Abstract
We report on 2 different types of left pulmonary artery sling (LPAS), types IA and IIB, diagnosed by fetal magnetic resonance imaging (MRI). We suggest that fetal MRI is an effective tool for accurately diagnosing LPAS and helping guide its perinatal management. Fetal MRI is relatively unaffected by the conditions that limit visualization by echocardiography. When prenatal ultrasound detects either a possible anomalous origin of the left pulmonary artery or a tracheobronchial anomaly, fetal MRI may provide additional information to confirm the LPAS diagnosis and classify its type. To our knowledge, these are the first reports of prenatally diagnosed LPAS by fetal MRI.
Keywords: Prenatal diagnosis, Left pulmonary artery sling, MRI, Bridging bronchus
Introduction
Left pulmonary artery sling (LPAS) is a rare vascular anomaly causing respiratory distress in which the left pulmonary artery (LPA) arises from the right pulmonary artery (RPA) and passes posteriorly, above the right main bronchus and between the trachea and esophagus, to the hilum of the left lung. The first reported case with definitive prenatal diagnosis was by echocardiography at 32 weeks' gestation in 2011 [1].
LPAS have been subdivided into 4 types: 2 main types based on the thoracic level of the carina, and 2 subtypes based on the presence or absence of an eparterial or tracheal right upper lobe bronchus [2].
We report 2 types of LPAS and discuss the value of fetal magnetic resonance imaging (MRI) to accurately diagnose LPAS as a complement to fetal ultrasound, and to help improve perinatal management. A hospital ethics committee approved the study. Written informed consent was obtained in both cases.
Case reports
Case 1: This case involved a 28-year-old woman whose routine ultrasound examination at 21 weeks' gestation revealed an abnormal right-sided heart location. Fetal MRI images were obtained on a non–contrast-enhanced 1.5T unit (PHILIPS Medical Systems, Amsterdam, Netherlands) using a 16-element phased-array body coil. To minimize claustrophobia, the supine, feet-first position was used. Balanced fast field echo (BFFE) and single-shot fast spin echo (SSFSE) sequences were used. The BFFE parameters were repetition time (TR), shortest; echo time (TE), shortest; field of view (FOV) 280 × 280 mm-350 × 350 mm; voxel size right-left (RL), 1.4; voxel size anterior-posterior (AP), 1.36; matrix, 216 × 218; thickness, 5.0-7.0 mm; gap, −5 to −3 mm; flap, 90°; number of signal average times (NSA), 3. The SSFSE parameters were TR, 12,000-15,000 ms; TE, 120 ms; FOV 280 × 280 mm-350 × 350 mm; voxel size RL, 1.0; voxel size AP, 1.37; matrix, 216 × 218; thickness, 5.0-7.0 mm; gap, −1 to 0 mm; flap, 80°. NSA, 1. Fetal MRI demonstrated that the RPA originated from the main pulmonary artery and the LPA originated from the RPA. The LPA then turned leftward posterior to the trachea to reach the left lung hilum. The distal RPA was not clearly displayed, and the right main bronchus was stenotic (Fig. 1). Also, the right lung was relatively small and the mediastinum was shifted toward the right. On the coronal view, the carina was obviously lower than the aortic arch, which was located at approximately the sixth or seventh thoracic vertebra (Fig. 2). Based on these findings, the diagnoses of LPAS, RPA stenosis, right main bronchus and right lung dysplasia, and possible bridging bronchus were made.
Fig. 1.
