Abstract
Objective
Hepatitis C virus (HCV) is associated with B cell lymphoproliferative disorders, including mixed cryoglobulinemia (MC) vasculitis and B cell non-Hodgkin’s lymphoma. The expansion of clonal and autoreactive rheumatoid factor–bearing CD21−/low marginal zone (MZ) B cells was demonstrated in patients with HCV-associated MC vasculitis. Fc receptor–like (FCRL) proteins comprise a family of immunoregulatory proteins preferentially expressed on B lineage cells. The goal of this study was to investigate the expression of FCRL proteins 1–5 on B cells from patients with HCV-associated MC vasculitis.
Methods
Expression of FCRL proteins 1–5 was assessed by flow cytometry on B cells from 15 HCV-infected patients with type II MC (7 of whom had B cell non-Hodgkin’s lymphoma), 20 HCV-infected patients without MC, and 20 healthy donors. To evaluate FCRL-5 as an immunotherapy target in HCV-associated MC vasculitis, 2 anti–FCRL-5 recombinant immunotoxins were produced using anti–FCRL-5 monoclonal antibodies and Pseudomonas exotoxin.
Results
Expression of FCRLs 2, 3, and 5 was markedly increased while expression of FCRL-1 was decreased on clonal CD21−/low MZ B cells, as compared with other B cell subsets, from HCV-infected patients and healthy donors. However, there was no difference in the pattern of FCRL expression between HCV-MC patients with lymphoma and those without lymphoma. The anti–FCRL-5 immunotoxins showed specific cytotoxicity against FCRL-5–expressing clonal CD21−/low MZ B cells isolated from HCV-infected patients as well as FCRL-5–transfected cell lines. No cytotoxicity against T cells or conventional B cells was observed.
Conclusion
These findings suggest that FCRL-5–targeting therapies could be a specific treatment for HCV-associated MC vasculitis and other FCRL-5–positive autoimmune B cell disorders.
Mixed cryoglobulinemia (MC) is a benign B cell proliferative disorder that can affect up to 50% of patients with hepatitis C virus (HCV) (1). HCV infection is also frequently associated with the development of B cell non-Hodgkin’s lymphoma (1–3). In accordance with these symptoms, the occurrence of abnormal clonal B cell populations in the liver and blood in HCV-infected patients has been demonstrated in several studies (4–7). Preferential use of a type of Ig heavy chain, characterized by VH1–69 and Igκ Vκ3 gene segments, has been noted in clonal B cells from patients with HCV-related B cell lymphoproliferation, and these segments encode rheumatoid factor of the Wa idiotype (8–12). In addition, the clonal B cells often harbor a peculiar marginal zone (MZ)–like phenotype characterized by IgM+Igκ+IgD−/lowCD27+ B cells expressing low or no surface CD21/CR2 (herein referred to as CD21−/low MZ B cells) (11).
We and others have recently described the expansion of CD21−/low MZ B cells in the peripheral blood of patients with HCV-associated MC vasculitis, and found that these cells are prone to anergy, with defective calcium signaling and poor proliferation in response to B cell receptor (BCR) stimulation (13,14). However, we also found that the CD21−/low MZ B cells remained in the blood of patients with HCV-MC vasculitis, instead of being eliminated, and that chronic antigenic stimulation through the Toll-like receptor pathway may create a favorable environment for breaking tolerance and activating these cells (14).
The uniform phenotypic characteristics of the abnormal B cells encouraged us to search for surface molecules that are specific to the cell population and can be used as targets of antibody-based therapeutic agents. Along this line, we previously performed a gene expression profile analysis and found a 2.0-fold up-regulation in expression of FCRL5/IRTA2 in CD21−/low MZ B cells as compared to conventional CD21+ MZ B cells from the same HCV-MC patients. FCRL5 expression was also up-regulated in CD21−/low MZ B cells from healthy donors, with a 2.2-fold increase compared to conventional CD21+ MZ B cells (14). In addition, a study by Isnardi et al demonstrated up-regulated FCRL5/IRTA2 expression in CD21−/low autoreactive unresponsive B cells from patients with rheumatoid arthritis and common variable immunodeficiency (15).
The family of FCRL proteins includes 6 trans-membrane proteins homologous to classic Fc receptors (16–18). Five members of the family (FCRL proteins 1–5) are preferentially and differently expressed in mature B cells at various differentiation stages, whereas FCRL-6 is highly expressed in T cells. The intracellular regions of FCRL proteins 1–6 possess different numbers of immunoreceptor tyrosine–based activation motif and/or immunoreceptor tyrosine–based inhibition motif (ITIM), suggesting that these proteins have regulatory functions on B cell activation through phosphorylation of the domains (19–23). Findings in previous experiments suggest that FCRL-1 promotes B cell activation and FCRL proteins 2–5 reversely inhibit BCR signaling. However, the exact physiologic function of FCRLs, beyond phosphorylation, has not been elucidated. Recent studies identified HLA–DR, a class II major histo-compatibility complex molecule, as a ligand of FCRL-6 (24). In addition, binding of the aggregated form of IgG and IgA to FCRL-5 and to FCRL-4, respectively, has been demonstrated (25). In a previous study, we found that stimulation with an anti–FCRL-5 antibody induced differentiation of B cells in an experimental condition (26). We also showed that FCRL-5 binds to the conformational form of IgG, suggesting that FCRL-5 is a new type of receptor that may enable B cells to sense Ig quality (27). Overall, it is speculated that binding of FCRLs to these ligands guides the lymphocytes for appropriate differentiation through the regulation of BCR signaling (28).
The stage-specific B cell expression and function of FCRL proteins 1–5 strongly suggest that the abnormal clonal B cells that develop in B cell lymphoproliferative disorders could express each FCRL molecule differentially in comparison with normal B cells. Indeed, we and other groups have reported that FCRL-5 is overexpressed on some malignant B cells in hairy cell leukemia, chronic lymphocytic leukemia, mantle cell lymphoma, and multiple myeloma (29). In addition, FCRL-5 was recently developed as a novel target in the treatment of multiple myeloma (30). In the present study, we analyzed the expression of FCRL proteins on B cells from HCV-infected patients with or without MC vasculitis, as well as on normal B cells from healthy donors, to explore the potential usefulness of FCRL-5–targeting therapy.
