Abstract
OBJECTIVES
The objective of this project was to assess a pediatric institution's use of infliximab and develop and evaluate electronic health record tools to improve safety and efficiency of infliximab ordering through auditing and improved communication.
METHODS
Best use of infliximab was defined through a literature review, analysis of baseline use of infliximab at our institution, and distribution and analysis of a national survey. Auditing and order communication were optimized through implementation of mandatory indications in the infliximab orderable and creation of an interactive flowsheet that collects discrete and free-text data. The value of the implemented electronic health record tools was assessed at the conclusion of the project.
RESULTS
Baseline analysis determined that 93.8% of orders were dosed appropriately according to the findings of a literature review. After implementation of the flowsheet and indications, the time to perform an audit of use was reduced from 60 minutes to 5 minutes per month. Four months post implementation, data were entered by 60% of the pediatric gastroenterologists at our institution on 15.3% of all encounters for infliximab. Users were surveyed on the value of the tools, with 100% planning to continue using the workflow, and 82% stating the tools frequently improve the efficiency and safety of infliximab prescribing.
CONCLUSIONS
Creation of a standard workflow by using an interactive flowsheet has improved auditing ability and facilitated the communication of important order information surrounding infliximab. Providers and pharmacists feel these tools improve the safety and efficiency of infliximab ordering, and auditing data reveal that the tools are being used.
Keywords: drug audit, EHR tool, EMR tool, Epic, infliximab
Introduction
The cost and high use of infliximab at our institution prompted an investigation into opportunities for improvement within ordering and verification processes. This project intended to review both the baseline utilization of infliximab at a freestanding children's hospital as well as the effect of electronic health record (EHR) tools on optimizing use of infliximab.
Investigation into current infliximab ordering practices at our institution revealed safety concerns and opportunities for errors. One nurse practitioner was responsible for ordering most outpatient infliximab orders, and she used a manual system for collecting dosage and monitoring information for each infusion encounter. In addition, inconsistent methods of communication existed between the primary gastroenterologist assessing the patient in clinic and this provider. For example, planned dose changes or the need for additional monitoring labs were communicated by email or informal paper notes. Multiple patient safety event reports at our institution were submitted as a result of the ordering of an incorrect infliximab dose or interval owing to these poor communication pathways. Implementation of tools in the EHR to streamline communication was selected as a way to improve continuity of care and support more efficient infliximab prescribing.
Limited information is published on the use of EHR tools to optimize prescribing in the ambulatory arena. Koopman et al1 published an article about a dashboard tool within the health record developed to more efficiently collect the data needed for ambulatory diabetes care appointments. After implementation, a time study showed a decrease in the mean time to obtain pertinent data from 5.5 minutes to 1.3 minutes (p < 0.001), a decrease in the mean mouse clicks to obtain data from 60 to 3 (p < 0.001), and improved ability to correctly identify the pertinent data (94% vs. 100%, p = 0.01). The use of a dashboard improved the efficiency and accuracy of acquiring data required for high-quality diabetes care. Although this article displayed success in improving data collection for outpatient appointments, it did not address creation of a tool to specifically impact medication ordering. From this information, we saw value in the creation of a similar tool for outpatient ordering of infliximab.
The initial objective of this project was to assess the current use of infliximab at our institution and determine if the dosing was appropriate in reference to best practices in the literature, expert opinion, and practices at other institutions. Once this information was determined, we developed, implemented, and assessed EHR tools to streamline order communication and auditing.
Materials and Methods
The design of the project contained both retrospective and prospective elements; however, the main initiative was performance improvement to determine if infliximab was being used appropriately based on published literature. Secondarily, we strove to measure improvements made to streamline ordering. The project site, Nemours/Alfred I. duPont Hospital for Children, is a 195-bed freestanding pediatric hospital in Wilmington, Delaware, with an outpatient infusion clinic serving patients 5 days per week. The inpatient pharmacy is responsible for acquisition and preparation of all medications used in the infusion clinic. Institutional review board approval was pursued however this initiative was deemed to be a performance improvement project, and therefore not human subject research.