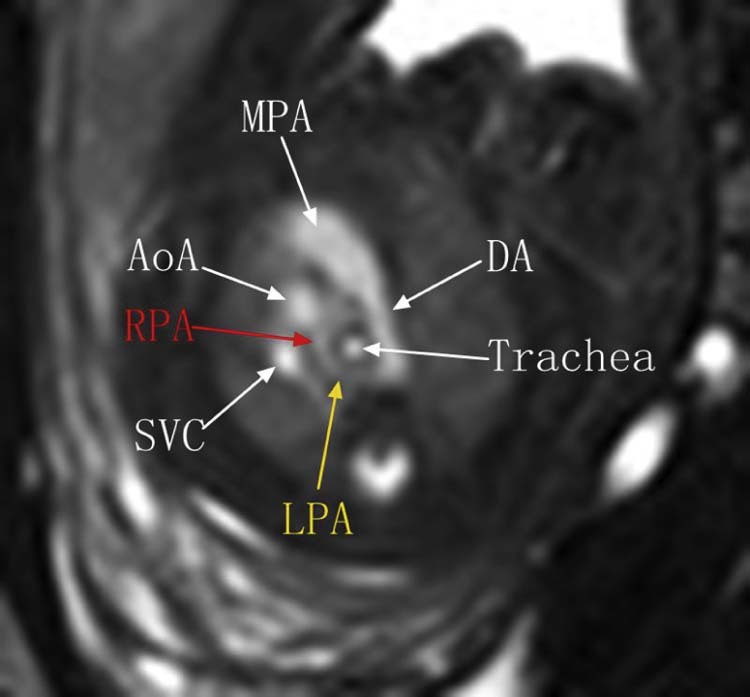
Fetal MRI BFFE sequence shows the left pulmonary artery (LPA) arising distally from the right pulmonary artery (RPA), then turning sharply around the right side of the trachea. AoA, arch of aorta; BFFE, balanced fast field echo; DA, ductus arteriosus; MRI, magnetic resonance imaging; SVC, superior vena cava.
Fig. 2.
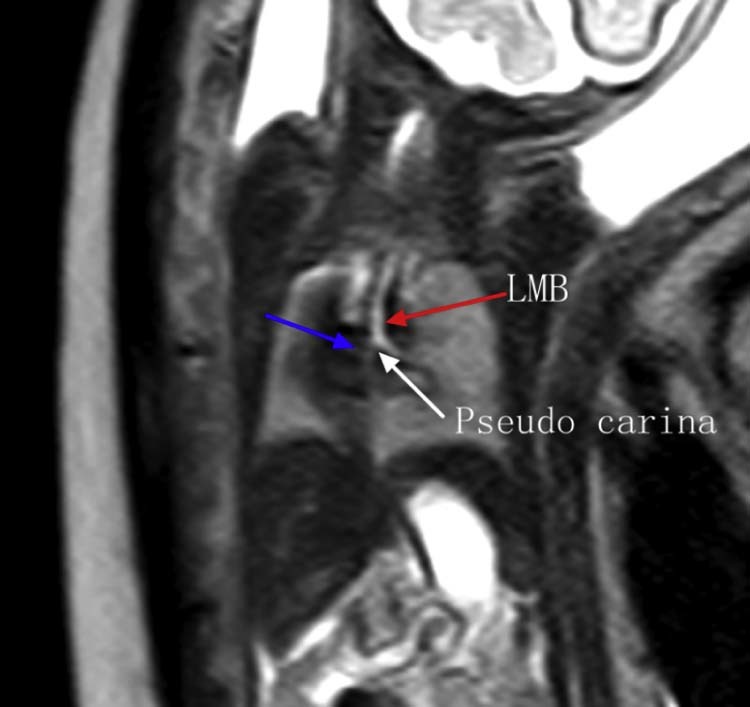
Fetal MRI SSFSE sequence shows the stenotic right main bronchus (blue arrow) comes from the sloping left main bronchus (red arrow), the position of pseudo carina is low (white arrow).which form a bridging bronchus in type II. LMB, left main bronchus; MRI, magnetic resonance imaging; SSFSE, single-shot fast spin echo.
Premature rupture of membranes occurred at 24 weeks, and the baby did not survive after birth. The parents requested an autopsy. The anatomic results were consistent with the prenatal diagnosis of LPAS type IIB, with presence of a bridging bronchus and absence of the right upper lobe bronchus. Furthermore, pseudo-carina position demonstrated approximately at T6, and the right lung was unilobar (Fig. 3, Fig. 4).
