Abstract
We describe a rare case of renal lymphangioma presenting as a focal unilateral multicystic renal mass and document the first reported use of triparametric ultrasound (B-mode, Doppler, and contrast-enhanced ultrasound) in its diagnosis and discrimination from other focal multicystic lesions. Renal lymphangiomas are rare, benign, typically developmental lesions composed of cystic dilatation of the lymphatic ducts, usually occurring bilaterally as perinephric collections or parapelvic cysts mimicking hydronephrosis. Radiologists have an important role in suggesting the diagnosis, as clinical presentation can be nonspecific. Management is usually conservative; however, nephron-sparing surgery may be recommended in symptomatic individuals.
Keywords: Renal lymphangioma, Contrast-enhanced ultrasound, Triparametric ultrasound
Case report
A 30-year-old man presented with a 3-month history of left abdominal pain. On examination, the man had a mild discomfort in his left flank, but his observations (heart rate, blood pressure, oxygen saturations, and temperature) were unremarkable. Full blood counts, urea and electrolytes, liver function tests, and C-reactive protein levels were all within normal range.
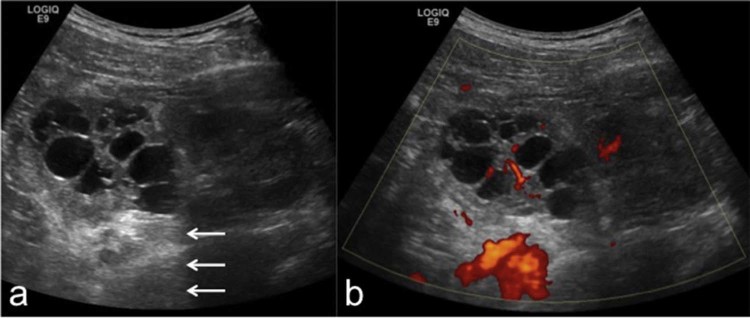
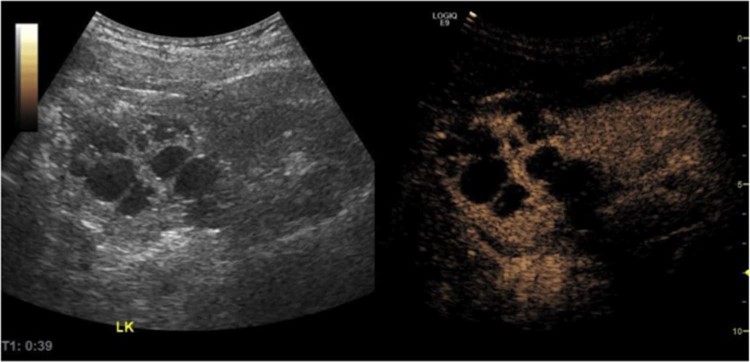
The patient underwent an ultrasound scan of the abdomen using B-mode and power Doppler on a General Electric Logiq E9 ultrasound machine, which showed multiple, variable-sized anechoic foci within the upper pole of the left kidney, with posterior acoustic enhancement and no evidence of internal echogenicity, appearances in keeping with a focal multilocular cystic lesion. Power Doppler showed evidence of hypervascularity (Fig. 1).
Fig. 1.
B-mode ultrasound (A) and power Doppler (B) of the left kidney showing a multilocular cystic lesion in the upper pole. A normal-appearing lower pole renal parenchyma is seen. The cysts are anechoic, and there is a posterior acoustic enhancement (arrows). The renal parenchyma between the cysts is hyperechoic with evidence of vascularity on Doppler.
