Abstract
S -palmitoylation is an abundant lipid post-translational modification (PTM) that is dynamically installed on and removed from target proteins to regulate their activity and cellular localization. A dearth of tools to study the activities and regulation of protein S-depalmitoylases – thioesterase “erasers” of protein cysteine S-palmitoylation – has contributed to an incomplete understanding of the role of dynamic S-palmitoylation in regulating proteome lipidation. Recently, we developed “depalmitoylation probes” (DPPs), small molecule probes that become fluorescent upon S-depalmitoylase enzymatic activity. In order to be suitable for application in live cells, the first-generation DPPs relied on a shorter lipid substrate (C8 vs. naturally occurring C16), which enhanced solubility and cell permeability. However, the use of an unnatural lipid substrate on the probes potentially limits the utility of the approach. Herein, we present a new member of the DPP family, DPP-5, which features an anionic carboxylate functional group that increases the probe water solubility. The enhanced water solubility of DPP-5 permits the use of a natural, palmitoylated substrate (C16), rather than a surrogate lipid. We show that DPP-5 is capable of monitoring endogenous S-depalmitoylases in live mammalian cells, and that it can reveal changes in S-depalmitoylation levels due to lipid stress. DPP-5 should prove to be a useful new tool to probe the regulation of proteome lipidation through dynamic S-depalmitoylation.
Graphical Abstract
Protein lipidation of cysteine residues by thioester formation with palmitate (S-palmitoylation), a C16 saturated fatty acid, is a prevalent post-translational modification (PTM) in mammalian systems1–4. Cysteine S-palmitoylation is unique among the other common lipid PTMs, such as N-myristoylation and S-prenylation, in that it is reversible and dynamic.5 The S-palmitoylation status of the human proteome is determined by twenty-three DHHC (Aspartate-Histidine-Histidine-Cysteine domain) palmitoyltransferase “writer” proteins3, 6, 7, and at least six S-depalmitoylase “eraser” proteins8, 9. The currently known S-depalmitoylase family includes the lysosomal Palmitoyl-Protein Thioesterase 1 (PPT1)10, the presumed cytosolic proteins acyl-protein thioesterase 1 (APT1) and acyl-protein thioesterase 2 (APT2)11, 12, and the α/β-Hydrolase domain-containing protein 17 members A, B, and C13.
The half-life of the S-palmitoyl group on a given protein target can vary from minutes14 to days15, depending on the activity of the S-depalmitoylases on the target. Moreover, the S-depalmitoylases are not statically active, but respond dynamically to cellular changes, such as growth factor signaling and neuronal activation16, 17. The S-depalmitoylases themselves have been found to be regulated through S-depalmitoylation18, resulting in a complex web of interconnected lipid signaling cascades. Cellular localization of the S-depalmitoylases is a key determinant of activity on a given target18, but other regulatory mechanisms are also likely at play. Therefore, methods to measure S-depalmitoylase activities in live cells are needed to uncover how proteome S-palmitoylation levels are regulated through dynamic S-depalmitoylases.
Current methods to monitor S-palmitoylation in biological systems include lipidation based proteomics8, 19, mass-tagging by Acyl PEG exchange20, and monitoring fluorescent substrate mimetics21–23. These methods reveal the balance between the “writers” and “erasers”, but cannot specifically detect dynamics in the S-depalmitoylases. The primary method to monitor the S-depalmitoylases directly involves activity-based protein profiling (ABPP)24. ABPP is a powerful approach for developing inhibitors, but does not reveal spatial information, which is likely a determinant of the cellular effects of S-depalmitoylases.