Fig. 3.
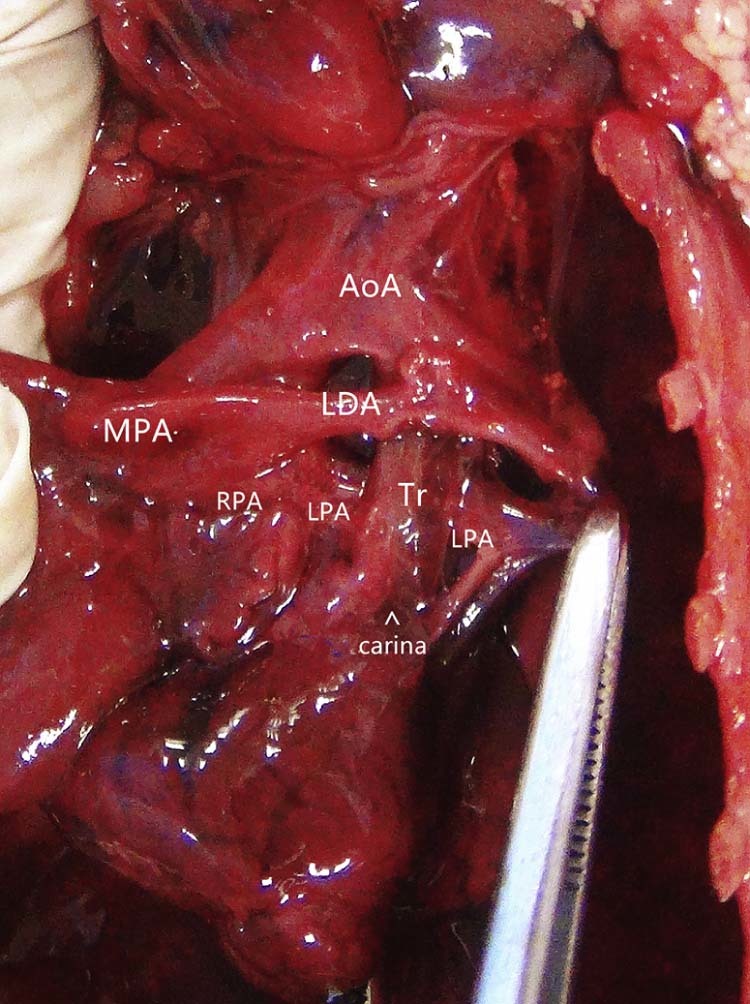
Autopsy image shows the left pulmonary artery (LPA) originating from the superior aspect of the right pulmonary artery (RPA), then turning leftward and coursing between the trachea and esophagus to reach the left lung hilum. AoA, arch of aorta; LDA, left ductus arteriosus; MPA, main pulmonary artery; Tr, Trachea.
Fig. 4.
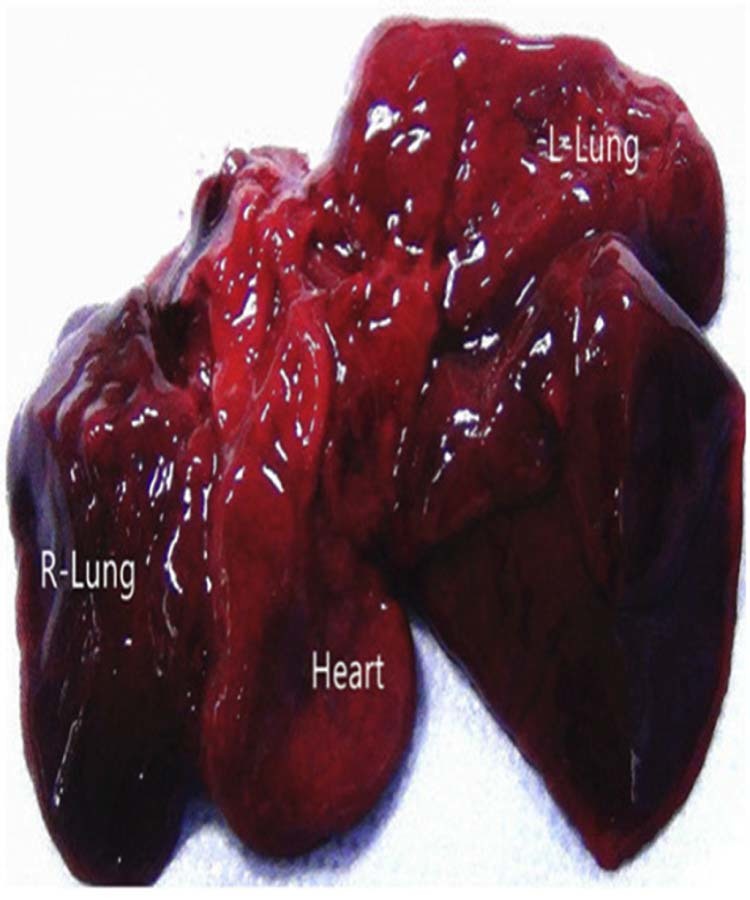
The right lung bronchus is stenotic (not shown), and the right lung was unilobar. The left lung was morphologically normal.
Case 2: A 29-year-old woman was referred for fetal MRI at 29 weeks' gestation because of an LPA origin abnormality on ultrasound. Fetal MRI showed that the LPA originated from the superior aspect of the RPA and then turned leftward posterior to the trachea (Fig. 5, Fig. 6). There was no obvious tracheal compression or narrowing. The carina position, bronchial anatomy, and lung volumes were normal (Fig. 7). The diagnosis of LPAS type IA was made and confirmed by thoracic computed tomography after birth (Fig. 8, Fig. 9).
Fig. 5.
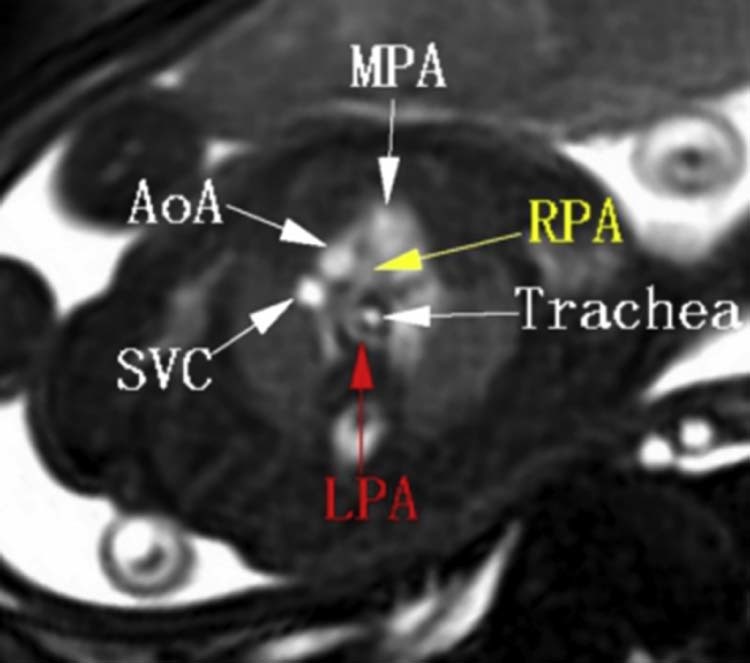
Fetal MRI. BFFE sequence shows that the RPA originates from the main pulmonary artery, and the LPA originates from the superior aspect of the RPA, then turns leftward posterior to the trachea. AoA, arch of aorta; BFFE, balanced fast field echo; MPA, main pulmonary artery; MRI, magnetic resonance imaging; LPA, left pulmonary artery; RPA, right pulmonary artery; SVC, superior vena cava.