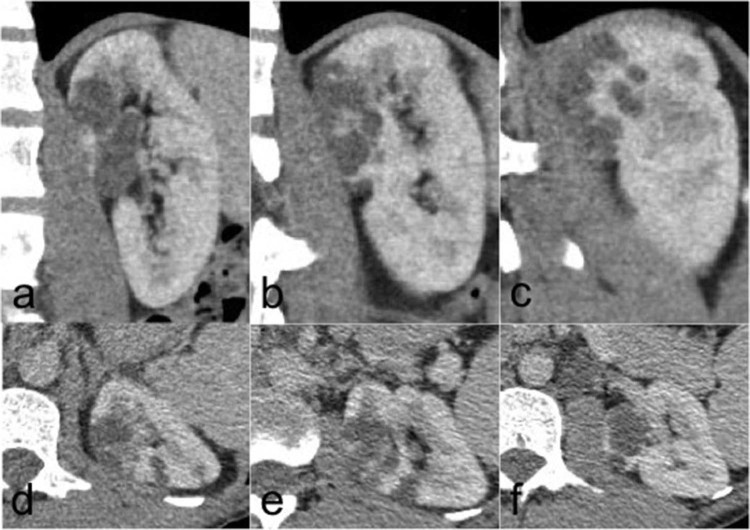
A subsequent computed tomography (CT) was acquired in the precontrast, corticomedullary, and nephrographic phases (3mm slices, General Electric). CT revealed a multiloculated cystic structure with no calcification or lipid density. The nephrographic phase showed an enhancement of the septations between the cysts with thickened walls but no intracystic mural nodule (Fig. 2).
Fig. 2.
Sagittal (A-C) anterior to posterior and axial (D-F) upper pole to interpolar regions of the left kidney in the nephrographic phase of a triple-phase renal protocol computed tomography. Pre- and corticomedullary phases are not shown. This shows a focal intrarenal multiloculated cystic lesion in the anteromedial upper pole cortex.
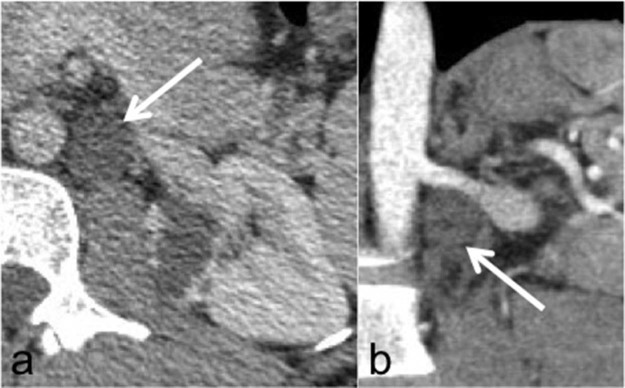
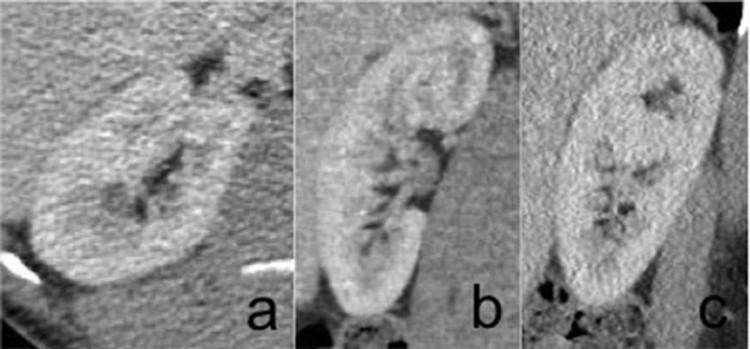
In addition, there was a cystic lesion (Fig. 3) in the retroperitoneal space (this was retrospectively interpreted as a dilated retroperitoneal lymphatic duct after the diagnosis was made histologically). The contralateral kidney showed no abnormality (Fig. 4). The rest of the abdomen and chest showed no abnormality.
Fig. 3.
Axial computed tomography in the nephrographic phase (A) and computed tomography reformat in the corticomedullary phase (B) showing a cystic lesion (arrows) in the left para-aortic region below the left renal artery not appreciated on initial review. In retrospect, this is most likely to represent a dilated retroperitoneal lymphatic duct, which could have helped in making the diagnosis of lymphangioma.
Fig. 4.
Axial (A), coronal (B), and sagittal (C) computed tomography in the nephrographic phase of the normal right kidney.
Further imaging was performed using a contrast-enhanced ultrasound (CEUS) examination with Sonovue microbubbles. A time-intensity curve (TIC) with quantitative analysis was generated using in-built software with the region of interest placed over the normal cortical renal parenchyma and the enhancing wall of the lesion for comparison. Postcontrast images following injection of 2.2 mL Sonovue showed an enhancement of the septations and walls to a similar extent to the adjacent normal renal parenchyma in both the arterial and portal venous phases (Fig. 5).