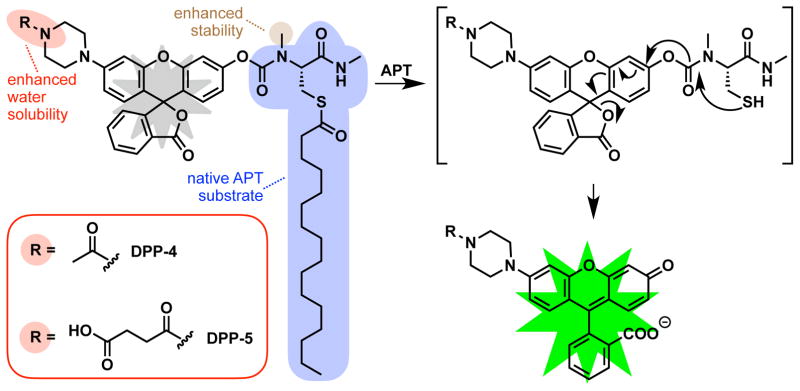
Recently, we unveiled depalmitoylation probes (DPPs), a new family of fluorescent probes that permit the analysis of S-depalmitoylases in real-time and in live cells25. The DPPs feature a pro-fluorescent molecule tethered to an S-acylated peptide substrate through a carbamate linkage. Thioesterase activity on the substrate results in a reaction cascade that leads to carbamate cleavage and release of a brightly fluorescent product (Figure 1). Importantly, the substrate portion of the DPPs utilizes a natural peptide-based substrate with a thioester-modified cysteine residue. We found that DPPs are capable of measuring endogenous levels of S-depalmitoylases in a range of cell types. Moreover, we used the DPPs to uncover rapid growth factor-mediated alterations in the S-depalmitoylases, revealing connections between receptor tyrosine kinases and dynamic lipidation signaling.
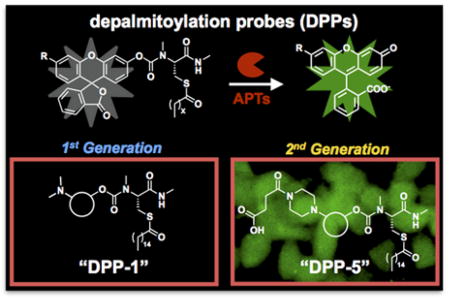
Figure 1.
Structure and mechanism of enzymatic activation of DPP-4 and DPP-5. An S-palmitoylated peptide substrate is tethered to a rhodol fluorophore through a carbamate linkage. Thioesterase activity on the substrate results in carbamate cleavage and release of a fluorescent product. A piperazine linker allows derivatization of the probes with additional functional groups to increase water solubility and utility in live cells.
Depalmitoylation probe 1 (DPP-1), the simplest DPP with a palmitoylated substrate, was quite insoluble and failed in live-cell experiments. In order to create DPPs capable of deployment in live cells, we found that we needed to instead use a non-natural surrogate acylation substrate mimetic, S-octanoylation (a C8 lipid) in our first generation S-depalmitoylase probes, DPP-2 and DPP-325. This shorter lipid modification increased water solubility and cell permeability, as indicated by the ability to perform in vitro biochemical assays without detergents and robust signal in live cells due to endogenous S-depalmitoylases, respectively. However, the usage of a shorter lipid modification is potentially problematic, as thioesterases that cannot act on a natural S-palmitoyl protein thioester and are not components of the S-depalmitoylation pathway could process these first-generation DPPs, resulting in false-positive signals. Therefore, the utility and specificity of the DPPs could be dramatically enhanced if they utilized a natural palmitoyl lipid modification as a substrate.
We reasoned that we could improve the design of the first-generation DPPs and allow for a palmitoyl modification by increasing the solubility of the probe scaffold. The rhodol-based scaffold that the DPPs are based upon affords several synthetically-tractable positions at which to install solubilizing groups. We chose to use a piperazine moiety on the xanthene portion of the rhodol scaffold, which has previously been used to make modified fluorescent probes26. We targeted two new molecules: 1) Depalmitoylation probe 4 (DPP-4), which has an acetyl amide-modification on the piperazine, and 2) Depalmitoylation probe 5 (DPP-5), which has a succinylated amide and therefore features a carboxylic acid. Both DPP-4 and DPP-5 utilize a simple palmitoylated cysteine residue with a methyl-amide modification and the substrate for the S-depalmitoylases (Figure 1). Importantly, the new modifications are not located at the key substrate recognition portion of the probe, which we reasoned would minimize interference with enzymatic activity by the S-depalmitoylases. To confirm this, we carried out flexible ligand-rigid receptor docking in Autodock Vina27 with the previously reported crystal structures of APT1 (PDB: 5SYM) and APT2 (PDB: 5SYN)28. As expected, analysis of the five lowest energy confirmations reveled that DPP-4 and DPP-5 docked to both APT1 and APT2 in a similar manner with most poses having the DPP palmitoylcysteine moiety in the active site and minimal interactions with the acetyl or succinylated amide (Figure S1–4; Table S1–4).
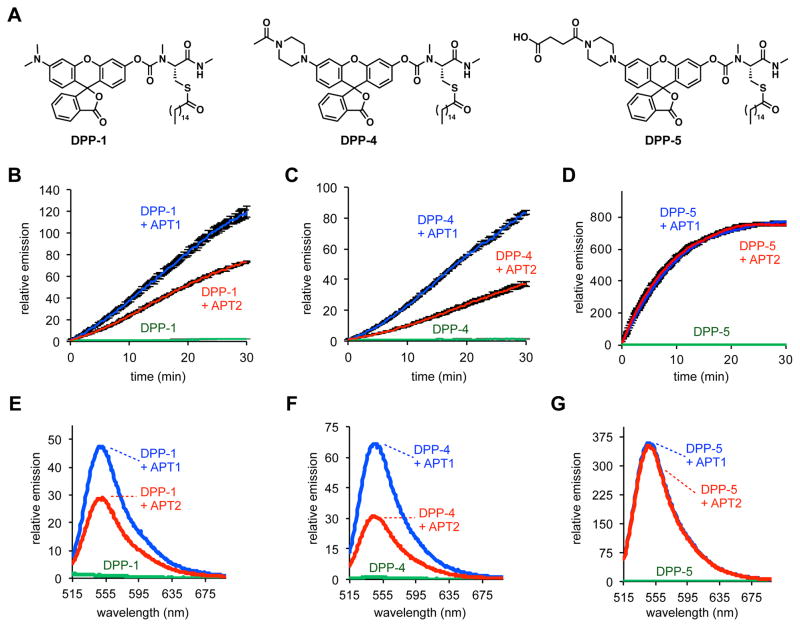
Synthesis of DPP-4 and DPP-5 each proceeded smoothly over four steps (Figure S5–6). Once in hand, we compared the new probes to DPP-1 in in vitro assays with recombinantly expressed human APT1 and APT2 (Figure 2A). Both DPP-1 (Figure 2B) and DPP-4 (Figure 2C) display very slow kinetics with APT1 and APT2 in the absence of detergents. After 30 min incubation with 1 μM probe and 50 nM of either APT1 or APT2, DPP-1 and DPP-4 showed only 50- or 30-fold and 70- or 30-fold enhancement in fluorescence signal, respectively (Figure 2E, F). DPP-5, however, showed a rapid turn-on response to both APT1 and APT2 (Figure 2D), with a 350-fold increase in fluorescent signal with both enzymes (Figure 2G), likely due to enhanced water solubility afforded by the additional carboxylate. Indeed, LogP analysis of DPP-1/4/5 corroborates the higher water solubility of DPP-5 compared to both DPP-1 and DPP-4 (Table S5). Kinetic analysis revealed DPP-5 has comparable kinetic parameters to previously reported N-Ras based semisynthetic substrates for APT1 and APT2 (Table 1, Figure S7)29. We were unable to perform quantitative analysis of DPP-1 and DPP-4 due to their slow kinetics in the absence of detergents. Given that DPP-5 showed robust activity with APT1 and APT2 in the absence of detergents, we next assessed whether the in vitro performance enhancement translated to live cells.
Figure 2.