Fig. 6.
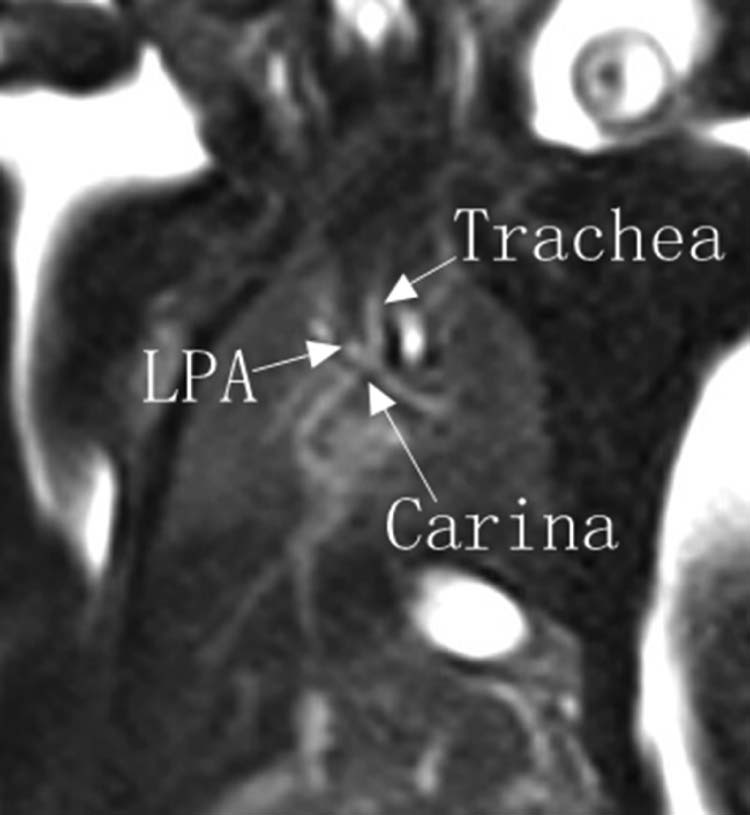
Fetal MRI. BFFE shows that there is no obvious trachea compression or narrowing and the carina position is normal. BFFE, balanced fast field echo; LPA, left pulmonary artery.
Fig. 7.
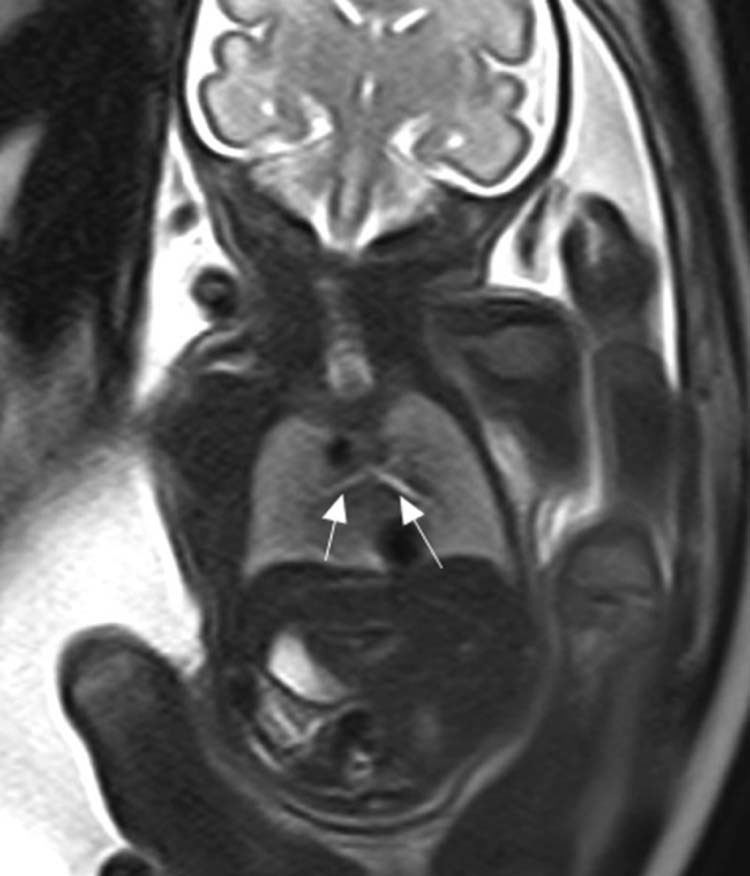
Fetal MRI SSFSE shows that the left and right lung volumes and the bronchi are normal. MRI, magnetic resonance imaging; SSFSE, single-shot fast spin echo.