Fig. 5.
Dual split screen still image in contrast mode, with the b-mode on the left and the contrast window on the right, 39 seconds after the injection of Sonovue contrast. There is avid enhancement of the renal parenchyma between the cysts qualitatively to a similar extent as the normal lower pole renal parenchyma. There is no internal enhancement of the cysts. LK, left kidney.
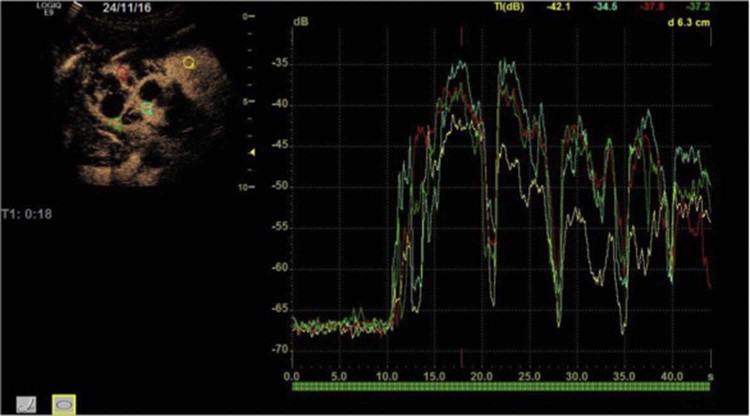
A TIC was generated, which showed a similar time-to-peak enhancement and enhancement intensity between the normal parenchyma and lesion septations (Fig. 6).
Fig. 6.
Time-intensity curve analysis. Four regions of interest of the same size (4 mm) have been placed, one on the normal renal parenchyma (yellow) and the other three (red, green, and blue) on the parenchyma between the cysts in the upper pole. All four curves have a very similar appearance, particularly the time to peak. We postulate that this is due to the renal parenchyma between the cysts made of normal tissue rather than malignant tissue, which one might expect in renal cell cancer.
The patient proceeded to a laparoscopic radical nephrectomy 2 months after initial imaging as a multicystic renal cell carcinoma (RCC) could not be excluded. The renal specimen was sent for histopathology analysis.
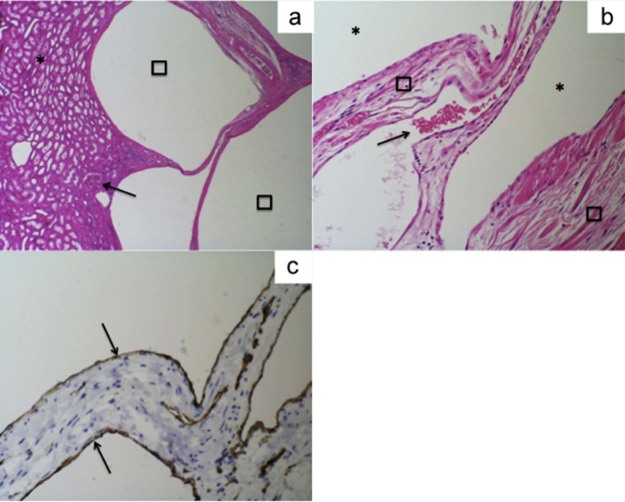
Microscopy showed a renal specimen without any primary glomerular pathology with a multicystic lesion situated mainly in the hilar fat but extending into the immediately adjacent kidney. The endothelial lining of the cystic spaces forming the lesion is compressed (Fig. 7) and stained positive for CD34 and CD31 but negative for pankeratin. The renal vein and artery were identified at the hilum and showed no significant pathology. The ureteric-pelvic margin showed no evidence of dysplasia or malignancy.
Fig. 7.
(A) Microscopic examination using hematoxylin and eosin staining at ×40 magnification shows a normal renal parenchyma (asterisk) and normal glomeruli (black arrow) with lymphocytic spaces (squares) representing the cystic spaces seen on radiological imaging. (B) Microscopic examination with magnification ×200 shows endothelial lined lymphovascular spaces (asterisks) with a normal intervening fibroconnective tissue (squares) and vascular channels containing red blood cells (black arrow). (C) Immunohistochemistry staining at ×200 magnification shows endothelial lining staining positive for CD31 (black arrows).
