Abstract
Background
Why are women more susceptible to multiple sclerosis, but men have worse disability progression? Sex differences in disease may be due to sex hormones, sex chromosomes, or both.
Objective
Determine whether differences in sex chromosomes can contribute to sex differences in multiple sclerosis using experimental autoimmune encephalomyelitis.
Methods
Sex chromosome transgenic mice, which permit the study of sex chromosomes not confounded by differences in sex hormones, were used to examine an effect of sex chromosomes on autoimmunity and neurodegeneration, focusing on X chromosome genes.
Results
T lymphocyte DNA methylation studies of the X chromosome gene Foxp3 suggested that maternal versus paternal imprinting of X chromosome genes may underlie sex differences in autoimmunity. Bone marrow chimeras with the same immune system but different sex chromosomes in the central nervous system, suggested that differential expression of the X chromosome gene Toll-like receptor 7 in neurons may contribute to sex differences in neurodegeneration.
Conclusion
Mapping the transcriptome and methylome in T lymphocytes and neurons in females versus males could reveal mechanisms underlying sex differences in autoimmunity and neurodegeneration.
Keywords: multiple sclerosis, genetics, immunology, animal model, neurodegeneration, sex differences
Multiple sclerosis and preclinical models
The National Institutes of Health (NIH) has recognized the importance of the study of sex differences and implemented new guidelines for NIH grants that prevent researchers from studying only one sex and ignoring the other.1 Implementation of this new NIH policy started in January of 2016.2
The study of both sexes can extend results found in one sex to the other sex making findings broader and more generalizable. Alternatively, results can differ revealing important sex differences. Sex differences in disease can illuminate naturally occurring disease modifiers which could be capitalized upon therapeutically.3
Multiple sclerosis (MS) has both autoimmune and neurodegenerative components. Autoimmune responses are thought to contribute to disease susceptibility and relapses. Gadolinium enhancement of white matter inflammatory lesions is the gold standard biomarker for assessing relapsing disease activity. On the other hand, progression of permanent disability is driven by neurodegeneration. Gray matter atrophy and other biomarkers correlate with disability progression. While inflammation is thought to trigger and contribute to neurodegeneration, robust immunosuppressive treatments do not halt disability progression, suggesting that mechanisms of neurodegeneration can become independent from inflammation at least in part. This has led to a search for MS treatments that are neuroprotective, as reviewed.4 All current disease modifying treatments for MS are designed to target the immune system, so are indirectly neuroprotective. What remains elusive are treatments that are lipophilic and target cells of the central nervous system (CNS) to provide direct neuroprotection.
MS pathogenesis is complex. Th1/Th17 T lymphocytes become activated in the peripheral immune system and enter the CNS with macrophages to cause white matter inflammatory lesions. Other cells also play a role including B lymphocytes and dendritic cells, to name a few. White matter inflammation leads to demyelination, glial (astrocyte and microglial) activation and axonal loss. Gray matter also shows pathology with synaptic and neuronal loss. Cortical demyelinating lesions occur which are characterized by a relative paucity of inflammation as compared to white matter lesions, as reviewed.5 Thus, while MS has been thought to be primarily a white matter disease, important involvement of gray matter has been shown by both neuroimaging and neuropathology.
There are several preclinical models for the study of MS. The relapsing-remitting model of experimental autoimmune encephalomyelitis (EAE) in SJL mice has been used extensively to study autoimmune responses and has led to many current anti-inflammatory treatments for RRMS. Other models are well suited for the study of other mechanisms in MS, such as the lysolecithin and cuprizone models to study demyelination and remyelination. Chronic progressive EAE models in C57BL/6 and NOD mice have been used to study neurodegenerative mechanisms in MS, going beyond spinal cord demyelination and axonal loss, to show astrocyte and microglial activation in white and gray matter, as well as neuronal and synaptic loss in gray matter.6–11 MRI has shown T2 lesions in white matter of spinal cord, brainstem and cerebellum of EAE, 12–15 with gray matter atrophy in cerebellar and cerebral cortex.12, 16 There is very little subcortical white matter in mice as compared to humans, so mice are not good models to study periventricular white matter lesions in MS. On the other hand, optic neuritis occurs in EAE with inflammation and demyelination in the optic nerve and thinning of the retinal nerve fiber layer using ocular coherence tomography (OCT).17–19
Here we will study sex differences in autoimmunity and neurodegeneration in MS using the chronic progressive EAE model.