In vitro activation of DPPs. (A) Structures of DPP-1 (first-generation probe) and the two new probes, DPP-4 and DPP-5. In vitro assays of 1 μM DPP-1 (B), DPP-4 (C), and DPP-5 (D) in HEPES (20 mM, pH 7.4, 150 mM NaCl) with or without 50 nM purified APT1 or 50 nM APT2 (λex 490/20 nm; λem 545/20 nm). Error bars are s.e.m. (n=4). Fluorescence emission spectra of DPP-1 (E), DPP-4 (F), and DPP-5 (G) in HEPES (20 mM, pH 7.4, 150 mM NaCl) after 30 min of treatment with or without 50 nM APT1 or 50 nM APT2 (λex 485 nm). All data normalized to the background of the probe alone.
Table 1.
Kinetic parameters of APT1 and APT2 with DPP-5
| KM (μM) | kcat (s−1) | kcat/KM (s−1 M−1) | |
|---|---|---|---|
| APT1 | 1.6 | 0.044 | 2.8 × 104 |
| APT2 | 2.1 | 0.066 | 3.1 × 104 |
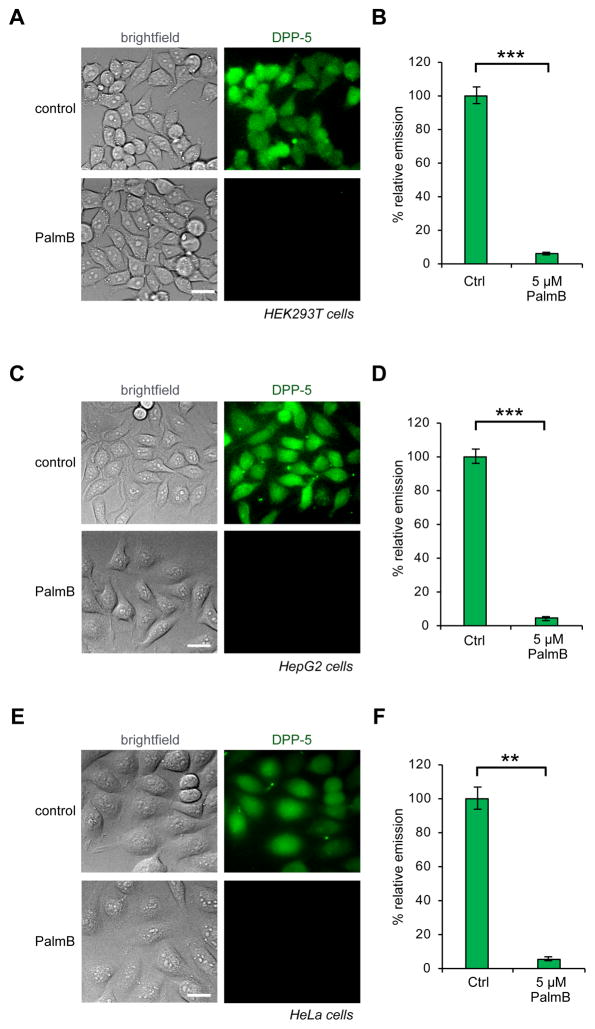
We loaded HEK293T cells with 1 μM DPP-5 for 20 min and then analyzed the fluorescent signal by fluorescence microscopy. Cells loaded with DPP-5 displayed bright, intracellular fluorescent signal (Figure 3A and S8). To determine whether the observed signal was due to endogenous S-depalmitoylases, we deployed palmostatin B (PalmB), a β-lactone-based pan-inhibitor of the S-depalmitoylases22. Pretreatment of the HEK293T cells prior to addition of DPP-5 abolished ~95% of the fluorescent signal (Figure 3B). We next sought to assay whether DPP-5 also functions in other cell lines. We repeated the PalmB imaging experiments in HepG2 (Figure 3C, D and S9) and HeLa cells (Figure 3E, F and S10). Treatment of both cell lines with 1 μM probe for 20 min resulted in robust intracellular fluorescent signal. Critically, pretreatment of the cells with 5 μM PalmB blocked over ~95% of the turn-on response, confirming DPP-5 is measuring endogenous enzyme-mediated S-depalmitoylation. Additionally, 10 μM ML348, an APT1-specific inhibitor, decreased the signal from DPP-5 by ~30% in HepG2, whereas 10 μM ML349, an APT2-specific inhibitor, resulted in no change in signal (Figure S11)24. Furthermore, treating cells with a combination of both inhibitors effects the DPP-5 to the similar levels as the APT1 inhibitor alone (Figure S11). The unaccounted for differential reduction in DPP-5 signal with PalmB and ML348 reinforces the general reactivity of DPP-5 with all possible S-depalmitoylases and suggests enzyme-targeted analogs of DPP-5 would be useful additions to the S-palmitoylation toolbox.