PATIENTS AND METHODS
Study subjects
We recruited 15 untreated patients with HCV infection and type II MC vasculitis (9 women and 6 men; mean age 47 years [range 25–73 years]) and 20 untreated patients with HCV infection without MC (7 women and 13 men; mean age 50 years [range 36–67 years]). All patients with HCV infection were positive for HCV RNA. Patients with HCV-MC had clinical manifestations of vasculitis (purpura or cutaneous ulcers, arthralgia, myalgia, peripheral neuropathy, renal involvement, cerebral vasculitis, gastrointestinal involvement, or cardiac involvement). Seven of the 15 patients with HCV-MC vasculitis had features of overt MZ B cell non-Hodgkin’s lymphoma, based on abnormal findings on bone marrow biopsy, an abnormal peripheral lymphocyte phenotype showing a clonal κ light chain–restricted CD19+ B cell population, and/or lymphoid organ enlargement. Four of the 7 patients with MZ B cell non-Hodgkin’s lymphoma had abnormal bone marrow biopsy findings in association with an abnormal clonal κ light chain–restricted CD19+ B cell population in the peripheral blood, whereas the 3 remaining patients had splenomegaly along with an abnormal clonal κ light chain–restricted CD19+ B cell population in the peripheral blood. As controls, blood samples from 20 healthy donors were obtained from the Etablissement Français du Sang (Hôpital Pitié-Salpêtrière).
The study was performed in accordance with the Declaration of Helsinki. All study subjects provided informed consent, with the approval of our Institutional Review Board.
Cell lines
AS283A cells expressing endogenous FCRL-5 were kindly provided by Dr. Giovanna Tosato (National Cancer Institute, National Institutes of Health, Bethesda, MD). A431 epidermoid carcinoma cells (CRL-1555; American Type Culture Collection) and DG44 (dihydrofolate reductase–deficient Chinese hamster ovary cells [Invitrogen]) were stably transfected with the plasmid encoding FCRL-5 complementary DNA (cDNA), as described previously (31).
Purification of peripheral blood mononuclear cells (PBMCs), B cells, and T cells
Fresh PBMCs from healthy donors, HCV-infected patients with MC, and HCV-infected patients without MC were obtained by standard density-gradient centrifugation. B cells from healthy donors were enriched from total PBMCs by negative magnetic bead selection using a B Cell Isolation Kit II (Miltenyi Biotech). The purity of the B cells was typically >95%. Clonal CD21−/low MZ B cells from patients with HCV-MC vasculitis were enriched from total PBMCs by magnetic bead selection using an IgM+ Memory B Cell Isolation Kit (Miltenyi Biotech) (results available from the corresponding author upon request). The purity of the CD21−/lowCD27+IgM+ MZ B cells, as assessed by flow cytometry using CD19, CD21, CD27, and IgM surface markers, was determined to be >90%. CD3+ T cells from HCV-infected patients with MC were enriched from the non–memory B cell fraction obtained after the first step of the selection process. The purity of CD3+ T cells was >85%.
Flow cytometry analysis of FCRL protein expression
Fresh PBMCs were stained with the following conjugated anti-human monoclonal antibodies (mAb): fluorescein isothiocyanate (FITC)–conjugated anti-CD21, energy-coupled dye–conjugated anti-CD19, allophyocyanin (APC)–conjugated anti-IgM, and phycoerythrin (PE)–Cy7–conjugated anti-CD27 (Beckman Coulter). PBMCs were also stained with PE-conjugated anti–FCRL-1, anti–FCRL-2, anti–FCRL-3, anti–FCRL-4, and anti–FCRL-5 mAb. We prepared these mAb as described previously (31–34). All of these FCRL-specific mAb were authorized as reference antibodies for FCRL proteins 1–5 (CD307a–e) in previous international human leukocyte differentiation antigens workshops (33,35). We also used PE-conjugated anti–FCRL-4 mAb (clone 413D12) from BioLegend. PE-conjugated IgG1 (Beckman Coulter) was used as an isotype control. Flow cytometry was performed on a Navios flow cytometer (Beckman Coulter) and analyzed using CXP analysis (Beckman Coulter) and FlowJo software (Tree Star).
Production of recombinant anti–FCRL-5 immunotoxins
Two anti–FCRL-5 recombinant immunotoxins, F56-IT and F25-IT, were produced based on the Fv sequences of F56 and F25 mAb specific to FCRL-5. We produced these anti–FCRL-5 mAb as previously described (31). Both of these mAb were authorized as reference antibodies for FCRL-5 in the 8th International Workshop on Human Leukocyte Differentiation Antigens (35). A new CD number (CD307e) was given to FCRL-5 based on these mAb. F56-IT and F25-IT were made using a standard protocol established in our laboratory (36,37). The Fv portions of the mAb were genetically fused with a 38-kd portion of Pseudomonas exotoxin A (PE38), which is of bacterial origin (38). Briefly, the VH and VL cDNAs were obtained from total cellular RNAs of F56 and F25 hybridoma cells using the rapid amplification of cDNA ends method. VH cDNAs were fused with PE38 in expression plasmids, and the expressed proteins in Escherichia coli were harvested as inclusion bodies. Recombinant VL chains were separately expressed, and the immunotoxin proteins were assembled by the refolding of the pairs of VH-PE38 and VL polypeptides into a disulfide-stabilized Fv fragment (dsFv) form. In the dsFv immunotoxins, each VH-PE38 is linked with the corresponding VL by a disulfide bond between 2 cysteine residues engineered in the framework region of each chain. Active monomeric protein was purified by ion-exchange and size-exclusion chromatography to near homogeneity.
The specific binding activity of F56-IT and F25-IT was confirmed by enzyme-linked immunosorbent assay and flow cytometry using FCRL-5-Fc fusion proteins and FCRL-5–expressing cells, respectively (29,32). The antigen-specific cytotoxic activities of the immunotoxins were confirmed based on the dehydrogenase activity in the cells, determined using a water-soluble tetrazolium 8–based cell-counting assay kit. We used anti-CD25 (LMB-2) immunotoxins as negative controls in the determinations of specific binding activity and antigen-specific cytotoxic activities. LMB-2 is composed of the Fv portion of the anti-Tac (anti-CD25) antibody, also fused to the 38-kd truncated form of Pseudomonas exotoxin A (39). LMB-2 has been previously used in clinical trials (40).