Overall appearances were in keeping with a renal lymphangioma.
Discussion
Renal lymphangioma is a rare benign disorder resulting from an abnormal dilatation of the lymphatic ducts of the kidney. Lymphangiectasia occurs more commonly in the neck and axilla. The exact pathogenesis is not yet established, and there is no age or sex predominance. On imaging investigations, lymphangiectasia usually appears as a fluid collection or a multicystic lesion in the kidney, either in the perinephric or in the parapelvic regions.
Most cases reported in the literature are found incidentally, but renal lymphangiomas can cause abdominal pain, hematuria, and bloating. Abdominal pain, as in our case, occurs in 42% of presentations [1]; however, there is no known association between the size of the lesion and the symptoms, and many large collections present asymptomatically [2]. Renal lymphangiomas can present as an abdominal mass particularly in pediatric patients [3].
When large, renal lymphangioma may present with renal impairment, hypertension, ascites, or pleural effusion, but these derangements have been seen to regress with treatment. Renal vein thrombosis has been reported in 1 case [4].
Typical imaging appearances depend on the location of the renal lymphangioma. Perinephric lymphangioma appears as fluid density collections with or without septation, enveloping the kidneys, and is the most common type.
Parapelvic lymphangioma is less common, and typical appearances are of fluid density cysts in the renal sinus, which may extend into the medial renal parenchyma. Parapelvic lymphangiomas can appear similar to hydronephrosis or other multicystic renal lesions, although no communication with the collecting system is demonstrated on postcontrast studies.
Intrarenal lymphangioma is an extremely rare occurrence and can appear as either a focal hyperechoic lesion in an adult patient [5] or as a nephromegaly in an infant [6].
Lymphatic drainage of the kidney occurs via parenchymal trunks in the renal pedicle into the para-aortic and paracaval lymph nodes. Case reports have indicated that dilated retroperitoneal lymphatics can support the diagnosis [4].
Lymphangiomas are bilateral in the majority of cases, and there may be coexisting perinephric and parapelvic collections [7], [8]. A review of the literature in the last decade showed that only 3 out of 21 reported cases were unilateral and only 1 of the 3 was focal and solid in appearance. Our case is the only reported case of a unilateral focal and multicystic lymphangioma (Table 1).
Table 1.
List of unilateral or bilateral cases of renal lymphangioma in the last 10 years.
| Paper | Unilateral | Bilateral | Notes | |
|---|---|---|---|---|
| 1 | Al Dofri 200924 | ✔ | ||
| 2 | Bansal et al. 201625 | ✔ | ||
| 3 | Blanc et al. 20142 | ✔ | ||
| 4 | Chaabouni et al. 201221 | ✔ | ||
| 5 | Elbanna et al. 201526 | ✔ | ||
| 6 | Gupta et al. 20074 | ✔ | Not focal | |
| 7 | Jeon et al. 201419 | ✔ | Not focal | |
| 8 | Karkouche et al. 201322 | ✔ | ||
| 9 | Kashgari et al. 20173 | ✔ | ||
| 10 | Kumar et al. 201527 | ✔ | ||
| 11 | Lal et al. 201628 | ✔ | Focal but not cystic | |
| 12 | Magu et al. 201029 | ✔ | ||
| 13 | Mayyappan et al. 20138 | ✔ | ||
| 14 | Nassiri et al. 201520 | ✔ | ||
| 15 | Pandya et al. 201630 | ✔ | ||
| 16 | Pianezza et al. 200631 | ✔ | ||
| 17 | Rastogi et al. 201032 | ✔ | ||
| 18 | Sarikaya et al. 200633 | ✔ | ||
| 19 | Singh et al. 201434 | ✔ | ||
| 20 | Sulthana et al. 201535 | ✔ | ||
| 21 | Valerio et al. 201223 | ✔ |
Gupta et al. and Jeon et al. described unilateral but not focal cases. Lal et al. described a unilateral focal case but had a noncystic appearance.
