Abstract
Working memory capacity, a critical component of executive function, expands developmentally from childhood through adulthood. Anomalies in this developmental process are seen in individuals with autism spectrum disorder (ASD), schizophrenia and intellectual disabilities (ID), implicating this atypical process in the trajectory of developmental neuropsychiatric disorders. However, the cellular and neuronal substrates underlying this process are not understood. Duplication and triplication of copy number variants of 22q11.2 are consistently and robustly associated with cognitive deficits of ASD and ID in humans, and overexpression of small 22q11.2 segments recapitulates dimensional aspects of developmental neuropsychiatric disorders in mice. We capitalized on these two lines of evidence to delve into the cellular substrates for this atypical development of working memory. Using a region- and cell-type-selective gene expression approach, we demonstrated that copy number elevations of catechol-O-methyl-transferase (COMT) or Tbx1, two genes encoded in the two small 22q11.2 segments, in adult neural stem/progenitor cells in the hippocampus prevents the developmental maturation of working memory capacity in mice. Moreover, copy number elevations of COMT or Tbx1 reduced the proliferation of adult neural stem/progenitor cells in a cell-autonomous manner in vitro and migration of their progenies in the hippocampus granular layer in vivo. Our data provide evidence for the novel hypothesis that copy number elevations of these 22q11.2 genes alter the developmental trajectory of working memory capacity via suboptimal adult neurogenesis in the hippocampus.
Introduction
One of the fundamental questions in developmental neurobiology centers on the development of various cognitive functions and how such development derails in mental disorders. The capacity of many cognitive functions develops from childhood to adulthood.1, 2, 3 Both cognition and working memory start to deteriorate at adolescence and predicts the onset of later schizophrenia.4, 5, 6, 7, 8, 9, 10 The developmental maturation of working memory starts to lag during a period from adolescence to adulthood in individuals with autism spectrum disorder (ASD).11, 12 Working memory correlates to IQ13, 14, 15 and is less developed, throughout development and in adulthood, in individuals with intellectual disabilities (ID).16, 17, 18, 19
Duplication, triplication and hemizygous deletion of a few hundred kb to a few Mb, collectively termed copy number variants, confer the most robust risk factors, to date, for developmental delays in cognitive function and developmental neuropsychiatric disorders.20, 21, 22 Carriers of a 1.5 Mb to 3 Mb duplication or triplication at human chromosome 22q11.2 exhibit high rates of developmental delays in cognitive capacities23, 24, 25 and ASD and ID.26 Duplication of this chromosomal locus is also enriched in individuals with ASD and ID.20, 21, 22 However, the precise genetic elements within 22q11.2 responsible for atypical cognitive development remain elusive due to their large duplication sizes in humans.
Using mouse models with constitutive overexpression of small 22q11.2 segments,27, 28 we showed that an arrest in developmental maturation of working memory capacity is recapitulated by copy number elevation of a 190 kb region, including human catechol-O-methyl-transferase (COMT), TXNRD2 and ARVCF.29 Moreover, constitutive copy number elevation of another 200 kb 22q11.2 segment, containing human SEPT5, GP1BB, TBX1 and GNB1L, resulted in impaired social behaviors and compulsively repetitive behaviors, the latter of which were attenuated by an antipsychotic drug, suggesting relevance of this chromosomal segment to developmental neuropsychiatric disorders.30
Among these 22q11.2-encoded genes, a high activity single nucleotide polymorphism of COMT is associated with poor working memory after, but not before, 10 years of age, compared with a low activity COMT allele in humans.31 Moreover, a gain-of-function mutation of TBX1 has been identified in individuals with developmental delays in cognition.32, 33 Overexpression of no other 22q11.2 genes has been shown to impair working memory. However, mutation carriers also have other copy number variants and mutations20, 34 and the impacts of single nucleotide polymorphisms are often inconsistent, presumably due to their weak effect sizes35 in humans. While Comt and Tbx1 contribute to working memory and other ASD-related behavioral phenotypes in mice,36, 37, 38 the precise neuronal and cellular mechanisms through which elevated levels of these genes contribute to developmental working memory maturation are not known in humans or mice.
The targeted cell type and developmental time points are justified for four reasons. First, excitotoxic lesions of the hippocampal dentate gyrus damage adult neural stem/progenitor cells as well as mature neurons, and impair working memory in mice (Supplementary Results 1 and Supplementary Figures 1a–c). Second, adult neural stem/progenitor cells have been functionally implicated in working memory in mice.39, 40, 41 Third, adult neural stem/progenitor cells in the hippocampus express both Tbx136 and COMT (Supplementary Results 2 and Supplementary Figure 2) in mice. As there is no reliable means to evaluate the proliferation and migration of adult neural progenitor cells in the brains of human carriers of 22q11.2 copy number elevation, we examined the mechanisms through which high gene doses of the two 22q11.2 genes COMT and Tbx1 affect adult neurogenesis in mouse and cell models. Fourth, constitutive overexpression of a 190 kb 22q11.2 segment, including COMT, attenuates working memory capacity at 2 months, but not at 1 month of age;29 these mouse ages correspond to adulthood and periadolescence, respectively.42 A similar phenomenon was observed from childhood to adulthood in humans who carry a high activity COMT allele.31
Our data show that copy number elevations of the two 22q11.2 genes, COMT and Tbx1, in adult neural stem/progenitor cells of the hippocampus arrest the developmental maturation of working memory capacity and adult neurogenesis.