Assay for anti–FCRL-5 immunotoxin cytotoxic effects on patient cells
The cytotoxic effects of the anti–FCRL-5 immunotoxins on purified B cells and T cells from HCV-infected patients with MC and healthy donors were measured by flow cytometry using 7-aminoactinomycin D (7-AAD) and annexin V staining. Prepared cell samples were seeded onto 96-well plates at 2 × 105 cells/well. Serial dilutions of F56-IT and F25-IT in the culture medium were added to the cells, at a final concentration of 0–10,000 ng/ml. After 72 hours of culture, the cells were stained with PE–Cy7–conjugated anti-CD19 or Alexa Fluor 700–conjugated anti-CD3 and FITC–conjugated anti-CD45 (Beckman Coulter), and then resuspended in annexin binding buffer and labeled with 7-AAD and PE-conjugated annexin V (BD PharMingen). Flow cytometric analyses were performed on a Navios flow cytometer, with results analyzed using Navios software (Beckman Coulter). Viability was assessed by setting the gates based on the light-scatter properties of the lymphocytes. Viable cells were those negative for 7-AAD and PE-conjugated annexin V. To compensate for spontaneous cell death, the relative percentage of viable cells at the end of the assay was calculated using the following formula: (no. of CD19+ or CD3+ viable cells recovered in the treated well/no. of CD19+ or CD3+ viable cells in the untreated well) × 100 (41). All experiments were performed in duplicate.
Statistical analysis
Data are expressed as the mean ± SD. Categorical variables were compared using Fisher’s exact test or a chi-square test, and continuous variables were compared using a t-test or Mann-Whitney U test, as appropriate. All tests were 2-sided at a 0.05 significance level. Graphing and statistical analyses were performed using GraphPad Prism software.
RESULTS
Expression of FCRL proteins on normal PBMCs and B cells from healthy donors
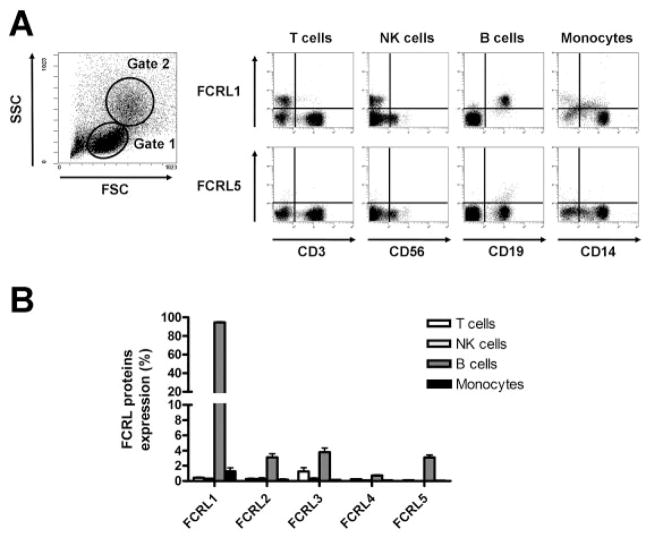
We first analyzed the expression of FCRL proteins 1–5 using anti-FCRL mAb on different cell fractions of normal PBMCs from healthy donors (Figures 1A and B). As expected from findings in the literature, FCRL-1 was widely expressed by CD19+ B cells, while FCRL proteins 2, 3, and 5 were expressed by ~4% of total peripheral B cells. FCRL-4 expression by CD19+ B cells was almost negligible. The proportions of cells expressing FCRL proteins among the subsets of CD3+ T cells, CD3−CD56+ natural killer (NK) cells, and CD14+ monocytes were mostly negligible, except for a small proportion of CD3+ T cells expressing FCRL-3 (mean ± SD 1.2 ± 1.1% of CD3+ T cells), as has been reported previously (34).
Figure 1.
Fc receptor–like (FCRL) protein expression by fresh peripheral blood mononuclear cells (PBMCs) from healthy donors. PBMCs from 20 healthy donors were stained with phycoerythrin-conjugated anti-FCRL monoclonal antibody and antibodies against lineage-specific markers. A, Left, Cells in the lymphocyte light-scatter gate (gate 1) were analyzed for CD3+ cells (T cells), CD56+ cells (natural killer [NK] cells), and CD19+ cells (B cells), while cells in the gate with larger scattering parameters (gate 2) were analyzed for CD14+ cells (monocytes). Right, Representative dot plots show expression of FCRL-1 and FCRL-5 on PBMCs from a healthy donor. FCRL-1 was expressed on the B cell fraction (CD19+). FCRL-5 was expressed on a small subpopulation (<4%) of B cells. B, The percentage of cells expressing FCRLs 1–5 was determined in the T cell, NK cell, B cell, and monocyte fractions. Bars show the mean ± SD.