The tick indicates whether the listed paper/author described a unilateral case or bilateral case of renal lymphangioma.
Imaging in our patient showed a focal unilateral multicystic lesion in an adult patient, which is extremely rare. The lesion consisted of variable-sized cysts with intervening thickened septations on ultrasound and CT imaging. This is a rare finding as almost all previous cases have shown involvement of the whole kidney rather than a focal lesion. In addition, a retrospective finding of a round fluid density in the retroperitoneum was thought to be dilated retroperitoneal lymphatics strongly suggesting the diagnosis (Fig. 3).
Septations can be seen on ultrasound, CT, and MRI with enhancement reported on both postcontrast CT and T1 postgadolinium MRI. We were able to demonstrate an enhancement of the thickened septations using CEUS (Sonovue microbubbles). We found that our TICs of the thickened septations were similar to the adjacent normal renal parenchyma.
This finding is in contrast to findings reported by Sparchez et al. and Dong et al., in which the majority of cases of RCC had a rapid wash-in or a rapid wash-out, or a rapid wash-in or a slow wash-out pattern of microbubble contrast [9], [10]. Aoki et al. also showed an enhancement of septations was faster for “time to peak” for patients with RCC [11].
We postulate in our case that the septations are in fact a normal renal parenchyma compressed between the focally enlarged lymph channels, giving it similar appearances on B-mode and CEUS. This pattern of intervening noncommunicating lymphangiectasia would fit with the typical description for renal lymphangioma presenting in the parapelvic region. The parapelvic pattern can mimic hydronephrosis or calyceal dilation, and only after a contrast enhancement of the pelvicaliceal system do parapelvic pattern show their separate nature.
Given the focal nature of the multicystic lesion in our case, the main differentials would include multilocular cystic cell renal carcinoma (MCRCC, also known as multilocular clear-cell RCC), multilocular cystic nephroma (MLCC, and its equivalent radiologically indistinguishable: cystic partially differentiated nephroblastoma), tubulocystic carcinoma, and multicystic dysplasia. While numerous studies have shown B-mode ultrasound and CT or MRI being unable to distinguish between focal multicystic renal lesions, there could be a role for CEUS with its improved spatial resolution and non-nephrotoxic profile.
Multicystic dysplasia is unlikely in our case as these tumors often present in the neonatal period as a unilateral multicystic mass involving most of the kidney and involute over time.
MCRCC is a variant of clear-cell RCC and is the main differential in our case. MCRCC has an excellent prognosis in comparison to clear-cell RCC, and patients with MCRCC could be offered a nephron-sparing resection. There is a male predominance, and MCRCC is usually seen between the second to the seventh decade. MCRCC is often unilateral and entirely composed of variable-sized cysts, and is difficult to distinguish from other multicystic lesions on imaging with the diagnosis made typically on histopathology. The cyst wall and the septa show contrast enhancement without an expansile tumor nodule, and asymmetric septa thickening may be seen [12].
There have been no direct studies examining the characteristics of CEUS in MCRCC; however, Aoki et al. [11] studied 20 patients with clear-cell RCC and found that the time to peak of the tumor was shorter than that of normal parenchyma in 100% of cases for both solid and cystic lesions, raising the possibility of using CEUS to discriminate MCRCC from renal lymphangioma. CEUS has been studied in RCC, and Sparchez et al. and Dong et al. have found clear-cell RCC has rapid wash-in and wash-out patterns, whereas papillary and chromophobe RCCs have slow wash-in and rapid wash-out patterns [9], [10]. One could postulate these findings may be seen in MCRCC; however, this would need further investigation.
MLCC can present with a focal mass containing irregular cysts with variable-sized enhancing septations with no solid component. Ultrasound and CT appearances generally show a multicystic mass with capsule and septa, which can enhance on postgadolinium MRI [13]. Typically, there is a bimodal age group with peaks in children younger than 4 years old and in adult women between the ages of 40-69 years old. Powell et al. in 1951 described eight criteria for MLCC: Unilateral, solitary, multilocular, noncommunication between cysts, noncommunication with the renal pelvis, loculi lined with the epithelium, and the interlocular septa devoid of normal renal parenchyma [14], [15].