Figure 3.
Activation of DPP-5 by endogenous S-depalmitoylases in live mammalian cells. (A) HEK293T cells treated with either DMSO or 5 μM PalmB for 30 min, washed, loaded with 1 μM DPP-5 with DMSO/5 μM PalmB for 20 min, and imaged by epifluorescence microscopy. (B) Quantification of experiment shown in (A). (C) HepG2 cells treated with either DMSO or 5 μM PalmB for 30 min, washed, loaded with 1 μM DPP-5 with DMSO/5 μM PalmB for 20 min, and imaged by epifluorescence microscopy. (D) Quantification of experiment shown in (C). (E) HeLa cells treated with either DMSO or 5 μM PalmB for 30 min, washed, loaded with 1 μM DPP-5 with DMSO/5 μM PalmB for 20 min, and imaged by epifluorescence microscopy. (F) Quantification of experiment shown in (E). Error bars are s.e.m., n = 3. Statistical analysis performed with a two-tailed Student’s t-test with unequal variance, ** P value < 0.01, *** P value < 0.005. 20 μm scale bars shown.
To compare the signal obtained with DPP-5 to DPP-1 and DPP-4, we performed imaging experiments in HEK293T cells with identical concentrations of each probe and identical microscope settings. While DPP-5 treatment resulted in robust fluorescent signal, DPP-1 and DPP-4 displayed very low, almost undetectable levels of fluorescence (Figure S12). Collectively, these results indicate that the additional carboxylate functional group dramatically improves the performance of DPP-5 both in vitro and in live cells.
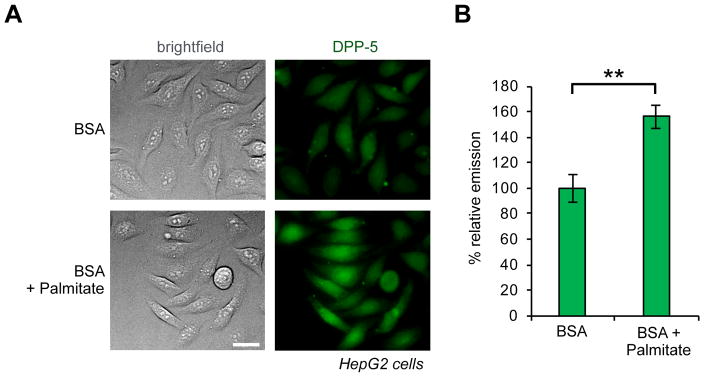
Finally, we sought to test whether DPP-5 could detect dynamic alterations in the S-depalmitoylases. Exogenous palmitate has been shown to cause ER stress and affects β-cell viability30, 31. Aberrant S-palmitoylation has been demonstrated to be one of the mechanisms by which excess palmitate induces ER stress30, but the role of S-palmitoylation dynamics in this process is largely unexplored. To this end, we chose to test whether lipid stress through palmitate treatment causes a response from the S-depalmitoylases. We found that pretreatment of HepG2 cells with 1 mM palmitate for 6 h post starvation resulted in a ~50 % increase in the S-depalmitoylase signal as measured by DPP-5 (Figure 4A, B and S13). Furthermore, pretreatment of HepG2 cells with PalmB reduces the DPP-5 signal, indicating that palmitate induces activation of S-depalmitoylase activity at the location DPP-5 is measuring the signal (Figure S14). To rule out the possibility that the changes in fluorescence signal are due to differential accumulation of DPP-5 in cells upon palmitate treatment, we carried out imaging with a control probe, diacetyl rhodol, which maintains the pro-fluorophore core. Once entering cells, the diacetyl rhodol probe will be rapidly activated by intracellular esterases, allowing us to measure cell permeability and uptake of a related, non-APT active compound (Figure S15a). We found no measurable effect on the fluorescence signal from HepG2 cells loaded with 1 μM diacetyl rhodol probe with 1 mM of palmitate addition in cells (Figure S15b–c). Collectively, these data show that the S-depalmitoylases respond to local lipid homeostatic levels. This observation also suggests that caution should be taken when adding exogenous lipids to metabolically label the proteome with lipids, as the lipid changes could cause alterations in the activities of the S-depalmitoylases, perturbing the system. Moreover, we have observed different responses to palmitate stress with other probes32, which are based on an intramolecular cyclization on electrophilic coumarin derivative33 to yield ratiometric response on APT activity, possibly due to differences in subcellular localization of the probes or APT preference, further illustrating the complexity of S-depalmitoylase regulation. Further investigation of these differential responses, and the downstream effects on proteome lipidation levels, is therefore warranted.
