Abstract
Objectives
Intervertebral disc (IVD) degenerates progressively with age and following injuries. In this study, we aimed to characterize early molecular events underlying disc degeneration using a mouse tail IVD injury model.
Design
We have established a transcutaneous minimally invasive approach to induce mouse tail IVD injury under fluoroscopic guidance. Morphological and molecular changes in the injured IVDs are compared with the baseline features of adjacent intact levels.
Results
Following needle puncture, tail IVDs exhibited time-dependent histological changes. The aggrecan neoepitope VDIPEN is evident from 2 days to 4 weeks post injury. A disintegrin and metalloproteinase domain-containing protein 8 (adam8) is a surface protease known to cleave fibronectin in the IVD. Gene expression of adam8 was elevated at all time points post injury, while the increase of C-X-C motif chemokine ligand (cxcl)-1 gene expression is statistically significant at 2 days and 2 weeks post injury. Type I collagen gene expression decreased initially at day 2, but increased at 2 weeks post injury, while no significant change in type II collagen gene expression was observed. The extracellular matrix gene expression pattern is consistent with fibrocartilage formation post injury.
Conclusion
Mouse tail IVDs degenerate post needle puncture, as demonstrated by histological changes and aggrecan degradation. The minimally invasive tail IVD injury model should prove useful to investigators studying mechanisms of IVD degeneration and repair.
Keywords: Intervertebral disc, mouse model, injury, degeneration, a disintegrin and metalloproteinase domain-containing protein 8 (Adam8)
BACKGROUND
In humans, because most patients do not receive medical attention for back pain during the early stages of intervertebral disc (IVD) degeneration, little is known about the changes that occur during the initial phases of such degeneration. Animal models of disc degeneration would be useful in understanding the events that initiate inflammation/repair, and in identifying critical steps that may be amenable to intervention.1 This work aims to define the early molecular changes during the initial 4 weeks, concurrent with histological changes following an injury to the tail IVD in the mouse.
The IVD injury model has been widely used to study the course of IVD degeneration, in large animals (ovine,2 porcine3), dogs4, and small animals (rabbit,5-8 rat9 and mouse10,11). One main advantage of the mouse model is the ability to perform genetic manipulations during IVD degeneration. Challenges of working with the mouse IVD mainly reflect its small size: surgical precision is crucial, and the amount of tissue for molecular and biochemical assays is limited. The IVD histology and molecular changes following injury with a 29 Gauge (G) needle were first described by Yang et al.11 The deterioration of biomechanical properties along with histological changes following tail IVD injury with a 26G needle were further described Martin et al.10 The needle puncture, with a 26G needle, was severe enough to induce IVD degeneration detectable with biomechanical measures.10 The advantages of the tail injury method include easy access to the tail disc, low morbidity, and a reproducible course of degeneration.
Primary objective
The main objective of this study was to further refine the surgical technique, including percutaneous puncture of the disc, thus shortening and simplifying the procedure and reducing mouse suffering. As a result of reduced surgical trauma, confounding factors are also reduced in the examination of inflammatory markers. Using this IVD injury model, we determined the time course of histopathological changes and changes in gene expression of Adam 8, which is an important molecule in inflammation12,13 and is up-regulated in degenerated IVDs in humans.14 We also examined cxcl-1 gene expression post injury since we have previously shown that interleukin (IL)-8, the human homologue of cxcl-1, is elevated in human IVD tissues surgically removed due to back pain.15
MATERIALS AND METHODS
Mice
All animal experimental procedures were approved Institutional Animal Care and Use Committee (IACUC) of the Corporal Michael J. Crescenz Veterans Affairs Medical Center in Philadelphia (approval #: 01517-0002). Fifteen young adult (8 week old) female C57BL/6j mice (the Jackson Laboratory, Bar Harbor, ME, USA) were used in this study. Mice were housed under pathogen-free conditions with environmental enrichment, with up to 5 animals per cage. Twenty animals were used for RNA extraction and histology, with 5 animals at each of 4 time points.
