Abstract
Background
Ferritins are ubiquitous multi-subunit iron storage and detoxification proteins that play a critical role in iron homeostasis. Ferrous ions that enter the protein's shell through hydrophilic channels are rapidly oxidized at dinuclear centers on the H-subunit before transfer to the protein's cavity for storage. The mechanisms of iron loading have been extensively studied but little is known about iron mobilization. Fe(III) reduction can occur via rapid reduction by suitable reducing agents followed by chelation of Fe(II) ions or via direct and slow Fe(III) chelation. Here, the iron release kinetics from ferritin by FMNH2 in the presence of various chaotropic agents are studied and their in-vivo physiological significance discussed.
Methods
The iron release kinetics from horse and human ferritins by FMNH2 were monitored at 522 nm where the Fe(II)–bipyridine complex absorbs. The experiments were performed in the presence of different concentrations of three chaotropic agents, urea, guanidine HCl, and triton.
Results and Conclusions
Under our experimental conditions, iron reductive mobilization by the non-enzymatic FMN/NAD(P)H system is limited by the concentration of FMNH2 and is independent on the type or amount of chaotropes present. Diffusion of FMNH2 through the ferritin pores is an unlikely mechanism for ferritin iron reduction. An iron mobilization mechanism involving rapid electron transfer through the protein shell is discussed.
General significance
Caution must be exercised when interpreting the kinetics of iron mobilization from ferritin using the FMN/NAD(P)H system. The kinetics are highly dependent on the amount of dissolved oxygen and the concentration of reagents used.
Introduction
Iron is a critical nutrient required for virtually all living organisms. It is a component of many cellular processes including respiration, electron transfer reactions, energy metabolism, DNA synthesis, and gene regulation 1. Due to the wide range of redox potentials (from approximately − 500 mV to + 600 mV), iron participates in a wide variety of oxidation–reduction reactions. At the same time, labile iron ions can participate in harmful free radical reactions through the generation of highly reactive hydroxyl radicals capable of causing permanent damage to DNA and proteins2-4. One of the means to protect cells from the potentially toxic effects of free iron and radical chemistry is ferritin, a ubiquitous and well-characterized iron storage and detoxification protein capable of sequestering thousands of iron atoms in the form of a biologically available iron inorganic core5-8. In bacteria, plants, fish and amphibians, ferritins are homopolymers composed of H-type subunits, while in animals, they typically consist of 24 similar subunits of two types, H and L. Exceptions are human mitochondrial ferritin, a homopolymer composed of 100% Mt-subunits similar to H-subunits9, 10, and human serum ferritin, a homopolymer composed of ∼100% L-subunits11. The H-subunit is responsible for the rapid oxidation of Fe(II) to Fe(III) at a dinuclear center, whereas the L-subunit helps iron clearance from the ferroxidase center of the H-subunit and supports iron nucleation and mineralization.
Iron(II) cations are shown to enter and exit the ferritin cavity via the approximately 4 Å wide eight hydrophilic three-fold channels (or pores)5, 7, 12. While the mechanism of iron uptake, oxidation and deposition is relatively well understood5, 6, 12, the process by which iron is mobilized from ferritin remains unclear and rather controversial. The degradation of the ferritin shell in lysosome or proteasome appears to play a major role in iron mobilization. The proteolysis of the ferritin shell exposes the inorganic iron core to the cellular environment where the presence of iron(III) reducing and iron(II) chelating species allow it to become part of the labile iron pool 13, 14. However, this degradation process is relatively slow when compared to the much faster and efficient release mechanisms (i.e. a few minutes) that are shown to rapidly mobilize iron cations from intact ferritin. While the ferritin iron core is an extremely stable entity in the absence of reducing agents, numerous in-vitro studies performed in cell-free conditions showed rapid mobilization of iron(III) cations through reduction by flavin mononucleotide (FMN) 15, 16 ascorbate, 17, 18 glutathione,16 sodium dithionite, 19 polyphenols, 20, 21 superoxide, 22, 23 and other agents8, 24, 25. In the absence of reducing agents, iron(III) cations can be released from ferritin by hydroxamates, 26 catechols, 21 and other synthetic chelators 27 with or without prior reduction to iron(II) cations. 28 Because FMNH2 is produced from FMN and nicotinamide adenine dinucleotide NAD(P)H by cellular flavin reductases, 16 one of the possible candidates or mechanisms through which iron is released from ferritin in-vivo is the reductive mobilization by reduced flavin mononucleotide (FMNH2).