Expression of FCRL proteins on subsets of B cells from healthy donors, HCV-infected patients without MC, and HCV-infected patients with MC vasculitis
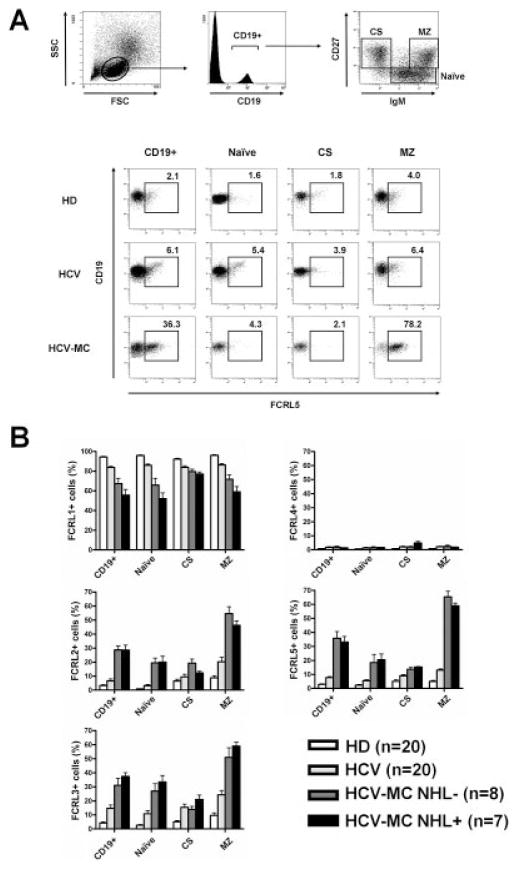
We analyzed the pattern of expression of FCRL proteins on the different B cell subsets obtained from HCV-infected patients with or without MC vasculitis, as well as from healthy donors. The main characteristics of the patients included in the study are summarized in Table 1. In this analysis, the CD19+ B cells were classified into 3 subpopulations depending on the expression of IgM and CD27 (IgM+CD27− naive B cells, IgM+CD27+ MZ B cells, and IgM−CD27+ class-switched B cells), and then further analyzed for the expression of FCRL proteins 1–5 on the gated cells (Figure 2A, top panels). The frequency of IgM+CD27+ B cells was similar between HCV-MC vasculitis patients with MZ B cell lymphoma and those without MZ B cell lymphoma (mean ± SD 41.0 ± 13.2% versus 38.6 ± 14.7% among all CD19+ B cells; P = 0.87). In contrast, the frequency of clonal CD21−/lowIgM+CD27+ B cells tended to be higher in HCV-MC vasculitis patients with MZ B cell lymphoma compared to those without MZ B cell lymphoma (44.2 ± 23.3% versus 24.3 ± 9.7% among all CD19+ B cells; P = 0.08).
Table 1.
Main characteristics of the patients and healthy donors*
| Characteristic | Healthy donors (n = 20) | HCV without MC (n = 20) | HCV with MC (n = 15) | HCV-MC without lymphoma (n = 8) | HCV-MC with lymphoma (n = 7) |
|---|---|---|---|---|---|
| Age, mean (range) years | – | 50 (36–67) | 47 (25–73) | 47 (39–73) | 51 (25–67) |
| Males, no. (%) | – | 13 (65) | 6 (40) | 2 | 4 |
| Type II MC, no. (%) | 0 (0) | 0 (0) | 15 (100) | 8 (100) | 7 (100) |
| MC vasculitis, no. (%) | 0 (0) | 0 (0) | 15 (100) | 8 (100) | 7 (100) |
| B cell NHL, no. (%) | 0 (0) | 0 (0) | 7 (47) | 0 (0) | 7 (100) |
| Cell subsets, mean ± SD % | |||||
| CD19+ B cells among lymphocytes | 11.3 ± 4.0 | 9.9 ± 5.4 | 5.6 ± 2.6 | 5.6 ± 2.9 | 5.7 ± 2.6 |
| IgM+CD27− among CD19+ B cells | 63.1 ± 4.8 | 64.1 ± 13.3 | 47.8 ± 16.0 | 46.2 ± 16.9 | 49.6 ± 15.9 |
| IgM+CD27+ among CD19+ B cells | 16.8 ± 4.6 | 16.5 ± 7.5 | 39.7 ± 13.6 | 38.6 ± 14.7 | 41.0 ± 13.2 |
| IgM−CD27+ among CD19+ B cells | 15.2 ± 5.1 | 15.1 ± 8.7 | 7.8 ± 4.9 | 10.4 ± 5.0 | 4.9 ± 2.7 |
| CD21−/lowIgM+CD27+ among CD19+ B cells | 1.7 ± 0.6 | 4.2 ± 2.1 | 33.6 ± 19.5 | 24.3 ± 9.7 | 44.2 ± 23.3 |
IgM+CD27− B cells were defined as naive B cells, IgM+CD27+ B cells were defined as marginal zone–like B cells, IgM−CD27+ B cells were defined as class-switched B cells, and CD21−/lowIgM+CD27+ B cells were defined as clonal marginal zone–like B cells. HCV = hepatitits C virus; MC = mixed cryoglobulinemia; NHL = non-Hodgkin’s lymphoma.
Figure 2.
Expression of Fc receptor–like (FCRL) proteins on peripheral blood mononuclear cell (PBMC) B cell subsets from healthy donors (HD), hepatitis C virus (HCV)–infected patients without mixed cryoglobulinemia (MC), and patients with HCV-MC vasculitis (with or without non-Hodgkin’s lymphoma [NHL]). PBMCs were stained with phycoerythrin (PE)–conjugated anti-FCRL proteins, energy-coupled dye–conjugated anti-CD19, allophycocyanin–conjugated anti-IgM, and PE–Cy7–conjugated anti-CD27. After gating on CD19+ cells, IgM+CD27+ naive B cells, IgM+CD27+ marginal zone (MZ)–like B cells, and IgM−CD27+ class-switched (CS) B cells were gated for analysis of expression of FCRL proteins 1–5. A, Representative dot plots show the gating strategy (top) and expression of FCRL-5 by gated B cells (bottom). B, Frequency of expression of FCRL proteins 1–5 by CD19+ B cells, naive B cells, MZ B cells, and CS B cells was compared. Bars show the mean ± SD.
Flow cytometry analysis revealed that B cells from HCV-MC vasculitis patients had decreased expression of FCRL-1 and increased expression of FCRL proteins 2, 3, and 5, when compared with B cells from HCV-infected patients without MC and healthy donors (Figures 2A and B). This unusual pattern of expression affected, in particular, IgM+CD27+ MZ B cells from patients with HCV-MC vasculitis, a cell subset known to be particularly enriched in clonal and autoreactive rheumatoid factor–bearing MZ B cells (Table 1). However, we did not find any difference in the pattern of expression of FCRL proteins between HCV-MC vasculitis patients with and those without lymphoma. We also analyzed the pattern of expression of FCRL proteins on conventional IgM+CD27+ MZ B cells from patients with primary Sjögren’s syndrome without lymphoma (n = 6), and observed a pattern of expression of FCRL-1, FCRL-2, FCRL-3, FCRL-4, and FCRL-5 (76.2%, 16.9%, 13.1%, 2.1%, and 16.5%, respectively) that was similar to that on IgM+CD27+ MZ B cells from HCV-infected patients without MC.