Figure 4.
Exogenous palmitate activates S-depalmitoylase activity. (A) HepG2 cells treated with 1% BSA ± 1 mM Palmitate for 6 hr post-starvation, washed, loaded with 1 μM DPP-5 for 20 min, and imaged by epifluorescence microscopy. (B) Quantification of experiment shown in (A). Error bars are s.e.m., n = 3. Statistical analysis performed with a two-tailed Student’s t-test with unequal variance, ** P value < 0.02. 20 μm scale bars shown.
To conclude, we presented the design, synthesis, and biological evaluation of DPP-4 and DPP-5, two new fluorescent probes to monitor S-depalmitoylase activities. We found that DPP-5 is capable of measuring S-depalmitoylase activity robustly in vitro and in live cells due to the enhanced solubility endowed by an additional carboxylic acid modification. DPP-5 improves upon the previously developed DPPs by utilizing a natural lipid substrate, rather than a surrogate synthetic substrate. DPP-5, along with future derivatives with additional substrate modifications to enhance S-depalmitoylase selectivity25, will provide important tools to study the regulation of proteome lipidation through dynamic S-depalmitoylases.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (R35 GM119840) to B.C.D. We thank C. He (University of Chicago) and Y. Krishnan (University of Chicago) for supplying materials and equipment.
Footnotes
R.S.K. and B.C.D. have filed a provisional patent on the DPPs.
The Supporting Information is available free of charge on the ACS Publications website.
Methods, synthetic details, supporting tables and figures (PDF)
References
- 1.Blanc M, David F, Abrami L, Migliozzi D, Armand F, Burgi J, van der Goot FG. F1000Res. 2015;4:261. doi: 10.12688/f1000research.6464.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanyon-Hogg T, Faronato M, Serwa RA, Tate EW. Trends Biochem Sci. 2017;42:566–581. doi: 10.1016/j.tibs.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Linder ME, Deschenes RJ. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 4.Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Nat Methods. 2011;9:84–89. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin DTS, Davis NG, Conibear E. Biochem Soc Trans. 2017;45:913–921. doi: 10.1042/BST20160303. [DOI] [PubMed] [Google Scholar]
- 6.Zheng B, DeRan M, Li X, Liao X, Fukata M, Wu X. J Am Chem Soc. 2013;135:7082–7085. doi: 10.1021/ja311416v. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb CD, Linder ME. Biochem Soc Trans. 2017;45:923–928. doi: 10.1042/BST20160304. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez JL, Majmudar JD, Martin BR. Curr Opin Chem Biol. 2013;17:20–26. doi: 10.1016/j.cbpa.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long JZ, Cravatt BF. Chem Rev. 2011;111:6022–6063. doi: 10.1021/cr200075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verkruyse LA, Hofmann SL. J Biol Chem. 1996;271:15831–15836. doi: 10.1074/jbc.271.26.15831. [DOI] [PubMed] [Google Scholar]
- 11.Duncan JA, Gilman AG. J Biol Chem. 2002;277:31740–31752. doi: 10.1074/jbc.M202505200. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda T, Sugimoto H, Yamashita S. Biochim Biophys Acta. 1999;1437:182–193. doi: 10.1016/s1388-1981(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin DT, Conibear E. Elife. 2015;4:e11306. doi: 10.7554/eLife.11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu JY, Hofmann SL. J Biol Chem. 1995;270:7251–7256. doi: 10.1074/jbc.270.13.7251. [DOI] [PubMed] [Google Scholar]