It has been reported that the rate of reductive mobilization of the inorganic ferritin iron core by FMNH2 is significantly increased in the presence of chaotropes such as urea, GndHCl, triton,29 or short peptides30, 31, and is significantly enhanced by local unfolding of the three-fold channels, an effect similar to that observed in three-fold channel mutants having structurally altered pores. However, earlier studies15, 16 have shown that, under anaerobic conditions, the rate of reduction of ferritin iron core by FMNH2 is about two orders of magnitude faster than in the presence of chaotropes, suggesting that the rate of iron mobilization is limited by the availability of FMNH2, raising doubts about the suggested ferritin-pore gating effects. This is further exacerbated by a recent study from our laboratory showing that the rate of iron reductive mobilization from ferritin by flavins is highly dependent on the concentration of dissolved oxygen in solution.32 To address these discrepancies, a series of kinetic experiments under tightly controlled oxygen atmospheres were performed to evaluate the effect of ferritin-pore gating as well as the effect of chaotropes on the reductive mobilization of iron from ferritin by reduced flavin mononucleotide (FMNH2). Our results indicate that the rate of iron release from ferritin is limited by the concentration of available FMNH2 and that chaotropes do not influence the rate of iron mobilization from ferritin. We found that encapsulation of FMN inside the ferritin cavity prevented its diffusion outside the protein shell, suggesting that diffusion of FMNH2 through the ferritin pores is an unlikely mechanism for ferritin iron reduction. An iron mobilization mechanism involving rapid electron transfer through the protein shell to the iron mineral core is discussed.
Materials and Methods
Flavin mononucleotide (FMN), nicotinamide adenine dinucleotide NADH, and iron-containing horse spleen ferritin (holo-HosF) were obtained from Sigma-Aldrich and used without further purification. Bipyridine, guanidine HCl, and triton X-100 (Sigma-Aldrich), and urea (J.T Baker Chemical Company) were purchased and used as received. Human recombinant heteropolymer apoferritin (∼ 20H- and 4L-subunits) was prepared as described elsewhere33, 34 and manually loaded with 500 iron atoms in the presence of oxygen in the form of 10 additions of 50 Fe(II)/shell with 10-15 minutes between additions. Unless otherwise stated, all experiments were conducted at 25 °C, in 100 mM MOPS buffer and 50 mM NaCl, pH 7.0. The iron release kinetics were performed on a Varian Cary 50 Bio, Cary 100 or Lambda 35 spectrophotopmeters. The concentration of the iron cations released from ferritin was measured by following the absorption of the Fe(II)-bipyridine complex at ∼ 520-525 nm (ε = 8650 M-1cm-1). The iron content of horse spleen ferritin was ∼ 2200 iron(III)/ferritin, as reported by the manufacturer.
Kinetics of reductive release of iron(III) from ferritin using the FMN/NADH system
The iron release kinetics were performed in either 1mm or 1cm UV-vis quartz cuvette under both anaerobic and aerobic conditions, respectively. Solutions of holo-horse spleen ferritin (0.02 μM) or recombinant holo-human heteropolymer ferritin (0.05 - 0.3 μM) were prepared in 100 mM MOPS, 50 mM NaCl, pH 7.0 and mixed with NADH (3.2 mM), 2,2′-bipyridine (3.2 mM), and FMN (3.2 mM), in the presence of different concentrations of chaotropic agents (i.e. 0.001 − 2 M urea, 0.1-10 mM guanidine HCl, or 0.1-10 % v/v triton) as indicated in the figure captions. Soon after the addition of all reagents in a 2 ml vial, the solution was rapidly inverted several times for thorough mixing. The 1 mm path UV-vis quartz cuvette (650 μL volume) was purged with argon, filled to the brim with the resultant solution, and then stoppered with a septum making sure that no air pockets or bubbles were trapped inside. For the anaerobic conditions, the protein samples were first degassed for 20-30 min using a degassing station from TA Instruments. For experiments involving FMNH2, a different degassing procedure was employed (more below). For comparison purposes, the same experiments were repeated aerobically in a 1 cm UV-vis quartz cuvette with a head space and a screw-on cap. For these aerobic experiments, the lag phase preceding the rise in absorbance at 522 nm varied in length (or time) and is likely due to the amount of dissolved oxygen present in solution. For the iron release experiments in the presence of urea, the ferritin solutions were either incubated at ambient temperature (or in the fridge) for 24 h, or within an hour of adding urea. In all cases, the results were identical within experimental errors, and all experiments were performed two to three times to ensure reproducibility.
Iron release kinetics from horse spleen ferritin by FMNH2 under anaerobic conditions
All experiments involving the direct use of FMNH2 were conducted under strictly anaerobic conditions and positive pressure of argon. All stock solutions were thoroughly degassed using at least five cycles of vacuuming (2 min each) while stirring. The stock solutions were intermittently flushed with pure argon to help with the deoxygenation procedure. The FMNH2 solution was prepared by catalytic hydrogenation of a solution of FMN (0.10 mmol) in water (200 mL) over 10% Pd/C (2 mg). After 24 hrs of hydrogenation, the solution contained about 67 % FMNH2 (i.e. 334 μM) and 33% FMN (i.e. 166 μM) as determined by UV-Vis spectroscopy and was used in the kinetic experiments without further purification.