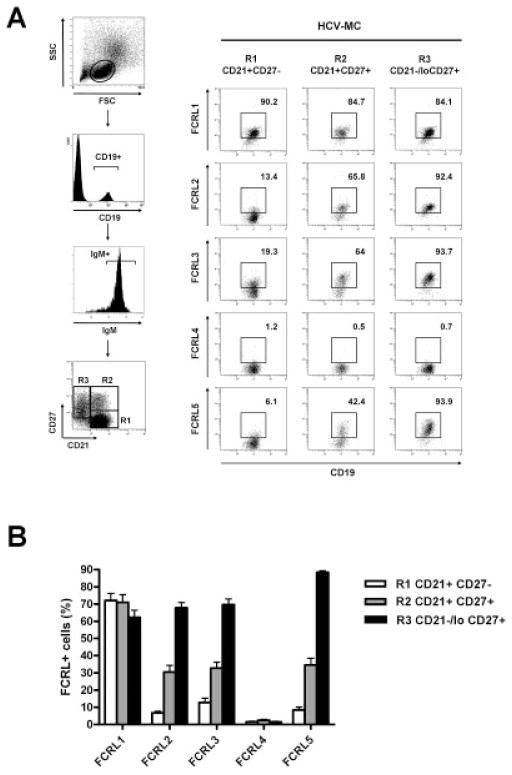
We next investigated FCRL expression on B cells from patients with HCV-MC vasculitis in detail. As shown in previous studies (11,42), peripheral B cells in these patients consist of 3 main subpopulations: CD21+IgM+CD27− naive B cells, conventional CD21+IgM+CD27+ MZ B cells, and clonal CD21−/low IgM+CD27+ MZ B cells (Figure 3A). As shown in Figures 3A and B, analysis of these subpopulations revealed that FCRL-2, FCRL-3, and FCRL-5 were expressed at elevated levels preferentially by the clonal CD21−/lowIgM+CD27+ MZ B cell population. Among these FCRLs, FCRL-5 was the most frequently (>90%) and specifically expressed by the clonal B cells from patients with HCV-MC vasculitis (Figure 3B).
Figure 3.
Pattern of FCRL protein expression by naive B cells, conventional CD21+ B cells, and clonal CD21−/low MZ B cells from patients with HCV-MC vasculitis. PBMCs from 15 patients with HCV-MC vasculitis were stained with PE-conjugated anti-FCRL proteins, fluorescein isothiocyanate–conjugated anti-CD21, energy-coupled dye–conjugated anti-CD19, allophycocyanin–conjugated anti-IgM, and PE–Cy7–conjugated anti-CD27. After gating on CD19+ and IgM+ cells, CD21+CD27− naive B cells (R1), CD21+CD27+ MZ B cells (R2), and clonal CD21−/low MZ B cells (R3) were gated for analysis of expression of FCRL proteins 1–5. A, Representative dot plots show gating strategy (left) and expression of FCRL proteins 1–5 by naive B cells, conventional CD21+ MZ B cells, and clonal CD21−/low MZ B cells (right) in a patient with HCV-MC. B, Expression of FCRL proteins 1–5 by naive B cells, conventional CD21+ MZ B cells, and clonal CD21−/low MZ B cells was assessed in all patients with HCV-MC vasculitis. Bars show the mean ± SD. See Figure 2 for definitions.
Preparation of anti–FCRL-5 immunotoxins
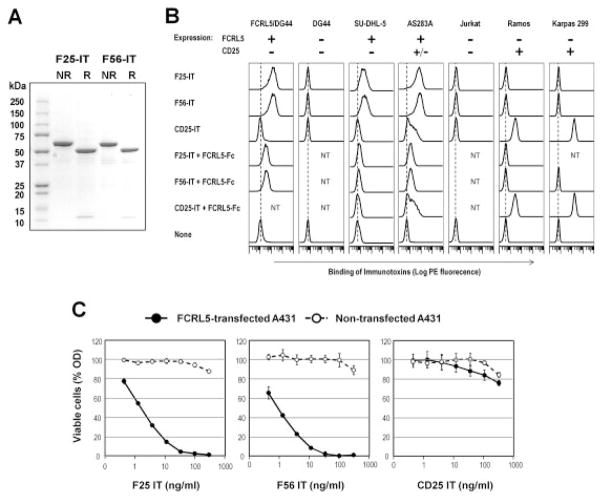
Our data indicated that FCRL-5 could be a useful target for immunotherapy in patients with HCV-associated MC vasculitis. Therefore, we made recombinant immunotoxins against FCRL-5 and examined their specific cytotoxicity on the patients’ cells. Results of sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of the anti–FCRL-5 immunotoxins F56-IT and F25-IT, after purification by ion-exchange and size-exclusion chromatography, revealed that both F56-IT and F25-IT migrated as single bands with the expected molecular weight (Mr 63,000) in nonreducing conditions (Figure 4A). Under reducing conditions, each band separated into 2 bands, which corresponded to VH-PE38 and VL, as expected.
Figure 4.
Characterization of anti–FCRL-5 immunotoxins. A, Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to analyze purified F25-IT and F56-IT. The purified disulfide-stabilized Fv immunotoxins (2 μg each) were analyzed in a 4–20% gradient gel under nonreducing (NR) and reducing (R) conditions. Coomassie blue staining shows that the nonreduced F25-IT and F56-IT migrated ~63 kd as single bands. With reduction, they dissociated into VH-PE38 fusion proteins (~51 kd) and VL chains (~12 kd). B, Specific binding of F25-IT and F56-IT to FCRL-5–positive cells was assessed by flow cytometry. F25-IT and F56-IT (with CD25-IT as negative control) were incubated with FCRL-5–stably transfected DG44, nontransfected (NT) DG44, or various human B or T cell lines, in the presence or absence of a 100-fold molar excess of FCRL-5–Fc fusion protein as the competitor. The cell-bound immunotoxins were detected using anti–Pseudomonas exotoxin rabbit antibody followed by PE-labeled anti-rabbit IgG. The expression status of FCRL-5 and CD25 is indicated as + or −. Solid lines represent cells stained with immunotoxins. Dotted lines represent the background fluorescence of cells without immunotoxins. C, F25-IT and F56-IT were assessed for FCRL-5–specific cytotoxicity. Different concentrations of F25-IT and F56-IT (with CD25-IT as negative control) were incubated for 40 hours with FCRL-5–stably transfected or nontransfected A431 human epithelial carcinoma cells. Viable cells were measured as the dehydrogenase activity in the cells, by a water-soluble tetrazolium 8–based cell-counting assay. Bars show the mean ± SD of 4 replicate wells. Error bars are missing when interfering with symbols. See Figure 2 for other definitions.
Figure 4B shows the FCRL-5–specific binding of F56-IT and F25-IT, as determined by flow cytometry, in the presence or absence of 100-fold molar excess of FCRL-5-Fc fusion protein as the competitor. Both F56-IT and F25-IT bound to the FCRL-5–transfected DG44 cell line and the AS283A and SU-DHL-5 human B cell lines that express endogenous FCRL-5 (43), whereas no binding was detected on the nontransfected DG44 cell line and the FCRL-5–negative cell lines Ramos, Jurkat, and Karpas 299 (35). In addition, CD25-IT did not bind to FCRL-5–positive cells, whereas binding to the Ramos and Karpas 299 cell lines was evident. FCRL-5-Fc–dependent competitions of the binding were observed only in the F56-IT and F25-IT stainings. These results confirmed the FCRL-5–specific reactivity of the prepared immunotoxins.
As shown in Figure 4C, the prepared F56 and F25 immunotoxins showed a strong and dose-dependent cytotoxic activity on FCRL-5–transfected A431 cell lines (50% inhibitory concentration of 1 ng/ml for F56-IT and 2 ng/ml for F25-IT). In contrast, no significant cytotoxicity was detected on FCRL-5–negative, nontransfected A431 cell lines up to the highest concentration tested (300 ng/ml). In addition, CD25-IT did not show any cytotoxic activity on FCRL-5–transfected A431 cell lines.
Specific cytotoxicity of anti–FCRL-5 immunotox-ins against the clonal CD21−/low MZ B cells in patients with HCV-MC vasculitis
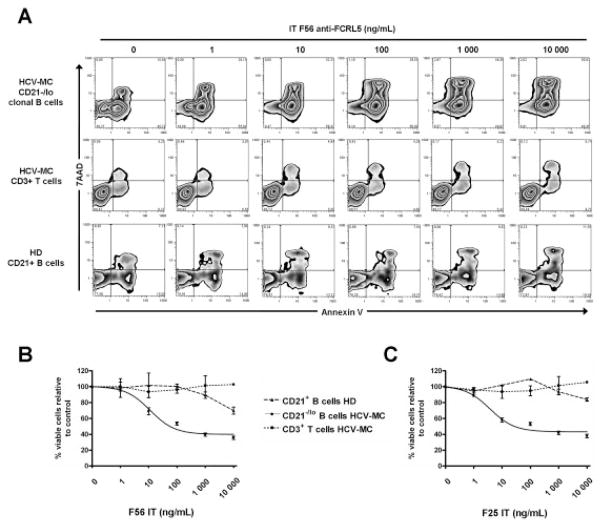
We next examined the cytotoxicity of F56 and F25 immunotoxins on PBMCs from patients with HCV-MC vasculitis and healthy donors. We purified IgM+CD27+ MZ B cells from total PBMCs from 3 patients with HCV-MC vasculitis, CD3+ T cells from the same patients with HCV-MC vasculitis, and total CD19+ B cells from 3 healthy donors. As shown in Figure 5A, F56-IT induced cell death, in a dose-dependent manner, in the clonal CD21−/low MZ B cells from a patient with HCV-MC vasculitis. In contrast, F56-IT showed no significant cytotoxicity on CD3+ T cells from the patients with HCV-MC vasculitis and on CD19+ B cells from healthy donors, confirming that the cytotoxicity was specific to FCRL-5–expressing cells. F25-IT had cytotoxic activity that was similar to that of F56-IT on clonal CD21−/low MZ B cells (Figures 5B and C). The specific cytotoxicity of the 2 anti–FCRL-5 immunotoxins was consistently detected in experiments with clonal CD21−/low MZ B cells from 3 different patients. The effective dose for killing 50% of the clonal CD21−/low MZ B cells was ~10 ng/ml for both immunotoxins, which was 1,000-fold lower than the noneffective doses of immunotoxins on T cells and normal CD19+ B cells.
Figure 5.
Anti–FCRL-5 immunotoxin cytotoxicity against clonal CD21−/low MZ B cells from patients with HCV-MC vasculitis. Clonal CD21−/low MZ B cells and CD3+ T cells from 3 patients with HCV-MC were enriched from PBMCs, as were CD21+ B cells from 3 healthy donors. Purified cells were incubated for 72 hours with serial dilutions of anti–FCRL-5 immunotoxins F56-IT and F25-IT. After incubation, cells were stained with PE–Cy7–conjugated anti-CD19 or Alexa Fluor 700–conjugated anti-CD3 and fluorescein isothiocyanate–conjugated anti-CD45, followed by staining with 7-aminoactinomycin D (7-AAD) and PE-conjugated annexin V to assess cell apoptosis. A, Representative dot plots from flow cytometry analysis of F56-IT cytoxicity are shown. B and C, Cytotoxicity of F56-IT (B) and F25-IT (C) was assessed in clonal CD21−/low MZ B cells from 3 patients with HCV-MC, CD3+ T cells from the same 3 patients, and CD21+ B cells from 3 healthy donors. Bars show the mean ± SD. See Figure 2 for other definitions.
DISCUSSION
Clonal expansion of autoreactive rheumatoid factor–bearing CD21−/low MZ B cells was shown to be associated with MC vasculitis in patients with HCV infection (11,13). The specific targeting of these cells is thus of particular interest for the development of novel therapies. In this study, we analyzed FCRL protein expression on B cells from HCV-infected patients with and those without MC vasculitis and from healthy donors. We showed that clonal CD21−/low MZ B cells from patients with HCV-MC vasculitis harbored an unusual pattern of FCRL protein expression that was different from that of normal B cells. Because a strong regulatory potential with regard to B cell activation has been shown for the different FCRL proteins (17,18), it is possible that this modulation of FCRL protein expression plays a role in controlling B cell activation as a self-limiting mechanism of activation.