Materials and methods
We used male C57BL/6J mice. We constructed lentiviral vectors carrying enhanced green fluorescence protein (EGFP), COMT-EGFP or Tbx1-EGFP, all under a murine stem cell virus promoter (MSCV), pseudotyped with vesicular stomatitis virus (VSV-G). These vectors achieved a high degree of selectivity to infect adult neural stem/progenitor cells (Supplementary Results 3 and Supplementary Figures 3 and 4). The vectors were bilaterally infused into the dorsal hippocampus and mice were evaluated 7–10 days later at 1 month or 2 months of age, in tasks designed to assess working memory, response to novelty, anxiety-like behaviors and motor behavior.29 Cell types infected by the vectors were identified using flow cytometry and double fluorescence immunohistochemistry. The position of transduced cells was histochemically determined after completion of all behavioral tests. We examined the cell-autonomous effects of COMT or Tbx1 overexpression on proliferation and apoptosis of adult neural stem/progenitor cells of the hippocampus in cell culture (see Supplementary Information, Methods).
Results
Copy number elevation of COMT and Tbx1 blunts the developmental expansion of working memory capacity
To examine the functional impact of COMT overexpression in adult neural stem/progenitor cells on the developmental trajectory of working memory, we infused the COMT vector 7–10 days before 1 or 2 months of age, corresponding with before and after adolescence, respectively,42 and tested mice in a battery of behavioral assays (Figure 1a).
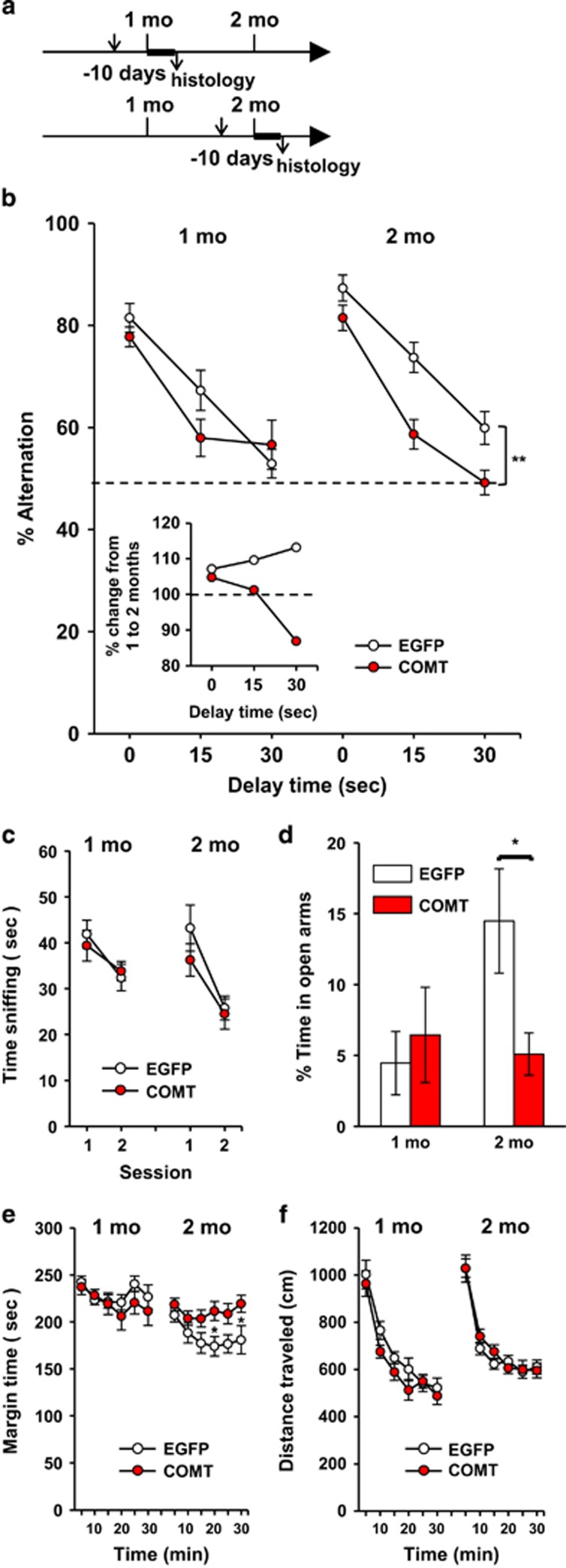
Figure 1.