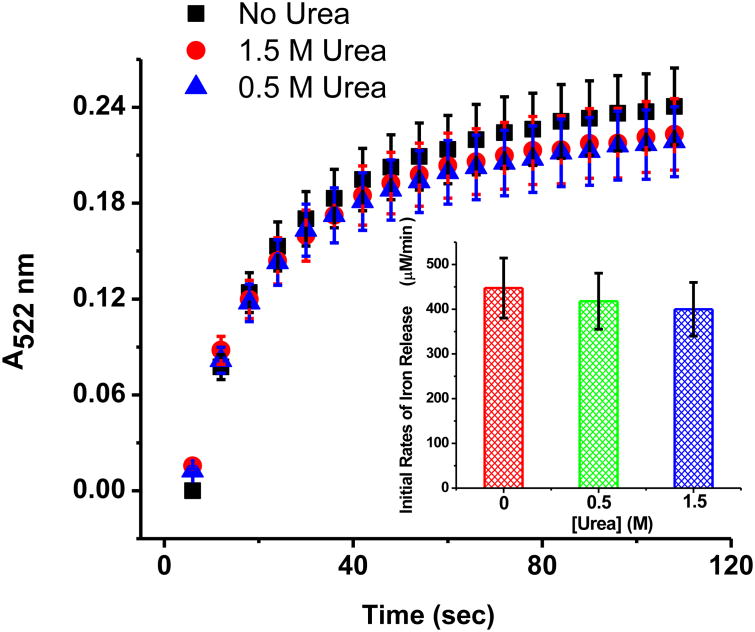
For the kinetic experiments, 0.5 ml of the FMNH2 stock solution was injected into a de-oxygenated 2 mm optical pass quartz cuvette containing 0.5 mL of a degassed holo-HosF solution, 2,2′-bipyridine, and urea, in 100 mM MOPS buffer, 50 mM NaCl buffer, pH 7.0. After mixing, the solution contained 167 μM FMNH2, 87 μM FMN, 0.35 μM horse spleen ferritin, 1 mM 2,2′-bipyridine, and various concentrations of urea as indicated in the caption of figure 3. The ferritin demineralization reaction was monitored for a total of 2 minutes (one scan every 5 seconds) using scanning kinetics between 350 nm - 600 nm. Each experiment in the absence or presence of 0.5 M or 1.5 M urea was repeated three to four times to ensure reproducibility.
Figure 3.
Kinetics of iron release from holo-HosF by FMNH2 under anaerobic conditions in the absence or presence of 0.5 M or 1.5 M urea. Conditions: 0.35 μM holo-HosF (∼ 2200 Fe/shell), 167 μM FMNH2 (initial concentration), 2 mm path length, 1 mM Bipy, 100 mM Mops, 50 mM NaCl, pH 7.0, 25 °C.
Encapsulation of FMN in horse spleen apoferritin
A mixture of apoferritin (2 mg in 50 μL water) and FMN (10 μL of 50 mM in water) was acidified to pH 2 with 0.02M HCl and shaken for 10 min. The pH was then adjusted to 7.0 using 20 μL of a saturated solution of NaHCO3 (0.96 g in 10 ml water). The resultant solution was set for 2 h at 4 °C before one-tenth aliquot was transferred onto ½″ Sephadex G-25 chromatography column for visualization of the different fractions of FMN@apoferritin, FMN, and a mixture of the two.
Results
The rates of reductive mobilization of iron from ferritin have been previously followed at 520-530 nm using the NADH/FMN/2,2′-bipyridine(bipy) system29, 32 where the Fe(II)-bipy3 complexes absorb (ε = 8650 M-1cm-1). In our experimental set up, a quartz UV cell with 1 mm optical path was filled to the brim with the protein solution containing all reagents. This procedure allowed us to exclude any effect from trapped oxygen bubbles on top of the solution and to minimize atmospheric oxygen diffusion inside the cell.
Effect of urea on the rates of iron release from ferritin
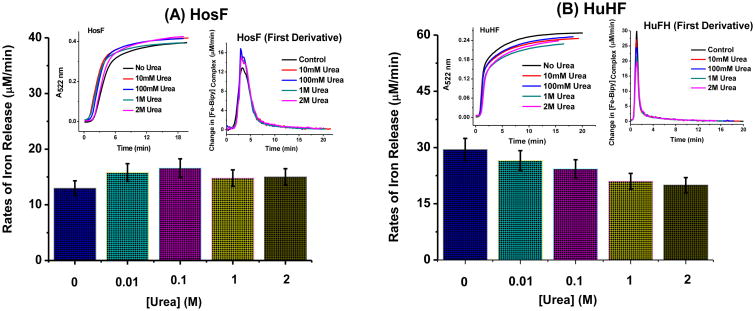
To evaluate the effects of chaotropes on the rates of iron release from ferritin, experiments were first performed in the presence of 0 to 2 M concentration of urea (Fig. 1). The induction period (or initial lag phase) varied from run to run and is dependent on the residual amount of dissolved oxygen initially present in solution. During that initial phase, the rate of iron release is extremely small due to the fact that almost all of the FMNH2 produced from FMN and NADH is immediately re-oxidized by dissolved oxygen. Soon after the oxygen is fully consumed, the rate of iron(II) release dramatically increases and has its maximum value at ∼ 125 s for HosF and ∼ 47 sec for HuHF (Figs. 1A, 1B, insets, respectively). The rates thereafter start to gradually decrease and level off at ∼ 8 min for HosF and ∼ 3 min for HuHF.
Figure 1.
Rates of iron release from holo-horse spleen ferritin (containing ∼ 2200 FeIII/shell) (A) and recombinant holo-human heteropolymer ferritin (containing ∼ 500 FeIII/shell) (B) in the presence of various concentrations of urea as indicated on the plot. The insets show changes in the absorbance of the Fe(II)-bipy3 complex at 522 nm versus time and the first derivatives or changes in the [Fe(II)-bipy3]complex formation rate with time for both holo-HosF and holo-HuHF. Each experiment was run in triplicate with the error bars corresponding to the standard deviation of the three trials shown. Conditions: 0.02 μM holo-HosF or 0.05 μM holo-HuHF, 3.2 mM NADH, 3.2 mM FMN, 3.2 mM bipyridine, in 100 mM MOPS buffer and 50 mM NaCl, pH 7.0, 25 °C, 1 cm quartz UV cuvette.
Similar experiments were performed under anaerobic conditions using a 1 mm cuvette without a head space and degassed solutions. In these experiments, additions of NADH and 2,2′-bipyridine solutions were made to degassed solutions of holo-HosF after incubation for 24 h with 0 - 2 M urea. The resultant solution was mixed with a small amount of oxygen-free solution of FMN and rapidly transferred through a septum to a UV-cell pre-flushed with argon. Very similar patterns of iron release kinetics were observed in the absence or presence of various concentrations of urea, whether the closed system consisted of experiments conducted under anaerobic experiments in the 1 mm cuvette or in the 1 cm cuvette with a screw-on cap and a head space.
Effect of guanidine/HCl and triton
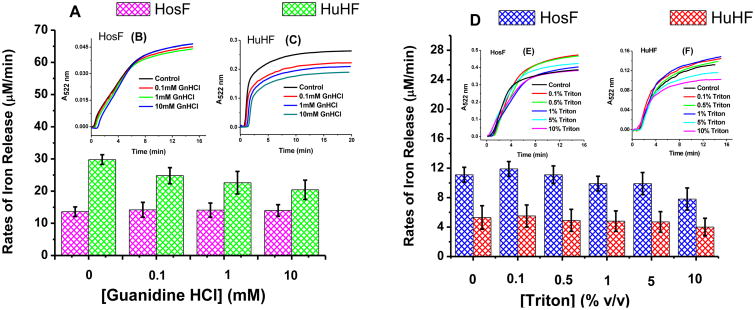
To examine the effect of other chaotropes on the kinetics of iron release from ferritin, the above experiments were repeated in the presence of various concentrations of guanidine/HCl and triton (Fig. 2). Here again, the rates of iron release from the two ferritin samples (holo-HosF and holo-HuHF) were similar within experimental uncertainties.
Figure 2.
Rates of iron release from holo-HosF and holo-HuHF in the presence of various concentrations of guanidine HCl (0-10 mM) (A) and triton (0.1% - 10% v/v) (D). The insets show changes in the absorbance of the Fe(II)-bipy3 complex versus time. Conditions: (B) 0.02 μM holo-HosF (1 mm quartz UV cuvette) and (C) 0.05 μM holo-HuHF (1 cm UV cuvette); (E) 0.02 μM holo-HosF and (F) 0.02 μM holo-HuHF (1 cm quartz UV cuvette), 3.2 mM NADH, 3.2 mM FMN, 3.2 mM bipyridine, in 100 mM MOPS buffer, 50 mM NaCl, pH 7.0, and 25 °C. Each experiment was run in triplicate with the error bars corresponding to the standard deviation between the three trials shown.
Kinetics of iron release from ferritin by FMNH2 under anaerobic conditions
To circumvent potential problems with the re-oxidation of FMNH2 produced in-situ by the reaction of FMN with NADH in the presence of oxygen, the iron release kinetics from ferritin were repeated under strictly anaerobic conditions using a solution of FMNH2 prepared by catalytic hydrogenation of a FMN solution, as explained above. The reaction progress was monitored by scanning kinetics following the addition of a solution of FMNH2 to a de-oxygenated quartz UV-vis cuvette containing a de-oxygenated solution of horse spleen ferritin, 2,2′-bipyridine, and urea. Figure 3 showed very rapid iron release kinetics upon addition of FMNH2 with similar initial rates (i.e. 422 ± 24 μM/min) in the absence or presence of 0.5 M or 1.5 M urea. It should be mentioned that the observed t½ in these fast reactions is approximately 30 sec, a result very similar to previously published data on the reduction of holo-horse spleen ferritin by FMNH2 in the absence of chaotropes.16
Encapsulation of FMN inside the ferritin cavity
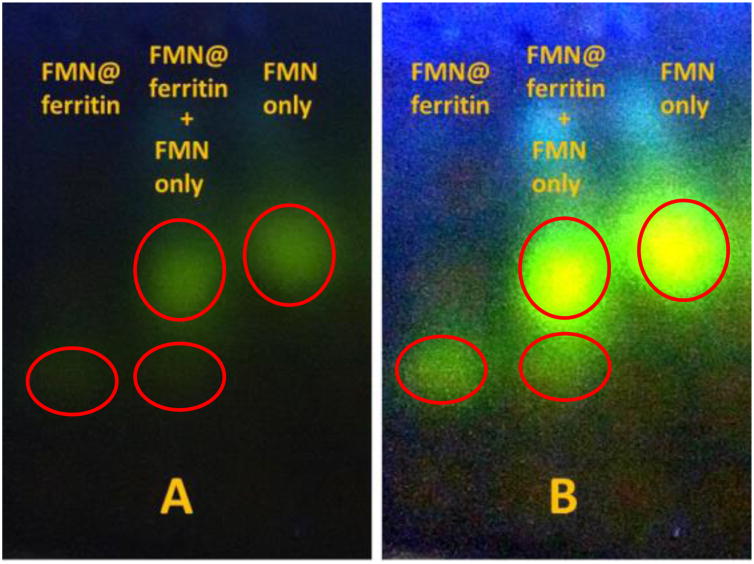
While reduction of the ferritin inorganic iron core by FMNH2 was reported a long time ago, its mechanism is a subject of controversies. Initially, a mechanism based on diffusion of FMNH2 into the interior of ferritin through either three- or four-fold channels (pores) was proposed.16 However, multiple recent reports painted a much different picture and casted doubt about the validity of the proposed pore diffusion mechanism in light of the inability of small molecules including cisplatin,34 anthocyanin,36 beta-carotene,37 and curcumin38 to diffuse through the ferritin pores and be trapped inside. Given the comparable size of flavin mononucleotide to the aforementioned molecules, we explored the ability of FMN to diffuse out of the ferritin pores following its encapsulation inside the protein's cavity. To that end, solutions of FMN and horse spleen apoferritin (iron-free) were acidified to pH 2 resulting in the dissociation of the ferritin nano-structure into its individual subunits. When the pH of the solution was adjusted to 7, the ferritin molecule reconstituted with FMN molecules trapped inside. The reaction mixture was then subjected to separation by thin layer Sephadex gel chromatography 39 (Fig. 4). Two distinct yellow fluorescent bands were observed with the first fraction exhibiting UV-vis spectral features consistent with a mixture of ferritin and FMN while the second fraction was exclusively composed of pure FMN. The results of figure 4 show no diffusion of FMN outside the ferritin shell suggesting stable entrapment of FMN inside the protein's cavity.
Figure 4.
Photo of thin layer Sephadex G-25 chromatographic separation of FMN@ferritin fraction (left), FMN fraction (right), and its mixture (middle) under 365 nm UV light irradiation. (A) Original photo (raw data). (B) Contrast enhancement using Image J software (0.4% saturated pixels settings).
Discussion
Our study of the effect of chaotropes on the reductive release of iron cations from ferritin by the non-catalytic FMN/NADH/2,2′-bipyridine system employed very similar experimental conditions to those reported in earlier studies 29, 30 to allow direct comparison of results. The one major difference is that our experimental protocol excluded the possibility of oxygen diffusion into the reaction mixture by using a quartz cuvette fitted with a tightly screwed-on cap without trapped air pockets. In all our experiments, we observed an initial induction period (i.e. a lag phase with no or minimal iron release) followed by an instantaneous release of iron and then a gradual decrease in the release rate due to complete dissolution of the iron hydroxide core inside ferritin. As determined in one of our earlier studies32, the initial induction period is due to the oxidation of the generated FMNH2 by dissolved oxygen since the rate of such reaction is extremely high.40 For solutions exposed to air, the rates of iron reduction can be influenced by the oxidation of FMNH2 by oxygen which can easily diffuse into the upper layers of the solution that is in direct contact with air, while the deeper inner layers remain oxygen free. Such scenario might create different absorbance readouts and thus different rates of iron release, depending on the stirring rate and where the incident monochromatic beam hits the cuvette containing the ferritin solution.
Under our experimental conditions where diffusion of oxygen is excluded, the similarity of the iron release kinetics in the absence and presence of urea (0-2 M) suggests a lack of any appreciable effect of urea on the rate of iron core reduction in human or horse spleen ferritin (Fig. 1). Our results are in disagreement with earlier studies showing a considerable increase in the rates of iron release from ferritin by urea. 29 Although recombinant frog ferritin was employed in these experiments, it is highly unlikely that the ferritin source is a contributing factor to the differences in the iron release kinetics given the high similarity of the overall 3D-structure and sequence identity between frog, horse and human ferritins. We believe that the discrepancy in our findings could be due to recombinant frog ferritin experiments being conducted in a reaction cell that is fully exposed to air. Under these conditions, the continuous supply and diffusion of oxygen into the solution contribute to potentially large variations in the concentrations of available FMNH2 since the latter is known to rapidly react with dissolved oxygen32, 40, thus affecting the overall rate of the reductive release of iron in areas where diffusion takes place. Furthermore, the uncertainty of the oxygen solubility in solutions of chaotropes, and the solution stirring rate are additional parameters that could further complicate the analysis of the iron release kinetics particularly when the protein solutions are exposed to air, as we suspect was the case in the earlier studies 29, 30. In contrast, our methodology precludes direct contact with oxygen in the air, and ensures fully homogeneous reaction mixtures thus providing direct information about the iron release kinetics. Even in the case of no direct contact with oxygen in air, the experimental rates of iron release depends on both the rate of generation of FMNH2 by the non-catalytic FMN/NADH reaction and the rate of FMNH2 reaction with ferritin. Thus, the complexity of this system could obscure direct observations of the actual influence of chaotropes on the rate of ferritin iron core reduction. To eliminate these uncertainties, we studied the effect of urea concentration on the rate of iron reduction in ferritin using pure FMNH2 prepared by catalytic hydrogenation as described in the Material and Methods section. As can be seen in Fig. 3, the iron release kinetics were essentially identical in the absence or presence of urea (0.5 M or 1.5 M), again confirming our hypothesis that urea does not exhibit any measurable effect on the rates of iron release from ferritin. Thus, we conclude that the kinetics of the reductive mobilization of iron from ferritin by FMNH2 are not affected by the presence of chaotropes (i.e. urea, GnHCl, or triton).
These results prompted us to re-examine the reaction mechanism pertaining to FMNH2 diffusion across the ferritin shell that was originally proposed many years ago 16. The stable encapsulation of FMN molecules inside horse spleen apo-ferritin, as with other previously entrapped molecules, suggests that the rate of diffusion of these molecules (including FMNH2) outside of the ferritin shell is extremely slow and in stark contrast with the rapid reductive mobilization of iron from ferritin by flavins. Significantly, diffusion of reduced flavins through the ferritin shell was originally proposed15 based on a single experiment showing that agarose-immobilized FMNH2 generated from a reaction of agarose-immobilized FMN with NADH is unable to reduce ferritin. These experiments not only failed to account for ferritin diffusion through cross-linked agarose beads, but fell short of considering possible changes in reactivity of reduced flavins caused by agarose binding. The inability of the diffusion-based mechanism to explain the high rates of iron release from ferritin prompted us to consider a more relevant mechanism involving rapid electron transfer across the protein shell.41 Notably, a recent study42 provided strong evidence for rapid electron transfer across the ferritin shell during iron deposition into L-chain ferritin, in support of electron transport mechanisms being at work during the reductive mobilization of iron from ferritin. Because the rapid reduction of iron(III) cations in ferritin by FMNH2 has been shown to occur in a variety of ferritins, the seemingly electron transfer mechanism appears to be a general feature for iron deposition and mobilization in ferritin and is likely to play an important role in iron homeostasis as well. However, despite its high potential to reduce ferritin iron core, the rapid oxidation of FMNH2 by dissolved oxygen32 preclude it from being a physiologically relevant reducing agent. Indeed, the physiological relevance of that process is a subject of controversy (as discussed in ref. 32) but should not affect our results in terms of the influence of chaotropes on the iron release kinetics from ferritin. Additional studies aimed at identifying other intracellular reducing agents that are capable of reducing the ferritin mineral core are needed.
In summary, our results showed that the presence of chaotropes had no effect on the reductive mobilization of iron from ferritin by FMNH2 prepared either by catalytic hydrogenation or by in-situ reaction of FMN with NADH under conditions of controlled oxygen concentrations, and are in marked contrast to earlier reports29. We found that encapsulation of FMN inside the ferritin cavity prevented its diffusion outside the protein shell, suggesting that diffusion of FMNH2 through the ferritin pores is an unlikely mechanism for ferritin iron reduction. Our data is in support of an iron mobilization mechanism through which electrons are rapidly transferred through the protein shell to the iron mineral core.
Highlights.
Chaotropes do not affect rates of iron mobilization from ferritin by FMNH2
The rates of iron release from ferritin are heavily dictated by molecular oxygen
Flavin mononucleotide cannot diffuse through ferritin pores to reduce the iron core
Iron mobilization by FMNH2 likely involves electron transfer via the ferritin shell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silva B, Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015;1852(7):1347–1359. doi: 10.1016/j.bbadis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Imlay JA. Pathways of oxidative damage. Annual Review of Microbiology. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 3.Turrens JF. Mitochondrial formation of reactive oxygen species. Journal of Physiology-London. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Bou-Abdallah F. The iron redox and hydrolysis chemistry of the ferritins. Biochimica et Biophysica Acta (BBA)-General Subjects. 2010;1800(8):719–731. doi: 10.1016/j.bbagen.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 7.Chasteen ND. Ferritin. Uptake, storage, and release of iron. Metal ions in biological systems. 1998;35:479. [PubMed] [Google Scholar]
- 8.Hynes MJ, Coinceanainn MiÌnÃ. Investigation of the release of iron from ferritin by naturally occurring antioxidants. Journal of inorganic biochemistry. 2002;90(1):18–21. doi: 10.1016/s0162-0134(02)00383-5. [DOI] [PubMed] [Google Scholar]
- 9.Drysdale JW, Adelman TG, Arosio P, Casareale D, Fitzpatrick P, Harzard JT, Yokota M. In Human isoferritins in normal and disease states. Seminars in hematology. 1977;1977:71–88. [PubMed] [Google Scholar]
- 10.Rucker P, Torti FM, Torti SV. Recombinant ferritin: modulation of subunit stoichiometry in bacterial expression systems. Protein engineering. 1997;10(8):967–973. doi: 10.1093/protein/10.8.967. [DOI] [PubMed] [Google Scholar]
- 11.Bou-Abdallah F, Santambrogio P, Levi S, Arosio P, Chasteen ND. Unique iron binding and oxidation properties of human mitochondrial ferritin: a comparative analysis with human H-chain ferritin. Journal of molecular biology. 2005;347(3):543–554. doi: 10.1016/j.jmb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Theil EC, Takagi H, Small GW, He L, Tipton AR, Danger D. The ferritin iron entry and exit problem. Inorganica Chimica Acta. 2000;297(1):242–251. [Google Scholar]
- 13.Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. American Journal of Physiology-Cell Physiology. 2006;291(3):C445–C455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- 14.Mehlhase J, Sandig G, Pantopoulos K, Grune T. Oxidation-induced ferritin turnover in microglial cells: role of proteasome. Free Radical Biology and Medicine. 2005;38(2):276–285. doi: 10.1016/j.freeradbiomed.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Jones T, Spencer R, Walsh C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogs. Biochemistry. 1978;17(19):4011–4017. doi: 10.1021/bi00612a021. [DOI] [PubMed] [Google Scholar]
- 16.Sirivech S, Frieden E, Osaki S. The release of iron from horse spleen ferritin by reduced flavins. Biochemical Journal. 1974;143(2):311–315. doi: 10.1042/bj1430311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer RF, Grabill TW, Petrovich RM. Reductive release of ferritin iron: a kinetic assay. Analytical Biochemistry. 1988;174(1):17–22. doi: 10.1016/0003-2697(88)90513-1. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai K, Nabeyama A, Fujimoto Y. Ascorbate-mediated iron release from ferritin in the presence of alloxan. Biometals. 2006;19(3):323–333. doi: 10.1007/s10534-005-1300-x. [DOI] [PubMed] [Google Scholar]
- 19.Funk F, Lenders JP, Crichton RR, Schneider W. Reductive mobilisation of ferritin iron. The FEBS Journal. 1985;152(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09177.x. [DOI] [PubMed] [Google Scholar]
- 20.Ji X, Huang L, Lin Q, Huang H. Characteristics and Kinetics of Iron Release from the Ferritin under the EGCG reduction. Biological trace element research. 2012;146(1):134–140. doi: 10.1007/s12011-011-9225-4. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez P, Gálvez N, Colacio E, Minones E, Dominguez-Vera J. Catechol releases iron (III) from ferritin by direct chelation without iron (II) production. Dalton Transactions. 2005;(4):811–813. doi: 10.1039/b416669h. [DOI] [PubMed] [Google Scholar]
- 22.Bolann BrJ, Ulvik RJ. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochemical Journal. 1987;243(1):55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteiro HP, Winterbourn CC. The superoxide-dependent transfer of iron from ferritin to transferrin and lactoferrin. Biochemical Journal. 1988;256(3):923–928. doi: 10.1042/bj2560923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oteiza PI, Kleinman CG, Demasi M, Bechara EJH. 5-Aminolevulinic acid induces iron release from ferritin. Archives of Biochemistry and Biophysics. 1995;316(1):607–611. doi: 10.1006/abbi.1995.1080. [DOI] [PubMed] [Google Scholar]
- 25.Kontoghiorghes GJ. Iron mobilization from ferritin using α-oxohydroxy heteroaromatic chelators. Biochemical Journal. 1986;233(1):299–302. doi: 10.1042/bj2330299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvez N, Ruiz B, Cuesta R, Colacio E, Dominguez-Vera JM. Release of iron from ferritin by aceto-and benzohydroxamic acids. Inorganic Chemistry. 2005;44(8):2706–2709. doi: 10.1021/ic048840s. [DOI] [PubMed] [Google Scholar]
- 27.Bou-Abdallah F, McNally J, Liu XX, Melman A. Oxygen catalyzed mobilization of iron from ferritin by iron(III) chelate ligands. Chemical Communications. 2011;47(2):731–733. doi: 10.1039/c0cc03454a. [DOI] [PubMed] [Google Scholar]
- 28.Watt RK, Hilton RJ, Graff DM. Oxido-reduction is not the only mechanism allowing ions to traverse the ferritin protein shell. Biochimica et Biophysica Acta (BBA)-General Subjects. 2010;1800(8):745–759. doi: 10.1016/j.bbagen.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Jin W, Theil EC. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proceedings of the National Academy of Sciences. 2003;100(7):3653–3658. doi: 10.1073/pnas.0636928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu XS, Patterson LD, Miller MJ, Theil EC. Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. Journal of Biological Chemistry. 2007;282(44):31821–31825. doi: 10.1074/jbc.C700153200. [DOI] [PubMed] [Google Scholar]
- 31.Theil EC, Liu XS, Tosha T. Gated pores in the ferritin protein nanocage. Inorganica Chimica Acta. 2008;361(4):868–874. doi: 10.1016/j.ica.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melman G, Bou-Abdallah F, Vane E, Maura P, Arosio P, Melman A. Iron release from ferritin by flavin nucleotides. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(10):4669–4674. doi: 10.1016/j.bbagen.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Santambrogio P, Levi S, Cozzi A, Rovida E, Albertini A, Arosio P. Production and characterization of recombinant heteropolymers of human ferritin H and L chains. Journal of Biological Chemistry. 1993;268(17):12744–12748. [PubMed] [Google Scholar]
- 34.Mehlenbacher M, Poli M, Arosio P, Santambrogio P, Levi S, Chasteen ND, Bou-Abdallah F. Iron Oxidation and Core Formation in Recombinant Heteropolymeric Human Ferritins. Biochemistry. 2017;56:3900–3912. doi: 10.1021/acs.biochem.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pontillo N, Pane F, Messori L, Amoresano A, Merlino A. Cisplatin encapsulation within a ferritin nanocage: a high-resolution crystallographic study. Chemical Communications. 2016;52(22):4136–4139. doi: 10.1039/c5cc10365g. [DOI] [PubMed] [Google Scholar]
- 36.Zhang T, Lv C, Chen L, Bai G, Zhao G, Xu C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Research International. 2014;62:183–192. [Google Scholar]
- 37.Chen L, Bai G, Yang R, Zang J, Zhou T, Zhao G. Encapsulation of beta-carotene within ferritin nanocages greatly increases its water-solubility and thermal stability. Food chemistry. 2014;149:307–312. doi: 10.1016/j.foodchem.2013.10.115. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Bai G, Yang S, Yang R, Zhao G, Xu C, Leung W. Encapsulation of curcumin in recombinant human H-chain ferritin increases its water-solubility and stability. Food Research International. 2014;62:1147–1153. [Google Scholar]
- 39.Morris C. Thin-layer chromatography of proteins on Sephadex G-100 and G-200. Journal of Chromatography A. 1964;16:167–175. doi: 10.1016/s0021-9673(01)82451-1. [DOI] [PubMed] [Google Scholar]
- 40.Gibson QH, Hastings JW. The oxidation of reduced flavin mononucleotide by molecular oxygen. Biochemical Journal. 1962;83(2):368. doi: 10.1042/bj0830368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watt GD, Jacobs D, Frankel RB. Redox reactivity of bacterial and mammalian ferritin: is reductant entry into the ferritin interior a necessary step for iron release? Proceedings of the National Academy of Sciences. 1988;85(20):7457–7461. doi: 10.1073/pnas.85.20.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmona U, Li L, Zhang L, Knez M. Ferritin light-chain subunits: key elements for the electron transfer across the protein cage. Chemical Communications. 2014;50(97):15358–15361. doi: 10.1039/c4cc07996e. [DOI] [PubMed] [Google Scholar]