Sex differences in MS
There is increased susceptibility of females compared to males in MS and many autoimmune diseases.20 Also, immune responses are more robust in healthy females compared to males, including autoantigen specific responses in MS.21–24 In addition, a higher relapse rate was observed in females compared with males who have RRMS throughout the entire duration of disease and at any age.25 Also, in the first five years of disease, RRMS patients with least 4 relapses per year have a greater female to male ratio (3.3:1) than patients who experience no relapses (2.3:1).25
In contrast, the risk of long term disability progression is higher in males.26, 27 A natural history study of untreated MS patients found that male gender was associated with a shorter time to, and a younger age for, conversion to SPMS,28 while another showed that males have a more severe disease phenotype resulting in faster accumulation of disability.29 Another showed that being male and of older age at onset predicted more rapid disability progression.30 Finally, a registry-based study of more than 14,000 patients revealed that male relapse-onset progressive patients accumulated disability faster than female relapse-onset progressive patients.31
For years it had been known that the characteristic profile of primary progressive MS was being male, being older and having spinal cord lesions.32 A more recent study identified predictors to develop primary progressive MS in a population of subjects with radiologically isolated syndrome (RIS). They found that male sex, older age and lesions predominantly in spinal cord were the three predictors of evolution from RIS to primary progressive MS.33 The male propensity to have more disability progression in MS is consistent with males being predisposed to other neurodegenerative diseases such as Parkinson’s Disease (PD)34 and amyotrophic lateral sclerosis (ALS).35
Here we will address mechanisms underlying the observation that women are more susceptible to MS and have a more relapsing disease course, but men have worse disability progression. This suggests that mechanisms underlying sex differences in susceptibility and relapses differ from mechanisms underlying sex differences in disability progression.36
A model to ascertain sex chromosome effects on disease
Sex differences in MS can be due to sex hormones, sex chromosomes or both. Roles for sex hormones in sex differences have been studied extensively in preclinical MS models37 and are being investigated in MS clinical trials.38–40 A role for sex chromosomes has been less well studied in EAE and is not mutually exclusive of a role for sex hormones. In preclinical models of disease, gonadectomy to remove effects of sex hormones does not suffice to study sex chromosome effects since differences between gonadectomized female and male mice are confounded by organizational effects of sex hormones from birth to the time of gonadectomy. Instead, the Four Core Genotypes (FCG) have been used to study sex chromosome effects not confounded by a difference in sex hormones.41 In FCG mice the Sry gene, which is responsible for testis development, has been deleted from the Y chromosome. Mice which have a deletion of the Sry gene on the Y chromosome are phenotypic females (ovary bearing). They are denoted XY− since they have other Y genes remaining, with only the Sry gene deleted. A comparison between XX and XY−reveals the effect of sex chromosomes on a given outcome measure in a setting of identical gonadal sex, both female. In addition, some mice have the Sry gene added back at an autosomal location resulting in XXSry and XY−Sry mice, again revealing the effect of sex chromosomes in a setting of identical gonadal sex, this time both male.42
Sex chromosome effects on autoimmunity
We used the FCG model to determine if there was a sex chromosome contribution to the increased susceptibility of SJL females compared to males with EAE.43, 44 We found that immune responses were more encephalitogenic in XX compared to XY- mice during adoptive EAE,45, 46 and that experimental lupus in SJL mice and spontaneous lupus in NZM2328 mice were each more severe in XX compared to XY-.46, 47 These preclinical observations were consistent with the female predisposition to several autoimmune diseases including MS and systemic lupus erythematosus (SLE). While the findings demonstrated a role for sex chromosomes in sex differences in immune responses, the genes and mechanisms involved remained unknown.
Possible explanations for observed differences between XX versus XY− mice include: a) Y gene presence or absence, b) differences in X gene dosage, or c) differences in parental X imprinting of X chromosome genes. Differential imprinting occurs because XY- mice have X genes that are all maternally imprinted, while XX mice have one maternally and one paternally imprinted X chromosome.48, 49 Because X inactivation is random, theoretically half of cells express X genes from the maternally imprinted X chromosome and the other half express genes from the paternally imprinted X chromosome. The X chromosome contains a multitude of genes, while the Y chromosome has shortened over evolution, conserving primarily genes related to reproduction.50 Indeed, several X chromosome genes are known to be involved in immune responses, one of which is Forkhead box p3 (Foxp3). Foxp3 is a transcription factor found in CD4+CD25hi T regulatory (Treg) cells and is considered the “master-regulator” of Treg development and function, with the induction of Foxp3 eliciting downstream immunosuppressive responses.51, 52 In EAE, Foxp3+ Tregs have been shown to regulate myelin protein specific effector T cells.53, 54 FOXP3 expression during the induction of Treg function is controlled by epigenetic mechanisms at the transcriptional level that involve Foxp3 DNA methylation.55–57 Perhaps most interestingly, it was previously shown that Tregs contribute to the relative resistance to EAE in males, as compared to females,58 but the underlying mechanism for this sex difference remained unknown.