Fig. 8.
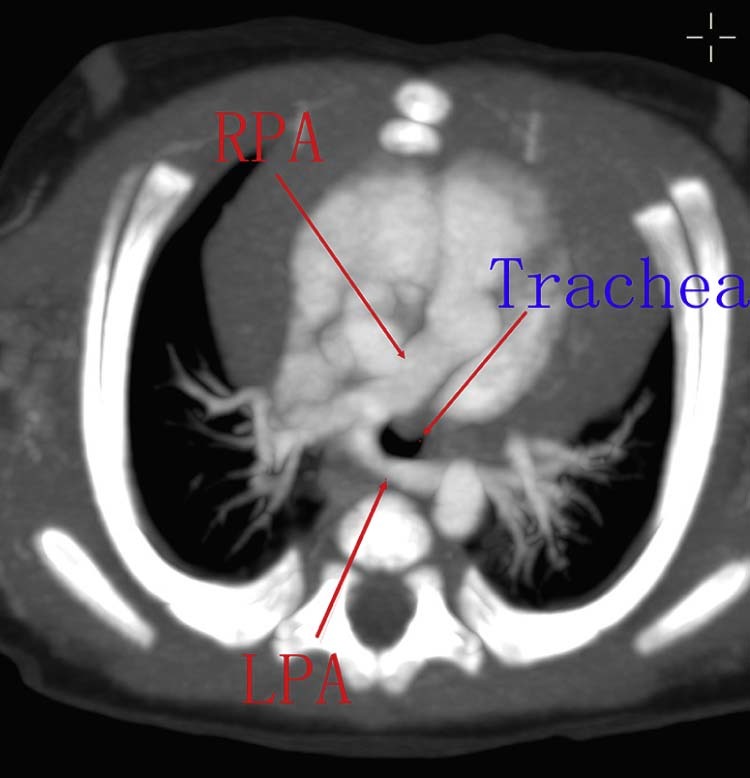
CT scans of LPAS type IA demonstrate the LPAS coursing posterior to the trachea, passing between the trachea and esophagus. CT, computed tomography; LPA, left pulmonary artery; LPAs, left pulmonary artery sling; RPA, right pulmonary artery.
Fig. 9.
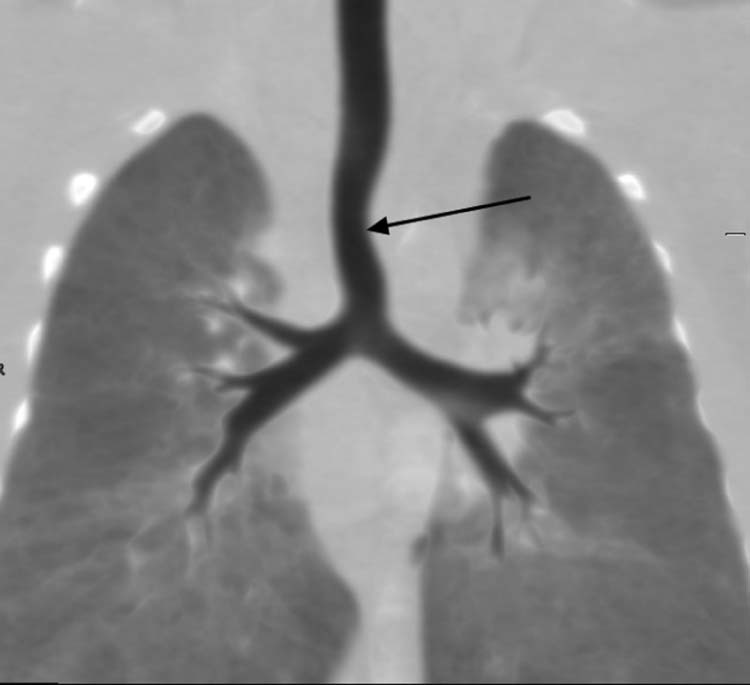
A coronal reconstructed image demonstrating a mild narrowing of the trachea (black arrow), which is left pulmonary artery sling type IA.