Phase I. This phase of the project focused on defining appropriate dosing regimens and analyzing baseline infliximab dosing. A literature review of existing evidence for use of infliximab in pediatrics, expert opinion discussions, results from a survey, and analysis of baseline use of infliximab at our institution were used to achieve a consensus definition of appropriate use. A comprehensive search of English-language sources including PubMed, Google Scholar, and EMBASE was used to determine the appropriate dose, interval, and indications for use of infliximab. Information gathered from this search was discussed with a panel of pediatric gastroenterologists at our institution to determine if current institutional practices differ from the principles established in the literature. Phase I also included distribution and analysis of a survey in research electronic data capture (REDCap)2 to a target audience of pediatric and adult pharmacists. The survey was distributed via 3 professional pharmacy organization forums and listserves (American Society of Health-System Pharmacists, American College of Clinical Pharmacy, Pediatric Pharmacy Advocacy Group), reaching hundreds of pharmacist members. Targeted information collected through the survey included prescribing patterns of infliximab; current use of EHR tools in ordering or verifying infliximab; sterile products preparation or infusion center workflow for infliximab; and any weight-based, drug concentration, or antibody-based monitoring protocols in place. In regard to the baseline analysis, data were collected on patients who received infliximab between July 1, 2013 and July 22, 2015. The data obtained include number of patients, number of prescribers, prescribing divisions, weight-based dosing of orders, weight-based dosing of orders per prescribing division, and inpatient versus outpatient administration.
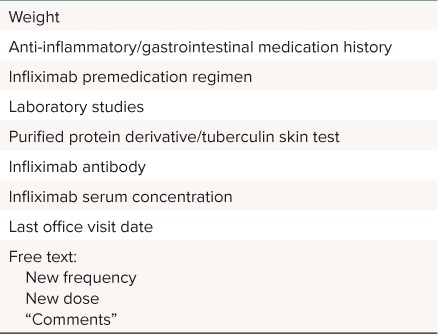
Phase II. Phase II focused on optimizing ordering through implementation of mandatory indications in the order pathway of infliximab and creation of a novel data collection tool. The indication field was a mandatory drop-down box in the medication order. Diagnoses included inflammatory bowel disease, rheumatologic diagnosis, and oncologic/transplant diagnosis. “Other” with a freetext entry was an option if a patient's indication was not well described by the list of three choices. The data collection tool was in the form of a questionnaire and flowsheet built within EPIC (Verona, WI) (Table 1). During the development phase of the project, stakeholders identified the need for a consistent method of relaying free-text patient data with the ability for storage in the EHR. Within EPIC, flowsheets are tools that typically collect numeric or categorical data from the chart, while questionnaires are tools that permit the collection of free-text patient data. In this combined tool, discrete data elements (i.e., weight, recent labs, and current prescriptions) automatically populated into the flowsheet, reducing the need for manual transcription. The new frequency, new dose, and comments sections of the questionnaire allowed prescribers to communicate future changes to the prescribing practitioner, as the data entered through those fields populated into the initial flowsheet. The questionnaire and flowsheet were developed in close collaboration with the division of gastroenterology to mimic their current paper data collection process. One important requirement for the success of this flowsheet was that it needed to display data across all patient encounters to provide a running log of all doses of infliximab and relevant labs, whether they occurred during a hospital stay, clinic visit, or an orders-only encounter. The tools were created by clinical informatics specialists and were reviewed and validated by pharmacy and gastroenterology stakeholders, with rapid feedback and changes as omissions or ideas for improvement arose. The initial electronic interventions were available to be viewed by anyone with electronic record access, however training and education in tool use and data entry was initially provided only to gastroenterology providers.
Table 1.
Data Included in the Infliximab Cross-Encounter Flowsheet
Phase III. This portion of the investigation assessed the usage and satisfaction with the EHR tools implemented during the previous phases. The mandatory indication field was assessed via a medication use evaluation of the prescribing disciplines and indications post intervention in comparison to the manually performed baseline analysis. Usage of the questionnaire and cross-encounter flowsheet were assessed by quantifying the frequency of data entry through the questionnaire compared to the total number of infliximab orders during the data collection period of December 1, 2015 to April 1, 2016. A postimplementation survey of prescribers and pharmacists regarding tool value was distributed in April 2016. Descriptive statistics were used for the analysis of data in this project.
Results
Evidence Review. A review of the literature and discussion with the attending and fellow physicians of the division of gastroenterology were used to define appropriate weight-based dosing criteria. Infliximab is a chimeric monoclonal IgG1 anti-TNFa antibody given by multiple intravenous infusions.3 It carries US Food and Drug Administration–labeled indications in pediatrics for Crohn's disease and ulcerative colitis, and its use is supported by evidence and clinical practice guidelines in both gastroenterology and rheumatology.4–10 In adult patients, evidence exists to support further benefit with doses up to 10 mg/kg/dose; and data from a trial of 21 patients, as well as a survey of 113 gastroenterologists, support the use of infliximab dosing greater than 5 mg/kg/dose as safe and effective in pediatric patients.10,11 Institutional dosing criteria are reported in Table 2.
Table 2.
Institutional Criteria for Dosing of Infliximab

The evidence review supported an appropriate weight-based dosing range of 3 to 10 mg/kg/dose. Prescribers suggested considering dose rounding in patients receiving intended doses of 10 mg/kg/dose with minor fluctuations in weight, resulting in doses up to 11 mg/kg/dose. Thus, our final appropriate weight-based dosing range was defined as 3 to 11 mg/kg/dose.
Baseline Analysis. Manual chart review of 2 years of data (July 1, 2013 to July 22, 2015) revealed a total of 3043 orders on 275 unique patients, with 33 unique prescribers ordering infliximab during this period. The division of gastroenterology prescribed 84.3% of the orders, followed by rheumatology (14.7%) and hematology and oncology (1%). Ninety-seven percent of the orders were administered in the outpatient setting (n = 2958). Weight-based dosing was reconciled and calculated on each order by using the dosing weight associated with the order (Table 3). The mean and median weight-based doses for the full data set were 7.74 mg/kg/dose and 7.81 mg/kg/dose, respectively. In gastroenterology patients, the mean dose was 7.66 mg/kg/dose and the median dose was 7.92 mg/kg/dose. According to the predefined appropriate weight-based dosing range of 3 to 11 mg/kg/dose, 93.8% of the orders for infliximab for any indication were appropriately dosed. In the subgroup of gastroenterology patients, 95.7% of orders were appropriately dosed.
Table 3.
Baseline Weight-Based Dosing of Infliximab

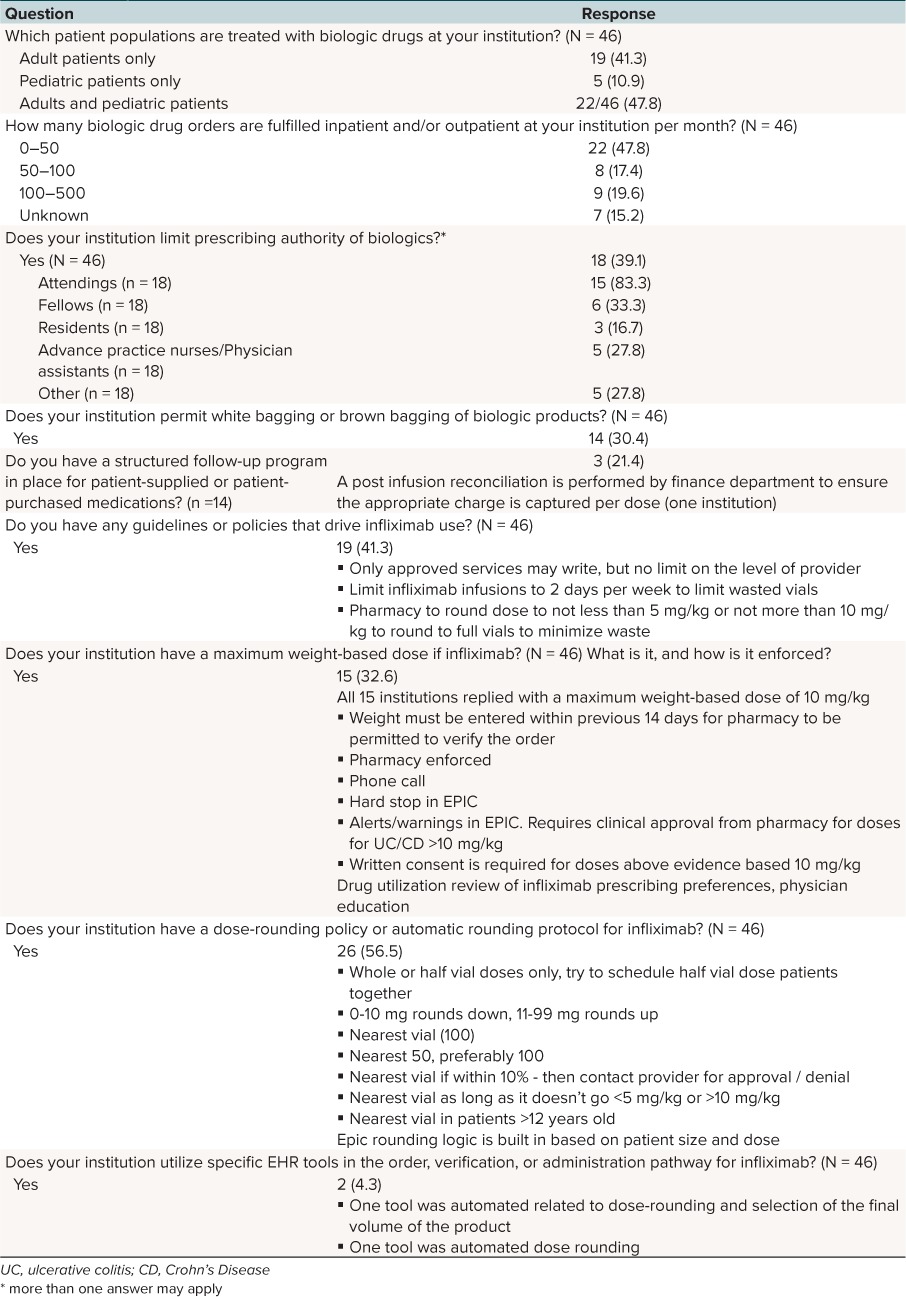
Survey to Other Institutions. Pharmacists from a total of 46 institutions completed the survey (Table 4). Forty-eight percent of the responses were from institutions caring for children and adults; 41% cared for only adults, and 11% cared for only pediatric patients. Respondents were asked about any institutional guidelines or policies that drive infliximab use: 19 institutions stated they have policies in place, including restriction of approved prescribing services, consolidating patients with doses that require a partial vial of infliximab, and pharmacy-driven dose-rounding protocols. Fifteen institutions responded to a question regarding a maximum weight-based dose, and the answers were unanimous at 10 mg/kg/dose. Enforcement of this weight-based parameter was variable, with institutions citing methods such as requirement of a weight within 14 days of infusion for pharmacy to verify the order, a hard stop alert in the health record, or pharmacy chart review and phone follow-up. Finally, 2 of the 46 institutions used specific EHR tools in the ordering, verification, or administration pathway for infliximab; both institutions used tools related to automatic dose rounding and volume standardization. There were no tools identified through the survey that addressed the appropriateness of the infliximab order.
Table 4.
Survey to Other Inpatient Pharmacies
Indications Audit Post Intervention. Mandatory indications for use of infliximab were implemented into the order pathway in October 2015. The indications list was developed and vetted by providers and pharmacists at both of our hospital sites and included Inflammatory Bowel Disease; Rheumatologic Diagnosis; Oncologic/Transplant Diagnosis; and Other Indication. An abbreviated list was approved to minimize the impact on prescriber workflow yet facilitate auditing of inappropriate indications for use. To minimize impact on workflow for the primary infliximab prescribers, “inflammatory bowel disease” was built as the default indication in the gastroenterology infliximab order set. During the period of October 22, 2015, and April 1, 2016, a total of 628 doses of infliximab were ordered and administered at our institution, and audit results showed that most orders were for patients with inflammatory bowel disease (92%), with 7% for rheumatology and 1% for hematology/oncology. Six orders were placed with an indication of “other” with no order comment. Upon manual chart review, all 6 orders were for patients with a history of inflammatory bowel disease, prescribed by gastroenterologists. We determined that it would not be possible to force a free-text entry for prescribers choosing “other” owing to limitations of the EPIC build. Follow-up education was provided, and no further erroneous indications were observed within the project period. The orders were predominantly administered in the outpatient areas (98%, n = 615). Evaluation for appropriate indications was facilitated by the mandatory indications compared to the previous method of manual chart review.
Flowsheet and Questionnaire Usage. A cross-encounter quality improvement flowsheet with an embedded “infliximab therapy update” for free text was developed (Figures 1 and 2). One important clarification was that this tool was independent of the workflow required to order infliximab. This tool was accessible during order entry and ideally would be used side-by-side during the ordering and verification processes. The tool was initially designed for use by the Division of Gastroenterology, but was freely searchable and available for use by other specialties prescribing infliximab.
Figure 1.

Data entry into interactive questionnaire.
Figure 2.

Free text and discrete data in the cross-encounter flowsheet.
During the period of December 1, 2015, through April 1, 2016, a total of 107 data entries were made by using the infliximab quality improvement questionnaire. These data were entered for 15.8% of patients who received infliximab during that period (27 of 171) and 14.9% of encounters for infliximab administration (74 of 495). Of the 15 gastroenterology prescribers trained on using the data entry tool, 60% entered data during the period. The prescribers who did not use the data entry tool during the pilot were infrequent infliximab prescribers and were provided additional education to encourage future use.
Usage of the tool was also assessed via a post-intervention survey. Providers were encouraged to participate in the survey via multiple scheduled email reminders over a 1-month period. Eleven of 21 targeted providers completed the survey (52.3%), representing 7 physicians (63.6%), 1 advanced practice nurse (0.9%) and 3 pharmacists (27.2%). The physician group consisted of gastroenterology prescribers, and the pharmacists included those who frequently encountered infliximab in their daily practices. All respondents noted that they know how to access the infliximab quality improvement questionnaire and flowsheet. All but 1 respondent noted they use the tool frequently (n = 6) or sometimes (n = 4), but all reported they planned to continue using the tool.
Of the providers who completed the survey, 82% stated that the infliximab quality improvement questionnaire and flowsheet frequently improve the safety (n = 9) and efficiency (n = 9) of infliximab prescribing. All of the respondents stated that they planned to continue to use the tools in their daily practices. Respondents provided feedback into specific patient scenarios where errors in dose or interval were avoided owing to the streamlined communication tools.
Time to Audit. An assessment of the time to perform an audit of infliximab prescriptions was performed pre and post implementation of the indications, questionnaire, and flowsheet. Before implementation, the time to assess 1 month of infliximab encounters and orders for appropriate dosing and indications took over 1 hour. Post tool development and validation, the time required for a pharmacist to audit 1 month of infliximab prescribing data was reduced to about 5 minutes owing to all of the pertinent data being assembled in 1 location in the EHR.
Discussion
Although the original intent of this project was to reduce inappropriate use of infliximab, our audits revealed overall appropriate indications and doses. In regard to communication flow, we noted several instances pre implementation where the primary gastroenterologist's plans to alter a patient's dose or infusion frequency were not implemented which could have negatively impacted patient outcomes. Through a literature search and survey results from multiple institutions we confirmed that this is the first reported questionnaire and flowsheet that allows free-text entry and discrete data within a single EHR tool. The creation of this tool has created a standard workflow for communication of pertinent patient and medication information, as well as planned changes between patient encounters. The ordering providers may access a single part of the chart to see all of the relevant information, continuously updated as new labs, weights or free text notes are added. Consistent usage of this standard workflow decreases opportunities for errors due to missed information and improves the transparency of the decisions surrounding infliximab ordering, allowing providers and pharmacists to appropriately intervene if a prescribing error has occurred. Compared with the previous practice of using paper forms to manually collect relevant patient data, there are fewer opportunities for transcription errors with a significant decrease in the time required to collect data. The combination of the communication tools and mandatory indications has decreased the time burden for auditing infliximab prescribing—the auditing provider or pharmacist has access to straightforward reports of utilization based on therapeutic indication, and the single-page flowsheet for further analysis of questionable or non-traditional use.
Some limitations exist to our approach of improving infliximab prescribing. The mandatory indications list has limited utility owing to the few, broad choices present for providers to select. Stakeholders were concerned about the impact of a lengthy indication list on prescriber workflow, as well as the utility of very specific indications for auditing (e.g., comparing and contrasting the utilization of infliximab for Crohn's disease versus ulcerative colitis and the possibility that a patient's disease may not clearly fit 1 or more of the chosen indications). Additionally, the major electronic intervention of this research was only piloted within 1 division (gastroenterology) which represented most, but not all, of the orders for infliximab pre and post intervention.
Another concern among institutions that use EPIC is the value of the free text data for the gastroenterology provider's plans. Some institutions address this situation by using “sign and hold” functions for the actual infliximab order. This is indeed a feasible alternative. Currently, the responsibility of ordering is delegated to 1 advanced practice nurse, and requiring gastroenterologists to place the order in “sign and hold” would disrupt their current workflow. If our institution were to begin using the “sign and hold” function, the gastroenterology providers would simply access the EHR tool before placing the “sign and hold” orders, the advanced practice nurse would still confirm the most recent lab results and prescriptions before order release, and nursing and pharmacy would still benefit from review of the flowsheet before verifying or administering the dose. In December 2016, the pharmacy department set an expectation that all pharmacists must review the flowsheet before verifying infliximab orders. We identified scenarios where the ordered dose was appropriate as based on the typical dosing range, but the actual dose for the patient may have been entered incorrectly when compared to the intended prescriber plan. The current workflow provides the added safety of a double-check; the gastroenterologist determines the plan and the advanced practice nurse enters the order based on that plan, and the pharmacist refers to the plan before verifying. Finally, we were unable to fully capture data regarding the use of the questionnaire and flowsheet owing to the manner in which the tools were built in the health record. The data collected reflect the frequency of data entry through the tools. Unfortunately, we could not audit how frequently prescribers, pharmacists, and nurses used the flowsheet in a read-only mode, which we would expect to be one of the most frequent ways this tool would be used.