Although the imaging appearances are very similar to our case, we were able to demonstrate on CEUS an enhancement pattern in the tissue between the cysts and the loculi, the same as the adjacent normal renal parenchyma, thus making the diagnosis of MLCC unlikely according to the Powell criteria in 1951. In addition, the age group of our patient does not fit that described in the literature for MLCC. Shahzad et al. [16] investigated focal multicystic renal lesions with CEUS, and two patients had cystic nephroma on eventual histology. Although there is no description of the CEUS TIC in detail, Shahzad et al.'s table shows that these lesions demonstrate a “malignant CEUS pattern” rather than a normal renal parenchyma pattern as in our case.
Lastly, tubulocystic carcinoma is a relatively new entity recognized officially by the 2010 American Joint Committee on Cancer/Union for International Cancer Control Tumour, node and metastasis, seventh edition. Tubulocystic carcinoma originates from proximal convoluted tubules and distal nephrons with aggressive features, but rarely metastasizes. Eighty-five percent are found in men with a mean age of 54 years old. Less than 100 cases have been described in the literature and the prognosis is generally good. Immunohistochemistry reveals CD10 and P504S positivity [17]. Radiologically one typically sees a well-defined multilocular cystic mass with thick septations on CT imaging, occasionally with solid nodules. However, on ultrasound, the typical appearance is more of focal hyperechogenicity with a posterior acoustic enhancement due to small cystic spaces. There is no vascular Doppler signal. Cornelis et al. studied 3 patients with CEUS and found slow enhancement within the septa with a spongiform-honeycomb appearance, again a description not in keeping with our findings [18].
From each of the differentials previously mentioned, it may be possible to distinguish renal lymphangioma, when presenting as a focal multicystic renal mass, from other multicystic lesions with contrast-enhanced ultrasound. This is vital for the patient as renal lymphangioma can be managed conservatively in the absence of severe symptoms or complications. Generally, multicystic focal renal masses tend to have a good prognosis; however, further investigation to characterize MCRCC on CEUS is still required.
If imaging characteristics are not diagnostic, histopathology can be used to suggest the diagnosis. Fluid sampling (where possible in perinephric cases) has been shown to contain either serous or chylous fluid with lymphocyte predominance [3], [19], [20]. Microscopy typically demonstrates endothelial lined spaces with no glomerular or tubular abnormality and positive staining for factor VIII, D2-40 antibody, CD34, and weakly for CD31, but negative staining for keratin and pancytokeratin [19], [21], [22]. Our patient's cell staining was positive for CD34 and CD31 but negative for pancytokeratin in keeping with previous reports.
The management in the majority of cases is conservative if the patient is asymptomatic, and there are no complications. Recurrent fluid collections after percutaneous drainage have been treated with marsupialization by surgically creating a communication between the cystic lesion and the peritoneal cavity [2]. There have been 2 reported uses of sclerotherapy in patients who had a symptomatic painful perinephric renal lymphangioma and were not suitable for surgical intervention [3], [23].
In summary, we describe a case of renal lymphangioma presenting with the unique appearance of a focal unilateral multicystic renal mass in an adult man. The diagnosis was confirmed with histopathologic analysis. We report the first description of renal lymphangioma using triparametric ultrasound (B-mode, Doppler, and contrast enhanced) and suggest its use when differentiating from other focal multicystic renal masses. We acknowledge that more investigation using CEUS is required, especially for a multilocular cystic RCC and other variants of clear-cell RCC, which can mimic appearances. The patient was well and asymptomatic at 5 months' follow-up.