(a) Experimental procedure. Upper and lower arrows indicate the time of surgical viral infusion and animal death for histological analysis, respectively. Thick horizontal lines indicate the time of behavioral assays. (b) Percentage of alternations (means±standard error of the mean (s.e.m.)), an index of working memory, in a T-maze (Age × Treatment, F(1,55)=5.45, P=0.0232; Treatment × Delay, F(2,110)=3.64, P=0.0294). EGFP-infused mice performed better at 2 months than at 1 month of age (Age, F(1,24)=6.20, P=0.0201), but not COMT-infused mice (Age, F(1,31)=0.126, P=0.7245). COMT overexpression impaired performance at all delays at 2 months (Treatment, F(1,33)=15.32, P=0.0004; Treatment × Delay, F(2,66)=1.64, P=0.2019; see ** at bracket), but not 1 month of age (Treatment, F(1,22)=1.38, P=0.2525). The dashed line indicates 50% (that is, chance level). Inset: % change (%= (2 month/1 month) × 100). The dashed line indicates no change from age 1 to 2 months. (c) Time spent (means±s.e.m.) in novel object approach. COMT overexpression had no effect on performance (Treatment, F(1,45)=0.88, P=0.3537). (d) Percentage of time (means±s.e.m.) spent in the open arms of the elevated plus maze. COMT overexpression obliterated increase in time from age 1 to 2 months spent in the open arms (Age × Treatment, F (1,43)=4.11, P=0.049). * indicates a significant (5%) difference between EGFP and COMT groups of 2-month-old mice, as determined by Newman–Keuls post-hoc comparison. (e) Thigmotaxis, as time (means±s.e.m.) spent in the margin area of an inescapable open field. Control mice spent less time in the margin (that is, a reduction in anxiety-like behavior) at age 2 months, compared to 1 month, but COMT-expressing mice did not (Age × Treatment, F (1,55 =9.55, P=0.0031). * indicates significantly (5%) different time points between EGFP and COMT groups of 2-month-old mice, as determined by Newman–Keuls post-hoc comparison. (f) Motor activity, as determined by horizontal distance traveled (means±s.e.m.), is not altered by COMT overexpression (Treatment, F (1,55)=0.83, P=0.3653). 1 month: EGFP, n=12; COMT-EGFP, n=12. 2 months: EGFP, n=14; COMT-EGFP, n=21. *P<0.05; **P<0.01. COMT, catechol-O-methyl-transferase; EGFP, enhanced green fluorescence protein.
Copy number elevation of COMT reduced working memory accuracy at 2 months of age, but not at age 1 month (Figure 1b). EGFP-infused mice showed 7, 10 and 13% higher rates of working memory at 0, 15 and 30 s delays, respectively, at age 2 months compared to 1 month (Figure 1b inset), thereby recapitulating the developmental maturation of human working memory capacity during this period in mice. In contrast, COMT-infused mice showed only 5 and 1% points higher and 13% points lower rates at 0, 15 and 30 sec delays, respectively, during the same developmental time span (Figure 1b inset); working memory with a heavy load was more severely affected. COMT copy number elevation reduced time spent in the open arms of the elevated plus maze (Figure 1d) and increased time spent in the margin area of the open field (Figure 1e), indicative of heightened anxiety, at age 2 months, but not 1 month. In contrast, COMT copy number elevation had no effect on the exploration of a novel object (Figure 1c) or motor activity in an inescapable open field(Figure 1f).
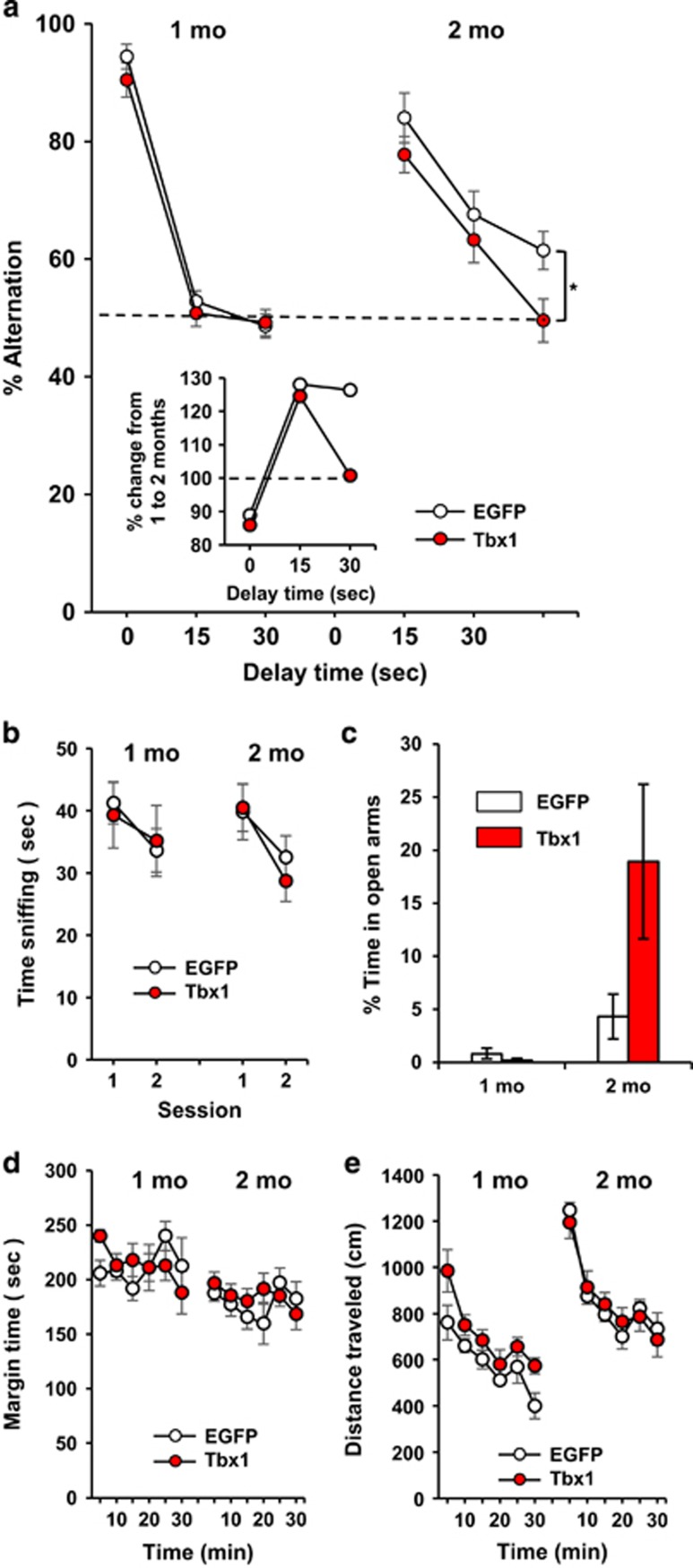
We next tested the effects of copy number elevation of Tbx1 in adult neural stem/progenitor cells on working memory capacity at 1 and 2 months of age. Such treatment reduced working memory with a long delay at 2 months but not 1 month of age (Figure 2a), but had no detectable effect on responses to a novel object (Figure 2b), anxiety-like behavior in the elevated plus maze (Figure 2c), and thigmotaxis (Figure 2d) or locomotor activity (Figure 2e).