Here, we determined whether differential parental imprinting of X genes could be the source of sex chromosome effects on autoimmunity. Specifically, DNA methylation was quantified in a CG-rich Foxp3 upstream enhancer region using bisulfite sequencing, as described,59 in CD4+ T cells from lymph node cells of PLP 139-151 immunized SJL mice which carried either the maternal or the paternal imprint. To create mice that had either the maternal or paternal imprint we used a third genotype XY*x.60 Mice that are XY*x have one intact X chromosome and, instead of a full Y chromosome, have one pseudoautosomal region with no male-specific region of the Y chromosome.61–63 XmY*x mice with a maternal X chromosome were generated by crossing XX with XY* mice.60 XpY*x mice with a paternal X chromosome were generated by crossing XmY*x females with wild type XY males. Age 8–12 week XmY*x mice (maternal X chromosome) and XpY*x (paternal X chromosome) were then used to determine if there were maternal versus paternal imprinting differences for Foxp3 in autoantigen specific T lymphocytes.
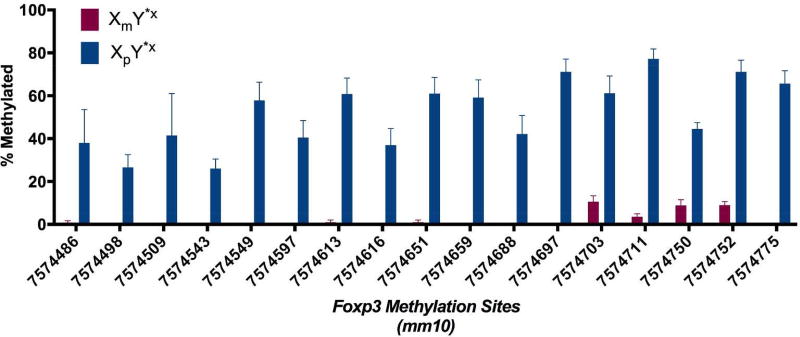
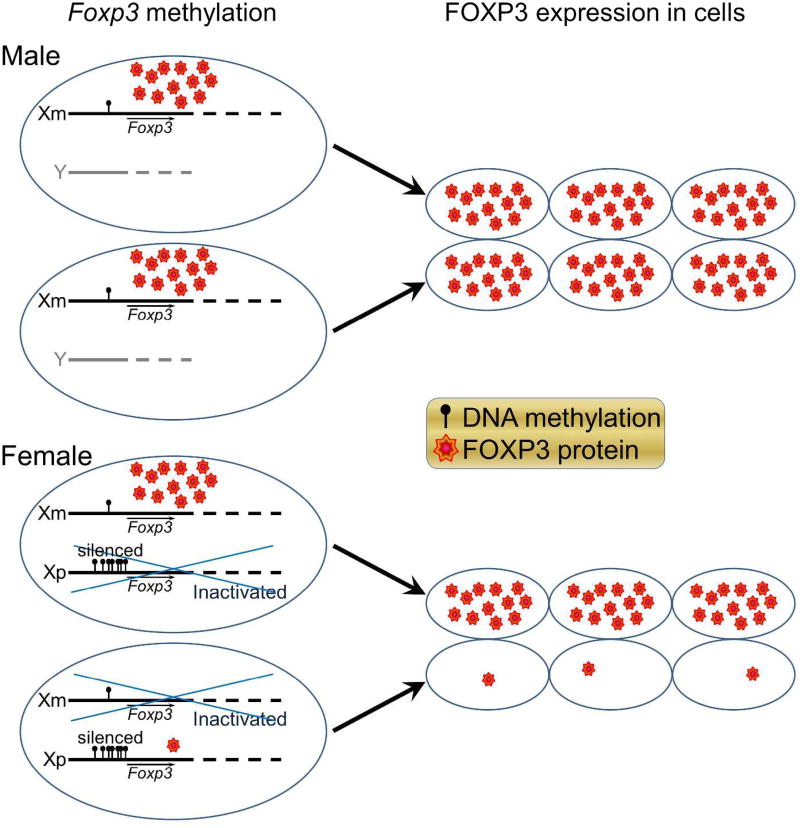
We found that CD4+ T cells from XmY*x mice demonstrated lower percentage methylation of the Foxp3 enhancer region, as compared to those from XpY*x (Fig. 1). Methylation causes gene silencing, so this would lead to more FoxP3 expression in males (XY) who express Xm genes in all cells as compared to females (XX) who express Xm genes in half their cells and Xp genes in the other half. Since CD4+CD25+FoxP3+ T cells are immunoregulatory, this would be consistent with less autoimmunity in males (Fig. 2). These FoxP3 data warrants further DNA methylation studies of other X chromosome genes as well. Also, imprinting effects are not mutually exclusive of X gene dosage effects. However, the majority of X genes undergo X-inactivation, with less than 5% escaping X-inactivation for higher gene dosage in XX compared to XY genotypes. At least for FoxP3, this is not the case since a higher dosage of FoxP3 in XX would be immunoregulatory, opposite from the direction of more autoimmunity in XX. That said, other X chromosome genes could be subject to X-dosage effects. Mapping the methylome and transcriptome of autoantigen specific CD4+ T lymphocytes as well as other critical immune cells such as macrophages and B lymphocytes in females and males and in mice of the FCG are needed to study sex differences in autoimmunity in MS.
