Cells (DCs) are unique immune cells as they possess the capacity to initiate and regulate both innate and adaptive immunity. As such DCs play a pivotal role in tumor immune-surveillance. Under normal circumstances DCs are maintained in an immature and inactivated state until exposed to optimal stimuli such as inflammatory cytokines, microbial factors or endogenous alarmins (see glossary) [1]. Once activated, DCs rapidly mature and process antigens to be presented to T cells on their major histocompatibility (MHC) molecules (Box1). Although all DCs are professional antigen presenting cells (APCs) specific subsets of DCs posses specialized antigen-processing machinery and excel at activating either CD4+ or CD8+ T cells. Classical conventional DCs are broadly classified into two subsets, namely, CD1c+ DCs and CD141+ DCs. The CD1c+ DCs are migratory cells and mainly recognized for activating CD4+ T cells. CD141+ DCs, on the other hand, are primarily lymph node (LN) resident with enhanced ability to perform antigen cross-presentation (see glossary) and activation of CD8+ T cells [2–4] (Box 2). Interestingly, in addition to traditional modes of antigen acquisition (Box2), DCs also expand the breadth of immune response by “transferring” their antigens to LN resident DCs usingthe process of “cross-dressing” (see glossary) [5, 6] or by acquiring antigens from exosomes, infected APCs or apoptotic cells [7, 8]. In fact, mice lacking endogenous DCs are unable to mount a T cell response when injected with antigen loaded exogenous DCs, thus, underscoring the requirement for antigen transfer and cross-presentation by endogenous DCs for raising antigen specific T cell immunity [9].
Box1. T cell activation by DCs.
Activation of immature DCs is characterized by MHC up-regulation, increased expression of lymph node (LN) homing chemokine receptors such as CCR7, T cell co-stimulatory molecules like CD80 and CD86 and secretion of cytokines like IL12, IL15 and type I IFNs [144]. DCs may take up antigens from malignant cells through multiple mechanisms namely, phagocytosis, pinocytosis and receptor mediated endocytosis and migrate to the draining LNs [145]. Within the LNs, guided by CCR7 and LN stroma, both DCs and naïve T cells make their way into the paracortex region where DCs and T cells are crowded together [146]. The use of advanced imaging techniques has demonstrated that migratory DCs tend to disperse in the peripheral paracortex whereas the LN-resident DCs tend to accumulate in the central paracortex [147]. Complimenting this observation, migratory DCs bearing viral antigens were found to travel to the LN, activate CD4+ T cells in the paracortex and recruit active CD4+ T cells to promote cross-presentation by XCR1+ DCs to CD8+ T cells in the deep cortex [148]. DC-mediated T cell priming is a three-step process. In phase I, naïve T cells sample DCs in short bursts. In the phase II, T cells establish and maintain prolonged contact with DCs, initiating activation and generation of memory CD8+ T cells [149]. In the phase III, T cells resume transient DC contact and commence proliferation [150]. This entire process may take up to days accounting for the delay in appearance of adaptive immune response. However, a dedicated subset of LN-DCs within the lymphatic sinus epithelium (LS-DCs) is speculated to capture; LN draining antigens, vaccine components or microbial factors and activate T cells rapidly [151].
Box 2. DC cell subsets and classifications.
The CD1c+ DCs in humans (equivalent to CD11b+ DCs in mice) are migratory cells characterized by high expression of CD11c, CD172α (Sirpα) and TLRs1-8, and are the most common type of DCs outnumbering CD141+ DC by several folds in peripheral tissues. CD141+ DCs in humans (comparable to CD103+CD8+ DCs in mice), while present in peripheral tissues, are primarily resident in the lymph nodes (LNs), and are marked by expression of XCR1, Clec9a, CADM1 and TLRs 3 and 8. In addition, monocyte derived inflammatory DCs (iDCs), are characterized by CD1c, CD11c, CD11b, CD172α (Sirpα) and CD206. Recently single cell RNA sequencing on cells derived from healthy donor blood indicated six DCs subsets; DC1 (Clec9A+), DC2 (CD1c+_A), DC3 (CD1c+_B), DC4 (CD1c−CD141-CD11c+), DC5 (Axl+SIGLEC6+) and DC6 (pDCs). However, the exact physiological role of these subgroups in vivo remains to be determined [2]. The iDCs and CD1c+ DCs are exceptional at MHC-II antigen presentation and CD4+ T cell activation [4, 152, 153] where as the CD141+ DCs, are recognized for producing type I IFNs and cross-presenting antigens to CD8+ T cells [2–4].
Dendritic cell vaccines against tumor antigens remain an exciting arm of immunotherapy that aim at boosting patient’s own immune response against their tumors [10]. Moreover, cell-based therapies are particularly desirable as they pose low risk of toxicity and hold the potential of activating other immune modulators such as Natural Killer (NK) cells in addition to T cells in anti-cancer mechanisms. Preclinical studies in 1990s first introduced the concept of using autologous bone marrow derived DCs as a viable vaccination option [11]. These studies laid the bedrock for DC vaccines and argued in favor of using ex-vivo generated DCs over peptide vaccination for generating successful CD4+ and CD8+ T cell mediated tumor immunity [12–14]. However, it was not until protocols were established for generation of DCs from monocyte precursors, MoDCs (see glossary) in humans that the use of ex-vivo DCs pulsed with tumor associated antigens (TAA) could really be exploited for clinical intervention [15].
Sipuleucel-T (see glossary) was the first DC-based anti-tumor vaccine to be approved by the FDA for use against asymptomatic or minimally symptomatic castration resistant prostrate cancer. Overall the therapy did significantly reduce the risk of death and evidence of immunity against the immunizing protein was observed. However, correlation with an immune response against the PA2024 antigen (a fusion protein between PAP and GM-CSF; see glossary for Sipuluceul-T) was not significantly strong and when compared to the fusion protein, substantially less native PAP specific immunity could be established [16, 17]. Thus, a lack of clear clinical benefits, especially in late stage cancer, lead to a rapid drop in the prescription of Sipuleucel-T in this setting. Deeper analysis of tumor immunobiology indicates that the initial lack-luster performance of Sipuleucel-T can be attributed to the multitude of immune evasion mechanisms deployed by tumor cells in advanced disease. In this review we will describe the immunosuppressive mechanisms that actively dampen DC function in cancer. Furthermore we will provide insights on innovative improvements in DC targeted vaccine platforms and how combination of immunotherapies can be used to overturn tumor-induced immune-suppression and prompt induction lasting anti-tumor responses.
DENDRITIC CELL DYSFUNCTION IN CANCER
The tumor microenvironment (TME) is a specialized niche created by the confluence of tumor cells, supporting stroma and infiltrating immune cells. Within this niche tumor cells adapt their environment to support maximal tumor growth and impede immune detection [18]. Type-I interferon (IFN) signaling and antigen cross-presentation are both considered key functions of DCs in driving anti-tumor immunity in the LNs and in the TME [19]. Indeed, mice deficient for Batf3, a transcription factor intricately involved in differentiation of cross-presenting DCs, are unable to evade tumor establishment [20]. Likewise, Flt3L (see glossary) and PolyI:C driven expansion and activation of CD103+ DCs is critical for tumor regression in response to immunotherapy [21]. Moreover, DCs isolated from cancer patients often lack maturation markers and fail to activate T cells [22]. These reports are in line with a recent observation made by Lavin et al, reporting a decrease in CD141+ DCs accompanied by a low number of activated CD8+ T cells in the tumors of patients with early stage lung adenocarcinoma [23]. Hence, tumor-derived factors appear to actively suppress normal DC function and recruitment to the TME and additionally have a direct effect on the efficacy of DC vaccines [24]. Below we discuss some tumor-derived factors that can impact DC function in the TME.
Suppressive alarmins
Matrix metalloproteinase-2 (MMP-2) is a gelatinase intricately involved in digesting the extracellular matrix [25]. Increase in MMP-2 expression is found to correlate with progressive disease and poor prognosis in cancer patients [26]. Our group identified a novel role for MMP-2, as a “suppressive” alarmin that inhibits IL12 secretion and Th1 T cell differentiation by facilitating IFN-alpha Receptor 1 cleavage and Toll Like Receptor-2 (TLR-2) stimulation on DCs [27–30]. Another TLR-2 alarmin, Versican, has been reported to induce immunosuppression within DCs [31] and macrophages in the TME [32]. However, targeted disruption of Versican or MMP-2 specifically as a means of cancer intervention has not been tested in humans as yet.
Antigen masking
The TME has been known to alter tumor antigens so as to avoid immune detection. A prime example of this evasion mechanism is the post-translational hypo-glycosylation of Mucin-1 (MUC1) secreted by tumorigenic cells. DCs are unable to process and present the hypo-glycosylated MUC1 (hgMUC1) to T cells [33, 34]. Moreover, hgMUC-1 can act as a chemo-attractant for immature DCs and interfere with DC differentiation and Th1 skewing [35]. A vaccine (TG4010) employing vaccinia virus engineered to express MUC1 and IL2 elicited immunological responses in some patients with metastatic renal clear cell carcinoma but showed no over-all benefit compared to standard treatments [36]. However, immunization of healthy subjects (with a history of adenomatous polyps) with synthetic long peptides corresponding to immunogenic epitopes in hgMUC-1 along with PolyI:C adjuvant lead to a robust vaccine-specific immune response [37]. A clinical trial is underway to compare immune outcome in pancreatic cancer patients vaccinated with autologous DCs pulsed with tumor lysate or MUC1 peptide (NCT03114631). Thus it would seem that hgMUC1 targeting vaccines may have the potential for generating prophylactic and possibly therapeutic tumor-immunity particularly when administered with the right adjuvant and in combination with other therapies.