Figure 2.
Effects of Tbx1 overexpression on behaviors at age 1 and 2 months. (a) Percentage of alternations (means±s.e.m.) in a T-maze. Tbx1 overexpression decreased working memory (Treatment, F(1,36)=5.80, P=0.021). Interaction of Age × Delay was significant (F(2,72)=11.99, P<0.0001), and exploratory ANOVA applied within each age indicated Treatment was significant at 2 months (F(1,23)=6.967, P=0.015 see * at bracket; Treatment × Delay, F(2,46)=0.531, P=0.592), but not at 1 month (F(1,13)=1.68, P=0.218). Inset: % change (%= (2 month/1 month) × 100). (b) Novel object approach (means±s.e.m.). Tbx1 overexpression had no effect (Treatment, F(1,36)=0.055, P=0.817). (c) Elevated plus maze. Tbx1 overexpression had no effect on the relative time (means±s.e.m.) spent in the open arms of the maze (Treatment, F(1,36)=1.85, P=0.182; Treatment × Age, F(1,36)=2.19, P=0.147). (d) Tbx1 overexpression had no effect on thigmotaxis (Treatment, F (1,36)=0.181, P=0.673; Treatment × Age, F(1,36)=0.04, P=0.848; Treatment × Age × Time F(5,180)=0.803, P=0.549). (e) Tbx1 overexpression had no effect on motor activity (means±s.e.m.) (Treatment, F (1,36)=1.67, P=0.205). 1 month, EGFP, n=8; Tbx1-EGFP, n=7. 2 months, EGFP, n=12; Tbx1-EGFP, n=13. ANOVA, analysis of variance; EGFP, enhanced green fluorescence protein.
Copy number elevation of COMT and Tbx1 arrests migration of adult neural stem/progenitor cells
We examined the distribution of transduced cells after completing behavioral testing, when some newly generated progenies of transduced adult neural stem/progenitor cells are expected to migrate into the granular layer from the subgranular zone. The majority of transduced cell bodies were located in and near the subgranular zone of the granular layer; their dendrites and axons reached the molecular layer of the dentate gyrus outward and extended toward the hilus and CA3 inward, respectively (Figures 3a–d, upper images), a pattern consistent with that of maturing, migrating cells derived from adult neural stem/progenitor cells.
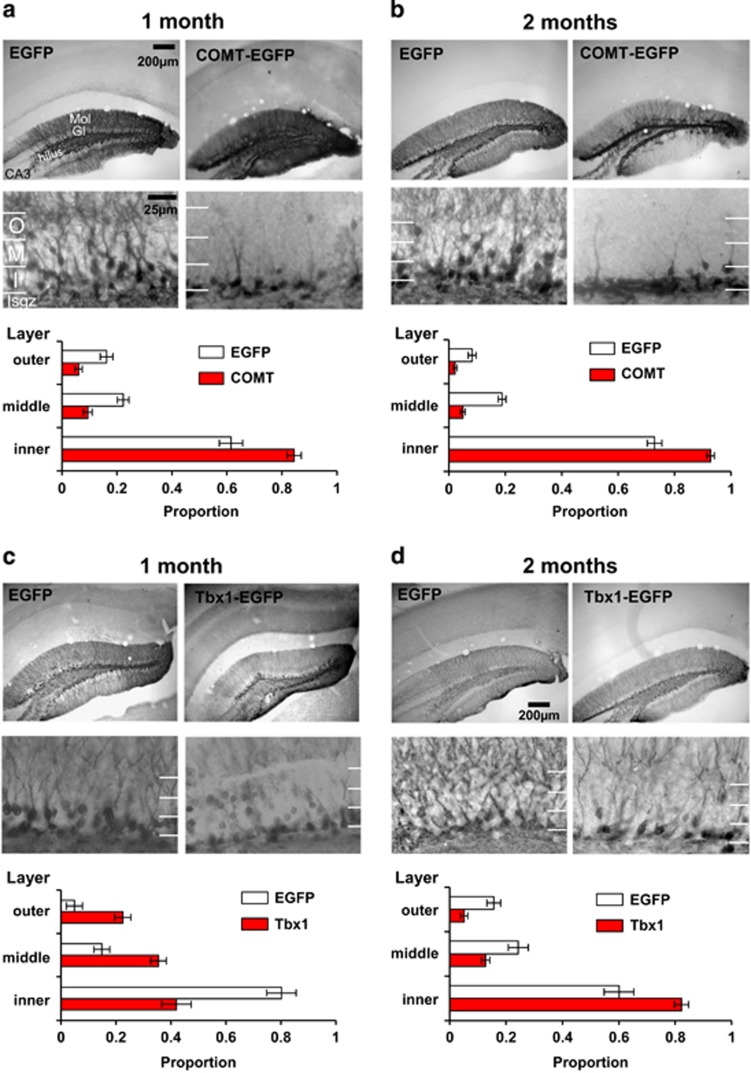
Figure 3.
Effects of COMT and Tbx1 overexpression on the localization of transduced cells in the hippocampal granular layer. The inner third zone includes the subgranular zone. Proportions (means±s.e.m.) of transduced cells are shown below representative images of COMT groups at 1 (a) and 2 months of age (b). More cells remained in the inner third zone following COMT overexpression at age 2 months than 1 month (Treatment, F(1,12584)=65.89, P< 0.0001; Age, F(1,12584)=10.24, P=0.0014). 1 month group: EGFP, N=42 section images; COMT-EGFP, N=34 section images; 2 months group: EGFP, N=35 section images; COMT-EGFP, N=31 section images. Proportions (means±s.e.m.) of transduced cells in EGFP and Tbx1-EGFP injected groups at 1 month (c) and 2 months of age (d) are shown. The distribution of transduced cells was dependent on age and treatment. Relatively more cells were found in the inner zone than middle and outer cell zones of both EGFP-infused mice and Tbx-1 infused mice in an age-dependent manner (Treatment × Age, F(1,8962)=46.16, P<0.0001). 1 month group: EGFP, N=42 section images; Tbx1-EGFP, N=29 section images; 2 months group: EGFP, N=13 section images; Tbx1-EGFP, N=22 section images. CA3, Region III of hippocampus proper; COMT, catechol-O-methyl-transferase; EGFP, enhanced green fluorescence protein; Gl, Granular layer of the dentate gyrus; I, inner third zone; Isgz, inner, subgranular zone; M, middle third zone; Mol, molecular zone of the dentate gyrus; O, outer third zone.
The total number of cell counts is not suitable to evaluate the impact of COMT or Tbx1 on proliferation, as the numbers are affected by the uncontrollable extent of infused viral vectors in the hippocampus. We thus used the proportion of cell numbers among the three zones for analysis. This index is independent of the extent of initial random infection. Even if proliferation is additionally affected, the proportion would not change if the same proportion of cells migrate to mid and outer zones. Thus, the ratio is a selective index for evaluation of the migration process. Transduced cells were distributed with a clear gradient from the subgranular/inner third zone to the outer third zone (see Figures 3a–d, lower images). COMT-transduced cells remained in the subgranular/inner third zone more so at age 2 months than 1 month (Figures 3a and b, bar graphs).
We observed the general gradient from the subgranular/inner third zone to the outer third zone in Tbx1-transduced cells at both 1 and 2 months of age. However, Tbx1-transduced cells remained more in the subgranular/inner third zone than EGFP-transduced cells at 2 months of age (Figure 3d, bar graphs), but we observed a more dispersed distribution of Tbx1-transduced cells than EGFP-transduced cells at 1 month of age (Figure 3c, bar graphs).
These data are consistent with our hypothesis that the migration of progenies of adult neural stem/progenitor cells is reduced by copy number elevation of COMT and Tbx1 in the hippocampal granular layer at 2 months of age, when these gene over-dosages impaired working memory.
Copy number elevation of COMT and Tbx1 arrests proliferation of adult neural stem/progenitor cells
It is not possible to unequivocally evaluate how COMT and Tbx1 overexpression in adult neural stem/progenitor cells alone affects the proliferation and apoptosis of transduced cells in vivo. To determine the cell-autonomous effects of gene overexpression on proliferation and apoptosis, we isolated adult neural stem/progenitor cells and transfected them with a plasmid containing either EGFP, COMT-EGFP or Tbx1-EGFP (Figure 4a). COMT copy number elevation significantly blunted and Tbx1 overexpression completely blocked the proliferation of adult neural stem/progenitor cells (Figure 4b). The proportions of annexin+ cells were indistinguishable among the three groups (Figure 4c), indicating that COMT and Tbx1 overexpression reduced the proliferation of adult neural stem/progenitor cells in a cell-autonomous manner without altering apoptosis rate.
Figure 4.
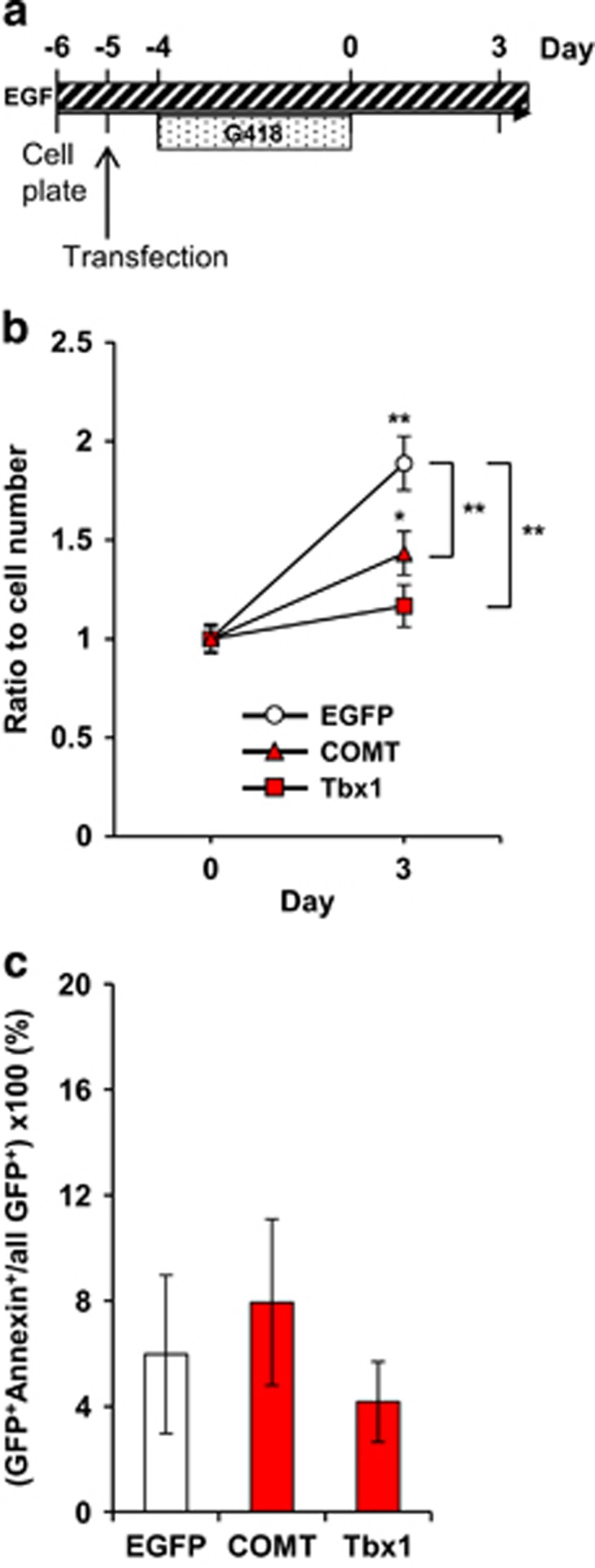
(a) In vitro experimental design. (b) Gene overexpression had differential effects on adult neural stem/progenitor cell proliferation (Gene × Day, F (2,66)=5.89, P=0.0044). Means (±s.e.m.) are shown. ** beside brackets indicates significantly (1%) different from EGFP group at day 3; * and ** above symbols indicate significantly (5 and 1%, respectively) different from Day 0. N=12 cell clones derived from 12 different pups/gene groups. (c) Tbx1 and COMT overexpression had no effect on apoptosis of adult neural stem/progenitor cells in vitro (P=0.764). N=4 cell clones per gene group derived from four pups. COMT, catechol-O-methyl-transferase; EGF, epidermal growth factor; G418, the antibiotic Geneticin.