References
- 1.Restrepo J.M., Amaya J.E.L., Sepulveda N.A., Velez M.U., Massaro M. Renal lymphangiectasia. MDCT and MRI findings. Rev Colomb Radiol. 2011;22:1–8. [Google Scholar]
- 2.Blanc M., Schmutz G., Belzile F., Sabbagh R. Renal lymphangiectasia presenting with hypertension and polycythemia. Can Urol Assoc J. 2014;8:e163–6. doi: 10.5489/cuaj.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashgari A., Ozair N., Al Zahrani A., Al Otibi M.O., Al Fakeeh K. Renal lymphangiomatosis, a rare differential diagnosis for autosomal recessive polycystic kidney disease in pediatric patients. Radiol Case Rep. 2017;12:70. doi: 10.1016/j.radcr.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R., Sharma R., Gamanagatti S., Dogra P.N., Kumar A. Unilateral renal lymphangiectasia: imaging appearance on sonography, CT and MRI. Int Urol Nephrol. 2007;39:361–364. doi: 10.1007/s11255-006-9039-z. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.K., Ahn H.J., Kim K.R., Cho K.S. Renal lymphangioma manifested as a solid mass on ultrasonography and computed tomography. J Ultrasound Med. 2002;21:203–206. doi: 10.7863/jum.2002.21.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Merguerian P.A., Sargent S.K., Dunn J.L. Unilateral lymphangiectasis of the kidney: an unusual cause of renal enlargement in an infant. J Urol. 1995;153:447–449. doi: 10.1097/00005392-199502000-00053. [DOI] [PubMed] [Google Scholar]
- 7.Gorantla R., Yalapati A., Dev B., Joseph S. Case report: perinephric lymphangiomatosis. Indian J Radiol Imaging. 2010;20:224–226. doi: 10.4103/0971-3026.69364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyyappan R.M., Ravikumar S., Gopinath M. Laparoscopic management in a rare case of bilateral perirenal lympangiomatosis. Indian J Urol. 2013;29:73. doi: 10.4103/0970-1591.109992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparchez Z., Radu P., Sparchez M., Crisan N., Kacso G., Petrut B. Contrast enhanced ultrasound of renal masses. A reappraisal of EFSUMB recommendations and possible emerging applications. Med Ultrason. 2015;17:219–226. doi: 10.11152/mu.2013.2066.172.fsum. [DOI] [PubMed] [Google Scholar]
- 10.Dong X.Q., Xu L.W., Xu C.M., Wang X.M. Contrast-enhanced ultrasound for detection and diagnosis of renal clear cell carcinoma. Chin Med J. 2009;122:1179–1183. [PubMed] [Google Scholar]
- 11.Aoki S., Hattori R., Yamamoto T., Funahashi Y., Matsukawa Y., Gotoh M. Contrast-enhanced ultrasound using a time-intensity curve for the diagnosis of renal cell carcinoma. BJU Int. 2010;108:349. doi: 10.1111/j.1464-410X.2010.09799.x. [DOI] [PubMed] [Google Scholar]
- 12.Freire M., Remer E.M. Clinical and radiologic features of cystic renal masses. AJR Am J Roentgenol. 2008;192:1367. doi: 10.2214/AJR.08.1468. [DOI] [PubMed] [Google Scholar]
- 13.Granja M.F., O'Brien A.T., Trujillo S., Mancera J., Aguirre D.A. Multilocular cystic nephroma: a systematic literature review of the radiologic and clinical findings. AJR Am J Roentgenol. 2015;205:1188–1193. doi: 10.2214/AJR.15.14548. [DOI] [PubMed] [Google Scholar]
- 14.Singh S., Chowdury V., Dixit R., Manchanda A. Multilocular cystic nephroma of the kidney: a case report. Indian J Radiol Imaging. 2006;16(4):901–904. [Google Scholar]
- 15.Powell T., Shackman R., Johnson H.D. Multilocular cysts of the kidney. Br J Urol. 1951;23:142. doi: 10.1111/j.1464-410x.1951.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 16.Shahzad K., Simms M.S., Byass O. Evaluation of contrast enhanced ultrasound for investigation of complex cystic renal masses. Br J Med Surg Urol. 2011;4:253. [Google Scholar]
- 17.Khalaf I., El-Badawy N., Shawarby M.A. Tubulocystic renal cell carcinoma, a rare tumor entity: review of literature and report of a case. Afr J Urol. 2013;19:1–6. [Google Scholar]