Immunosuppressive cytokines
Expression of suppressive cytokines such as VEGF (See glossary) [38], Transforming growth Factor beta (TGFβ) [39], Macrophage colony stimulating factor (MCSF) [40] and IL10 [41] in the TME has been directly correlated with advanced tumor stages. These cytokines exert their immunosuppressive influence by; inhibiting DC differentiation and maturation thus hindering Th1 T cell differentiation, promoting expression of IDO (see glossary) and PDL1 on DCs thus promoting T cell anergy [42, 43], prompting DCs to differentiate T cells towards a regulatory phenotype [44] and preventing DCs from exiting the TME [45]. Indeed, pharmaceutical interruption of VEGF [38, 46], MCSF [47], TGFβ [48] and IL10 (NCT02731742) by way of using small molecule inhibitors or blocking antibodies is being actively explored to treat cancers.
However, it should be noted that mice deficient for IL10 spontaneously develop colitis, characterized by excessive secretion of inflammatory cytokines like IFNγ, IL17 and IL12, thus emphasizing the role of IL10 in limiting pathological inflammation and promoting immune tolerance towards commensal microorganisms in the gut [49]. Furthermore, IL10 has also been reported to promote CD8+ T cell activation and proliferation in the TME. Indeed low dose administration of PEGylated IL10 (AM0010) in combination with anti-PD-1 antibody in cancer patients elicited immune activation, tumor shrinkage and good tolerability [50, 51]. Overall, since IL10 appears to play a dual role in tumor immunity, it is unclear at this time under what circumstances IL-10 targeted therapy will be efficacious, as this is likely to be context and dose dependent.
Metabolic Stress
The TME alters the metabolic pathways in tumor associated immune cells to facilitate tumor cell escape and immune detection. A study by Herber et al in 2009 first reported that high lipid accumulation could render CD11c+CD8+DCs and classical tumor associated DCs defective in antigen presentation and T cell activation in both murine models of cancers as well as in cancer patients [52]. Along the same lines of investigation, Cubillos-Ruiz and colleagues demonstrated that an inhospitable TME can cause DCs to accumulate endoplasmic reticulum (ER) stress in the form of reactive oxygen species and lipid peroxidation resulting in divergent activation of the unfolded protein response (UPR). Aberrant UPR activation in turn induces overt expression of transcription factor XBP-1 and consequent inhibition of antigen processing and presentation by DCs. Interestingly, treatment with antioxidants and inhibition of XBP-1 expression using nano-particles successfully relieved the ER stress on DCs restoring their potential to activate T cell and resulting in tumor regression [53, 54]. Taken together these findings indicate that agents that restore DC metabolic health should be developed and tested as a combination therapy cocktails along with immune checkpoint blockade (ICB).
Hypoxia
The unremitting hypoxia in the TME promotes excessive accumulation of immunosuppressive factors like VEGF, adenosine and IDO. Physiological role of adenosine is to reduce overt inflammation in case of tissue injury until homeostasis is restored. However, in a consistently hypoxic TME, adenosine continues to accumulate and interferes with the functions of all anti-tumor immune cells. For DCs in particular, adenosine induces immunosuppressive cytokines and enhances expression of IDO [55] promoting apoptosis, anergy and T cell tolerance [56]. Multiple clinical and pre-clinical studies are underway to investigate the clinical efficacy of adenosine signaling inhibitors [57] and IDO inhibitors in combination with other therapies [58].
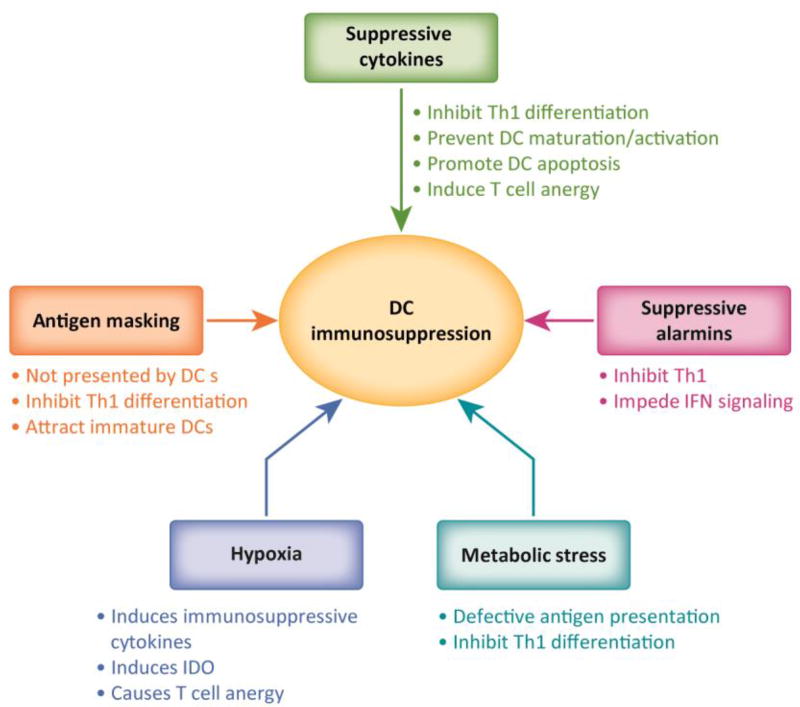
In summary multiple TME generated factors collectively contribute to suppress DC function by inhibiting DC recruitment, egress, activation, antigen presentation and Th1 differentiation (Figure 1). Thus DC vaccine platforms will have to accommodate interventions against these factors as a combination in order to maximize their therapeutic potential, achieve tumor regression and generate immunological memory.
Fig 1. DC dysfunction in the tumor microenvironment.
The tumor microenvironment is enriched in immune suppressive elements that serve to promote tumor growth by directly or indirectly inhibiting DC functions. These elements include suppressive cytokines, suppressive alarmins, hypoxia, metabolic stress and evasive mechanisms like antigen masking. Cumulatively these elements impair multiple aspects of DC functions and result in absence of adequate Th1 differentiation in the tumor microenvironment.
RE-EMERGENCE OF DENDRITIC CELL TARGETING VACCINES
Over the last few years renewed interest in harnessing DCs for therapeutic purposes has rekindled the interest in improving DC vaccine pipeline. These improvements take into the account the severe immune-suppression induced by the TME. As a result the current DC vaccine platforms have innovated means of improving DC maturation, antigen presentation, antigen loading, antigen selection and proliferation. Some of the areas under rapid optimization are listed below.
Alternatives to using MoDCs
Generating MoDCs is a time consuming process with significant logistical challenges. Moreover, the ex-vivo matured MoDCs do not correlate well with in-vivo cross-presenting CD141+ DCs [59, 60]. Use of natural or conventional (cDCs), that is DCs isolated directly from peripheral blood, is one way to avoid using MoDCs. cDC vaccines can be prepared quickly as the cells do not need to undergo exvivo differentiation. Following PBMC isolation, cDCs of the preferred subset maybe enriched using commercially available kits or by cell sorting. Thereafter the cells may be matured, activated, loaded with antigens ex-vivo and injected back into the patients. A study testing intra-nodal administration of isolated and purified CD1c+ DCs, activated and loaded with HLA-A2.1-restricted tumor derived peptides in patients with stage IV melanoma (NCT01690377) concluded that cDC vaccines were indeed safe and capable of generating tumor specific cytotoxic T lymphocyte (CTL) immunity that correlated with improved progression free survival (PFS) [61]. Similar results were reported using blood isolated plasmacytoid DC vaccinations by the same group [62]. However, no kits are commercially available to enrich for CD141+ DC subset and the low yield of pure DC subsets in peripheral blood remains a major roadblock in using blood isolated DCs for immunotherapy.
Another approach is to differentiate cross-presenting XCR1+Clec9A+ DCs (Box2) or Langerhans cells (LCs, a specialized subset of DCs) from CD34+ hematopoietic stem cells (HSC) isolated from cord blood [63]. CD34+ HSC-derived DCs have been tested in clinical trials in the past with promising success in generating tumor specific immunity [64–66]. Currently a clinical trial is underway (NCT01995708) to specifically test the efficacy of vaccinating with autologous LCs derived from CD34+ progenitors transfected with mRNA encoding TAA in patients with multiple myeloma [67]. Lentiviral transduction is also being used to generate CD1d expressing human pluripotent stem cells that could be differentiated into CD1d+ DCs with the capacity to activate not just CD8+ T cells but also iNKT cells [68]. Advances such as these make it conceivable to use stem cells to generate any DC subset in large numbers to be used for immunotherapy.
The ideal solution for replacing MoDCs as vaccines would be use of off-the-shelf DC-like immortalized cell lines. Access to such cell lines would allow for extensive testing, avoid issues of low cell number, enable easy handling and increase reproducibility. MUTZ-3 cell line, derived from human leukemic myeloid cells, comes close to mimicking a DC-like phenotype and function [69] but has not been tested clinically. A major problem with using a cell line is that these cells are allogeneic and may fail entirely as a vaccine or elicit major adverse events. Having said that, allogeneic DCs have been tested in clinical trials in patients with acute myeloid leukemia (AML) (NCT01373515) and allogeneic DCs generated from cord blood were reported to be immunogenic [70]. Thus, use of allogeneic DCs as vaccines is feasible but further testing is required to optimize this platform.
Optimizing DC maturation Stimuli
The maturation stimulus is a major factor that dictates the success or failure of DC vaccines. TLR agonists (TLR-4 agonist LPS, TLR-3 agonist PolyI:C, TLR-7/8 agonist resiquimod), cytokines (TNFα, IL1β, IL6, IFNα, IFNγ), co-stimulatory receptor ligands (CD40L), prostaglandin E2 (PGE2), have been used either alone or in various cocktail formulations to mature and program DCs. The desired maturation outcome is to induce high expression of MHC-I and MHC-II; co-stimulatory molecules CD40, CD80 and CD86; secretion of Th1 inflammatory cytokines like IL12 and IFNs and expression of chemokines such as CCR7 in order to polarize DCs towards Th1 activation [71–76]. More recently mRNA transfection-based delivery of a cocktail of co-stimulatory molecules (CD40L, CD70 and constitutively active TLR-4) called TriMix has emerged as a novel means of maturing DCs (TriMix-DCs) with enhanced T cell activation potential [77–79]. Vaccination with autologous TriMix-DCs co-electroporated with mRNA coding for melanoma-associated antigens was reported to be feasible, safe and has yielded antigen specific immune responses (NCT00074230, NCT01066390) [80, 81]. Combination therapy administering TriMix-DCs in combination with other therapeutics such as anti-CTLA-4 antibody are now being explored (NCT01302496).
Optimizing Antigen Selection
Introducing only select TAAs presents the inherent barrier of activating T cells against a narrow range of antigens in a MHC restricted fashion. Incubating DCs with whole tumor lysate or killed tumor cells allows for a wide range of TAAs to be presented on DCs. In addition, secretion of alarmins and cytokines from tumor cells serves as natural maturation stimuli [82, 83]. A study performed to explore the efficacy of vaccinating with MoDCs loaded with autologous tumor lysate and cytokine induced killer cells (CIKs) in patients with stage IV breast cancer reported a significant increase in PFS and overall survival (OS) over a 10-year follow-up [84]. However, another study reported no particular clinical benefit despite induction of immunological responses [85]. Oxidization of whole tumor cells using hypochlorous acid (HPO) has lately been suggested to improve DC vaccination outcome substantially. Although still awaiting clinical verification, oxidizing tumor lysates with HPO was demonstrated to improve antigen uptake, cross-presentation by DCs and tumor specific competent CD8+ T cell response ex-vivo [86]. Another approach to target DC in-vivo is using the GVAX vaccine platform that consists of irradiated tumor cells modified to secret GM-CSF. GM-CSF serves to attract and maintain DCs thus promoting tumor immunity [87, 88]. It was demonstrated that dual blockade of both PD1 and CTLA4 along with GVAX administration lead to remarkable tumor rejection in murine tumor models [89].
Fusion of tumor cells with DCs is yet another technique to allow DCs access to all tumor antigens through creation of a tumor-DC hybrid using chemicals such as polyethylene glycol. Tested in a small trial, irradiated tumor cells from resected gliobastoma multiforme (GBM) tumor were fused with autologous MoDCs. Subsequently the fused cells were transfected with PolyI:C and siRNA for IL10 and injected in the patients along with standard chemotherapy. The therapy was tolerated well and appeared to improve patient response to standard chemotherapy [90, 91]. In another clinical trial (NCT01096602) AML patients under remission following ICB therapy were vaccinated with autologous MoDCs fused with their own cancer cells. The results from the trial revealed a successful expansion of helper and CD8+T cells specific for TAAs and a remarkable lack of remission in all patients within a median of 57 months of follow-up [92].
Neo-antigens are generated either due to a tumor-related or spontaneous mutations and give rise to novel antigens that differentiate healthy “self” cells from cancerous cells [93, 94]. Several studies are exploring neo-antigens for immunotherapy (as examples, NCT02035956, NCT01970358, NCT02149225, NCT 02348320, NCT02316457) [95]. Although not all neo-antigens are immunogenic, the concept that mutational load correlates with ICB response has been supported in a number of independent studies and clinical trials [93, 96–98]. For example, high number of mutations accrued due to MSI (see glossary) in solid tumors has been positively correlated with an increased tumor immunogenicity and improved over all response to PD1 blockade [99, 100]. As a result the FDA has approved use of PD1 inhibitor for treatment in adult in patients with unrespectable and MSI-high and MMR-deficient solid tumors who do not respond to other treatments [101]. However, not all tumors with high mutation load respond to ICB and inversely patients with modest mutation frequency may respond robustly. Emerging data suggests that high mutation burden alone is not sufficient to ensure ICB response. Other factors such as CTLA4 and PDL1 expression on the tumor cells or immune cells as well tumor cell intrinsic signaling mechanisms like the Wnt/βCatenin pathway (reported to inhibit infiltration and activation of CD103+ DC and T cells in the TME) may also influence a patient’s ability to respond to ICBs [102–104] Perhaps the most important aspect of neo-antigen vaccine is the vast difference in neo-antigen repertoires between patients. Therefore, efforts are being made to generate vaccines that target patient-specific mutations. Indeed, vaccination with DCs loaded with personalized neo-antigenic epitopes has been shown to elicit clear evidence of priming and boosting of CD8+ T cells in cancer patients [105] (Box3). Our group has recently launched a clinical trial (NCT02721043) to test the safety and immunogenicity of a personalized vaccine in patients with advanced solid tumors. Under this platform tumors will be sequenced to identify unique mutation associated neo-antigens to generate personalized peptide vaccine. Thereafter the patients will be vaccinated with their personalized genomic peptide vaccine along with PolyIC:LC adjuvant and monitored for adverse events and anti-tumor immunity. It is hoped that the data will provide novel insights into how neo-antigens maybe predicted and used to design anti tumor vaccines.
Box3. Personalized neo-antigen Vaccines.
Carreno et al performed whole exome sequencing on tumors from three patients with Stage III resected cutaneous melanoma to identify somatic mutations and generated HLA-A*02:01 restricted neo-antigenic peptides. Autologous MoDCs loaded with selected neo-antigen peptides were injected into the patients. The results showed evidence of priming and augmentation of CD8+T cell response to multiple neo-antigens and provided a proof of concept for the future of personalized neo-antigen based therapies [105]. Following their remarkable success with first three patients, the authors have extended their study to include 17 patients (NCT00683670). Two recent clinical studies have explored the efficacy of vaccinating with predicted personalized HLA matched neo-antigens with favorable results in stage II and IV melanoma patients. One of these studies utilized an RNA based vaccine by engineering the ten highest-ranking neo-antigens into two RNAs and administering intranodally (NCT02035956) where as the second study explored the benefits of vaccinating with long peptides representing twenty neo-antigens along with TLR-3 agonist, PolyIC:LC as an adjuvant (NCT01970358). In both studies (conducted over a period of 12–26 months), the patients displayed an immune response to the vaccine and most patients registered significantly reduced recurrence of melanoma either due to vaccination alone or in combination with PD1 blockade [154, 155].
Optimizing Antigen Loading on DCs
The nature of T cell immunity generated by DCs depends heavily upon the mode of antigen uptake [106]. Antigens bound to antibodies against endocytosis receptors such as C type Lectin Receptors (like Clec9A [107] and DEC-205 [108]), Mannose Receptors and CD40 [109] are more likely to undergo cross-presentation. Indeed a vaccines comprising of a fusion protein of DEC-205 and TAA NY-ESO-1 (CDX-1401) is being tested to enhance NY-ESO-1 cross-presentation by DCs in-vivo (NCT02166905, NCT01834248). Vaccination with a combination of systemic Flt3L (CDX-301) and CDX-1401) along with PolyIC:LC (NCT02129075) has shown evidence of priming T cell immunity to the vaccine antigen and makes a case for using Flt3L for mobilizing DCs in-vivo to improve vaccine response [110]. Similarly, Mannose Receptor engaging vaccine, CDX1307 has yielded promising results [111]. CD40L, either in the form of recombinant protein, targeting antibodies, electroporated mRNA or in fusion with tumor lysate, is being investigated for facilitating tumor lysate uptake and cross-presentation by DCs in-vivo (NCT00053391) [112, 113]. Furthermore, anti-CD40 antibodies were demonstrated to substantially improve T cell immunity in non-human primates [114] and CD40 antibodies are now being explored in clinic as a tumor therapeutic (NCT02376699, NCT02482168, NCT01103635). Lentiviral transduction is being used to genetically over-express desired tumor antigens in DCs differentiated from hPSCs to avoid the additional step of antigen loading [115]. In addition, monocytes are being modulated by lentiviral transduction to express growth cytokines like GM-CSF and IL4 along with TAA thus giving rise to “smart DCs” with capacity to self-differentiate (not requiring exogenous addition of cytokines) and express TAAs on MHC molecules [116]. A particularly exciting new avenue for DC vaccine therapy is the advent of RNA based DC vaccine such as TriMix-DCs discussed above [78]. Under this module DCs are transfected with mRNA coding for selected TAA and cytokines so that each DC naturally expresses, process and presents self-antigens on its MHCs [117, 118].
Optimizing DC mobilization
Once a DC vaccine is administered, the extent of immunological response is strongly influenced by capacity of the antigen bearing DCs to traffic to the draining LNs and tumor sites. Mitchel et al demonstrated that pre-conditioning the tumor site with tetanus/diphtheria (Td) toxoid vaccine significantly improved the survival and antigen specific T cell responses in GBM patients receiving autologous MoDCs vaccines loaded with GBM antigen pp65. The authors surmised that as most people have received Td toxoid vaccines in their childhood, re-exposure would recall the CD4+ memory response thus activating immunity and aiding in DC migration to LNs [119]. GM-CSF is known for its function in DC recruitment and maturation. In addition, it also facilitates homing of cytotoxic T lymphocytes (CTLs) to the tumor site [87, 88]. GM-CSF secreting genetically modified tumor cells under the GVAX vaccine platform, (GVAX-Pancreas NCT00084383 or Melanoma-GVAX-NCT01435499) have yielded promising antigen specific protective immune responses specially when administered along with supporting drugs like cyclophosphamide or innate immune ligands (STINGVAX) [120–123]. Flt3L is also being actively tested for DC expansion both ex-vivo and in-vivo with encouraging results [21]. Future trials are set to explore the reach of rhFLT3L as a combination therapy for treating cancers (NCT01811992, NCT02839265). Incoming data from early clinical trials (NCT0219075 and NCT01976585) suggests promising future for combination rhFLT3L therapy in early stage tumors (unpublished, personal communications with Dr. Brody).
Combing DC vaccine with other cell-based therapies
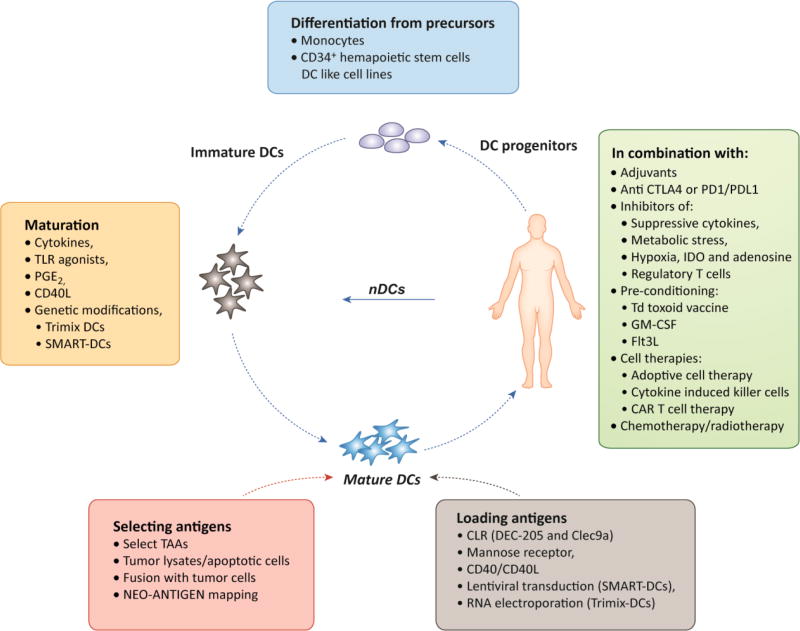
DC vaccination protocols are being designed to include other cell-based therapies such as cytokine induced killer cells (CIK) therapy and adoptive cell transfer therapy (ACT) therapy. CIK are autologous T cells, NK cells and NKT cells activated and expanded ex-vivo under the influence of anti-CD3 stimulation and cytokines. The DC-CIK combined vaccine has been shown to elicit far less adverse events as compared to standard chemotherapy and has shown promising potential in terms of improving over all survival and quality of life when tested in patients [124, 125]. Adoptive transfer of tumor specific T cells or T cells with engineered T cell receptor [126] in combination with TAA loaded DCs is yet another attractive immunotherapy option that has gained momentum due to its anti-tumor effects and usefulness even in advanced tumors [127]. A summary of multiple variables in DC vaccine platforms is depicted in Figure 2 (Key Figure).
Fig 2. Variables in DC vaccine platform.
A summary of current aspects of DC vaccine pipeline undergoing optimization. A) Differentiating immature DCs from precursors: patient derived monocytes and CD34+ Hematopoietic stem cells could be used to generate MoDCs or XCR1+Clec9a+ DCs or CD1d DCs or Langerhan cells, respectively. Or natural/conventional DCs could be directly isolated from patient blood. In future perhaps DC like cell lines could be optimized for generating a universal immature DC line. B) Maturing DCs: DCs would be matured using cytokines, TLR ligands, PGE2, CD40 ligand or a combination of above. Maturation signals can be provided as exogenous stimuli or transfected/transduced into the immature DCs as in the case of Trimix DCs and SMART DCs. C) Selecting and loading antigens: Whole tumor lysates or tumor cell fusion with DCs may be used. TAAs or neo-antigens (personalized or shared) may be selected. Antigens are loaded on DCs in form of short or long peptides through traditional DC priming, as DNA through lentiviral transduction or in RNA form through electroporation. Antigens could be introduced with CLRs, mannose receptors, CD40L or CD40 activating antibodies to improve cross-presentation. D) Combinations: To maximize DC vaccine clinical efficacy the vaccine should be administered with most suitable adjuvant in combination with, i) CTLA4 or PD1/PDL1 inhibitor, ii) inhibitors of immune suppression, iii) facilitators of DC mobilization like Td toxoid vaccine, GM-CSF or Flt3L, iv) other cell based therapies like CIK, ACT and CAR T cell therapy and v) in combination with standard chemotherapy and radiation therapy. MoDCs: Monocyte derived DCs; nDCs: natural DCs; PGE2: prostaglandin E2; TAAs: Tumor associated antigens; CLR: C type lectin receptors.
Concluding Remarks
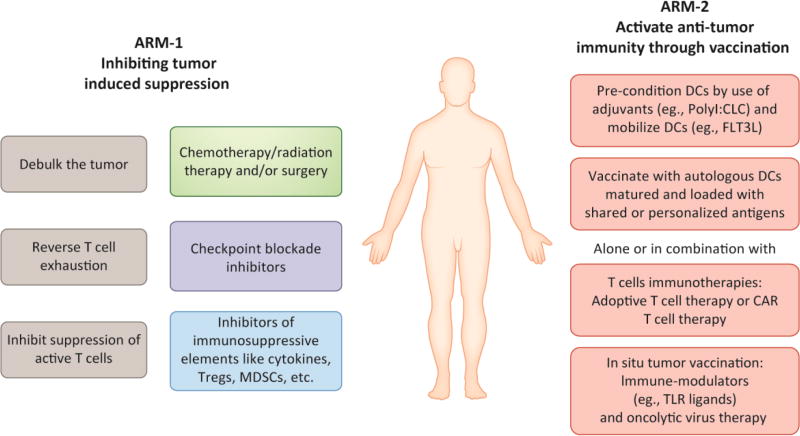
Many modern immunotherapy platforms have been developed to treat established cancers such as the ICBs and more recently, personalized CAR T cell therapy for treatment of B-cell acute lymphoblastic leukemia [128]. However, none of these therapies have been effective in all patients, indicating a need for combining different immunotherapy platforms. The future of cancer immunotherapy is likely to be built upon combination of two arms. One arm shall focus on inhibiting tumor-induced immunosuppression by using inhibitors of checkpoint molecules CTLA4 and PD/PDL1; blocking antibodies against immunosuppressive cytokines like VEGF, inhibitors of hypoxia, IDO inhibitors, adenosine antagonists and inhibitors of regulatory T cells like anti-CD25 antibodies and cyclophosphamide. The second arm shall be aimed at activating anti-tumor immunity through DC vaccines utilizing novel techniques of DC generation, activation, maturation, in-vivo targeting, antigen loading and personalized neo-antigen mapping (Figure 3). In line with this view many cancer immunotherapy platforms are currently testing DC vaccinations in combination with therapies such as ICB, chemotherapy, radiation therapy, IDO inhibitors, etc (Table1). Indeed, the Sipuleucel-T vaccine approach that had initially failed to garner clinical efficacy is now being re-assessed as a combination therapy, and also being tested in earlier stage disease (Table2). Another example favoring the two-arm approach or combination therapy is the promising early results of DC targeting Flt3L combination therapy (with TLR ligands and TAAs-fused with DEC-205) over Flt3L monotherapy (Table3).
Fig 3. Two-arm approach for treating cancer with anti-tumor DC targeted vaccines.
A “two-arm” approach enables DC vaccines to overcome tumor-induced immune-suppression and successfully induce anti-tumor immunity. Under “ARM-1” of this approach patients would first undergo tumor de-bulking by undergoing surgery and/or followed by radiation/chemotherapy. Thereafter, patients would be treated with checkpoint blockade inhibitors to reverse T cell exhaustion. At the same time other T cell inhibitory elements like regulatory T cells and myeloid derived suppressor cells (MDSCs) would need to be neutralized by use of drugs such as cyclophosphamide or anti-CD25 antibodies, or agents that modify the TME e.g. IDO inhibitors, adenosine antagonists etc. Under ARM-2, the patient would be pre-conditioned with adjuvants and with agents like Flt3L to mobilize DCs before being vaccinated with autologous DCs loaded with shared or personalized antigens. At this stage other immunotherapies such as adoptive T cell transfer, CAR T cell therapy, insitu tumor vaccination with TLR ligands and Oncolytic virus therapy could also be co-administered to boost the efficacy of DC vaccines.
Table1.
Current clinical trials testing DC vaccines in combination with other therapies for treating cancer
| Study Start |
Brief Title | Condition | NCT Identifier |
Intervention | Phase | Status as of December 2017 |
|---|---|---|---|---|---|---|
| 2004 | Vaccine Therapy With Either Neoadjuvant or Adjuvant Chemotherapy and Adjuvant Radiation Therapy in Treating Women With p53-Overexpressing Stage III Breast Cancer | Breast Cancer | NCT00082641 | Drug: Doxorubicin and cyclophosphamide, Drug: Paclitaxel Procedure: Surgery Drug: Radiotherapy Biological: Autologous dendritic cell-adenovirus p53 vaccine | 1 and 2 | Active, not Recruiting |
| 2004 | Vaccination of Patients With Renal Cell Cancer With Dendritic Cell Tumor Fusions and GM-CSF | Renal Cancer | NCT00458536 | Drug: GM-CSF Biological: Dendritic Cell Tumor Fusion Vaccine | 1 and 2 | Active, not Recruiting |
| 2006 | Lymphodepletion Plus Adoptive Cell Transfer With or Without Dendritic Cell Immunization in Patients With Metastatic Melanoma | Melanoma | NCT00338377 | Drug: Chemotherapy Biological: T-Cells infusion + high dose IL2 + MART-1 loaded DC vaccine. | 2 | Recruiting |
| 2008 | To Immunize Patients With Extensive Stage SCLC Combined With Chemo With or Without All Trans Retinoic Acid | Small Cell Lung Cancer | NCT00617409 | Drug: Paclitaxel Biological: Ad.p53-DC vaccines Drug: All -trans Retinoic Acid (ATRA) | 2 | Ongoing but not Recruiting |
| 2009 | Dendritic Cell (DC)-Based Vaccines Loaded With Allogeneic Prostate Cell Lines in Combination With Androgen Ablation in Patients With Prostate Cancer | Prostate Cancer | NCT00970203 | Biological: Androgen ablation (AA). Biological: DC1 vaccine (alpha-type-1-polarized dendritic cells loaded with apoptotic allogeneic tumor) | 2 | Recruiting |
| 2009 | Study of Gene Modified Immune Cells in Patients With Advanced Melanoma (F5) | Metastatic Melanoma | NCT00910650 | Drug: Chemotherapy Biologic: Autologous MART-1 TCR CTLs + MART-1 peptide pulsed dendritic cells | 2 | Recruiting |
| 2010 | Vaccine Therapy and 1-MT in Treating Patients With Metastatic Breast Cancer | Breast Cancer | NCT01042535 | Drug: 1-methyl-dtryptophan (IDO inhibitor) Biological: adenovirus-p53 transduced dendritic cell vaccine | 1 and 2 | Ongoing but not Recruiting |
| 2010 | Blockade of PD-1 in Conjunction With the Dendritic Cell/Myeloma Vaccines Following Stem Cell Transplantation | Multiple Myeloma | NCT01067287 | Drug: CT-011 (anti-PD1 antibody) Biological: Dendritic Cell Fusion Vaccine | 2 | Ongoing but not Recruiting |
| 2012 | Gene and Vaccine Therapy in Treating Patients With Advanced Malignancies | Malignant Neoplasm | NCT01697527 | Drug: Chemotherapy Biological: IL2+ NYESO-1 reactive TCR retroviral vector transduced autologous PBL B+NY-ESO-1 peptide pulsed dendritic cell | 2 | Recruiting |
| 2013 | Dendritic Cell Vaccines + Dasatinib for Metastatic Melanoma | Metastatic Melanoma | NCT01876212 | Drug: Dasatinib (tyrosine kinase inhibitor) Biological: autologous type-1 polarized Dendritic Cell pulsed with HLA-A2-presented tumor blood vessel antigen (TBVA)-derived peptides (DLK1310-318, EphA2883-891, HBB31-39, NRP1433-441, RGS55-13 and TEM1691-700) | 2 | Recruiting |
| 2014 | Dendritic Cell-based Immunotherapy for Advanced Solid Tumours of Children and Young Adults | Sarcoma, Central Nervous System Tumor | NCT02496520 | Procedure: Surgery Drug: Chemotherapy Radiation: Radiation therapy Biological: Autologous dendritic cells pulsed with tumor lysate | 1 and 2 | Recruiting |
| 2014 | Treatment of Patients With Progressive and/or Refractory Solid Malignancies | Progressive Solid Malignancies Refractory Solid Malignancies | NCT02224599 | Drug: Cyclophosphamide Biological:Tumor associated peptide antigens (TAPA) - pulsed DCs | 1 and 2 | Recruiting |
| 2014 | Phase III Study of DCVAC Added to Standard Chemotherapy for Men With Metastatic Castration Resistant Prostate Cancer (VIABLE) | Metastatic Castrate Resistant Prostate Cancer | NCT02111577 | Drug: Docetaxel Drug: Taxotere Biological: Dendritic Cells vaccine-DCVAC | 3 | Ongoing but not Recruiting |
| 2014 | αDC1 Vaccine + Chemokine Modulatory Regimen (CKM) as Adjuvant Treatment of Peritoneal Surface Malignancies | Peritoneal Surface Malignancies | NCT02151448 | Drug: Experimental chemokine modulatory regimen (Interferon Alfa-2b+Celecoxib+Rintatol imod Biological: Autologous type-1 polarized Dendritic Cell pulsed with tumor antigen | 1 and 2 | Recruiting |
| 2015 | MiHA-loaded PD-L-silenced DC Vaccination After Allogeneic SCT (PSCT19) | Hematological Malignancies | NCT02528682 | Biological: PD1/PDL1 silenced and Minor histocompatibility antigens (MiHA)-loaded DC Vaccination | 1 and 2 | Recruiting |
| 2015 | myDC/pDC in Stage III Melanoma Patients | Melanoma | NCT02574377 | Combination of peptide loaded myeloid and plasmacytoid DCs | 1 and 2 | Ongoing but not Recruiting |
| 2016 | Dendritic Cell/Myeloma Fusion Vaccine for Multiple Myeloma (BMT CTN 1401) | Multiple Myeloma | NCT02728102 | Procedure: Autologous Stem Cell Transplant Biological: DC-Myeloma fusion vaccine with GM-CSF | 2 | Recruiting |
| 2016 | Autologous Dendritic Cell-Vaccination in Mesothelioma (MESODEC) | Malignant Pleural Mesothelioma | NCT02649829 | Drug: Chemotherapy Biological: Dendritic cell vaccination | 1 and 2 | Recruiting |
| 2016 | Sequential Intranodal Immunotherapy (SIIT) Combined With Anti-PD1 (Pembrolizumab) in Follicular Lymphoma (Lymvac-2) | Follicular Lymphoma | NCT02677155 | Drug: Radiotherapy Biological: Rituximab(anri-CD20 antibody) Biological: Autologous dendritic cells Biological: GM-CSF Biological: Pembrolizumab | 2 | Recruiting |
| 2016 | Adjuvant Dendritic Cellimmunotherapy Plus Temozolomide in Glioblastoma Patients (ADDIT-GLIO) | Glioblastoma Multiforme of Brain | NCT02649582 | Drug: Chemotherapy Biological: Dendritic cell vaccine | 1 and 2 | Recruiting |
| 2017 | Avelumab Plus Autologous Dendritic Cell Vaccine in Pre-treated Metastatic Colorectal Cancer Patients (AVEVAC) | Colorectal Carcinoma | NCT03152565 | Biologic: Autologous DCs vaccine Biologic: Avelumab (anti-PD-L1 antibody) | 1 and 2 | Not Recruiting |
| 2017 | Autologous Dendritic Cells Pulsed With Tumor Lysate Antigen Vaccine and Nivolumab in Treating Patients With Recurrent Glioblastoma | Glioblastoma | NCT03014804 | Biologic: Autologous dendritic cells pulsed with tumor lysate antigen Vaccine Biological: Nivolumab (anti-PD-1 antibody) | 2 | Not open for Recruiting |
| 2017 | Vaccination With Dendritic Cells Pulsed With Autologous Tumor Homogenate in Combination With HD-IL2 and Immunomodulating Radiotherapy in Metastatic RCC (RENALVax-2) | Metastatic Renal cell carcinoma | NCT03226236 | Radiation: boost radiotherapy (XRT) Biological: Autologous DC vaccine Drug: High-Dose IL-2 | 2 | Recruiting |
| 2017 | Dendritic Cell Therapy After Cryosurgery in Combination With Pembrolizumab in Treating Patients With Stage III-IV Melanoma That Cannot Be Remove by Surgery | Cutaneous Melanoma | NCT03325101 | Procedure: Cryosurgery Biological: Pembrolizumab (anti-PD-1 antibody) Biologic: Therapeutic Autologous Dendritic Cells | 1 and 2 | Recruiting |
| 2017 | Dendritic Cell Therapy, Cryosurgery, and Pembrolizumab in Treating Patients With Non-Hodgkin Lymphoma | Non-Hodgkin lymphoma | NCT03035331 | Procedure: Cryosurgery Biological: Pembrolizumab (anti-PD-1 antibody) Biologic: Therapeutic Autologous Dendritic Cells Biologic: Prevnar | 1 and 2 | Recruiting |
Source: clinicaltrials.gov. List of current combination trials on clinicaltrials.gov under search terms "Dendritic cells", "Phase 2–3 " and "Interventions". Sipuleucel and Flt3L trials are not included in this list and can be found in Table2 and Table3, respectively. Trials that have not been updated in past one year were excluded.
Table2.