Discussion
Our data demonstrate that copy number elevations of the two 22q11.2 genes, COMT and Tbx1, in the hippocampal dentate gyrus reduced developmental maturation of working memory capacity and the proliferation—and migration of the progenies—of adult neural stem/progenitor cells. Whereas association between a COMT high activity allele and working memory deficits has been amply and elegantly demonstrated,43 our data show that overexpression of these two genes in adult neural stem/progenitor cells does not affect working memory per se, but the developmental expansion of its capacity from 1 to 2 months of age in mice.
COMT, but not Tbx1, overexpression in the adult, but not peri-adolescent, hippocampus exacerbated anxiety-related behaviors. This was a rather selective effect, as neither gene treatment altered novel response or motor activity. Ibotenic acid lesions severely impaired working memory, reduced response to a novel object and increased motor activity, but had no effect on anxiety-like behaviors (see Supplementary Figures 1c–g). While all the treatments equally reduced working memory capacity, these variable effects of COMT and Tbx1 overexpression and excitotoxic lesions on other behaviors indicate that working memory deficits cannot be adequately accounted for by the indirect effects of these treatments on the other behaviors.
We previously demonstrated that transgenic mice constitutively overexpressing a 190 kb human chromosomal 22q11.2 segment, including COMT, TXNRD2 and ARVCF, exhibited no maturation deficit of working memory in a no-delay version of spontaneous alternation at any age but showed deficits in working memory with delays in rewarded alternation at 2 months but not at 1 month.29 Another report showed that the developmental maturation of working memory capacity is blunted from adolescence to adulthood in individuals with a high activity allele of COMT in humans.31 Our current data expand these mouse and human studies in three ways. First, we identified COMT and Tbx1 in hippocampal adult stem/neural progenitor cells as a functional contributor, although we do not rule out the possibility that mature neurons in the hippocampus and elsewhere are also involved. Second, working memory with delay, as measured by spontaneous alternation, was also affected by increased COMT dose. Third, working memory with delay is affected by Tbx1 overexpression in the hippocampus at 2, but not 1, months of age.
Our data are unique in that specific contributory genes are identified but are consistent with studies that showed the detrimental impact of inhibition of adult stem/progenitor cells on working memory in rodents.39, 40 However, some aspects of our data are seemingly inconsistent with others. One study showed inhibition of adult neurogenesis did not impair working memory tasks where no delay is imposed in rats.44 However, in this study, rats did not show a robust delay-dependent performance reduction in the non-matching-to-place task in this study,44 raising the possibility that the task might not have been as sensitive to working memory load as our task. Our own data are consistent with this observation in that gene elevation of COMT and Tbx1 did not affect working memory with no delay as severely as working memory with delay (Figures 1a and 2a).29 The interval between inhibition of adult neurogenesis and testing of working memory is another parameter that could account for diverse results. Our mice were tested 7–10 days after gene overexpression. Other studies similarly showed that while working memory is severely impaired at 1 and 3 weeks after irradiation of adult stem cells,40 performance is normal at 7 weeks40 and 4–8 weeks44 or enhanced at 2.5–3 months41 after inhibition of adult neurogenesis. Such normal performance might reflect a recovery of adult stem/progenitor cells or compensatory processes.40 Moreover, as it takes approximately 2 months for adult stem/progenitor cells to differentiate into mature neurons, stem cells or their immediate progenies (for example, immature neurons) might mediate the detrimental effect of gene overdose in or functional inhibition of adult stem/progenitor cells. Taken together, these seemingly inconsistent data collectively suggest that working memory with a heavy load is more severely affected by a functional inhibition or reduction of adult stem/progenitor cells and their immediate progenies (that is, immature neurons) than of mature neurons derived from adult stem/progenitor cells.
Overexpression of the protein products of these 22q11.2 genes might non-specifically reduce the functional capacity of adult stem cells in our mouse models and in human carriers of duplication and triplication. However, this possibility does not explain why the same gene overexpression did not affect working memory at 1 month of age. Certain intrinsic properties of adult neural stem/progenitor cells might underlie the age-dependent effect. There is a drastic decline in the neurogenesis rate in the hippocampal subgranular zone from age 1 month to 3 months in mice45, 46 and from infancy to adulthood in humans.47, 48, 49 Given that COMT and Tbx1 overexpressing cells tended to remain at the inner third of the granular layer more so at age 2 months than 1 month, the endogenously diminished proliferation and migration at age 2 months might make it increasingly difficult for stem cells to overcome the detrimental effects of increased gene dose.
Not incompatible with this possibility, an additional potential mechanism is a developmental change in the properties of COMT and its substrates. Developmental maturation of working memory capacity from age 1 to 2 months occurred when endogenous COMT enhanced its capacity to methylate substrates in the hippocampus, presumably reflecting a higher activity ratio of membrane bound-COMT to soluble-COMT at age 2 months, compared to 1 month (Supplementary Results 4, Supplementary Figure 5 and Supplementary Table 1). Catecholamine depletion reduces the proliferation rate of adult neural stem/progenitor cells in the rodent subgranular zone under certain conditions.50, 51 As COMT also methylates any protein that contains catechol, catecholamines and other catechol-carrying molecules could serve as functional substrates for this age-dependent effect of COMT.
There might even be a distinct mechanism through which Tbx1 overexpression did not impair working memory at 1 month of age. Our data suggest that Tbx1 overexpressed at 1 month facilitated migration, compared to EGFP; Tbx1 overexpression at 2 months induced the opposite pattern. It could be that newly generated cells at 1 month might have compensated for the impact of Tbx1 by facilitating migration of transduced cells due to their more active proliferation capacity, thus less detrimental effect on working memory.
One unique property of our study is that we evaluated the impact of gene overexpression on working memory and other behaviors following a 7–10-day recovery period after surgical gene transduction. Some newly dividing cells can start to differentiate into neuroblasts within a few days and into neurons after a few weeks in the adult mouse brain, although it takes more than 1 week for differentiation into astrocytes.46 However, this time period is not sufficient for the majority of nascent neurons to mature to extend their axons to target neurons in the hilus and CA352 and their dendrites to express spines.53 Thus, the impact of cells newly generated from transduced stem cells during adulthood would be expected to remain relatively localized under our experimental protocol. As immature and maturing neurons bear a slow excitatory response to GABA inputs, COMT and Tbx1 overexpression might impair working memory by such local effects within the granular layer. A future challenge is to identify a novel substrate through which copy number variants impact working memory by altering local electrophysiologic properties of newly generated immature neurons at the time when mice exhibit blunted working memory capacity.
Molecules and their associated cascades in neonatal and adult neural stem/progenitor cells can be further exploited to better understand the developmental trajectories of cognitive and affective anomalies associated with ASD, ID and schizophrenia.37, 54 The ultimate validation of our observations in mouse and cell models will be achieved only when it is shown that potential therapies developed from such hypothetical mechanisms provide beneficial effects on the atypical developmental maturation of working memory in humans.
Acknowledgments
We thank Dr Arthur Nienhuis of St. Jude Children’s Research Hospital for providing us with pCL20c-MSCV-EGFP plasmid. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH099660 (NH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R21HD053114 and U54HD090260 (NH), National Cancer Institute of the National Institutes of Health under Award Number P30CA013330 (JZ), a NARSAD Independent Investigator Award and a Maltz Foundation award (NH), the Uehara Memorial Foundation and Senshin Medical Research Foundation (SB) and Senshin Medical Research Foundation (HN).The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr Takeshi Hiramoto for his critical comments on an early version of the manuscript, and Drs J. Roy-Chowdhury and Xia Wang of the Gene Therapy Core, Albert Einstein College of Medicine for preparation of high titer viral particles.
Author contributions
The first three authors (SB, TI and SA) contributed equally to this work. SB, TI, SA, YN, HN, AN, TT, GK, AH, MN, SE and NH contributed to the overall design and execution of experiments and analyses. SB, TI, YN and NH wrote the manuscript. GD-T and PTM conducted Comt enzyme assays. GK, TI, TT conducted behavioral analyses. YN and KT carried out proliferation and apoptosis analyses in vitro. SA, HN, YN, JZ and NH conducted FACS. SK and KK provided the template plasmid of viral vectors and SB designed viral vectors. SB, TI, SA, AN, TT, AH, SE and MN carried out histochemical analyses.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol 2004; 40: 177–190. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology 2012; 26: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev 2004; 75: 1357–1372. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry 2008; 165: 579–587. [DOI] [PubMed] [Google Scholar]
- David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med 1997; 27: 1311–1323. [DOI] [PubMed] [Google Scholar]
- Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry 1999; 156: 1328–1335. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry 2002; 159: 1183–1189. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry 2010; 167: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry 2014; 171: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand 2014; 130: 1–15. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry 2007; 61: 474–481. [DOI] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE et al. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology 2013; 27: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: the same or different constructs? Psychol Bull 2005; 131: 30–60. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci 2003; 7: 547–552. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci 2006; 17: 172–179. [DOI] [PubMed] [Google Scholar]
- Danielsson H, Henry L, Ronnberg J, Nilsson LG. Executive functions in individuals with intellectual disability. Res Dev Disabil 2010; 31: 1299–1304. [DOI] [PubMed] [Google Scholar]
- Danielsson H, Henry L, Messer D, Ronnberg J. Strengths and weaknesses in executive functioning in children with intellectual disability. Res Dev Disabil 2012; 33: 600–607. [DOI] [PubMed] [Google Scholar]
- Schuchardt K, Gebhardt M, Maehler C. Working memory functions in children with different degrees of intellectual disability. J Intellect Disabil Res 2010; 54: 346–353. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJ, Van Luit JE, Jongmans MJ, Van der Molen MW. Memory profiles in children with mild intellectual disabilities: strengths and weaknesses. Res Dev Disabil 2009; 30: 1237–1247. [DOI] [PubMed] [Google Scholar]
- Girirajan S, Eichler EE. Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet 2010; 19: R176–R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Rees E, Walters JT, Escott-Price V, Georgieva L, Richards AL et al. The penetrance of copy number variations for schizophrenia and developmentaldelay. Biol Psychiatry 2013; 75: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell 2012; 148: 1223–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel C, Fernstrom M, Ohrner Y, Anneren G, Thuresson AC. Clinical variability of the 22q11.2 duplication syndrome. Eur J Med Genet 2008; 51: 501–510. [DOI] [PubMed] [Google Scholar]
- Yobb TM, Somerville MJ, Willatt L, Firth HV, Harrison K, MacKenzie J et al. Microduplication and triplication of 22q11.2: a highly variable syndrome. Am J Hum Genet 2005; 76: 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz SO, Pires R, Pires LM, Carreira IM, Anjos R, Maciel P et al. A unique phenotype in a patient with a rare triplication of the 22q11.2 region and new clinical insights of the 22q11.2 microduplication syndrome: a report of two cases. BMC Pediatr 2015; 15: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger TL, Miller JS, DePolo LM, de Marchena AB, Clements CC, Emanuel BS et al. 22q11.2 duplication syndrome: elevated rate of autism spectrum disorder and need for medical screening. Mol Autism 2016; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Nishi A. Dimensional deconstruction and reconstruction of CNV-associated neuropsychiatric disorders. In: Pletnikov MV, Waddington JL (eds). Modeling the Psychopathological Dimensions of Schizophrenia: From Molecules to Behavior. Handbook of Behavioral Neuroscience, Vol. 23. Academic Press, Elsevier: London, UK, 2015, pp 285–302. [Google Scholar]
- Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy Number Variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry 2013; 18: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Harper KM, Hiramoto T, Funke B, Lee M, Kang G et al. Over-expression of a human chromosome 22q11.2 segment including TXNRD2, COMT, and ARVCF developmentally affects incentive learning and working memory in mice. Hum Mol Genet 2009; 18: 3914–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Zhu H, Lee M, Funke B, Arai M, Itokawa M et al. A 200- kb region of human chromosome 22q11.2 confers antipsychotic-responsive behavioral abnormalities in mice. Proc Natl Acad Sci USA 2005; 102: 19132–19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J et al. Influence of the COMT genotype on working memory and brain activity changes during development. Biol Psychiatry 2011; 70: 222–229. [DOI] [PubMed] [Google Scholar]
- Torres-Juan L, Rosell J, Morla M, Vidal-Pou C, Garcia-Algas F, de la Fuente MA et al. Mutations in TBX1 genocopy the 22q11.2 deletion and duplication syndromes: a new susceptibility factor for mental retardation. Eur J Hum Genet 2007; 15: 658–663. [DOI] [PubMed] [Google Scholar]
- Zweier C, Sticht H, ydin-Yaylagul I, Campbell CE, Rauch A. Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am J Hum Genet 2007; 80: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R et al. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One 2014; 9: e91598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry 2008; 64: 137–144. [DOI] [PubMed] [Google Scholar]
- Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y et al. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum Mol Genet 2011; 20: 4775–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Okabe S, O'Broin P, Nishi A, Ye K, Beckert M et al. Structure and function of neonatal social communication in a genetic mouse model of autism. Mol Psychiatry 2016; 21: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci 2008; 28: 8709–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Suzuki K, Wakuda T, Seki N, Thanseem I, Matsuzaki H et al. Irradiation in adulthood as a new model of schizophrenia. PLoS One 2008; 3: e2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 2003; 119: 635–642. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV et al. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci USA 2007; 104: 4642–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH, Dagg CP, Snell GD. Reproduction. In: Green EL (ed). Biology of the Laboratory Mouse, 2nd edn. Dover Publications: New York, 2007 (oneline publication).
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 2006; 60: 141–151. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 2009; 159: 59–68. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol Biochem Behav 2007; 86: 327–333. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011; 8: 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013; 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 2010; 5: e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol 2016; 42: 621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur J Neurosci 2002; 16: 2008–2012. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 2004; 7: 726–735. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 2008; 11: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 2006; 26: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boku S, Toda H, Nakagawa S, Kato A, Inoue T, Koyama T et al. Neonatal maternal separation alters the capacity of adult neural precursor cells to differentiate into neurons via methylation of retinoic acid receptor gene promoter. Biological Psychiatry 2014; 77: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.