Tail injury surgery
The mouse was anesthetized with Ketamine (90mg/kg) and Xylazine (10mg/kg) subcutaneously. Under anesthesia, the mouse tail IVDs were injured with a 26-G needle inserted under fluoroscopic guidance with a mini C-arm (OrthoScan FD Pulse Mini C-Arm, Orthoscan Inc., Scottsdale, AZ). Specifically, the skin was cleaned with betadine, the mouse coccygeal (Co) IVDs were identified, and a 26G needle was inserted into the IVD space until the needle tip reached 2/3 of the disc thickness (Fig. S1). Gelatinous tissues were often found on the needle tip after this had been removed, suggeting that the needle puncture induces an acute herniation of the gelatinous nucleus pulposus (NP) of the IVD. An arrow indicates the coccygeal disc between the third and fourth coccygeal vertebrae (Co3/4; arrows in Fig. S1). This procedure has been modified from previously published work,10 where a skin incision was made in the tail over the IVD prior to needle insertion into the disc. In the current study, the Co3/4 and Co5/6 IVDs in each mouse were injured, while Co4/5 and Co6/7 served as intact controls (Fig. S1). The percutaneous approach reduced procedure time and minimized risk to the animals. The animals behaved normally (by observing breathing, eating, ambulation, etc.) and did not require medication for pain on the day after surgery. No adverse event such as infection or bleeding was noted. Five animals were sacrificed by exposure to CO2 at each of the following time points: 2 days, 1 week, 2 weeks and 4 weeks after tail disc injury. From each mouse tail, Co3/4 (injured) and Co4/5 (intact control) discs were isolated individually for RNA extraction. Co5/6 (injured) and Co6/7 (intact control) were isolated enbloc (Fig. 1) for histological examination and immunostaining (Fig. 3).
Figure 1. Histological changes (low magnification) 2 days, 1, 2 and 4 weeks after coccygeal intervertebral disc (IVD) injury.
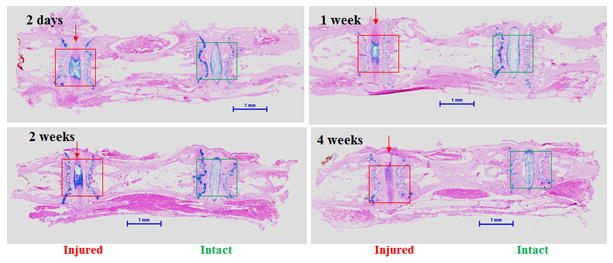
Injured (outlined in red) and the adjacent intact (outlined in green) IVDs were stained with Alcian blue, and counterstained with hematoxylin and eosin (H&E). Scale bars represent 1 millimeter (mm).
Figure 3. Aggrecan neoepitope 1 and 4 weeks after coccygeal intervertebral disc (IVD) injury.
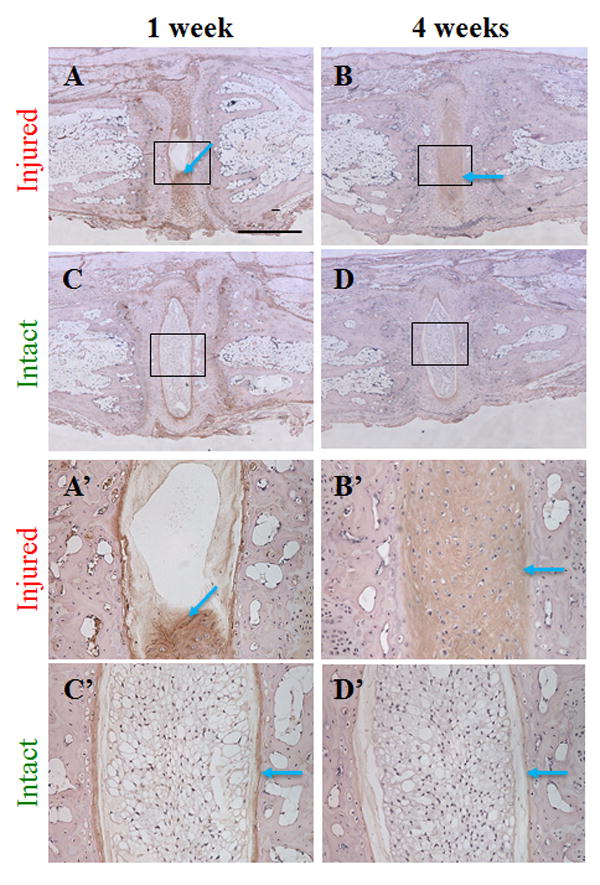
Injured and adjacent intact IVDs were stained with antibody that recognizes the aggrecan neoepitope VDIPEN, and were counterstained with hematoxylin. The scale bar represents 625 micrometers in panels A-D, 125 micrometers in panels A’-D’.