- 18.Cornelis F., Helenon O., Correas J.M., Lemaitre L., Andre M., Meuwly J.Y. Tubulocystic renal cell carcinoma: a new radiological entity. Eur Radiol. 2016;26:1108–1115. doi: 10.1007/s00330-015-3923-9. [DOI] [PubMed] [Google Scholar]
- 19.Jeon T.G., Kong D.H., Park H.J., Kim S., Park W.Y., Kim S.D. Perirenal lymphangiomatosis. World J Mens Health. 2014;32:116–119. doi: 10.5534/wjmh.2014.32.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassiri A.A., Lotfollahi L., Bakhshayeshkaram M., Kiani A., Haghighi S., Alavi I. Renal lymphangiectasia: a curious cause of pleural effusion. Tanaffos. 2015;14:213–216. [PMC free article] [PubMed] [Google Scholar]
- 21.Chaabouni A., Rebai N., Fourati M., Rekik S., Chabchoub K., Slimen M.H. Cystic lymphangioma of the kidney: diagnosis and management. Int J Surg Case Rep. 2012;3:587–589. doi: 10.1016/j.ijscr.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karkouche R., Rocher L., Guettier C., Corcos G., Benoit G., Fernandez H. Bilateral renal lymphangiomatosis: imaging and histopathologic findings. Abdom Imaging. 2013;38:858–862. doi: 10.1007/s00261-012-9977-0. [DOI] [PubMed] [Google Scholar]
- 23.Valerio M., Meuwly J.Y., Tawadros C., Jichlinski P. Percutaneous drainage and sclerotherapy as definitive treatment of renal lymphangiomatosis. Can Urol Assoc J. 2012;6:e3–7. doi: 10.5489/cuaj.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Dofri S.A.A. Renal lymphangiectasia presented by pleural effusion and ascites. Radiol Case. 2009;3:5–10. doi: 10.3941/jrcr.v3i10.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal K., Sureka B., Pargewar S., Arora A. Renal lymphangiectasia: one disease, many names! Indian J Neprhol. 2016;26:57–58. doi: 10.4103/0971-4065.155730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbanna K., Almutairi B.M., Zidan A.T. Bilateral renal lymphangiectasia. radiological findings by ultrasound, CT and MRI. J Clin Imaging Sci. 2015;5:6. doi: 10.4103/2156-7514.150449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar K., Ahmad A., Singh M., Kumar A., Singh R.P., Hussain M. Bilateral renal lymphangiectasia in a thirty-two-year-old woman. Neprho Urol Mon. 2015;7:e21736. doi: 10.5812/numonthly.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lal H., Agrawal V., Naik S. Renal lymphangioma. J Integr Nephrol Androl. 2016;3:130–132. [Google Scholar]
- 29.Magu S., Agrawal S., Dalaal S.K. Bilatearl renal lymphangioma—an incidental finding. Indian J Nephrol. 2010;20:114–115. doi: 10.4103/0971-4065.65309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandya V.K., Shah M.K., Gandhi S.P., Patel H.V. Bilateral renal lymphangiectasia. J Clin Diagn Res. 2016;10:TD1–2. doi: 10.7860/JCDR/2016/19475.8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pianezza M.L., Mokhtassi A., Wu L., Honey J.D.A. Case report: renal lymphangiectasia. Can J Urol. 2006;13:3204. [PubMed] [Google Scholar]
- 32.Rastogi R., Rastogi U.C., Sarikwal A., Rastogi V. Renal lymphangiectasia associated with chronic myeloid leukemia. Saudi J Kidney Dis Transpl. 2010;21:724. [PubMed] [Google Scholar]
- 33.Sarikaya B., Akturk Y., Bekar U., Topaloglu S. Bilateral renal lymphangiomatosis mimicking hydronephrosis: multidetector CT urographic findings. Abdom Imaging. 2006;31:732. doi: 10.1007/s00261-005-8014-y. [DOI] [PubMed] [Google Scholar]
- 34.Singh S.N., Sundar G., Satishchandra H. An unusual case of bilateral renal lymphangiectasia. S Afr J Rad. 2014;18 Art.#587. [Google Scholar]
- 35.Sulthana P.M., Kumar S.S., Malathi V., Sekhar K.S. Bilateral renal lymphangiectasia. {online} 2015. http://www.eurorad.org/case.php?id=13018 Available from: