Abstract
BACKGROUND
Magnetic resonance imaging (MRI) has been performed safely in patients without MRI-conditional cardiac implantable electronic devices (CIED), but experience specifically with cardiac magnetic resonance imaging (CMR) is limited in this patient population.
OBJECTIVE
Evaluate the safety of CMR in non-MRI-conditional CIED and the interpretability of images using wideband sequences.
METHODS
We performed 114 consecutive CMR studies in 111 patients (mean age 59±14 years with 12 pacemakers, 73 implantable cardioverter defibrillators, 29 biventricular defibrillators) utilizing a wideband pulse sequence for late gadolinium enhancement (LGE) imaging. A standardized protocol for device management and patient monitoring was followed. Patients were evaluated for major clinical adverse events and device parameter changes immediately after CMR and at clinical follow-up.
RESULTS
A total of 111 CMR studies were completed successfully. There were no patient deaths, new arrhythmias, immediate generator or lead failures, electrical resets, or pacing capture failures in dependent patients. Right atrial, right ventricular, and left ventricular lead impedances were significantly lower post-CMR, median difference −7Ω (IQR −20 to 0Ω) (p<0.0001), 0Ω (IQR −19 to 0Ω) (p=0.0001), and −10Ω (IQR −30 to 0Ω) (p=0.023), respectively. These changes persisted through follow-up with median difference −18.5Ω (IQR −41 to −66Ω) (p=0.007) and −19Ω (IQR −44 to −7Ω) (p=0.006), and −30Ω (IQR −130 to 0Ω) (p = 0.003), respectively. Ninety-seven (87%) studies had no artifact limiting interpretation.
CONCLUSIONS
CMR can be performed safely in non-MRI-conditional CIEDs using a standardized protocol. Use of a wideband pulse sequence for LGE imaging yields a high rate of studies unaffected by artifact.
Keywords: Cardiac Magnetic Resonance Imaging, Cardiac Implanted Electronic Device, Imaging Protocol, Ventricular Tachycardia, Viability Assessment
INTRODUCTION
Over the past decade, there have been many advances in the use of magnetic resonance imaging (MRI) in patients with cardiac implanted electronic devices (CIEDs). Since 2010, several MRI-conditional CIEDs have been developed and approved for use with multiple MRI imaging modalities1–4. However, the large majority of current CIED patients have non-MRI- conditional devices implanted. Protocols have been developed for the safe performance of MRI studies in patients with non-MRI-conditional devices5, 6. Whereas two decades ago, CIEDs were viewed as absolute contraindications to performing MRI7, many MRI studies are now being performed safely utilizing published protocols5, 8–11. However, in most centers, the presence of a CIED is still considered a contraindication to MRI.
The biggest concern arises in thoracic imaging, and particularly cardiac MRI (CMR) imaging, where the focus of the gradient magnetic field and the radiofrequency energy is over the area of the device generator and leads. Device and lead artifact is also a major concern, potentially limiting image interpretability and clinical applicability12–14. In patients with ventricular tachycardia (VT) being considered for catheter ablation, CMR may be pivotal in defining scar location for pre-procedural planning and risk stratification, making this a particularly important area to clarify as many of these patients will have implantable cardioverter defibrillators (ICD)15–18.
In this study, we present our single center experience with CMR imaging in patients with non-MRI-conditional CIEDs, considered the highest risk study in these patients. We assessed imaging quality utilizing a wideband technique for late gadolinium enhancement (LGE) sequences that has been previously described12.
METHODS
We retrospectively evaluated all consecutive patients with non-MRI-conditional CIEDs who underwent CMR between April 2013 and October 2016 at the Ronald Reagan Medical Center, a tertiary care hospital. The study was approved by the hospital institutional review board. All patients were referred for a clinically indicated scan where the referring physician confirmed that no other imaging modality could provide essential information available from MRI. Each patient was evaluated by a cardiac electrophysiologist and reviewed with an MRI radiologist prior to undergoing CMR. All patients provided informed consent prior to undergoing CMR.
A standard protocol, adopted from that previously outlined by the European Society of Cardiology6, was followed for all cases (Figure 1). All patients underwent pre-procedural device interrogation to evaluate baseline device settings and parameters. The pacing mode was changed to asynchronous pacing in pacemaker dependent patients, and programmed off in others. All tachyarrhythmia detections and therapies were programmed off in patients with ICDs. A recent chest radiograph was reviewed to check for abandoned leads. Intraprocedural monitoring by an advanced resuscitation certified nurse practitioner with expertise in device management was performed by means of verbal communication with continuous electrocardiographic, pulse oximetry, and non-invasive blood pressure measurements (In-Vivo Systems, Canberra Industries, Meriden, CT). Following completion of the CMR study, device interrogation was performed to reevaluate parameters and reprogram original settings.
Figure 1. Protocol for cardiac magnetic resonance imaging in patients with cardiac implanted electronic devices.
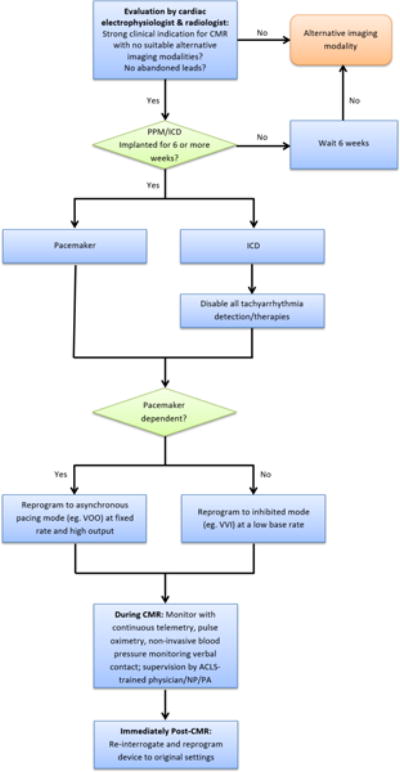
Abbreviations: CMR: cardiac magnetic resonance imaging, PPM: permanent pacemaker, ICD: implantable cardioverter defibrillator, ACLS: advanced cardiac life support, NP: nurse practitioner, PA: physician assistant
Devices were re-interrogated within 1-6 months following the study or as clinically indicated for other concerns in patients followed longitudinally at our center. Major clinical adverse events for which we evaluated included clinical deterioration or death during the CMR study, device generator failure requiring replacement, lead failure requiring replacement, new onset atrial or ventricular arrhythmia, loss of capture in pacemaker dependent patients, or electrical reset. In addition to evaluating for statistically significant device parameter changes, we also evaluated for clinically significant changes in device parameters, which were defined as: 1) 0.5 V increase in capture threshold or any increase in pulse width, 2) change in pacing lead impedance ≥ 50Ω or change in high voltage lead impedance ≥ 3Ω, 3) decrease in sensing amplitudes ≥ 50%, or 4) decrease in battery voltage ≥ 0.04V, similar to the secondary clinical outcomes defined by the Magnasafe Registry study10.
CMR studies were performed on a 1.5 Tesla MRI scanner (Avanto, Siemens Healthcare, Erlangen, Germany). Standard cardiac cine MRI was performed using a spoiled gradient recalled echo (SGRE) sequence to evaluate cardiac function. Subsequently, gadolinium contrast agent (Gadobenate Dimeglumine, MultiHance, Bracco Diagnostics, Italy) was injected intravenously at a typical dose of 0.15 (range 0.1-0.2) mmol/kg body weight. Ten minutes following gadolinium injection, LGE images were obtained using a wideband inversion pulse sequence19, which is a hyperbolic secant pulse with a bandwidth of 3.8 kHz and B1 amplitude of 11.2 uT (Supplementary Figure 1). All studies adhered to a specific absorption rate (SAR) limit of 2W/kg. The actual SAR for the wideband LGE sequences varied from 0.07 – 0.1 W/kg, and the scan time for each wideband LGE slice was 10-12 seconds. The gradient slew rate used in the wideband LGE sequence ranged from 96-122 mT/m/ms among the three encoding axes.
Statistical analysis
Patient variables are expressed as mean +/− SD. Device variables are expressed as medians with interquartile range (IQR). Comparisons of post-CMR and longer-term follow up device parameters to those pre-CMR were performed using Wilcoxon signed-rank test. Agreement between changes in device parameters was compared between the post and follow-up measurements using McNemar’s test and numerical correlation using simple linear regression. Statistical significance was determined if p < 0.05. All statistical tests were two-sided. Correction for multiple comparisons was not performed due to the potential for discounting differences in device parameters following CMR where they may truly exist. Statistical analysis was performed using JMP Pro 13 software (SAS Institute Inc, Cary, NC).
RESULTS
One hundred fourteen CMR studies were performed in 111 patients, with mean age 59 ± 14 years (Table 1), out of 333 MRI studies in non-MRI-conditional CIED patients during the same time period. Three patients underwent 2 studies each. A pacemaker was implanted in 12 (11%), ICD in 73 (64%), and cardiac resynchronization therapy defibrillator (CRT-D) in 29 (25%) with 114 right ventricular (RV) leads, 29 left ventricular (LV) leads, and 85 right atrial (RA) leads. Three patients (3%) were pacemaker dependent (1 each with pacemaker, ICD, CRT-D). The most common indications for CMR were pre-procedural imaging for VT catheter ablation evaluation in 83 (73%) patients, evaluation of cardiac structure and/or function in 17 (15%), and myocardial viability for revascularization in 6 (5%) (Table 1). Of the patients undergoing evaluation for VT ablation, 13 (16%) had had a prior ablation and 6 (46%) underwent a subsequent ablation procedure. Of the other 70 patients without prior ablation, 36 (51%) subsequently underwent ablation and 7 (10%) subsequently underwent cardiac sympathetic denervation.
Table 1.
Patient Demographics
| Age (n = 111) | 59 +/− 14 |
| Device | |
| Pacemaker | 12 (11%) |
| ICD | 73 (64%) |
| CRT-D | 29 (25%) |
| Right-sided implant | 4 (4%) |
| Manufacturer | |
| Medtronic | 30 (26%) |
| St. Jude Medical | 39 (34%) |
| Boston Scientific | 34 (29%) |
| Biotronik | 11 (10%) |
| Pacemaker Dependent | 3 (3%) |
| Structural Heart Disease | 92 (83%) |
| Cardiac Sarcoidosis | 2 (2%) |
| ARVC | 4 (4%) |
| Other NICM | 52 (46%) |
| ICM | 27 (24%) |
| Mixed ICM/NICM | 2 (2%) |
| HCM | 4 (4%) |
| Channelopathy (Brugada) | 1 (1%) |
| Structurally Normal Heart | 19 (17%) |
| Indications | |
| VT/Scar evaluation | 83 (73%) |
| Structure/function evaluation | 17 (15%) |
| Viability evaluation | 6 (5%) |
| Pre-AF ablation | 5 (4%) |
| Sarcoidosis evaluation | 3 (3%) |
Abbreviations. ICD: implantable cardioverter defibrillator, CRT-D: cardiac resynchronization therapy defibrillator, ARVC: arrhythmogenic right ventricular cardiomyopathy, NICM: nonischemic cardiomyopathy, HCM: hypertrophic cardiomyopathy, VT: ventricular tachycardia, AF: atrial fibrillation
Three studies were stopped prematurely: one due to anginal chest pain, the second due to anxiety (both resolved once the scan was terminated), and a third due to frequent runs of non-sustained VT prior to the initiation of any imaging sequences. Four other patients had concerns during the scan which did not lead to termination of the study: 2 - chest pain, 1 - headache, and 1 - intermittent episodes of slow hemodynamically stable VT (not a new arrhythmia). No patient deaths, new arrhythmias, generator or lead failures, electrical resets, or loss of capture in pacemaker dependent patients occurred.
Pre and post-CMR measurements for sensing parameters and capture thresholds were not available in all patients, but impedances were recorded for all studies (Table 2). Battery measurements were not available in 3 studies. There were no statistically significant differences in sensed P or R wave amplitude, shock lead impedance, capture thresholds, or battery voltage/longevity before and after CMR (Table 3). RA, RV, and LV lead impedances were statistically significantly lower post-CMR as compared to pre-CMR, median difference −7Ω (IQR −20 to 0 Ω) (p < 0.0001), 0Ω (IQR −19 to 0 Ω) (p = 0.0001; indicating overall more changes in the negative direction), and −10Ω (IQR −30 to 0 Ω) (p = 0.023), respectively. A significant increase in capture threshold was seen in one of each lead type, and a significant decrease in P wave amplitude was seen after 2 studies (Table 4). The highest number of significant device changes were noted in impedance measurements, particularly high voltage lead impedance with 21/69 (30%) significant changes, 17 (81%) of which were decreases in impedance of greater than or equal to 3Ω.
Table 2.
Device Parameter Measurements Before and After Cardiac Magnetic Resonance Imaging Study
| Parameter† | Pre-CMR‡ | Post-CMR‡ | Follow-up‡ |
|---|---|---|---|
| P wave amplitude (mV) n = 73/66/44 |
2.9 (1.9 to 5.0) | 3.2 (1.7 to 4.8) | 2.6 (1.9 to 5.0) |
| R wave amplitude (mV) n = 97/88/58 |
10.7 (7.2 to 12.0) | 11.0 (7.2 to 12) | 10.1 (7.2 to 12.0) |
| Atrial lead impedance (Ω) n = 85/85/49 |
478 (417 to 522) | 462 (405 to 513) | 475 (402 to 523) |
| Right ventricular lead impedance (Ω) n = 114/114/62 |
449 (400 to 549) | 456 (400 to 540) | 458 (397 to 538) |
| Left ventricular lead impedance (Ω) n = 29/29/18 |
590 (481 to 861) | 590 (465 to 857) | 519 (392 to 773) |
| Shock lead impedance (Ω) n = 70/69/54 |
52 (46 to 68) | 52 (45 to 66) | 53 (46 to 68) |
| Atrial capture threshold (V) n = 75/75/41 |
0.75 (0.625 to 1) | 0.75 (0.625 to 1) | 0.75 (0.625 to 0.95) |
| Right ventricular capture threshold (V) n = 109/110/60 |
0.9 (0.75 to 1.1) | 0.9 (0.75 to 1.2) | 0.95 (0.7 to 1.25) |
| Left ventricular capture threshold (V) n = 29/28/16 |
1 (0.725 to 1.6) | 1 (0.7 to 1.7) | 1 (0.7 to 1.5) |
| Battery Voltage (V) n = 44/44/19 |
3.0 (2.8 to 3.1) | 3.0 (2.8 to 3.1) | 2.9 (2.6 to 3.1) |
| Battery Longevity (years) n=67/67/43 |
6.0 (4.1 to 8.2) | 6.4 (4.1 to 8.5) | 5.9 (3.7 to 8.0) |
n values denote the number of values available for pre/post/follow-up measurements
values are presented as median (interquartile range lower limit to upper limit)
Abbreviations: CMR – cardiac magnetic resonance imaging
Table 3.
Device Parameter Changes Immediately after Cardiac Magnetic Resonance Imaging Study and at Follow-up Compared to Pre-scan Values
| Parameter | Post change | Post percent change | p-value | Follow-Up change | Follow-up percent change | p-value |
|---|---|---|---|---|---|---|
| P-wave amplitude (mV) | 0 (−0.3 to 0.1) | 0 (−11 to 5) | 0.256 | 0 (−0.4 to 0.7) | 2.7 (−11 to 37) | 0.182 |
| R-wave amplitude (mV) | 0 (−0.4 to 0.3) | 0 (−5 to 3) | 0.492 | 0 (−0.6 to 0.4) | 0 (−8 to 5) | 0.331 |
| Atrial-lead impedance (Ω) | −7 (−20 to 0) | −2 (−4 to 0) | <0.0001 | −18.5 (−41 to 6) | −4 (−8 to 1) | 0.007 |
| Right-ventricular lead impedance (Ω) | 0 (−19 to 0) | 0 (−4 to 0) | 0.0001 | −19 (−44 to 7) | −2.7 (−9 to 2) | 0.006 |
| Left-ventricular lead impedance (Ω) | −10 (−30 to 0) | −3 (−6 to 0) | 0.023 | −30 (−130 to 0) | −8 (−18 to 0) | 0.003 |
| Shock lead impedance (Ω) | 0 (−2.5 to 1) | 0 (−3 to 2) | 0.100 | −2 (−4 to 1.3) | −3 (−7 to 2) | 0.219 |
| Atrial capture threshold (V)† | 0 (0 to 0) | 0 (0 to 0) | 0.847 | 0 (−0.1 to 0.125) | 0 (−12.5 to 20) | 0.440 |
| Right-ventricular capture threshold (V)† | 0 (0 to 0) | 0 (0 to 0) | 0.072 | 0 (0 to 0.175) | 0 (0 to 17) | 0.100 |
| Left ventricular capture threshold (V)† | 0 (0 to 0.175) | 0 (−18 to 6) | 0.4875 | −0.05 (−0.22 to 0.10) | −3 (−10 to 13) | 0.336 |
| Battery‡ | ||||||
| Voltage (V) | 0 (0 to 0) | 0 (0 to 0) | 0.1147 | |||
| Longevity (years) | 0 (0 to 0) | 0 (0 to 0) | 0.116 |
Direct comparisons not performed if pulse width were different
Battery parameters were not evaluated at follow-up due to many other unrelated intervening factors which may affect this parameter
Table 4.
Significant Device Parameter Changes Immediately Post-Cardiac Magnetic Resonance Imaging
| Parameter | |
|---|---|
| Capture threshold† | |
| Right atrial (n = 75) | 1 (1%) |
| Right ventricular (n = 109) | 1 (1%) |
| Left ventricular (n = 28) | 1 (4%) |
| Pacing Lead Impedance‡ | |
| Right atrial (n = 85) | 2 (2%) |
| Right ventricular (n = 114) | 8 (7%) |
| Left ventricular (n = 29) | 6 (21%) |
| High Voltage Lead Impedance§ (n = 69) | 21 (30%)* |
| Sensing Amplitude¶ | |
| P wave (n = 66) | 2 (3%) |
| R wave (n = 88) | 0 (0%) |
| Battery Voltage# (n = 44) | 0 (0%) |
0.5 V increase in capture threshold or requirement for increase in pulse width
Change in pacing lead impedance ≥ 50Ω
Change in high voltage lead impedance ≥ 3Ω
Decrease in sensing amplitudes ≥ 50%
Decrease in voltage ≥ 0.04V
Significant high voltage lead impedance changes include 17 decrease and 4 increase
Device follow-up was obtained in 63 (55%) of patients, occurring at a median of 129 days (IQR 63 to 238 days). The RA, RV, and LV lead impedances were statistically significantly lower compared to pre-CMR measurements with median difference −18.5Ω (IQR −41 to −66 Ω) (p = 0.007) and −19Ω (IQR −44 to −7Ω) (p = 0.006), and −30Ω (IQR −130 to 0 Ω) (p = 0.003), respectively. A drop in impedance in the RA lead immediately following CMR was well-correlated with a persistent drop in impedance at follow-up (McNemar’s p = 0.782), though the amount of change in impedance immediately following CMR poorly predicted long-term impedance change (r2 = 0.16). The same was true of RV lead impedance drop (McNemar’s p = 0.240, r2 = 0.16) and LV lead impedance drop (McNemar’s p = 0.739), though a moderate linear relationship was found for left ventricular impedance changes (r2 = 0.57). All other parameters were similar to pre-CMR measurements at long-term follow-up. Two lead failures occurred during subsequent follow-up: one RA lead dislodgement and one RV lead with non-capture, both following catheter ablation in the same chamber. One other RV lead had a significant change in capture threshold from a baseline of 1V at 0.4ms, to 1.5V at 0.5ms at 3 months, and 1.75V at 0.6ms at 4 months post-MRI. It remained stable through further follow-up including a second cardiac MRI; the lead impedance remained unchanged during this period. No device generators were replaced unexpectedly following CMR.
Overall, 14 (13%) studies were affected by some artifact, 3 (3%) with severe artifact: susceptibility artifact from ICDs obscured the entire heart in one study and the anterior, anteroseptal, and anterolateral walls in a second (Figure 2C). In a third study, interpretation was severely limited by a combination of artifact from an artificial tricuspid valve, sternotomy wires, and device generator (CRT-D). Four (4%) other studies were affected by motion or respiratory artifact only. Nine (8%) in total were affected by susceptibility artifact from the device generator (4 CRT-D, 5 ICD), 1 by device lead artifact. In the 6 cases with susceptibility artifact not deemed severe, the anterior wall and apex were obscured in 4 and 3 of the studies, respectively.
Figure 2. Late gadolinium enhancement sequences utilizing a wideband protocol.
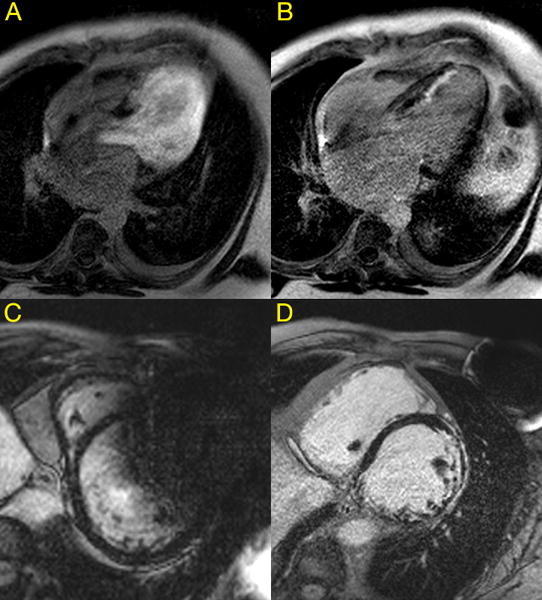
Panel A) Image obtained without wideband protocol showing significant hyperintensitivity artifact obscuring the myocardium, B) the same study as in panel A now using a wideband protocol revealing late gadolinium enhancement of the septal endocardial myocardium, C) susceptibility artifact remains despite utilizing wideband protocol due to proximity of implantable cardioverter defibrillator generator to the heart, obscuring the anterior wall and parts of the anteroseptal and anterolateral walls, D) utilizing a wideband protocol, susceptibility artifact from the cardiac resynchronization therapy defibrillator device generator is sufficiently decreased to allow full view of the myocardium revealing diffuse areas of late gadolinium enhancement. This is representative of the late gadolinium enhancement images obtained in 87% of our study population.
DISCUSSION
In this study, which to our knowledge is the largest series of CMR in CIED patients with 111 completed studies, we found that performing CMR studies in patients with CIEDs using a wideband technique for LGE sequences is both safe for patients and the implanted device, and can produce a high rate of fully interpretable LGE images.
Lead impedances for all leads, aside from the high voltage lead, were shown to decrease statistically significantly immediately after completion of CMR, all of which persisted through follow-up. The largest number of significant device changes was noted in decreases in the high voltage lead impedance as defined by a change of 3Ω or greater, though none of these leads required replacement. The most clinically significant increase in capture threshold was noted in an RV lead with increase from 1.0V at 0.4ms to 1.75V at 0.6ms by 4 months post-CMR, though no further changes occurred with a second CMR study.
The low rate of significant device or lead parameter changes is consistent with both those studies that included and excluded thoracic MRI scans. Nazarian et al. also showed in their study, which included thoracic and non-thoracic MRI studies, a significant drop in all lead impedances immediately post-MRI, though only RV impedance remained persistently decreased at follow-up5. Studies on 51 CMR scans by Dandamudi et al. and 95 CMR studies limited to LGE sequences by Horwood et al. also showed small decreases in lead impedances post-CMR20, 21. We did not find significant decreases in battery voltage post-MRI or decreases in sensing parameters as reported by other studies5, 9. Currently, there are no agreed-upon criteria for what constitutes a significant change in device parameters after MRI studies, and no consensus on what changes are considered ‘clinically’ significant or when to intervene clinically with lead replacement or revision. The etiology of the impedance changes is unclear, as is whether they are cumulative over multiple MRI studies. Further studies are required to elucidate the putative mechanisms and determine if multiple MRI studies, particularly CMR, can be performed safely in non-MRI-conditional CIED patients.
In order to be clinically useful, CMR images in this patient population must also be free of major artifact. The presence of ferromagnetic material in the device generator interferes with the transmission and reception of the radiofrequency signal that forms the basis for MRI. This effect can result in susceptibility (metal) artifact over a radius of 5-12cm surrounding the generator and is more problematic on LGE images19, 22, which are the most important for VT ablation planning and assessment of myocardial viability for revascularization.
We have previously shown that using a wideband inversion pulse for LGE imaging can greatly reduce susceptibility artifact19 (Figure 2 Panels A/B/D). Using this sequence, we found that only 14/111 studies (13%) had any artifact limiting interpretation of the sequences including LGE images, 4 of which were caused by motion artifact which could limit any MRI study. Only 3 (3%) studies had significant artifact severely limiting or undermining interpretation of the study. Much higher rates were reported by Dandamudi et al and Buendia et al with 14/51 (27%) and 11/39 (28.2%) CMR studies, respectively, having significant or severe artifact20, 23. An overall rate of 87% of studies being completely interpretable, and 87% in left sided-ICD/CRT-D devices also compares favorably to 18/55(32.7%) reported by Sasaki et al.14 and 0/22(0%) by Dickfeld et al.24. Hilbert et al. reported a recent smaller study of 28 patients, with proportionately more pacemakers and right sided implants, which cause much less to no MR imaging artifact obscuring the LV. Using a modified broadband sequence, 85.7% of their LGE studies were completely interpretable, identical to our result of 87%. They reported similar limitations including close proximity of the device generator to the heart19, and artifact primarily obscuring the anterior LV wall25. Other studies of CMR in CIED patients have acknowledged the presence of artifact but only report that most images were interpretable1, 21, 22 without mention of the degree of artifact, so direct comparison is not possible.
Our study also emphasizes the importance of vigilant patient monitoring throughout the imaging period, given the potential for this high-risk patient population to have recurrent ventricular arrhythmias during the study when tachy-therapies are disabled.
Limitations
Some parameters, particularly sensing amplitude, were not obtainable for all cases. Battery voltage was also not reported in many devices, instead being reported only as estimated longevity, which is not as precise and may limit our ability to detect small changes in battery life. However, for those cases where voltage information was available, there was no apparent change with the entire interquartile range of changes at 0V. We also could not obtain follow-up device data in all patients, primarily due to the patients having device follow-up elsewhere, though rates of follow-up are similar to other series at tertiary care centers5.
Conclusions
CMR can be performed safely in non-MRI-conditional CIEDs using a standardized protocol. Right atrial, ventricular and left ventricular lead impedances decreased immediately following the scan and persisted through follow-up, but did not result in the need for lead replacement or revision. Use of a wideband technique for LGE sequences yielded artifact-free myocardial images in 87% of studies.
Supplementary Material
Acknowledgments
Dr. Duc Do is supported by an award from the UCLA Specialty Training and Advanced Research (STAR) Program. Dr. Peng Hu is supported by NIH R21HL118533. Dr. Kalyanam Shivkumar is supported by NIH R01 HL084261 & NIH OT2OD023848.
Footnotes
Conflicts of Interest: PH is the inventor of the wideband sequence used in this study, patent US 20150346304 A1
References
- 1.Wollmann CG, Thudt K, Kaiser B, Salomonowitz E, Mayr H, Globits S. Safe performance of magnetic resonance of the heart in patients with magnetic resonance conditional pacemaker systems: the safety issue of the ESTIMATE study. Journal of Cardiovascular Magnetic Resonance. 2014;16:30. doi: 10.1186/1532-429X-16-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey WM, Rosenthal L, Fananapazir L, Gleva M, Mazur A, Rinaldi CA, Kypta A, Merkely B, Woodard PK. Clinical safety of the ProMRI pacemaker system in patients subjected to head and lower lumbar 1.5-T magnetic resonance imaging scanning conditions. Heart Rhythm. 2015;12:1183–1191. doi: 10.1016/j.hrthm.2015.02.010. 6// [DOI] [PubMed] [Google Scholar]
- 3.Gold MR, Sommer T, Schwitter J, Al Fagih A, Albert T, Merkely B, Peterson M, Ciuffo A, Lee S, Landborg L, Cerkvenik J, Kanal E. Full-Body MRI in Patients With an Implantable Cardioverter-DefibrillatorPrimary Results of a Randomized Study. Journal of the American College of Cardiology. 2015;65:2581–2588. doi: 10.1016/j.jacc.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff BL, Bello D, Taborsky M, Vymazal J, Kanal E, Heuer H, Hecking K, Johnson WB, Young W, Ramza B. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm. 2011;8:65–73. doi: 10.1016/j.hrthm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Nazarian S, Hansford R, Roguin A, et al. A Prospective Evaluation of a Protocol for Magnetic Resonance Imaging of Patients With Implanted Cardiac Devices. Annals of Internal Medicine. 2011;155:415–424. doi: 10.7326/0003-4819-155-7-201110040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roguin A, Schwitter J, Vahlhaus C, Lombardi M, Brugada J, Vardas P, Auricchio A, Priori S, Sommer T. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. EP Europace. 2008;10:336–346. doi: 10.1093/europace/eun021. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, Kanal E, Manning WJ, Martin ET, Smith JM, Wilke N. Safety of Magnetic Resonance Imaging in Patients With Cardiovascular Devices An American Heart Association Scientific Statement From the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: Endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–2891. doi: 10.1161/CIRCULATIONAHA.107.187256. [DOI] [PubMed] [Google Scholar]
- 8.Martin ET, Coman JA, Shellock FG, Pulling CC, Fair R, Jenkins K. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla. Journal of the American College of Cardiology. 2004;43:1315–1324. doi: 10.1016/j.jacc.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Naehle CP, Strach K, Thomas D, Meyer C, Linhart M, Bitaraf S, Litt H, Schwab JO, Schild H, Sommer T. Magnetic resonance imaging at 1.5-T in patients with implantable cardioverter-defibrillators. Journal of the American College of Cardiology. 2009;54:549–555. doi: 10.1016/j.jacc.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 10.Russo RJ, Costa HS, Silva PD, et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. New England Journal of Medicine. 2017;376:755–764. doi: 10.1056/NEJMoa1603265. [DOI] [PubMed] [Google Scholar]
- 11.Strom JB, Whelan JB, Shen C, Zheng SQ, Mortele KJ, Kramer DB. Safety and utility of magnetic resonance imaging in patients with cardiac implantable electronic devices. Heart Rhythm. 2017;14:1138–1144. doi: 10.1016/j.hrthm.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, Hu P. Improved Late Gadolinium Enhancement MR Imaging for Patients with Implanted Cardiac Devices. Radiology. 2014;270:269–274. doi: 10.1148/radiol.13130942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesubi O, Ahmad G, Jeudy J, Jimenez A, Kuk R, Saliaris A, See V, Shorofsky S, Dickfeld T. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing and Clinical Electrophysiology. 2014;37:1274–1283. doi: 10.1111/pace.12405. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Hansford R, Zviman MM, Kolandaivelu A, Bluemke DA, Berger RD, Calkins H, Halperin HR, Nazarian S. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter-defibrillators. Circulation: Cardiovascular Imaging. 2011;4:662–670. doi: 10.1161/CIRCIMAGING.111.965764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreu D, Ortiz-Pérez JT, Boussy T, Fernández-Armenta J, de Caralt TM, Perea RJ, Prat-González S, Mont L, Brugada J, Berruezo A. Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. European Heart Journal. 2014 doi: 10.1093/eurheartj/eht510. [DOI] [PubMed] [Google Scholar]
- 16.Njeim M, Yokokawa M, Frank L, Crawford T, Good E, Morady F, Bogun F. Value of cardiac magnetic resonance imaging in patients with failed ablation procedures for ventricular tachycardia. Journal of cardiovascular electrophysiology. 2016;27:183–189. doi: 10.1111/jce.12848. [DOI] [PubMed] [Google Scholar]
- 17.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreu D, Penela D, Acosta J, et al. Cardiac magnetic resonance–aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm. 2017;14:1121–1128. doi: 10.1016/j.hrthm.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Stevens SM, Tung R, Rashid S, Gima J, Cote S, Pavez G, Khan S, Ennis DB, Finn JP, Boyle N, Shivkumar K, Hu P. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter-defibrillators and ventricular tachycardia: late gadolinium enhancement correlation with electroanatomic mapping. Heart Rhythm. 2014;11:289–298. doi: 10.1016/j.hrthm.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandamudi S, Collins JD, Carr JC, et al. The Safety of Cardiac and Thoracic Magnetic Resonance Imaging in Patients with Cardiac Implantable Electronic Devices. Academic Radiology. 2016;23:1498–1505. doi: 10.1016/j.acra.2016.08.016. 12// [DOI] [PubMed] [Google Scholar]
- 21.Horwood L, Attili A, Luba F, Ibrahim E-SH, Parmar H, Stojanovska J, Gadoth-Goodman S, Fette C, Oral H, Bogun F. Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace. 2016:euw122. doi: 10.1093/europace/euw122. [DOI] [PubMed] [Google Scholar]
- 22.Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, Weiss RG, Berger RD, Bluemke DA, Halperin HR. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277–1284. doi: 10.1161/CIRCULATIONAHA.105.607655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buendía F, Cano Ó, Sánchez-Gómez JM, Igual B, Osca J, Sancho-Tello MJ, Olagüe J, Salvador A. Cardiac magnetic resonance imaging at 1.5 T in patients with cardiac rhythm devices. Europace. 2011;13:533–538. doi: 10.1093/europace/euq501. [DOI] [PubMed] [Google Scholar]
- 24.Dickfeld T, Tian J, Ahmad G, Jimenez A, Turgeman A, Kuk R, Peters M, Saliaris A, Saba M, Shorofsky S. MRI-guided ventricular tachycardia ablation integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circulation: Arrhythmia and Electrophysiology. 2011;4:172–184. doi: 10.1161/CIRCEP.110.958744. [DOI] [PubMed] [Google Scholar]
- 25.Hilbert S, Weber A, Nehrke K, Börnert P, Schnackenburg B, Oebel S, Spampinato R, Rogge C, Richter S, Hindricks G. Artefact-free late gadolinium enhancement imaging in patients with implanted cardiac devices using a modified broadband sequence: current strategies and results from a real-world patient cohort. EP Europace. 2017:eux016. doi: 10.1093/europace/eux016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