Although an in-depth review of these errors was not the focus of our investigation, investigation of the safety benefits of this EHR tool is the focus of an ongoing project. A repeated audit 10 months after implementation revealed a total of 387 data entries through the questionnaire, with 14 of 15 gastroenterologists actively using the questionnaire and flowsheet. In addition, in-depth chart review during the same period revealed that the EHR tool was used, leading to correct and timely dosing in 78% (125/165) of patient encounters for infliximab infusion. Repeated survey of participating providers supplied feedback that the tool provided timely, successful implementation of instructed changes made by the primary gastroenterologists.
Future directions for this research include repeated audits of the appropriate dosing of infliximab, as well as utilization of these tools after they have been in place for some time. Development of a more accurate method of assessing the use of the communication workflow will provide more information into the tool's strengths, limitations, and opportunities for refinement. To further optimize the dosing of infliximab at our institution, we are pursuing the opportunity to insert weight-based dosing criteria or alerts within the order pathway of infliximab to improve adherence to our institutional infliximab dosing guidelines, as well as gain information about the provider and patient-specific factors motivating doses exceeding those recommended in the literature. Finally, the expansion of the communication workflow to other divisions is a future area of interest, with priority areas including rheumatology, solid organ transplant, and outpatient parenteral nutrition.
In conclusion, the creation of mandatory indications and an interactive cross-encounter flowsheet has improved the ability to assess appropriateness of infliximab use and facilitated the communication of important order information. Prescribers and pharmacists believe that these tools improve the safety and efficiency of infliximab ordering, and auditing data reveal that the tools are being used.
Acknowledgments
This report was presented as a platform presentation at the 2016 Eastern States Conference in Hershey, Pennsylvania. The authors thank Syed Abidi and the Nemours Health Informatics team for technical assistance in building the electronic health record tools. The authors also thank the Division of Gastroenterology of Nemours/Alfred I. duPont Hospital for Children, Wilmington, Delaware, for validation of the tools.
ABBREVIATIONS
- EHR
electronic health record
Footnotes
Disclosures The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1. Koopman R, Kochendorfer K, Moore J, . et al. A diabetes dashboard and physician efficiency and accuracy in accessing data needed for high-quality diabetes Care. Ann Fam Med. 2011; 9 5: 398– 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris P, Taylor R, Thielke R, . et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42 2: 377– 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. REMICADE (infliximab) [prescribing information]. 2015. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/REMICADE-pi.pdf. Accessed November 19, 2017.
- 4. Clark M, Colombel J, Feagan B, . et al. American Gastroenterological Association Consensus Development Conference on the Use of Biologics in the Treatment of Inflammatory Bowel Disease, June 21–23, 2006. Gastroenterology. 2007; 133 1: 312– 339. [DOI] [PubMed] [Google Scholar]
- 5. Turner D, Levine A, Escher J, . et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012; 55 3: 340– 361. [DOI] [PubMed] [Google Scholar]
- 6. Regan B, Bousvaros A.. Pediatric ulcerative colitis: a practical guide to management. Pediatr Drugs. 2014; 16 3: 189– 198. [DOI] [PubMed] [Google Scholar]
- 7. Sandhu B, Fell J, Beattie R, . et al. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010; 50 suppl 1: S1– S13. [DOI] [PubMed] [Google Scholar]
- 8. Colombel J, Sandborn W, Reinisch W, . et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362 15: 1383– 1395. [DOI] [PubMed] [Google Scholar]
- 9. Park K, Sin A, Wu M, . et al. Utilization trends of anti-TNF agents and health outcomes in adults and children with inflammatory bowel diseases. Inflamm Bowel Dis. 2014; 20 7: 1242– 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldassano R, Braegger C, Escher J, . et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003; 98 4: 833– 838. [DOI] [PubMed] [Google Scholar]
- 11. Nattiv R, Wojcicki JM, Garnett EA, . et al. High-dose infliximab for treatment of pediatric ulcerative colitis: a survey of clinical practice. World J Gastroenterol. 2012; 18 11: 1229– 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruperto N, Lovell D, Cuttica R, . et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007; 56 9: 3096– 3106. [DOI] [PubMed] [Google Scholar]