Sipuleucel-T combination therapies in clinical trials for Prostrate Cancer
| Study Start |
Brief Title | Condition | NCT Identifier | Intervention | Phase | Status as of December 2017 |
|---|---|---|---|---|---|---|
| 2012 | Phase II Study of Sipuleucel-T and Indoximod for Patients With Refractory Metastatic Prostate Cancer | Metastatic Prostrate Cancer | NCT01560923 | Sipuleucel-T + Indoximod (IDO pathway inhibitor) | 2 | Active, not recruiting |
| 2013 | A Study of Sipuleucel-T With Administration of Enzalutamide in Men With Metastatic Castrate-Resistant Prostate Cancer | Metastatic Prostrate Cancer | NCT01981122 | Sipuleucel-T + Enzalutamide (Synthetic non-steroidal antiandrogen) | 2 | Active, not recruiting |
| 2013 | Sipuleucel-T With or Without Radiation Therapy in Treating Patients With Hormone-Resistant Metastatic Prostate Cancer | Adenocarcinoma of the Prostate; Bone Metastases; Hormoneresistant Prostate Cancer; Recurrent Prostate Cancer; Soft Tissue Metastases Stage IV Prostate Cancer | NCT01807065 | Sipuleucel-T + External beam radiation therapy | 2 | Active, not recruiting |
| 2013 | Sipuleucel-T and Stereotactic Ablative Body Radiation (SABR) for Metastatic Castrate-resistant Prostate Cancer (mCRPC) | Metastatic castrationresistant Prostate Cancer | NCT01818986 | Sipuleucel-T + Stereotactic Ablative Body Radiation | 2 | Recruiting |
| 2013 | Provenge With or Without pTVG-HP DNA Booster Vaccine in Prostate Cancer | Prostrate Cancer | NCT01706458 | Sipuleucel-T + DNA Vaccine (Plasmid DNA encoding human prostatic acid phosphatase) | 2 | Active, not recruiting |
| 2013 | Radiation Therapy in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Receiving Sipuleucel-T | Hormone-Resistant Prostate Cancer; Metastatic Malignant Neoplasm in the Bone; Recurrent Prostate Carcinoma; Stage IV Prostate Cancer | NCT01833208 | Sipuleucel-T + Radiation Therapy | Recruiting | |
| 2014 | A Randomized Phase 2 Trial of Combining Sipuleucel-T With Immediate vs. Delayed CTLA-4 Blockade for Prostate Cancer | Prostrate Cancer | NCT01804465 | SipT Treatment + Ipilimumab (Anti-CTLA-4 antibody) | 2 | Recruiting |
| 2015 | Ph 2 Study of Sipuleucel-T W/ or W/O Radium-223 in Men With Asymptomatic or Minimally Symptomatic Bone-MCRPC | Prostrate Cancer | NCT02463799 | Sipuleucel-T + Radium-223 | 2 | Recruiting |
| 2015 | Men With Metastatic Castrate-Resistant Prostate Cancer Treated With Either Sipuleucel-T (Provenge®), Abiraterone Acetate (Zytiga®) or Enzalutamide (Xtandi®) Undergoing Cardiopulmonary EXercise Testing | Prostrate Cancer | NCT02353715 | Sipuleucel-T + Enzalutamide (Synthetic non-steroidal antiandrogen) or Abiraterone acet ate (Androgen synthesis inhibitor) | 1 | Recruiting |
| 2017 | Clinical Study of Atezolizumab (Anti-PD-L1) and Sipuleucel-T in Patients Who Have Asymptomatic or Minimally Symptomatic Metastatic Castrate Resistant Prostate Cancer | Metastatic Prostrate Cancer | NCT03024216 | Sipuleucel-T + Atezolizumab (Anti-PDL-1 antibody) | 1 | Recruiting |
Source: clinicaltrials.gov.
Table3.
Flt3L combination therapies in clinical trial for cancer
| Study Start |
Brief Title | Condition | NCT Identifier | Intervention | Phase | Status as
of December 2017 |
Results |
|---|---|---|---|---|---|---|---|
| 2013 | Combined Cytotoxic and Immune-Stimulatory Therapy for Glioma | Malignant Glioma; Glioblastom a Multiforme | NCT01811992 | Ad-hCMV-Flt3L + AdhCMV-TK | 1 | Recruiting | N/A |
| 2013 | In Situ Vaccine for Low-Grade Lymphoma: Combination of Intratumoral Flt3L and Poly-ICLC With Low-Dose Radiotherapy | Low-Grade Bcell Lympho ma | NCT01976585 | rhuFlt3L/CDX-301 + Poly-ICLC | 2 | Recruiting | N/A |
| 2014 | CDX-1401 and Poly-ICLC Vaccine Therapy With or Without CDX-301in Treating Patients With Stage IIB-IV Melanoma | Resected Melanoma | NCT02129075 | rhuFlt3L/CDX-301 + DEC-205/NY-ESO-1 + PolyIC:LC | 2 | Completed | Higher tumor specific immune responses observed in subjects who received FLT3L (unpublished data, personal communications with Dr. Nina Bhardwaj) |
| 2016 | FLT3 Ligand Immunotherapy and Stereotactic Radiotherapy for Advanced Non-small Cell Lung Cancer | Nonsmall Cell L ung Cancer | NCT02839265 | rhuFlt3L/CDX-301 + Stereotactic Bo dy Radiotherap y | 2 | Recruiting | N/A |
Source: clinicaltrials.gov. Ad-hCMV-Flt3L: Replication defective adenoviral vector expressing soluble Flt3L under transcriptional control of the CMV promoter. Ad-hCMV-TK: replication defective adenoviral vector expressing Herpes Simplex Virus thymidine kinase gene under transcriptional control of the CMV promoter. rhuFlt3L: Recombinant human Flt3L.
With constant influx of new information the above-mentioned parameters will need to be accordingly tweaked and adjusted (see Outstanding Questions) [129–131]. Indeed, a host of novel adjuvants are being rapidly developed and stand to further improve the DC vaccine platform [10]. Moreover, past few years have witnessed technological advancements making it possible for patients to receive treatment best suited to complement his/her immune system. Furthermore new technologies such as DC differentiation from stem cells and deep sequencing technology and CRISPR/Cas9 genome editing are predicted to change the landscape of personalized immunotherapy.
Highlights.
Dendritic cells are key mediators of tumor immunity due to their unique capacity for cross-presenting self-tumor antigens to CD8+ T cells.
Tumor-derived factors actively suppress normal DC function and have a direct effect on the efficacy of DC vaccines.
Effective anti-cancer vaccines will need to be administered as a combination of two main components; a) inhibitors of TME-induced immunosuppression and b) improved DC vaccines loaded with most immunogenic tumor associated antigens.
Open Questions.
Despite the progress in the field of therapeutic cancer vaccines, there is a pervasive need for preventive cancer vaccines. How to accurately detect and define pre-diagnostic cancer markers and how to target these markers for prophylactic cancer vaccines?
Manufacturing personalized neo-antigen vaccines is a time consuming and expensive process. Hence, it is worth considering, if the use of better adjuvants and improved DC targeting may improve the clinical response to shared antigen vaccines thereby making cancer vaccines more accessible.
As discussed in the review how to generate and/or target the most beneficial DC subset, ie, XCR1+ DCs in humans for DC vaccines is still an open question.
Furthermore, it is still unclear whether exogenous DC vaccines perform better than in situ DC vaccines.
In view of recent findings indicating that checkpoint molecules prevent effective T cell priming and activation [129, 130], it might be worth combining vaccination with ICB to enhance immune responsiveness [131]. Finally there needs to be a better understanding of which combinations of ARM1 and ARM2 treatments best complement a patients’ immune constitution to ensure tumor regression.
Acknowledgments
This Work was supported by the National Institutes of Health (RO1CA201189,R01CA180913 and R01AI081848), Cancer Research Institute and the Melanoma Research Alliance. NB is a member of the Parker Institute for Cancer Immunotherapy, which supported Icahn School of Medicine at Mount Sinai, NY, Cancer Immunotherapy Program.
Glossary
- Alarmins
Alarmins are endogenous ligands secreted by dying, necrotic or stressed tumor cells that can activate immune cells even in the absence of infections. Examples of activating alarmins include HMGB1, HSPs and ATP [1]. Several RNA species are now emerging as novel alarmins. Long non-coding RNA such as HSATII (up regulated in human epithelial cancers such as pancreatic cancer) was shown to act as an alarmin by inducing secretion of IL12 and TNFα in human monocyte derived DCs [132]. Similarly tumor secreted exosomes laden with non-coding Y RNA, hY4, were demonstrated to activate TLR-7 on monocytes and elicit inflammation [133]. Furthermore, pharmacological epigenetic modulators have been shown to enhance expression of “activating” alarmins such as double stranded (ds) RNA encoded by endogenous retroviruses [134, 135].
- Antigen Cross-presentation
Antigen Cross-presentation is a unique ability of DCs to internalize exogenous antigens into phagosomes and present them on the MHC-I molecules to activate CD8+ T cells [136, 137]. Interestingly, TLR-4 engagement has been shown to induce cross-presentation by facilitating lysosome clustering and delaying cargo degradation in the phagosomes [138, 139]. In addition, early TLR stimulation has also been reported to promote transfer of reserve MHC-I molecules from the endosomal recycling compartment to the phagosomes to support cross-presentation [140].
- Cross-Dressing
Cross-dressing is a cellular process through which receiver LN-DCs may directly take up pre-antigen loaded MHC-I complexes from donor migratory DCs and express this complex on their cell surface [141].
- Flt3L
Fms-related tyrosine kinase 3 ligand is a cytokine needed for DC mobilization and proliferation.
- IDO
Indoleamine 2,3-dioxygenase is an enzyme that induces depletion of tryptophan, a metabolite important for T cell activation.
- MoDCs
Monocyte derived dendritic cells are generated by isolating CD14+ monocytes from patient blood and differentiating these into immature DCs under the influence of IL4 and GM-CSF.
- MSI
Microsatellite instability is manifested as aberrant repetitions in DNA sequences that are introduced when the tumor cells harbor mutations in DNA mismatch repair (MMR) genes.
- Sipuleucel-T
Sipuleucel-T is a vaccine comprising of an enriched preparation of white cells containing a significant fraction of antigen presenting cells, including DCs. These are pulsed with prostatic acid phosphatase (PAP) fused with GM-CSF (PA2024) ex-vivo and then re-introduced in the patient intravenously to induce immunity [142, 143].
- VEGF
Vascular endothelial growth factor is a cytokine induced in response to stresses such as hypoxia, under the regulation of hypoxia inducible factor and aids in tissue healing and wound repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nie Y, et al. Alarmins and Antitumor Immunity. Clinical therapeutics. 2016;38:1042–1053. doi: 10.1016/j.clinthera.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villani AC, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356 doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granot T, et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017;46:504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segura E. Review of Mouse and Human Dendritic Cell Subsets. Methods in molecular biology. 2016;1423:3–15. doi: 10.1007/978-1-4939-3606-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Carbone FR, et al. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends in immunology. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Pitt JM, et al. Dendritic cell-derived exosomes for cancer therapy. The Journal of clinical investigation. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery C, et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature immunology. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 9.Yewdall AW, et al. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PloS one. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena M, Bhardwaj N. Turbocharging vaccines: emerging adjuvants for dendritic cell based therapeutic cancer vaccines. Current opinion in immunology. 2017;47:35–43. doi: 10.1016/j.coi.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porgador A, Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. The Journal of experimental medicine. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayordomo JI, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nature medicine. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 13.Zitvogel L, et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. The Journal of experimental medicine. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamand V, et al. Vaccination with tumor-antigen-pulsed dendritic cells induces in vivo resistance to a B cell lymphoma. Advances in experimental medicine and biology. 1993;329:611–616. doi: 10.1007/978-1-4615-2930-9_102. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill D, Bhardwaj N. Generation of autologous peptide- and protein-pulsed dendritic cells for patient-specific immunotherapy. Methods in molecular medicine. 2005;109:97–112. [PubMed] [Google Scholar]
- 16.Huber ML, et al. Interdisciplinary critique of sipuleucel-T as immunotherapy in castrationresistant prostate cancer. Journal of the National Cancer Institute. 2012;104:273–279. doi: 10.1093/jnci/djr514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantoff PW, et al. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. Journal of the National Cancer Institute. 2012;104:1107–1109. doi: 10.1093/jnci/djs279. author reply 1109–1112. [DOI] [PubMed] [Google Scholar]
- 18.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrales L, et al. Innate immune signaling and regulation in cancer immunotherapy. Cell research. 2017;27:96–108. doi: 10.1038/cr.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmon H, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verronese E, et al. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. Oncoimmunology. 2016;5:e1100791. doi: 10.1080/2162402X.2015.1100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavin Y, et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell. 2017;169:750–765 e717. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Current opinion in immunology. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khokha R, et al. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nature reviews. Immunology. 2013;13:649–665. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 26.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. The Journal of pathology. 2015;237:273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Gugel E, et al. Modulation of innate immunity in the tumor microenvironment. Cancer immunology, immunotherapy : CII. 2016;65:1261–1268. doi: 10.1007/s00262-016-1859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godefroy E, et al. Activation of toll-like receptor-2 by endogenous matrix metalloproteinase-2 modulates dendritic-cell-mediated inflammatory responses. Cell reports. 2014;9:1856–1870. doi: 10.1016/j.celrep.2014.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godefroy E, et al. Matrix metalloproteinase-2 conditions human dendritic cells to prime inflammatory T(H)2 cells via an IL-12- and OX40L-dependent pathway. Cancer cell. 2011;19:333–346. doi: 10.1016/j.ccr.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godefroy E, Bhardwaj N. Dysregulation of anti-tumor immunity by the matrix metalloproteinase-2. Oncoimmunology. 2012;1:109–111. doi: 10.4161/onci.1.1.17994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M, et al. Toll-like Receptor 2 Activation Promotes Tumor Dendritic Cell Dysfunction by Regulating IL-6 and IL-10 Receptor Signaling. Cell reports. 2015;13:2851–2864. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 32.Hope C, et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128:680–685. doi: 10.1182/blood-2016-03-705780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiltbold EM, et al. Naturally processed class II epitope from the tumor antigen MUC1 primes human CD4+ T cells. Cancer research. 1998;58:5066–5070. [PubMed] [Google Scholar]
- 34.Hiltbold EM, et al. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. Journal of immunology. 2000;165:3730–3741. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- 35.Carlos CA, et al. Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. Journal of immunology. 2005;175:1628–1635. doi: 10.4049/jimmunol.175.3.1628. [DOI] [PubMed] [Google Scholar]
- 36.Oudard S, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer immunology, immunotherapy : CII. 2011;60:261–271. doi: 10.1007/s00262-010-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohmueller JJ, et al. Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and immunotherapeutic potential. Scientific reports. 2016;6:31740. doi: 10.1038/srep31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nature reviews. Drug discovery. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Ten Dijke P. Immunoregulation by members of the TGFbeta superfamily. Nature reviews. Immunology. 2016;16:723–740. doi: 10.1038/nri.2016.112. [DOI] [PubMed] [Google Scholar]
- 40.Chockalingam S, Ghosh SS. Macrophage colony-stimulating factor and cancer: a review. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:10635–10644. doi: 10.1007/s13277-014-2627-0. [DOI] [PubMed] [Google Scholar]
- 41.Mannino MH, et al. The paradoxical role of IL-10 in immunity and cancer. Cancer letters. 2015;367:103–107. doi: 10.1016/j.canlet.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Marti LC, et al. Vascular endothelial growth factor-A enhances indoleamine 2,3-dioxygenase expression by dendritic cells and subsequently impacts lymphocyte proliferation. Memorias do Instituto Oswaldo Cruz. 2014;109:70–79. doi: 10.1590/0074-0276130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song S, et al. Dendritic cells with an increased PD-L1 by TGF-beta induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. International immunopharmacology. 2014;20:117–123. doi: 10.1016/j.intimp.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Ghiringhelli F, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. The Journal of experimental medicine. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber F, et al. Transforming growth factor-beta1 immobilises dendritic cells within skin tumours and facilitates tumour escape from the immune system. Cancer immunology, immunotherapy : CII. 2005;54:898–906. doi: 10.1007/s00262-004-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallin JJ, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nature communications. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ries CH, et al. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Current opinion in pharmacology. 2015;23:45–51. doi: 10.1016/j.coph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Herbertz S, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug design, development and therapy. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goto Y, et al. IL-10-producing CD4(+) T cells negatively regulate fucosylation of epithelial cells in the gut. Scientific reports. 2015;5:15918. doi: 10.1038/srep15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naing A, et al. Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016 doi: 10.1200/JCO.2016.68.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durable Responses Achieved with AM0010. Cancer discovery. 2016;6:OF4. doi: 10.1158/2159-8290.CD-NB2016-125. [DOI] [PubMed] [Google Scholar]
- 52.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nature medicine. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cubillos-Ruiz JR, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cubillos-Ruiz JR, Glimcher LH. Targeting abnormal ER stress responses in tumors: A new approach to cancer immunotherapy. Oncoimmunology. 2016;5:e1098802. doi: 10.1080/2162402X.2015.1098802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young A, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer discovery. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 56.van Baren N, Van den Eynde BJ. Tumoral Immune Resistance Mediated by Enzymes That Degrade Tryptophan. Cancer immunology research. 2015;3:978–985. doi: 10.1158/2326-6066.CIR-15-0095. [DOI] [PubMed] [Google Scholar]
- 57.Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert review of anticancer therapy. 2017;17:527–535. doi: 10.1080/14737140.2017.1316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mondanelli G, et al. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Current opinion in pharmacology. 2017;35:30–39. doi: 10.1016/j.coph.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Lundberg K, et al. Transcriptional profiling of human dendritic cell populations and models--unique profiles of in vitro dendritic cells and implications on functionality and applicability. PloS one. 2013;8:e52875. doi: 10.1371/journal.pone.0052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collin M, et al. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreibelt G, et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22:2155–2166. doi: 10.1158/1078-0432.CCR-15-2205. [DOI] [PubMed] [Google Scholar]
- 62.Tel J, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer research. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 63.Balan S, Dalod M. In Vitro Generation of Human XCR1(+) Dendritic Cells from CD34(+) Hematopoietic Progenitors. Methods in molecular biology. 2016;1423:19–37. doi: 10.1007/978-1-4939-3606-9_2. [DOI] [PubMed] [Google Scholar]
- 64.Di Nicola M, et al. Boosting T cell-mediated immunity to tyrosinase by vaccinia virus-transduced, CD34(+)-derived dendritic cell vaccination: a phase I trial in metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:5381–5390. doi: 10.1158/1078-0432.CCR-04-0602. [DOI] [PubMed] [Google Scholar]
- 65.Mackensen A, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. International journal of cancer. 2000;86:385–392. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 66.Banchereau J, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer research. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 67.Ratzinger G, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. Journal of immunology. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 68.Zeng J, Wang S. Human dendritic cells derived from embryonic stem cells stably modified with CD1d efficiently stimulate antitumor invariant natural killer T cell response. Stem cells translational medicine. 2014;3:69–80. doi: 10.5966/sctm.2013-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santegoets SJ, et al. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. Journal of leukocyte biology. 2008;84:1364–1373. doi: 10.1189/jlb.0208092. [DOI] [PubMed] [Google Scholar]