Discussion
Value of prenatal diagnosis and classification of LPAS
Early diagnosis of this rare malformation is important because it may prevent the occurrence of recurrent pulmonary infections and other postnatal complications [1]. The classification of LPAS can be made using the system proposed by Wells et al. (Fig 10) [3], [4]. Type I LPAS, as in case report 2, can be found incidentally in asymptomatic adults. It has also become apparent that type II slings tend to cause greater morbidity and mortality. This subtype cannot be treated by reanastomosis of the pulmonary artery alone, usually because of concurrent tracheal stenosis due to complete cartilage rings and absence of the pars membranacea [5]. Instead, the narrow airway generally also requires repair [3].
Fig. 10.
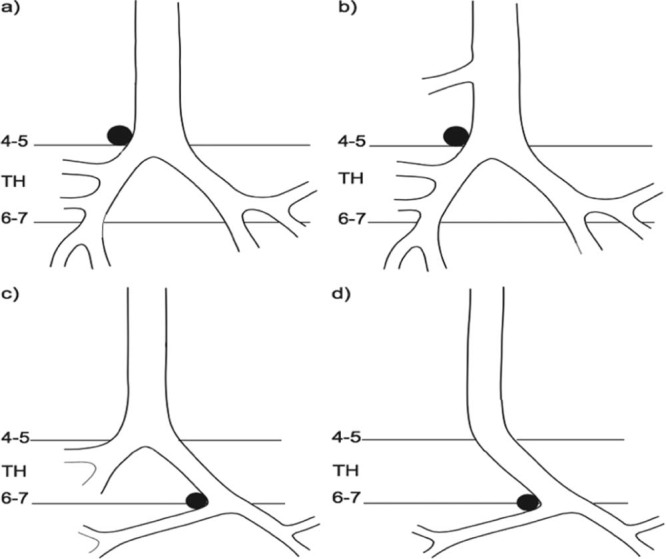
Tracheobrochial arborization disorders associated with left pulmonary artery sling (LPAS), the LPAS crosses over the right bronchus in type I, and the bridging bronchus in type II. The four LPAS types are shown in the diagrams: (a) type IA, (b) type IB, (c) type IIA, and (d) type IIB. Type IA (a) refers to cases with normal tracheobronchial anatomy and a normal bifurcation at the level of the fourth or fifth thoracic vertebrae (TH). In type IB (b), there is an additional tracheal bronchus to the right upper lobe. A bridging bronchus is present in type II LPAS cases. In type IIA (c), a bridging bronchus originates from a pseudo-carina at the level of the sixth or seventh thoracic vertebra, with a right upper lobe bronchus originating from the carina at the level of the fourth or fifth thoracic vertebra. In type II B (d), the right upper lobe bronchus is absent.