Histological Evaluation
The IVDs and portions of the adjacent bony vertebral bodies were isolated immediately after euthanasia. The disc with its surrounding vertebral bodies was fixed with 4% paraformaldehyde for 24 hours. The bone-disc-bone segments were decalcified with a solution consisting of 10% citric acid with 22% formic acid for 24 hours until the bony portion was completely decalcified, as previously described.16 The tissues were then dehydrated and embedded in paraffin and sectioned to 5μM thickness, and the sections were stained with Alcian Blue with Haematoxylin and Eosin (H&E) counter stain, or with H&E only. Specifically, the tissue sections were stained with 1% Alcian Blue Solution (Poly Scientific R&D Corp., Bay Shore, N.Y.) for 5 minutes, followed by Haematoxylin for 5 minutes and Eosin for approximately 20 seconds. All samples were examined under a light microscope (Nikon) and photographed. Representative sections from each time point were chosen for presentation.
Histological Grades
A modified histological grading scale for mouse discs was developed based on the previous grading methods.11,17 As shown in Table 1, a numeric scale for quantification of the degree of degeneration was designed. Analysis included the status of the AF organization and the extracellular matrix and cell morphology in the NP. Total scores ranged from 0 to 8. Scores of 0–2.0 represent essentially normal tissue; IVDs with mild disc degeneration scored 2.1–4.0 points; IVDs with moderate degeneration scored 4.1–6.0; IVDs with severe degeneration scored 6.1–8.0. Sections of injured and intact discs from 12 mice were evaluated by three experienced clinician-scientists in a double blinded manner. The total scores for NP and AF were calculated and inter-observer variability was assessed by a weighted Kappa using SAS software (Version 9.4, SAS Institute, Cary, NC). Average scores of all 3 raters were used to compare injured and intact disc histological grades.
Table 1.
Histological Scoring Criteria.
| Grade | Annulus Fibrosus (AF) | Nucleus Pulposus (NP) |
|---|---|---|
| 0 | Discrete and well-opposed lamellae | Oval shape, highly hydrated, many notochordal cells |
| 1 | Moderately serpentine with rupture | Irregular shape; decreased hydration |
| 2 | Severely serpentine with rupture; reversed contour | Large defects; cell clusters present |
| 3 | Indistinct border of NP and AF | Minor clefts; cell clusters decrease |
| 4 | Clefts formed; fragmentation of lamellae | Occupied by connective tissue |
Immunohistochemical staining
Aggrecan neoepitope was examined with antibody to its cleavage site, with the VDIPEN neoepitope at the cleavage site. Specifically, the paraffin sections were pre-treated with 0.1% pepsin with 0.02N HCl, and incubated with primary antibodies (a generous gift from Dr. J. Mort, Shriner’s Hospital) at 1:1000 dilution. The antibodies were visualized by incubation with appropriate biotinylated antibodies (Vector Laboratories, Burlingame, CA, USA) followed by the color development method using the ImmpPCAT DAB Peroxidase Substrate kit (Vector Laboratories, Burlingame, CA, USA). The sections were counterstained with hematoxylin.
RNA isolation and quantitative Real-Time PCR
IVD tissues were separated from their adjacent cartilaginous endplates and bone with a scalpel, under a dissecting microscope (VistaVision, VWR International, Radnor, Pennsylvania; Fig. S2). Total cellular RNA was isolated by the Trizol method as described previously.8 Specifically, the isolated IVD tissues were soaked in RNALater (Ambion, Foster City, CA) overnight, and stored at -80°C until extraction. On the day of RNA extraction, RNALater was removed and the tissues were snap frozen with liquid Nitrogen, then transferred into Trizol (Invitrogen, Carlsbad, CA). The tissues were homogenized with a homogenizer, with disposable OmniTip probes for hard tissue (Omin International, Kennesaw, GA). Phenol was then extracted with chloroform 3 times and discarded. RNA was precipitated with 70% ethanol, and was further purified using an RNeasy Micro Kit (Qiagen), according to the manufacturer’s protocol. RNA concentration was determined using a Synergy H4 Hybrid Reader (BioTek, Winooski, VT, USA). To generate cDNA, all RNA from each IVD (7-20ng/μl, total volume 50μl per sample) was used as template in a reverse transcriptase reaction using the SuperScript VILO cDNA synthesis kit (Life Technologies, Carlsbad, CA, USA) containing random hexamers and added polyDT primers (Invitrogen, Carlsbad, CA, USA). CDNA sequences were retrieved from Ensembl (release 84, March 2016). Primers for real-time PCR (Table 2) were designed using Primer-BLAST,18 and synthesized by Invitrogen (Carlsbad, CA, USA; Table 2). For each PCR reaction, cDNA, SYBR Select master mix (Life Technologies, Carlsbad, CA, USA), and primers (working concentration 0.5μM) were mixed, and deionized water was added to a total volume of 20μL per reaction. MicroAmp Optical 96-well reaction plates (Applied Biosystems, Foster City, CA) with 20μl of reaction mix/well were sealed with optical adhesive film (Life Technologies, Frederick, MD) and run in a ViiA7 real-time PCR system (Applied Biosystems, Foster City, CA) using the following program: (1) 50°C for 2 min, (2) 95°C for 2 min, (3) 95°C for 15 seconds, (4) 58°C for 1 min, (5) repeat steps 3 and 4 a total of 40 times. Single products were confirmed by determining melting curves at the conclusion of the reaction. Relative expression was calculated using the 2-∆∆Ct method19,20 normalized to gapdh (endogenous loading control).