- 15.Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, Dey A, Hannoush RN, Fairbrother WJ, Cunningham CN. Structure. 2016;24:179–186. doi: 10.1016/j.str.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 16.El-Husseini Ael D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 17.Ponimaskin E, Dityateva G, Ruonala MO, Fukata M, Fukata Y, Kobe F, Wouters FS, Delling M, Bredt DS, Schachner M, Dityatev A. J Neurosci. 2008;28:8897–8907. doi: 10.1523/JNEUROSCI.2171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong E, Peng S, Chandra G, Sarkar C, Zhang Z, Bagh MB, Mukherjee AB. J Biol Chem. 2013;288:9112–9125. doi: 10.1074/jbc.M112.421073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng T, Thinon E, Hang HC. Curr Opin Chem Biol. 2016;30:77–86. doi: 10.1016/j.cbpa.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Percher A, Ramakrishnan S, Thinon E, Yuan X, Yount JS, Hang HC. Proc Natl Acad Sci U S A. 2016;113:4302–4307. doi: 10.1073/pnas.1602244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creaser SP, Peterson BR. J Am Chem Soc. 2002;124:2444–2445. doi: 10.1021/ja017671x. [DOI] [PubMed] [Google Scholar]
- 22.Dekker FJ, Rocks O, Vartak N, Menninger S, Hedberg C, Balamurugan R, Wetzel S, Renner S, Gerauer M, Scholermann B, Rusch M, Kramer JW, Rauh D, Coates GW, Brunsveld L, Bastiaens PI, Waldmann H. Nat Chem Biol. 2010;6:449–456. doi: 10.1038/nchembio.362. [DOI] [PubMed] [Google Scholar]
- 23.Gormer K, Burger M, Kruijtzer JA, Vetter I, Vartak N, Brunsveld L, Bastiaens PI, Liskamp RM, Triola G, Waldmann H. Chembiochem. 2012;13:1017–1023. doi: 10.1002/cbic.201200078. [DOI] [PubMed] [Google Scholar]
- 24.Adibekian A, Martin BR, Chang JW, Hsu KL, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, Ferguson J, Hodder PS, Rosen H, Cravatt BF. J Am Chem Soc. 2012;134:10345–10348. doi: 10.1021/ja303400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kathayat RS, Elvira PD, Dickinson BC. Nat Chem Biol. 2017;130:150–152. doi: 10.1038/nchembio.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickinson BC, Chang CJ. J Am Chem Soc. 2008;130:9638–9639. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trott O, Olson AJ. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won SJ, Davda D, Labby KJ, Hwang SY, Pricer R, Majmudar JD, Armacost KA, Rodriguez LA, Rodriguez CL, Chong FS, Torossian KA, Palakurthi J, Hur ES, Meagher JL, Brooks CL, 3rd, Stuckey JA, Martin BR. ACS Chem Biol. 2016;11:3374–3382. doi: 10.1021/acschembio.6b00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusch M, Zimmermann TJ, Burger M, Dekker FJ, Gormer K, Triola G, Brockmeyer A, Janning P, Bottcher T, Sieber SA, Vetter IR, Hedberg C, Waldmann H. Angew Chem Int Ed Engl. 2011;50:9838–9842. doi: 10.1002/anie.201102967. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. Am J Physiol Endocrinol Metab. 2012;302:E1390–1398. doi: 10.1152/ajpendo.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. J Cell Sci. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck MW, Kathayat RS, Cham CM, Chang EB, Dickinson BC. Chem Sci. 2017 doi: 10.1039/C7SC02805A. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho AY, Choi K. Chem Lett. 2012;41:1611–1612. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.