Figure 1. The maternal X imprint, compared to the paternal X imprint, has less Foxp3 methylation.
DNA methylation was quantified in a CG-rich Foxp3 upstream enhancer region using bisulfite sequencing, as described1 in autoantigen specific T lymphocytes from mice with a maternal X chromosome (XmY*x) or a paternal X chromosome (XpY*x). The position of each CG site is given on the x-axis using the mouse genome version mm10.
Figure 2. Less methylation of the maternal X chromosome is protective for autoimmunity in XY mice since T lymphocytes of XY mice express more FOXP3 due to having the maternal X allele exclusively, as compared to XX who have the maternal X allele expression in only half of their T lymphocytes.
Maternal X (Xm): lower Foxp3 DNA methylation, less silencing, higher FOXP3 protein expression, less autoimmunity in XmY males. Paternal X (Xp): higher Foxp3 DNA methylation, more silencing, less FOXP3 protein expression, more autoimmunity, in XmXp females. Blue crossing over one allele in females indicates random X-inactivation.
Sex chromosome effects on neurodegeneration
A challenge in MS and EAE involves discerning whether effects on neurodegeneration are merely a consequence of effects on the immune system. Bone marrow chimeras are widely used to study compartmental effects in vivo in EAE. This entails irradiating mice to abolish their immune system followed by reconstitution of their immune system using bone marrow from a donor mouse. Sex chromosome effects on neurodegeneration during EAE can be determined if disease is induced in bone marrow chimeric mice that have an immune system of the same genetic sex, while having a CNS with a different genetic sex (XX or XY).
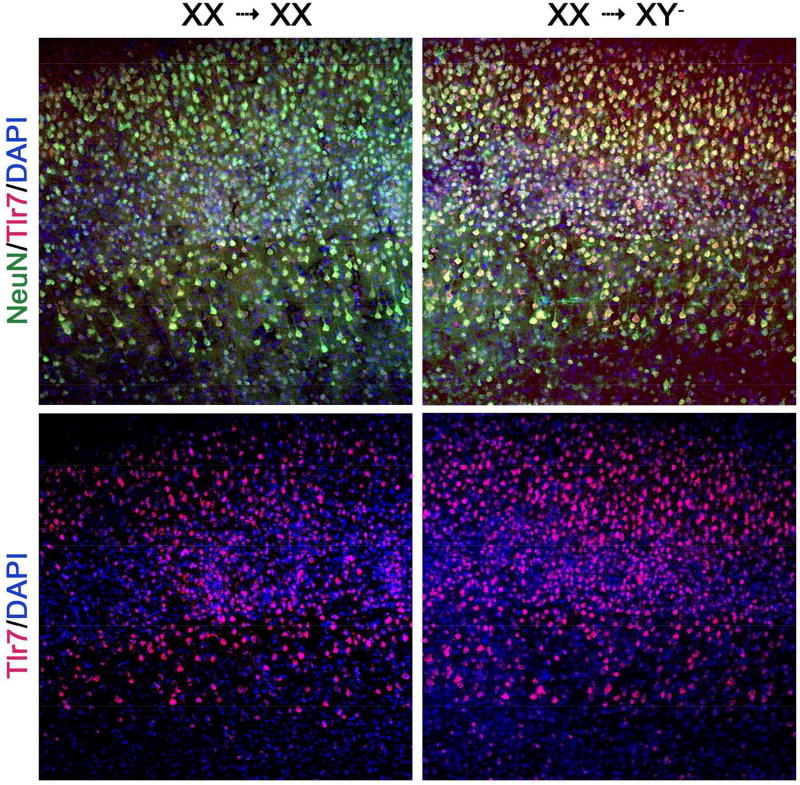
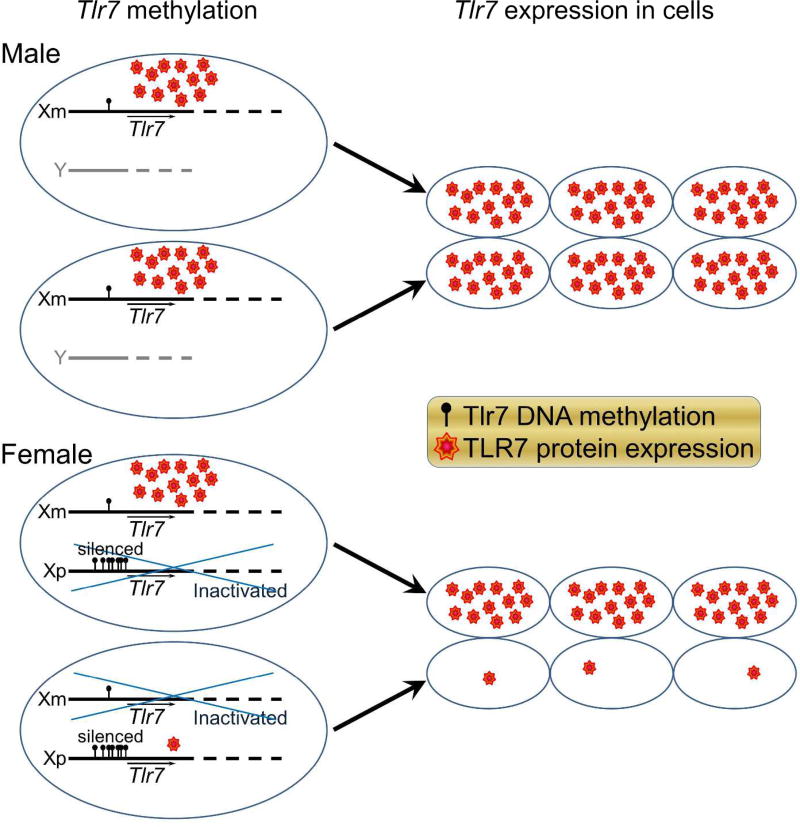
We used the Four Core Genotype model to study sex differences due to sex chromosomes, not confounded by differences in sex hormones. We also used bone marrow chimeras to study sex chromosome effects in the CNS, not confounded by differences in the immune system. Specifically, XX versus XY- bone marrow chimeras were reconstituted with a common immune system of one sex chromosome complement. We found that EAE mice with XY- sex chromosome complement in the CNS, compared with XX, demonstrated worse EAE clinical disease severity with more neuropathology in spinal cord (axonal and myelin loss), cerebellum (Purkinje cell and myelin loss) and cerebral cortex (synaptic loss).7 A candidate gene on the X-chromosome, Toll-like receptor 7 (Tlr7), was then examined since it had been shown to mediate neuronal degeneration in cortical neurons.64 TLR7 protein expression in cortical neurons was higher in mice with XY-, compared with XX, CNS (Fig. 3). This publication was the first demonstration of a direct effect of sex chromosome complement on neurodegeneration in a neurological disease,7 and findings were consistent with worse neurodegeneration in XY males as compared to XX females (Fig. 4). Notably, higher TLR7 in neurons of XY mice could not be due to X-dosage effects since Tlr7 is an X gene so would be higher, not lower, in XX if that were the case. That said, without methylation studies, it remains unclear whether the differential expression of TLR7 in neurons during EAE are due to direct effects of maternal versus paternal imprinting of Tlr7. Mapping of the methylome and the transcriptome of neurons as well as other critical CNS cells such as oligodendrocytes or astrocytes in females and males, as well as in mice of the FCG, are needed to study sex differences in disability progression in MS.
Figure 3. Mice with XY- CNS, compared with XX CNS, have more Tlr7 expressing cortical neurons in EAE.
Cerebral motor cortical layers I-VI stained with Tlr7 antibody, n = 5 mice per group. Double labeling of Tlr7 co-localizing with neuronal (NeuN) markers, previously quantified.2 DAPI = nuclear stain. Bone marrow chimeras: XX→XY- indicates XX donor cells into XY- recipient, XX→XX indicates XX donor cells into XX recipient. Shown are representative 10x capture of coronal sections.
Figure 4. Less methylation of the maternal X chromosome is deleterious for neurodegeneration in XY mice since neurons of XY mice express more TLR7 due to having the maternal X allele exclusively, as compared to XX who have the maternal X allele expression in only half of their neurons.
Tlr7 on Xm, lower methylation, less silencing, higher TLR7 expression, worse neurodegeneration in XmY males. Tlr7 on Xp, higher methylation, more silencing, less TLR7 expression, less neurodegeneration in XmXp females. Blue crossing over one allele in females indicates random X-inactivation.
Putting sex chromosome effects into perspective with past MS and EAE genetic studies
Sex chromosome effects on autoimmunity and neurodegeneration have been shown in he MS preclinical model, and intriguing differences in X chromosome gene methylation and expression have been observed when comparing XX versus XY- mice using the Four Core Genotypes. We suggest that genome wide methylation and transcriptome studies are needed in critical cell types in the immune and central nervous systems of females and males with EAE as a model for MS. This focus is new as compared to past genetic studies in MS and EAE. In MS, previous genome wide association studies (GWAS) confirmed a major role for HLA in disease susceptibility and identified numerous other susceptibility loci which contributed, including IL7 receptor and IL2 receptor alpha, to name a few.65–67 However, genetic association studies are limited to detection of polymorphisms and do not identify epigenetic effects that can influence gene expression. Also, X and Y chromosome genes were not assessed in GWAS studies. In EAE, F1 backcrosses confirmed a major role for MHC and also identified many other genes which contributed to susceptibility.68 Consomic studies showed that the Y chromosome of the SJL mouse strain, when bred into mice of another strain’s X chromosome and autosomes, conferred increased EAE susceptibility.69, 70 These studies focused on polymorphic differences in gene sequence. In contrast, mapping the methylome and transcriptome of X chromosome genes and their associated autosomes in females versus males focuses on differences in gene expression of the same sequence.
In MS, genetic factors underlying the increased susceptibility to disease in females have been attributed to maternal parent-of-origin disproportionate transmission of the major histocompatibility complex HLA-DRB1*15 (chromosome 6) to female offspring 71. Other studies have linked maternal parent-of-origin to higher risk of MS in female offspring based on half-sibling studies, avuncular pair studies, extended pedigrees of affected patients, and studies of interracial admixture populations.72–75 These clinical observations have prompted the hypothesis of a maternal genetic and female fetal environment interaction during pregnancy that favors the passing of MS susceptibility genes from mother to daughter. Our finding of differences in maternal versus paternal imprinting of X chromosome genes suggests that parental imprinting of X chromosome genes may play a role in maternal parent-of-origin effects.
Compared to disease susceptibility, less is known about genetic factors that lead to disease progression in MS. GWAS studies in MS found few genetic susceptibility loci in CNS genes.76–78 Gene expression array studies have determined that a significantly higher proportion of genes on the X chromosome, as compared to genes on autosomes, are preferentially expressed in the brain compared to other somatic tissues.79, 80 The accumulation of brain-specific genes located on the X chromosome over evolution puts them in a unique position to influence the CNS response to injury. In EAE or MS, that injury would be an immune attack.
Together this study of X genes in the Four Core Genotype mice during EAE warrants further study of X gene methylation and expression in cells of the immune system and the CNS to find new therapeutic targets to recapitulate sex differences in disease susceptibility and progression, respectively.
Acknowledgments
We wish to thank Professor Arthur Arnold, Ph.D. for insightful discussions and for providing transgenic mice for generating the XY*x genotypes. We thank Noriko Itoh, M.S. for generating mice of each sex chromosome genotype.
Funding
This work was supported by NIH grant R01NS096748, the Conrad N. Hilton Foundation (Grants #20130231 & #20150232), and California Community Foundation Grant #BAPP-15-118094 (each to R.V.), and the Tom Sherak MS Hope Foundation.
Footnotes
Conflicts of Interest
The Authors declare that there is no conflict of interest.
References
- 1.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2016;30:519–24. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voskuhl R. Preclinical studies of sex differences: a clinical perspective. Biol Sex Differ. 2016;7:7. doi: 10.1186/s13293-016-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ontaneda D, Thompson AJ, Fox RJ, Cohen JA. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–66. doi: 10.1016/S0140-6736(16)31320-4. [DOI] [PubMed] [Google Scholar]
- 5.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 6.Centonze D, Muzio L, Rossi S, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–52. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2014;111:2806–11. doi: 10.1073/pnas.1307091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacKenzie-Graham A, Tiwari-Wooddruff S, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48:637–51. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen S, Wang Y, Kivisakk P, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing remitting experimental autoimmune encephalomyelitis. Brain. 2007 doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 10.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–86. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh N, Kim R, Peng M, et al. Bedside to bench to bedside research: Estrogen receptor beta ligand as a candidate neuroprotective treatment for multiple sclerosis. J Neuroimmunol. 2017;304:63–71. doi: 10.1016/j.jneuroim.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKenzie-Graham A, Tinsley MR, Shah KP, et al. Cerebellar cortical atrophy in experimental autoimmune encephalomyelitis. Neuroimage. 2006;32:1016–23. doi: 10.1016/j.neuroimage.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48:637–51. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waiczies H, Millward JM, Lepore S, et al. Identification of cellular infiltrates during early stages of brain inflammation with magnetic resonance microscopy. PLoS One. 2012;7:e32796. doi: 10.1371/journal.pone.0032796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahrens ET, Laidlaw DH, Readhead C, Brosnan CF, Fraser SE, Jacobs RE. MR microscopy of transgenic mice that spontaneously acquire experimental allergic encephalomyelitis. Magn Reson Med. 1998;40:119–32. doi: 10.1002/mrm.1910400117. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie-Graham A, Rinek GA, Avedisian A, et al. Cortical atrophy in experimental autoimmune encephalomyelitis: in vivo imaging. Neuroimage. 2012;60:95–104. doi: 10.1016/j.neuroimage.2011.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn TA, Dutt M, Shindler KS. Optic neuritis and retinal ganglion cell loss in a chronic murine model of multiple sclerosis. Front Neurol. 2011;2:50. doi: 10.3389/fneur.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsunaga Y, Kezuka T, An X, et al. Visual functional and histopathological correlation in experimental autoimmune optic neuritis. Invest Ophthalmol Vis Sci. 2012;53:6964–71. doi: 10.1167/iovs.12-10559. [DOI] [PubMed] [Google Scholar]
- 19.Brambilla R, Dvoriantchikova G, Barakat D, Ivanov D, Bethea JR, Shestopalov VI. Transgenic inhibition of astroglial NF-kappaB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. J Neuroinflammation. 2012;9:213. doi: 10.1186/1742-2094-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 21.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 22.Kantarci OH, Hebrink DD, Schaefer-Klein J, et al. Interferon gamma allelic variants: sex-biased multiple sclerosis susceptibility and gene expression. Arch Neurol. 2008;65:349–57. doi: 10.1001/archneurol.2007.66. [DOI] [PubMed] [Google Scholar]
- 23.Moldovan IR, Cotleur AC, Zamor N, Butler RS, Pelfrey CM. Multiple sclerosis patients show sexual dimorphism in cytokine responses to myelin antigens. J Neuroimmunol. 2008;193:161–9. doi: 10.1016/j.jneuroim.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelfrey CM, Cotleur AC, Lee JC, Rudick RA. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J Neuroimmunol. 2002;130:211–23. doi: 10.1016/s0165-5728(02)00224-2. [DOI] [PubMed] [Google Scholar]
- 25.Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136:3609–17. doi: 10.1093/brain/awt281. [DOI] [PubMed] [Google Scholar]
- 26.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126:770–82. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 27.Runmarker B, Andersson C, Odén A, Andersen O. Prediction of outcome in multiple sclerosis based on multivariate models. Journal of Neurology. 1994;241:597–604. doi: 10.1007/BF00920623. [DOI] [PubMed] [Google Scholar]
- 28.Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:1039–43. doi: 10.1136/jnnp.2010.208173. [DOI] [PubMed] [Google Scholar]
- 29.Tomassini V, Pozzilli C. Sex hormones, brain damage and clinical course of Multiple Sclerosis. J Neurol Sci. 2009;286:35–9. doi: 10.1016/j.jns.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Shirani A, Zhao Y, Kingwell E, Rieckmann P, Tremlett H. Temporal trends of disability progression in multiple sclerosis: findings from British Columbia, Canada (1975–2009) Mult Scler. 2012;18:442–50. doi: 10.1177/1352458511422097. [DOI] [PubMed] [Google Scholar]
- 31.Ribbons KA, McElduff P, Boz C, et al. Male Sex Is Independently Associated with Faster Disability Accumulation in Relapse-Onset MS but Not in Primary Progressive MS. PLoS One. 2015;10:e0122686. doi: 10.1371/journal.pone.0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson A. Overview of primary progressive multiple sclerosis (PPMS): similarities and differences from other forms of MS, diagnostic criteria, pros and cons of progressive diagnosis. Mult Scler. 2004;10(Suppl 1):S2–7. doi: 10.1191/1352458504ms1024oa. [DOI] [PubMed] [Google Scholar]
- 33.Kantarci OH, Lebrun C, Siva A, et al. Primary Progressive Multiple Sclerosis Evolving From Radiologically Isolated Syndrome. Ann Neurol. 2016;79:288–94. doi: 10.1002/ana.24564. [DOI] [PubMed] [Google Scholar]
- 34.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–9. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris F, Shepherd R, Denys E, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci. 1993;118:48–55. doi: 10.1016/0022-510x(93)90245-t. [DOI] [PubMed] [Google Scholar]
- 36.Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 2012;8:255–63. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–15. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurth F, Luders E, Sicotte NL, et al. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. 2014;4:454–60. doi: 10.1016/j.nicl.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Giglio L, Marinelli F, Barletta VT, et al. Effect on Cognition of Estroprogestins Combined with Interferon Beta in Multiple Sclerosis: Analysis of Secondary Outcomes from a Randomised Controlled Trial. CNS Drugs. 2017;31:161–8. doi: 10.1007/s40263-016-0401-0. [DOI] [PubMed] [Google Scholar]
- 40.Voskuhl RR, Wang H, Wu TC, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:35–46. doi: 10.1016/S1474-4422(15)00322-1. [DOI] [PubMed] [Google Scholar]
- 41.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–4. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 42.Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitacre CC, Reingold SC, O'Looney PA Task Force Gender Multiple Sclerosis A. Biomedicine - A gender cap in autoimmunity. Science. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 44.Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nature Reviews Neurology. 2012;8:255–63. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A Yin-Yang Effect Between Sex Chromosome Complement and Sex Hormones on the Immune Response. Endocrinology. 2005 doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 46.Smith-Bouvier DL, Divekar AA, Sasidhar M, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasidhar MV, Itoh N, Gold SM, Lawson GW, Voskuhl RR. The XX sex chromosome complement in mice is associated with increased spontaneous lupus compared with XY. Ann Rheum Dis. 2012;71:1418–22. doi: 10.1136/annrheumdis-2011-201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capel B, Burgoyne PS, Mittwoch U. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse - Discussion. Philos T Roy Soc B. 1995;350:260–1. doi: 10.1098/rstb.1995.0159. [DOI] [PubMed] [Google Scholar]
- 49.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends in Endocrinology and Metabolism. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Charlesworth B. The evolution of sex chromosomes. Science. 1991;251:1030–3. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- 51.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 52.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 53.Yu P, Gregg RK, Bell JJ, et al. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. Journal of Immunology. 2005;174:6772–80. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- 54.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: Contribution of CD4(+)CD25(+) regulatory cells within the central nervous system. Journal of Immunology. 2005;175:3025–32. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 55.Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. Plos Biology. 2007;5:169–78. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polansky JK, Kretschmer K, Freyer J, et al. DNA methylation controls Foxp3 gene expression. European Journal of Immunology. 2008;38:1654–63. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 57.Lal G, Zhang N, van der Touw W, et al. Epigenetic Regulation of Foxp3 Expression in Regulatory T Cells by DNA Methylation. J Immunol. 2009;182:259–73. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy J, Waldner H, Zhang XM, et al. Cutting edge: CD4(+)CD25(+) regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. Journal of Immunology. 2005;175:5591–5. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 59.Coit P, Yalavarthi S, Zhao WP, Kaplan MJ, Sawalha AH. Epigenome Profiling Reveals Robust Hypomethylation of Interferon Signature Genes in Lupus Neutrophils. Arthritis Rheumatol. 2014;66:S32–S3. doi: 10.1016/j.jaut.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eicher EM, Hale DW, Hunt PA, et al. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–30. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- 61.Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenetics and Cell Genetics. 1998;80:37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and Y Chromosome Complement Influence Adiposity and Metabolism in Mice. Endocrinology. 2013;154:1092–104. doi: 10.1210/en.2012-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isles AR, Davies W, Burrmann D, Burgoyne PS, Wilkinson LS. Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner's syndrome. Hum Mol Genet. 2004;13:1849–55. doi: 10.1093/hmg/ddh203. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann SM, Kruger C, Park B, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–35. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 65.Baranzini SE, Wang J, Gibson RA, et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum Mol Genet. 2009;18:767–78. doi: 10.1093/hmg/ddn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nature Genetics. 2013;45:1353. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Encinas JA, Wicker LS, Peterson LB, et al. QTL influencing autoimmune diabetes and encephalomyelitis map to a 0.15-cM region containing Il2. Nat Genet. 1999;21:158–60. doi: 10.1038/5941. [DOI] [PubMed] [Google Scholar]
- 69.Case LK, Wall EH, Dragon JA, et al. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23:1474–85. doi: 10.1101/gr.156703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teuscher C, Noubade R, Spach K, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103:8024–9. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chao MJ, Herrera BM, Ramagopalan SV, et al. Parent-of-origin effects at the major histocompatibility complex in multiple sclerosis. Hum Mol Genet. 2010;19:3679–89. doi: 10.1093/hmg/ddq282. [DOI] [PubMed] [Google Scholar]
- 72.Herrera BM, Ramagopalan SV, Lincoln MR, et al. Parent-of-origin effects in MS: observations from avuncular pairs. Neurology. 2008;71:799–803. doi: 10.1212/01.wnl.0000312377.50395.00. [DOI] [PubMed] [Google Scholar]
- 73.Ebers GC, Sadovnick AD, Dyment DA, Yee IML, Willer CJ, Risch N. Parent-of-origin effect in multiple sclerosis: observations in half-siblings. Lancet. 2004;363:1773–4. doi: 10.1016/S0140-6736(04)16304-6. [DOI] [PubMed] [Google Scholar]
- 74.Hoppenbrouwers IA, Liu F, Aulchenko YS, et al. Maternal transmission of multiple sclerosis in a Dutch population. Arch Neurol-Chicago. 2008;65:345–8. doi: 10.1001/archneurol.2007.63. [DOI] [PubMed] [Google Scholar]
- 75.Ramagopalan SV, Yee IM, Dyment DA, et al. Parent-of-origin effect in multiple sclerosis Observations from interracial matings. Neurology. 2009;73:602–5. doi: 10.1212/WNL.0b013e3181af33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baranzini SE, Wang J, Gibson RA, et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum Mol Genet. 2009;18:767–78. doi: 10.1093/hmg/ddn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen DK, Disteche CM. High expression of the mammalian X chromosome in brain. Brain Research. 2006;1126:46–9. doi: 10.1016/j.brainres.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nature Genetics. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]