Table 2.
Primers for Real-Time PCR.
| Gene | Sequence | Expected Product | Transcript ID |
|---|---|---|---|
| Gapdh | F: 5’-AAG GGC TCA TGA CCA CAG TC-3’ | 133 bp | ENSMUST00000118875 |
| R: 5’-GGA TGA CCT TGC CCA CAG-3’ | NM_008084 | ||
|
| |||
| Adam8 | F: 5’-TTG GCA CAG GCA GCA ACA TT-3’ | 168 bp | ENSMUST00000106069 |
| R: 5’-CAC TCC CTC TTG TGG TTG CA-3’ | NM_007403 | ||
|
| |||
| Col1a1 | F: 5’-CTG ACG CAT GGC CAA GAA GA-3’ | 72 bp | ENSMUST00000001547 |
| R: 5’-ATA CCT CGG GTT TCC ACG TC-3’ | NM_007742 | ||
|
| |||
| Col2a1 | F: CTG GGA ATG TCC TCT GCG ATG | 135 bp | ENSMUST00000023123 |
| R: GCC CCT TTG GCC CTA ATT TTC | NM_031163 | ||
|
| |||
| Cxcl1 | F: 5’-TGCACCCAAACCGAAGTCAT-3’ | 177bp | ENSMUST00000031327 |
| R: 5’-TTGTCAGAAGCCAGCGTTCAC-3’ | NM_008176 | ||
F: forward; R: reverse: ENSMUST: Ensembl ID; NM: The National Center for Biotechnology Information (NCBI) reference sequence.
Statistics
The difference in genes of interest (adam8, cxcl1, col1a1 and col2a1) and house-keeping gene (gapdh) cycle thresholds (∆CT) was calculated for each injured/intact pair. To assess differences in ∆CT between injured and intact tissues for each time period, a 2-factor analysis of variance (ANOVA) in repeated measures was used, where time was a grouping factor and injured/intact was the repeated measure. Post-hoc t-tests using the ANOVA pooled variance were performed for injured/intact differences within each time period. A p-value of <0.05 was considered statistically significant. All analyses were performed using SAS statistical software (Version 9.4, SAS Institute, Cary, NC).
RESULTS
Histology of Injured Mouse Tail IVD
Following injury, the IVD tissue showed loss of normal NP architecture (Figs. 1 and 2). During the initial post injury period (2 days to 2 weeks), the empty spaces in the NP are most likely due to herniated NP, although this could also be the result of sectioning artifact (Fig 2, red squares in 2-day, 1-week and 2-week panels and Figs. 2A-2C and 2A’-2C’, arrows). The whole IVD changed from an oval shape (Fig. 2 E-H) to an irregular shape (Fig. 2 A-D), suggesting loss of NP turgor, as a result of decompression. By 4 weeks post injury, the IVD space was filled with chondrocyte-like cells (Figs. 2D and 2D’) positive to Alcian blue staining. The AF structure also changed from well-organized lamellae (Fig. 2 E-H, yellow squares E”-H”) to severely serpentine layers with clefts (Figs. 2 A-D, yellow squares and 2 A”-2D”).
Figure 2. Histological changes 2 days, 1, 2 and 4 weeks after coccygeal intervertebral disc (IVD) injury.