In human disorders, initial studies indicated that FCRL proteins could provide useful markers for the diagnosis and treatment of clonal B cell lymphoproliferation (18), including lymphoproliferation of hairy cell leukemia cells (31), chronic lymphocytic leukemia cells, and multiple myeloma cells (29). Along this line, we demonstrated an unusual pattern of FCRL protein expression on the clonal CD21−/low MZ B cell population in HCV-infected patients with MC vasculitis, with decreased expression of FCRL-1 and increased expression of FCRL-2, FCRL-3, and, more widely, FCRL-5. In comparison with other autoimmune diseases with B cell activation, MZ B cells from patients with primary Sjögren’s syndrome did not exhibit this unusual pattern of expression, but no conclusion could be drawn because of the absence of monoclonal B cell lymphoproliferation and, in particular, lymphoma in our patients with primary Sjögren’s syndrome.
This CD21−/low MZ B cell population was shown to be enriched in clonal and autoreactive anergic B cells (11,13), suggesting that the expression of FCRL-5 in this population could be associated with lymphomagenesis and/or functional anergy of autoreactive B cells. It was suggested that FCRL-5 is an inhibitory coreceptor of B cells that could play a role in the functional anergy of B cells, inhibiting BCR-mediated calcium mobilization, intracellular tyrosine phosphorylation, and Erk kinase activation through ITIM recruitment of the SH2 domain–containing protein tyrosine phosphatase 1 (SHP-1) (19). In addition, FCRL-1, which was found to be down-regulated on CD21−/low MZ B cells, was shown to be an activating coreceptor of B cells, enhancing BCR-induced Ca2+ mobilization and proliferation (20). These findings suggest that FCRL-5, as well as FCRL-1, could play a role in the induction of functional anergy of autoreactive rheumatoid factor–bearing MZ B cells. It should be noted that our findings regarding FCRL-5 expression are in accordance with those in a study by Isnardi et al, which also demonstrated up-regulation of FCRL5/IRTA2 expression in CD21−/low autoreactive unresponsive B cells from patients with rheumatoid arthritis and common variable immunodeficiency (15). The recent finding that FCRL-5 binds IgG (25) could provide insights with regard to the pathophysiology of the disease. It is probable that FCRL-5, when activated by IgG immune complex, inhibits BCR signaling by recruiting SHP-1 phosphatase to the ITIM motifs.
In the present study, we did not detect any significant expression of FCRL-4 on the cell surface of B cells from patients with HCV-MC vasculitis. This finding is in contrast to that in a recent study by Charles et al (13), in which high expression of FCRL-4 was detected on clonal MZ-like B cells, although the FCRL-4 gene expression, assessed by microarray and quantitative reverse transcription–polymerase chain reaction, was not detected at the messenger RNA (mRNA) level (13). We also did not detect FCRL-4 mRNA expression in B cells from patients with HCV-MC vasculitis (14).
In this study, we demonstrated that 2 recombinant anti–FCRL-5 immunotoxins showed a specific and strong cytotoxic effect against FCRL-5–expressing clonal CD21−/low MZ B cells from patients with HCV-MC vasculitis. Although in vivo trials will be necessary in the future, these findings suggest that, in the setting of HCV-associated MC vasculitis, anti–FCRL-5 immunotoxins could kill only clonal CD21−/low MZ B cells. This specifically targeted killing cannot be achieved with anti-CD20 mAb, which are used in daily clinical practice and are also cytotoxic to other CD20-expressing B cells such as pro/pre-B cells, naive B cells, and class-switched conventional B cells. A recent report describing the development of another anti–FCRL-5 mAb as the antibody-drug conjugate for treatment of multiple myeloma supports this idea (30).
Overall, our data show that FCRL-5 is frequently and specifically expressed on clonal CD21−/low MZ B cells from patients with HCV-MC vasculitis. Thus, these findings suggest that FCRL-5 has the potential to be an excellent immunotherapeutic target for treatment of HCV-infected patients with MC vasculitis.
Acknowledgments
Dr. Terrier’s work was supported by the Fondation pour la Recherche Médicale, the Agence Nationale pour la Recherche sur le Sida et les Hépatites, and the Société Nationale Française de Méde-cine Interne. Dr. Nagata’s work was supported by the Leukemia Research Foundation, the CLL Global Research Foundation, and the NIH (grant 1P20-RR-024219-01A2). Dr. Pastan’s work was supported by the NIH Intramural Research Program, National Cancer Institute, Center for Cancer Research.
We gratefully acknowledge Nathalie Ferry and Gaëlle Martin for providing technical and analysis support.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Terrier had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Terrier, Nagata, Saadoun, Cacoub.
Acquisition of data. Terrier, Nagata, Ise, Rosenzwajg, Pastan, Saa-doun, Cacoub.
Analysis and interpretation of data. Terrier, Nagata, Ise, Klatzmann, Saadoun, Cacoub.
References
- 1.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–5. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 2.Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 3.Saadoun D, Suarez F, Lefrere F, Valensi F, Mariette X, Aouba A, et al. Splenic lymphoma with villous lymphocytes, associated with type II cryoglobulinemia and HCV infection: a new entity? Blood. 2005;105:74–6. doi: 10.1182/blood-2004-05-1711. [DOI] [PubMed] [Google Scholar]
- 4.Franzin F, Efremov DG, Pozzato G, Tulissi P, Batista F, Burrone OR. Clonal B-cell expansions in peripheral blood of HCV-infected patients. Br J Haematol. 1995;90:548–52. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 5.Sansonno D, De Vita S, Iacobelli AR, Cornacchiulo V, Boiocchi M, Dammacco F. Clonal analysis of intrahepatic B cells from HCV-infected patients with and without mixed cryoglobulinemia. J Immunol. 1998;160:3594–601. [PubMed] [Google Scholar]
- 6.Racanelli V, Sansonno D, Piccoli C, D’Amore FP, Tucci FA, Dammacco F. Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus-infected patients. J Immunol. 2001;167:21–9. doi: 10.4049/jimmunol.167.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Vallat L, Benhamou Y, Gutierrez M, Ghillani P, Hercher C, Thibault V, et al. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–78. doi: 10.1002/art.20594. [DOI] [PubMed] [Google Scholar]
- 8.Ivanovski M, Silvestri F, Pozzato G, Anand S, Mazzaro C, Burrone OR, et al. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–42. [PubMed] [Google Scholar]
- 9.De Re V, De Vita S, Marzotto A, Rupolo M, Gloghini A, Pivetta B, et al. Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus-associated non-Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood. 2000;96:3578–84. [PubMed] [Google Scholar]
- 10.Carbonari M, Caprini E, Tedesco T, Mazzetta F, Tocco V, Casato M, et al. Hepatitis C virus drives the unconstrained monoclonal expansion of VH1–69-expressing memory B cells in type II cryoglobulinemia: a model of infection-driven lymphomagenesis. J Immunol. 2005;174:6532–9. doi: 10.4049/jimmunol.174.10.6532. [DOI] [PubMed] [Google Scholar]
- 11.Charles ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, et al. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–56. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight GB, Gao L, Gragnani L, Elfahal MM, De Rosa FG, Gordon FD, et al. Detection of WA B cells in hepatitis C virus infection: a potential prognostic marker for cryoglobulinemic vasculitis and B cell malignancies. Arthritis Rheum. 2010;62:2152–9. doi: 10.1002/art.27490. [DOI] [PubMed] [Google Scholar]
- 13.Charles ED, Brunetti C, Marukian S, Ritola KD, Talal AH, Marks K, et al. Clonal B cells in patients with hepatitis C virus–associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood. 2011;117:5425–37. doi: 10.1182/blood-2010-10-312942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terrier B, Joly F, Vazquez T, Benech P, Rosenzwajg M, Carpentier W, et al. Expansion of functionally anergic CD21−/low marginal zone-like B cell clones in hepatitis C virus infection-related autoimmunity. J Immunol. 2011;187:6550–63. doi: 10.4049/jimmunol.1102022. [DOI] [PubMed] [Google Scholar]
- 15.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21− human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–36. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maltais LJ, Lovering RC, Taranin AV, Colonna M, Ravetch JV, Dalla-Favera R, et al. New nomenclature for Fc receptor-like molecules. Nat Immunol. 2006;7:431–2. doi: 10.1038/ni0506-431. [DOI] [PubMed] [Google Scholar]
- 17.Ehrhardt GR, Cooper MD. Immunoregulatory roles for Fc receptor-like molecules. Curr Top Microbiol Immunol. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]
- 18.Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–60. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 19.Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci U S A. 2007;104:9770–5. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leu CM, Davis RS, Gartland LA, Fine WD, Cooper MD. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–6. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- 21.Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. FcR-like 2 inhibition of B cell receptor-mediated activation of B cells. J Immunol. 2010;185:7405–12. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Naka-mura Y, et al. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–10. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- 23.Ehrhardt GR, Davis RS, Hsu JT, Leu CM, Ehrhardt A, Cooper MD. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Natl Acad Sci U S A. 2003;100:13489–94. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreeder DM, Cannon JP, Wu J, Li R, Shakhmatov MA, Davis RS. FcR-like 6 is an MHC class II receptor. J Immunol. 2010;185:23–7. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson TJ, Fuchs A, Colonna M. Human FcRL4 and FcRL5 are receptors for IgA and IgG. J Immunol. 2012;188:4741–5. doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dement-Brown J, Newton CS, Ise T, Damdinsuren B, Nagata S, Tolnay M. Fc receptor-like 5 promotes B cell proliferation and drives the development of cells displaying switched isotypes. J Leukoc Biol. 2012;91:59–67. doi: 10.1189/jlb.0211096. [DOI] [PubMed] [Google Scholar]
- 27.Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, et al. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J Immunol. 2013;190:5739–46. doi: 10.4049/jimmunol.1202860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Z, Li R, Li H, Zhou T, Davis RS. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc Natl Acad Sci U S A. 2013;110:E1282–90. doi: 10.1073/pnas.1215156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ise T, Nagata S, Kreitman RJ, Wilson WH, Wayne AS, Stetler-Stevenson M, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia. 2007;21:169–74. doi: 10.1038/sj.leu.2404445. [DOI] [PubMed] [Google Scholar]
- 30.Elkins K, Zheng B, Go M, Slaga D, Du C, Scales SJ, et al. FcRL5 as a target of antibody–drug conjugates for the treatment of multiple myeloma. Mol Cancer Ther. 2012;11:2222–32. doi: 10.1158/1535-7163.MCT-12-0087. [DOI] [PubMed] [Google Scholar]
- 31.Ise T, Maeda H, Santora K, Xiang L, Kreitman RJ, Pastan I, et al. Immunoglobulin superfamily receptor translocation associated 2 protein on lymphoma cell lines and hairy cell leukemia cells detected by novel monoclonal antibodies. Clin Cancer Res. 2005;11:87–96. [PubMed] [Google Scholar]
- 32.Du X, Nagata S, Ise T, Stetler-Stevenson M, Pastan I. FCRL1 on chronic lymphocytic leukemia, hairy cell leukemia, and B-cell non-Hodgkin lymphoma as a target of immunotoxins. Blood. 2008;111:338–43. doi: 10.1182/blood-2007-07-102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matesanz-Isabel J, Sintes J, Llinas L, de Salort J, Lazaro A, Engel P. New B-cell CD molecules. Immunol Lett. 2011;134:104–12. doi: 10.1016/j.imlet.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J Immunol. 2009;182:7518–26. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal-Laliena M, Romero X, March S, Requena V, Petriz J, Engel P. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell Immunol. 2005;236:6–16. doi: 10.1016/j.cellimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–18. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 37.Nagata S, Onda M, Numata Y, Santora K, Beers R, Kreitman RJ, et al. Novel anti-CD30 recombinant immunotoxins containing disulfide-stabilized Fv fragments. Clin Cancer Res. 2002;8:2345–55. [PubMed] [Google Scholar]
- 38.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–37. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 39.Kreitman RJ, Wilson WH, Robbins D, Margulies I, Stetler-Stevenson M, Waldmann TA, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340–8. [PubMed] [Google Scholar]
- 40.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–36. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 41.Mussai F, Campana D, Bhojwani D, Stetler-Stevenson M, Steinberg SM, Wayne AS, et al. Cytotoxicity of the anti-CD22 immunotoxin HA22 (CAT-8015) against paediatric acute lymphoblastic leukaemia. Br J Haematol. 2010;150:352–8. doi: 10.1111/j.1365-2141.2010.08251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–41. doi: 10.1182/blood-2007-11-122713. [DOI] [PubMed] [Google Scholar]
- 43.Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–89. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]