- 70.Kumar J, et al. Umbilical cord blood-derived CD11c(+) dendritic cells could serve as an alternative allogeneic source of dendritic cells for cancer immunotherapy. Stem cell research & therapy. 2015;6:184. doi: 10.1186/s13287-015-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee AW, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 72.Scandella E, et al. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–1601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 73.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Seminars in immunology. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Napolitani G, et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature immunology. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boullart AC, et al. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer immunology, immunotherapy : CII. 2008;57:1589–1597. doi: 10.1007/s00262-008-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okada H, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benteyn D, et al. Single-step antigen loading and maturation of dendritic cells through mRNA electroporation of a tumor-associated antigen and a TriMix of costimulatory molecules. Methods in molecular biology. 2014;1139:3–15. doi: 10.1007/978-1-4939-0345-0_1. [DOI] [PubMed] [Google Scholar]
- 78.Van Lint S, et al. Optimized dendritic cell-based immunotherapy for melanoma: the TriMix-formula. Cancer immunology, immunotherapy : CII. 2014;63:959–967. doi: 10.1007/s00262-014-1558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pen JJ, et al. Modulation of regulatory T cell function by monocyte-derived dendritic cells matured through electroporation with mRNA encoding CD40 ligand, constitutively active TLR4, and CD70. Journal of immunology. 2013;191:1976–1983. doi: 10.4049/jimmunol.1201008. [DOI] [PubMed] [Google Scholar]
- 80.Wilgenhof S, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Annals of oncology : official journal of the European Society for Medical Oncology. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 81.Van Nuffel AM, et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer immunology, immunotherapy : CII. 2012;61:1033–1043. doi: 10.1007/s00262-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzalez FE, et al. Melanoma cell lysate induces CCR7 expression and in vivo migration to draining lymph nodes of therapeutic human dendritic cells. Immunology. 2014;142:396–405. doi: 10.1111/imm.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vandenberk L, et al. Exploiting the Immunogenic Potential of Cancer Cells for Improved Dendritic Cell Vaccines. Frontiers in immunology. 2015;6:663. doi: 10.3389/fimmu.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin M, et al. 2003–2013, a valuable study: Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in stage IV breast cancer. Immunology letters. 2017;183:37–43. doi: 10.1016/j.imlet.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 85.Miwa S, et al. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer. 2017 doi: 10.1002/cncr.30606. [DOI] [PubMed] [Google Scholar]
- 86.Chiang CL, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clancy-Thompson E, et al. Peptide vaccination in Montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells. Cancer immunology research. 2013;1:332–339. doi: 10.1158/2326-6066.CIR-13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee SJ, et al. Local administration of granulocyte macrophage colony-stimulating factor induces local accumulation of dendritic cells and antigen-specific CD8+ T cells and enhances dendritic cell cross-presentation. Vaccine. 2015;33:1549–1555. doi: 10.1016/j.vaccine.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duraiswamy J, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer research. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akasaki Y, et al. Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer immunology, immunotherapy : CII. 2016;65:1499–1509. doi: 10.1007/s00262-016-1905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koido S. Dendritic-Tumor Fusion Cell-Based Cancer Vaccines. International journal of molecular sciences. 2016;17 doi: 10.3390/ijms17060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenblatt J, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Science translational medicine. 2016;8:368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yarchoan M, et al. Targeting neoantigens to augment antitumour immunity. Nature reviews. Cancer. 2017;17:209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finnigan JP, Jr, et al. Mutation-Derived Tumor Antigens: Novel Targets in Cancer Immunotherapy. Oncology. 2015;29 [PubMed] [Google Scholar]
- 95.Bobisse S, et al. Neoantigen-based cancer immunotherapy. Annals of translational medicine. 2016;4:262. doi: 10.21037/atm.2016.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Passardi A, et al. Immune Checkpoints as a Target for Colorectal Cancer Treatment. International journal of molecular sciences. 2017;18 doi: 10.3390/ijms18061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cortes-Ciriano I, et al. A molecular portrait of microsatellite instability across multiple cancers. Nature communications. 2017;8:15180. doi: 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang L, et al. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Applied immunohistochemistry & molecular morphology : AIMM. 2017 doi: 10.1097/PAI.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 102.Van Allen EM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das M, et al. Tim-3 and its role in regulating anti-tumor immunity. Immunological reviews. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spranger S, et al. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 105.Carreno BM, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moeller I, et al. Uptake routes of tumor-antigen MAGE-A3 by dendritic cells determine priming of naive T-cell subtypes. Cancer immunology, immunotherapy : CII. 2012;61:2079–2090. doi: 10.1007/s00262-012-1272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schreibelt G, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–2292262. doi: 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- 108.Saluja SS, et al. Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen. International journal of nanomedicine. 2014;9:5231–5246. doi: 10.2147/IJN.S66639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhardwaj N, et al. A Phase II Randomized Study of CDX-1401, a Dendritic Cell Targeting NY-ESO-1 Vaccine, in Patients with Malignant Melanoma Pre-Treated with Recombinant CDX-301, a Recombinant Human Flt3 Ligand. Journal of Clinical Oncology. 2016;34:9589–9589. [Google Scholar]
- 111.Macri C, et al. Targeting dendritic cells: a promising strategy to improve vaccine effectiveness. Clinical & translational immunology. 2016;5:e66. doi: 10.1038/cti.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin W, et al. Dendritic cell targeting vaccine for HPV-associated cancer. Cancer cell & microenvironment. 2016;3 [PMC free article] [PubMed] [Google Scholar]
- 113.Nimanong S, et al. CD40 Signaling Drives Potent Cellular Immune Responses in Heterologous Cancer Vaccinations. Cancer research. 2017;77:1918–1926. doi: 10.1158/0008-5472.CAN-16-2089. [DOI] [PubMed] [Google Scholar]
- 114.Thompson EA, et al. Human Anti-CD40 Antibody and Poly IC:LC Adjuvant Combination Induces Potent T Cell Responses in the Lung of Nonhuman Primates. Journal of immunology. 2015;195:1015–1024. doi: 10.4049/jimmunol.1500078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeng J, et al. Antigenically Modified Human Pluripotent Stem Cells Generate Antigen-Presenting Dendritic Cells. Scientific reports. 2015;5:15262. doi: 10.1038/srep15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sundarasetty BS, et al. Lentivirus-induced 'Smart' dendritic cells: Pharmacodynamics and GMP-compliant production for immunotherapy against TRP2-positive melanoma. Gene therapy. 2015;22:707–720. doi: 10.1038/gt.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunological reviews. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 118.Hoffmann JM, et al. Next-generation dendritic cell-based vaccines for leukemia patients. Immunotherapy. 2017;9:173–181. doi: 10.2217/imt-2016-0116. [DOI] [PubMed] [Google Scholar]
- 119.Mitchell DA, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lipson EJ, et al. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. Journal of translational medicine. 2015;13:214. doi: 10.1186/s12967-015-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laheru D, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lutz E, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Annals of surgery. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fu J, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Science translational medicine. 2015;7:283ra252. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mosinska P, et al. Dual Functional Capability of Dendritic Cells - Cytokine-Induced Killer Cells in Improving Side Effects of Colorectal Cancer Therapy. Frontiers in pharmacology. 2017;8:126. doi: 10.3389/fphar.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang L, et al. Dendritic cell vaccine and cytokine-induced killer cell therapy for the treatment of advanced non-small cell lung cancer. Oncology letters. 2016;11:2605–2610. doi: 10.3892/ol.2016.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chodon T, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2457–2465. doi: 10.1158/1078-0432.CCR-13-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poschke I, et al. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer immunology, immunotherapy : CII. 2014;63:1061–1071. doi: 10.1007/s00262-014-1575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luskin MR, DeAngelo DJ. Chimeric Antigen Receptor Therapy in Acute Lymphoblastic Leukemia Clinical Practice. Current hematologic malignancy reports. 2017 doi: 10.1007/s11899-017-0394-x. [DOI] [PubMed] [Google Scholar]
- 129.Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei SC, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 2017;170:1120–1133 e1117. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tanne A, et al. Distinguishing the immunostimulatory properties of noncoding RNAs expressed in cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15154–15159. doi: 10.1073/pnas.1517584112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haderk F, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Science immunology. 2017;2 doi: 10.1126/sciimmunol.aah5509. [DOI] [PubMed] [Google Scholar]
- 134.Roulois D, et al. Pharmacological DNA demethylation: Implications for cancer immunotherapy. Oncoimmunology. 2016;5:e1090077. doi: 10.1080/2162402X.2015.1090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiappinelli KB, et al. Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer research. 2016;76:1683–1689. doi: 10.1158/0008-5472.CAN-15-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cho HJ, Bhardwaj N. Against the self: dendritic cells versus cancer. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2003;111:805–817. doi: 10.1034/j.1600-0463.2003.11107812.x. [DOI] [PubMed] [Google Scholar]
- 137.Cruz FM, et al. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annual review of immunology. 2017 doi: 10.1146/annurev-immunol-041015-055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alloatti A, et al. Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity. 2015;43:1087–1100. doi: 10.1016/j.immuni.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 139.Alloatti A, et al. Dendritic cell maturation and cross-presentation: timing matters! Immunological reviews. 2016;272:97–108. doi: 10.1111/imr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nair-Gupta P, et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 143.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 144.Sabado RL, et al. Dendritic Cell Vaccines. Methods in molecular biology. 2016;1403:763–777. doi: 10.1007/978-1-4939-3387-7_44. [DOI] [PubMed] [Google Scholar]
- 145.Alvarez D, et al. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Groom JR. Moving to the suburbs: T-cell positioning within lymph nodes during activation and memory. Immunology and cell biology. 2015;93:330–336. doi: 10.1038/icb.2015.29. [DOI] [PubMed] [Google Scholar]
- 147.Gerner MY, et al. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hor JL, et al. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4+ and CD8+ T Cell Activation to Localized Viral Infection. Immunity. 2015;43:554–565. doi: 10.1016/j.immuni.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 149.Henrickson SE, et al. Antigen availability determines CD8(+) T cell-dendritic cell interaction kinetics and memory fate decisions. Immunity. 2013;39:496–507. doi: 10.1016/j.immuni.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mempel TR, et al. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 151.Gerner MY, et al. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 152.Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends in immunology. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 153.Guilliams M, et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity. 2016;45:669–684. doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 155.Ott PA, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]