Fetal MRI protocol to diagnose and classify LPAS
The most useful sequence to observe the structure and course of the LPA and RPA in our protocol was BFFE. Because the blood in the heart and great vessels has high signal under normal circumstances, it is possible to see the main pulmonary artery dividing into the LPA and RPA in the 3 vessel-pulmonary artery branch view, usually in the axial plane. When the LPA originates from the RPA and loops behind the trachea, passing between the trachea and esophagus, the diagnosis of LPAS is confirmed. We used negative interval scanning from the thoracic inlet to the diaphragm (20 slices) with a slice thickness of 7 mm and overlap of 5 mm about 40 seconds per scan time. The overlap enhances visualization of anatomic detail of cardiovascular structures including the pulmonary artery and the other great thoracic vessels, as well as the cardiac chambers. After BFFE, we used the SSFSE sequence. In this sequence, the blood in the heart and great vessels has low signal, whereas the trachea (filled with amniotic fluid) has high signal, allowing us to observe the anatomic structure of the tracheobronchial tree. The SSFSE sequence technique also allows clear visualization of the aortic arch anatomy. To account for effects of fetal movement, we repeated sequences that were degraded by fetal motion [6]. Fetal MRI may also provide relevant information on lung volume and signal intensity, and is an accurate prenatal method for evaluating fetal lung maturity using the lung-to-liver signal intensity ratio on T2-weighted images [5].
Relevance for future diagnosis and management of LPAS
Fetal diagnosis of LPAS or bridging bronchus is rare. This is likely due to the rarity of both disease processes, lack of referring clinician and radiologist awareness, and previously limited capability of imaging technology to detect these anomalies. With increasing availability of high-resolution and motion-resistant fetal MR techniques, prenatal diagnosis is becoming more feasible. Although LPAS is rare, early diagnosis and classification is important. Early detection could allow stratification of high-risk patients (type II, more likely to have complete cartilage rings and airway stenosis), and perhaps direct at-risk neonates into higher level monitoring or earlier surgical intervention.
In conclusion, prenatal diagnosis of LPAS is possible and valuable, and may be worth including in routine fetal magnetic resonance evaluation.
Footnotes
Conflicts of Interest: The authors declare that they have no financial relationship with people or organizations that can inappropriately influence their work and that they have no interest of any nature or kind in any product, service, or company that could be construed as influencing the position presented in, or the review of, this article.
References
- 1.Yorioka H., Kasamatsu A., Kanzaki H. Prenatal diagnosis of fetal left pulmonary artery sling. Ultrasound Obstet Gynecol. 2011;37:245–246. doi: 10.1002/uog.8804. [DOI] [PubMed] [Google Scholar]
- 2.du Plessis A.M., Andronikou S., Goussaard P. Bridging bronchus and sling left pulmonary artery: a rare entity demonstrated by coronal CT with 3-D rendering display and minimal-intensity projections. Pediatr Radiol. 2008;38(9):1024–1026. doi: 10.1007/s00247-008-0913-y. [DOI] [PubMed] [Google Scholar]
- 3.Wells T.R., Gwin J.L., Landing B.H. Reconsideration of the anatomy of sling left pulmonary artery: the association of one form with bridging bronchus and imperforate anus. Anatomic and diagnostic aspects. J Pediatr Surg. 1988;23(10):892–898. doi: 10.1016/s0022-3468(88)80379-8. [DOI] [PubMed] [Google Scholar]
- 4.Baden W., Schaefer J., Kumpf M. Comparison of imaging techniques in the diagnosis of bridging bronchus. Eur Respir J. 2008;31(112):5–1131. doi: 10.1183/09031936.00045907. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Li X.L., Hu K. The value of cardiac magnetic resonance in the diagnosis of fetal aortic arch anomalies. J Matern Fetal Neonatal Med. 2016:1–6. doi: 10.1080/14767058.2016.1214126. [DOI] [PubMed] [Google Scholar]
- 6.Oka Y., Rahman M., Sasakura C. Prenatal diagnosis of fetal respiratory function: evaluation of fetal lung maturity using lung-to-liver signal intensity ratio at magnetic resonance imaging. Prenat Diagn. 2014;34(13):1289–1294. doi: 10.1002/pd.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]