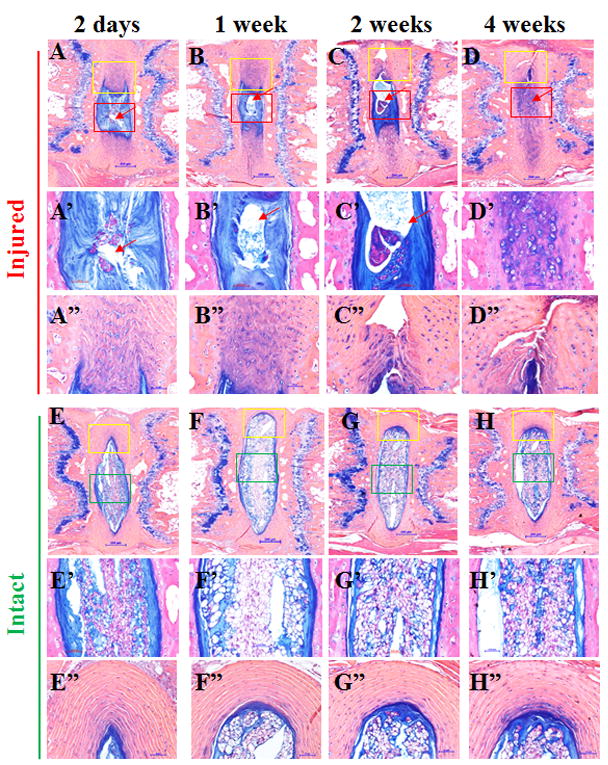
Injured and adjacent intact IVDs were stained with Alcian blue, and counterstained with hematoxylin and eosin (H&E); A’-D’ and A”-D” are magnified images in squares in A-D, respectively. E’-H’ and E”-H” are magnified images in squares in E-H, respectively. Scale bars in panels A-H represent 200μm; scale bars in panels A’-H’ and A”-H” represent 50 μm.
We evaluated histologic grade of the injured and control IVDs, modified from the grading system reported previously (Table 1).11 The scoring is based on microscopic sections, stained with Alcian blue and H&E (Figure 2), and with H&E only (images not shown). NP or AF showed grades ranging from 0 (normal) to 4 (severely degenerative), with the total score (NP+AF scores) ranging from 0 to 8 (Table 1). The scores of 3 graders correlated well, as measured by inter-rater variability (κ= 0.9311). The average scores for the intact IVDs ranged from 0.33 to 0.56, while those for the injured IVDs increased from 2.56 to 5.78 over time after the surgery (Table 3). The scores for intact discs were all in the normal range (≤2.0), while the scores for injured discs were in the mild (2.1-4.0) or moderate (4.1-6.0) range of degeneration according to the grading system described (Table 1). Differences between the histological scores of injured IVDs at 2 days, 1 and 2 weeks post injury, compared with those of intact IVDs, were not statistically significant (Table 3; n=3, P=0.102, 0.099, and 0.077, respectively; Table 3). The difference in histological score between injured and intact control IVDs was statistically significant at 4 weeks after injury (Table 3; n=3; p=0.0241).
Table 3.
Histological Scores.
| Scores/Time point | 2 Days | 1 Week | 2 Weeks | 4 Weeks |
|---|---|---|---|---|
| Injured | 2.56±0.48 | 3.11±0.97 | 3.56±1.11 | 5.78±0.73 |
| Intact | 0.56±0.29 | 0.56±0.11 | 0.33±0.19 | 0.44±0.11 |
| P-value | 0.1019 | 0.0987 | 0.0772 | 0.0241 |
Scores shown as mean ± standard error of the mean.
Increase in cleaved products of aggrecan associated with cartilaginous changes
Immunohistochemical examination was performed with an antibody that recognizes the aggrecan neoepitope, likely generated by MMPs.21-23 Immunoreactivity was found in the regions rich in chondrocyte-like cells in injured IVD at 1 and 4 weeks post injury (Fig. 3A, 3B, 3A’ and 3B’), while the staining was limited to the interface between the NP and AF in intact IVDs (Fig. 3, Intact), suggesting that proteoglycan remodeling becomes active in the cartilaginous lesion in injured IVD.
Adam8 Gene Expression is upregulated following injury
We have previously demonstrated that disc degeneration is associated with increased ADAM8 protease and its fibronectin cleavage products in humans.14 To determine whether this phenomenon is recapitulated in this IVD injury model, adam8 expression was measured via quantitative RT-PCR over a 4-week period following injury (Fig. 4A). Following injury, Adam8 gene expression increased compared with adjacent intact controls at all time points examined: day 2, p=0.004; 1 week, p=0.018; 2 weeks: p=0.003; 4 weeks, p=0.025 (Fig. 4A; n=5 at each time point).
Figure 4. Adam8, cxcl1, type I collagen (col1a1) and type II collagen (col2a1) gene expression following injury.
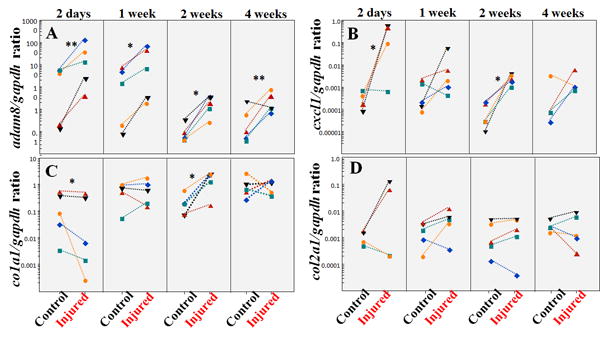
A: Adam8 to gapdh gene expression ratio; B: cxcl1 to gapdh gene expression ratio; C: Col1a1 to gapdh gene expression ratio; D: Type II collagen (col2a1) to gapdh gene expression ratio. Each individual symbol within a column represents injured and its adjacent intact IVD (control) from one mouse. **p<0.01; *P<0.05.
Cxcl-1 Gene Expression is Upregulated Following Injury
We have previously shown that interleukin (IL)-8, the human homologue of cxcl-1, is elevated in human IVD tissues surgically removed due to back pain.15 Cxcl-1 gene expression is elevated at 2 days and 2 weeks post injury (Fig. 4B; n=5, p=0.023 and 0.010, respectively). There is no statistically significant change at 1 week or 4 weeks post injury (Fig. 4B; n=5, p=0.850; n=4, p=0.879, respectively).
Extracellular Matrix Gene Expression representing Injury-repair Responses
To examine whether expression of genes encoding extracellular matrix molecules is altered in response to injury, we examined Collagen1 α1 chain (Col1a1) and Collagen2 α1 chain (Col2a1) gene expression. Col1a1 gene expression was downregulated at day 2 after injury (Fig. 4C, n=5, p=0.023), but upregulated at 2 weeks post injury (n=5, p=0.010). There is no statistically significant difference in Col1a1 gene expression at 1 week or 4 weeks post injury (Fig. 4C, n=5, p=0.850 or 0.879, respectively). Col2a1 gene expression comparing injured and intact controls did not reach statistical significance at any of the time points studied (Fig. 4D, n= 4-5, p>0.050).
DISCUSSION
The mouse model is attractive because of the opportunity for genetic manipulations. However, the mouse tail is non weight-bearing, thus the biomechanics of the vertebra-disc-vertebra motion segment differ from those of the human lumbar spine. Non-human primates are the only mammalian species that spend part of their time in an upright position, and share important physiological characteristics with the humans. However, rodent models provide opportunities for genetic manipulation, and a larger sample number can be achieved, thus are useful in spine research.
The needle injury resulted in a full thickness AF injury model with a relatively large needle (26G), which result in an acute NP herniation through the injured AF. While this is an important model to study disc herniation and the subsequent degeneration, we acknowledge that only a percentage of the human condition involves concurrent NP herniation while others only manifest as annular bulge. Furthermore, variability of the procedure has been taken into consideration, even with meticulous training and practice on the part of the surgeon. The degeneration induced by puncture with a 26G needle was mild to moderate, equivalent to grade 1 in the goat (grades 0 being normal, 2 being severe),17 and grade 2-4 in humans (grade 1 being normal, grade 5 being severe).24 Not surprisingly, injury induced in our model is more severe than that reported with a thinner needle.11 By 4 weeks post injury, the NP space was filled with chondrocyte-like cells (Figs. 1 and 2). It is worth noting that although histological features continue to be the gold standard for evaluating human disc degeneration, the scores tend to be subjective and insensitive to detect subtle differences between injured groups. Also, because the severity of injury in the mouse model can be controlled by using different sized needles, a smaller gauge needle may be used for experiments that aim to detect subtle changes.
The origin of the cells filling up the space left by NP herniation has not been determined yet: they may be proliferating residual NP cells or migrating from the AF or surrounding cartilaginous endplate as previously suggested.25 One important mechanistic question is the origin of cells contributing to repair. Cell tracing method with a fluorescent Cre reporter mouse crossed with Cre specifically expressed in different zones of the mouse IVD (e.g., Col1CreER/Col2CreER/Shh-creER) will definitively confirm which anatomical area these cells migrated from. Col1CreER,26 Col2CreER27 or ShhCreER28 would allow specific fluorescent activities in the outer AF, inner AF, or NP, respectively. Adopting these methodology would help to determine the cell origin and elucidate the mechanism of repair following a disc injury.
Aggrecanase and multiple MMPs are capable of cleaving aggrecan.22,23 The neoepitope VDIPEN resulting from MMP cleavage21 is detected consistently following disc injury (Fig. 3). The findings suggest that MMPs, at least in part, play a role in tissue remodeling, degeneration and repair following an IVD injury.
Although the ultimate goal of the study is to understand the pathophysiological events in human IVD degeneration, we acknowledge that there are significant differences between mouse and human spine both histologically16 and physiologically. Notochordal cells persist in the mouse IVD which play a significant role in development of IVD degeneration.29 Other significant differences in mechanical loading and composition and metabolism exist.1,30 The adult goat/sheep and chondrodystrophic dogs do not have persistent nortochord cells, and thus are more suitable for certain studies. But again, these animals lack the opportunity for genetic manipulations that the mouse model offers.
Adam8 (also known as CD156) belongs to the Adam family of cell surface proteases.13 Members of the Adam family participate in remodeling of extracellular matrix, cell migration, and processing of membrane-bound signaling molecules,31 and play important roles in the immune system. Adam8 is known to cleave fibronectin and promote cartilage degradation in articular cartilage.32 In human IVD tissue, Adam8 has been shown to cleave fibronectin, resulting in a bioactive fragment14 that could further accelerate disc degeneration.33,34 Adam8 gene expression is elevated at all time points examined, suggesting a key role in the injury/repair process, thus warranting further investigation. However, we have yet to confirm the key role of adam8 in the repair process following IVD injury. For example, Adam8 protein level and tissue distribution need to be determined by quantitative Western blot and immunostaining, in order to confirm that the messenger RNAs are indeed translated into protein. We have shown clear progression of IVD degeneration in response to tail IVD injury by histology, immunostaining and gene expression methods. One limitation is that although we found that aggrecan neoepitope was present in areas rich in chondrocyte-like cells, cellularity has not been quantified. To examine cell proliferation, we will use a method detecting the incorporation of 5-ethynyl-2’ -deoxyuridine (EdU), a thymidine analogue, into DNA,35 as previously reported.36, 37
In summary, we have established a minimally invasive approach to the mouse tail IVD-injury model that is easier to use than the previously described open surgical approach. Adam8 gene expression is upregulated at all time points after injury, suggesting a role of this protease in initiating injury/repair events. This IVD injury model may be suitable for elucidating the origin and nature of the cells appearing in injured NP and the role of Adam8 in cell migration/inflammation and aggrecan degradation.
Supplementary Material
A: the Co3/4 IVD was injured with a 26 Gauge needle (arrow) under fluoroscopic guidance. B: the Co3/4 and Co5/6 IVDs were injured (red arrows), and Co4/5 and Co6/7 served as intact controls.
Acknowledgments
Funding information: YZ has been supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, 1K08 HD049598). This work is supported, in part, by research grants from the North American Spine Society (NASS), a pilot grant from the Department of Veterans Affairs Healthcare Network-VISN 4, and a Penn Center for Musculoskeletal Disorders (PCMD) pilot grant (P30-AR050950-10 Pilot). All authors critically reviewed and approved the manuscript. The Thomas Jefferson University Sidney Kimmel Cancer Center Translational Research/Pathology Shared Resource Core Facility provided outstanding histology service. The authors thank Debra Pawlowski, Jeffrey House, and Pierre Conti, VMD for care of the animals. We gratefully thank Jason W. Ashley, PhD, Haoru Jia, MD and John T. Martin, PhD, for technical support, Martin F. Heyworth, MD, for critically editing the manuscript, and Carla R. Scanzello for valuable insights and discussions. None of the authors have any professional and financial affiliation that could be perceived to be biased in the presentation of the current work.
Footnotes
Author Contribution: Data acquisition, ZT; MY; XM; LQ; MEI; YZ; Research design and/or data interpretation, ZT; YZ; MP; MEI; LJS; RLM. Statistical analysis and research design: FSS. Drafting and revising manuscript: YZ; MP; MEI; LJS; RLM; LQ. YZ and ZT are responsible for storage and access of all primary data. All authors have read and approved the final submitted manuscript.
References
- 1.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melrose J, Shu C, Young C, et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa 1976) 2012;37(1):18–25. doi: 10.1097/BRS.0b013e31820cd8d5. [DOI] [PubMed] [Google Scholar]
- 3.Acosta FL, Jr, Metz L, Adkisson HD, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17(23-24):3045–3055. doi: 10.1089/ten.tea.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine (Phila Pa 1976) 2009;34(21):2297–2304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 5.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 6.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30(1):15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 7.Sowa G, Vadala G, Studer R, et al. Characterization of intervertebral disc aging: Longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33(17):1821–1828. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Chee A, Shi P, et al. Allogeneic articular chondrocyte transplantation downregulates interleukin 8 gene expression in the degenerating rabbit intervertebral disk in vivo. Am J Phys Med Rehabil. 2015;94(7):530–538. doi: 10.1097/PHM.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Liu L, Wang S, et al. Production of CCL20 on nucleus pulposus cells recruits IL-17-producing cells to degenerated IVD tissues in rat models. J Mol Histol. 2015 doi: 10.1007/s10735-015-9651-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013;31(8):1276–1282. doi: 10.1002/jor.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218(1):113–121. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol. 1990;2(6):585–591. doi: 10.1093/intimm/2.6.585. [DOI] [PubMed] [Google Scholar]
- 13.Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S. CD156 (human ADAM8): Expression, primary amino acid sequence, and gene location. Genomics. 1997;41(1):56–62. doi: 10.1006/geno.1997.4607. [DOI] [PubMed] [Google Scholar]
- 14.Ruel N, Markova DZ, Adams SL, et al. Fibronectin fragments and the cleaving enzyme ADAM-8 in the degenerative human intervertebral disc. Spine (Phila Pa 1976) 2014;39(16):1274–1279. doi: 10.1097/BRS.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Chee A, Shi P, et al. Intervertebral disc cells produce interleukins found in patients with back pain. Am J Phys Med Rehabil. 2016;95(6):407–415. doi: 10.1097/PHM.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Lenart BA, Lee JK, et al. Histological features of endplates of the mammalian spine: From mice to men. Spine (Phila Pa 1976) 2014;39(5):E312–7. doi: 10.1097/BRS.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Drapeau S, An HS, Markova D, Lenart BA, Anderson DG. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine (Phila Pa 1976) 2011;36(19):1519–1527. doi: 10.1097/BRS.0b013e3181f60b39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13 doi: 10.1186/1471-2105-13-134. 134-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267(2):1008–1014. [PubMed] [Google Scholar]
- 22.Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5(2):94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 2014;1(1) doi: 10.1186/s40634-014-0008-7. 8014-0008-7. Epub 2014 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003;28(10):982–990. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 26.Bedore J, Quesnel K, Quinonez D, Seguin CA, Leask A. Targeting the annulus fibrosus of the intervertebral disc: Col1a2-cre(ER)T mice show specific activity of cre recombinase in the outer annulus fibrosus. J Cell Commun Signal. 2016;10(2):137–142. doi: 10.1007/s12079-016-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo N, Yuasa T, Shimono K, et al. Intervertebral disc development is regulated by Wnt/beta-catenin signaling. Spine (Phila Pa 1976) 2011;36(8):E513–8. doi: 10.1097/BRS.0b013e3181f52cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: Implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237(12):3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCann MR, Seguin CA. Notochord cells in intervertebral disc development and degeneration. J Dev Biol. 2016;4(1):1–18. doi: 10.3390/jdb4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotz JC. Animal models of intervertebral disc degeneration: Lessons learned. Spine (Phila Pa 1976) 2004;29(23):2742–2750. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez I, Moreno JL, Zandueta C, Montuenga L, Lecanda F. Novel alternatively spliced ADAM8 isoforms contribute to the aggressive bone metastatic phenotype of lung cancer. Oncogene. 2010;29(26):3758–3769. doi: 10.1038/onc.2010.130. [DOI] [PubMed] [Google Scholar]
- 32.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54(9):2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30(11):1242–1246. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DG, Markova D, Adams SL, Pacifici M, An HS, Zhang Y. Fibronectin splicing variants in human intervertebral disc and association with disc degeneration. Spine (Phila Pa 1976) 2010;35(17):1581–1588. doi: 10.1097/BRS.0b013e3181c6ef1a. [DOI] [PubMed] [Google Scholar]
- 35.Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A. Detection of S-phase cell cycle progression using 5-ethynyl-2’-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2’-deoxyuridine antibodies. BioTechniques. 2008;44(7):927–929. doi: 10.2144/000112812. [DOI] [PubMed] [Google Scholar]
- 36.Candela ME, Cantley L, Yasuaha R, Iwamoto M, Pacifici M, Enomoto-Iwamoto M. Distribution of slow-cycling cells in epiphyseal cartilage and requirement of beta-catenin signaling for their maintenance in growth plate. J Orthop Res. 2014;32(5):661–668. doi: 10.1002/jor.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candela ME, Wang C, Gunawardena AT, et al. Alpha 5 integrin mediates osteoarthritic changes in mouse knee joints. PLoS One. 2016;11(6):e0156783. doi: 10.1371/journal.pone.0156783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: the Co3/4 IVD was injured with a 26 Gauge needle (arrow) under fluoroscopic guidance. B: the Co3/4 and Co5/6 IVDs were injured (red arrows), and Co4/5 and Co6/7 served as intact controls.


