Abstract
Rationale and objectives
Novel synthetic cannabinoid compounds continue to appear in the market advertised as legal alternatives to marijuana and the older synthetic cannabinoid compounds which are now controlled substances. Most of these newer compounds have been found to act at CB1 receptors, so the purpose of this study was to study the abuse liability of these compounds.
Methods
Five of these compounds (BB-22, FUB-PB-22, 5F-AMB, NM2201, and MAB-CHMINACA) were tested for their ability to produce discriminative stimulus effects similar to Δ9-tetrahydrocannabinol (Δ9-THC) in rats. The ability of the CB1 receptor inverse agonist rimonabant to antagonize the discriminative stimulus effects of the five test compounds was also tested.
Results
All five of the test compounds fully substituted for the discriminative stimulus effects of Δ9-THC at some dose, although MAB-CHMINACA produced an inverted-U shaped dose effect. Rimonabant fully antagonized the Δ9-THC-like discriminative stimulus effects of BB-22, 5F-AMB, NM2201, and MAB-CHMINACA, but only reduced the effects of FUB-PB-22 to 40–50% of Δ9-THC-appropriate responding.
Conclusions
These findings suggest that all 5 of the test compounds produced Δ9-THC-like effects and will likely have abuse liability similar to that of the controlled cannabinoid compounds.
Keywords: Cannabinoids, Drug discrimination, Locomotor activity, Abuse liability, Mouse, Rat
Introduction
Synthetic compounds that mimic the effects of controlled substances such as psychostimulants, cannabis, and hallucinogens have continued their increase in popularity among recreational users as many of these new synthetic compounds are not legally controlled and are not detectable by blood tests for illicit drug use (UNODC 2014). Before 2008, there was little or no observation of synthetic cannabinoids in the recreational drug market, but currently, they have become the mostly widely used class of recreational psychoactive compounds (Nelson et al. 2014), and comprise 28% of the designer drug market, greater than synthetic cathinones (25%), or tryptamine hallucinogens (4%) (UNODC, 2014). Several of these compounds, including BB-22 (QUCHIC, 1-(cyclohexylmethyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester), FUB-PB-22 (quinolin-8-yl 1-[(4-fluorophenyl)methyl]-1H-indole-3-carboxylate), 5F-AMB (5F-MMB-PINACA and 5F-AMB-PINACA, Methyl (2S)-2-{[1-(5-fluoropentyl)-1H-indazol-3-yl]formamido}-3-methylbutanoate), NM2201 (CBL-2201, naphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate), and MAB-CHMINACA (ADB-CHMINACA, N-[(2S)-1-amino-3,3-dimethyl-1-oxobutan-2-yl]-1-(cyclohexylmethyl)indazole-3-carboxamide), have seen increased recreational use. MAB-CHMINACA has been temporarily controlled as a schedule I compound in the United States (Drug Enforcement Administration 2016).
All five of these novel compounds have been found in seized materials in recent years in Europe, Asia, and the United States (Kondrasenko et al. 2015; Lobo Vincente et al. 2016; Odoardi et al. 2016; Shevyrin et al. 2014; Uchiyama et al. 2013), and MAB-CHMINACA is commonly found in forensic samples from impaired drivers, post-mortem exams, etc. (Tynon et al. 2016). Two of these compounds in particular have been associated with serious adverse effects following recreational use. 5F-AMB has been implicated in fatal poisonings (Hasegawa et al. 2015a; Shanks and Behonick 2016) and MAB-CHMINACA has been associated with toxicities including agitation and aggression, delirium and hallucinations, vomiting, convulsions, acute kidney injury, cardiotoxic effects, coma, and death (Adamozicz and Gieroń 2016; Hasegawa et al. 2015b; Katz 2016; Trecki et al. 2015). Taken together, these findings indicate that these synthetic cannabinoids produce serious risks to public health.
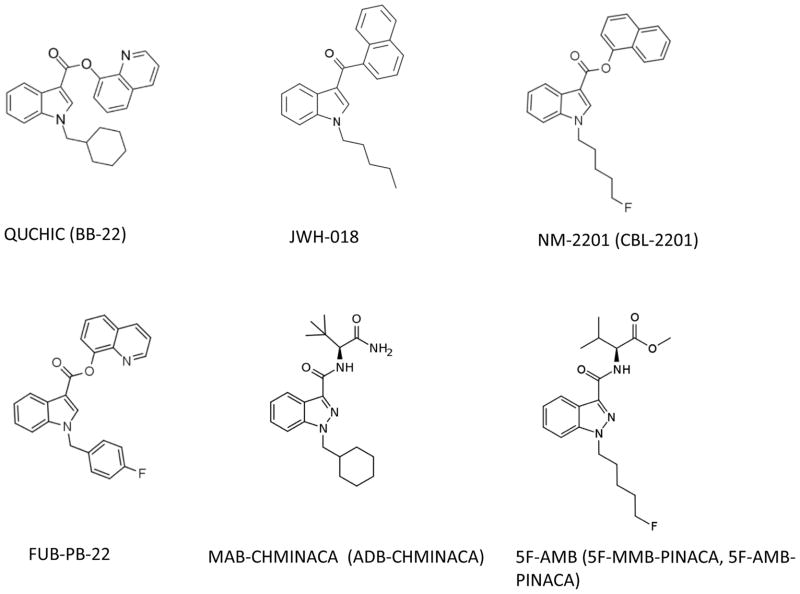
The steps in identifying abuse liability include determining whether the compounds, 1) have chemical structures closely related to known drugs of abuse, 2) share common pharmacological mechanisms of action with known drugs of abuse, 3) produce similar subjective effects as known drugs of abuse, and 4) produce reinforcing effects. These five compounds (BB-22, FUB-PB-22, 5F-AMB, NM2201, and MAB-CHMINACA) have structures closely related to abused synthetic cannabinoids such as JWH-018 (Figure 1). BB-22, FUB-PB-22, 5F-AMB, and NM2201 all bind to the CB1 and CB2 cannabinoid receptors and act as high potency, high efficacy receptor agonists (Banister et al. 2016; De Luca et al. 2016; Hess et al. 2016). Despite the large number of reports on the adverse effects of MAB-CHMINACA, there are no published reports on its mechanism of action. Steps 1 and 2 have mostly been addressed for these compounds, but because there is a dearth of behavioral studies investigating these compounds, steps 3 and 4 remain unanswered.
Fig. 1.
Chemical structures of the five cannabinoid test compounds and of the controlled synthetic cannabinoid compound JWH-018.
The purpose of the present study was to identify whether these compounds produce similar subjective effects as Δ9-THC. The drug discrimination assay is a widely-used animal model of the subjective effects of psychoactive compounds. Because Δ9-THC does not produce consistent, reliable conditioned place preference or self-administration in rodents (see review by Tanda, 2016), drug discrimination remains the best model for the abuse liability of cannabinoids. Fortunately, drug discrimination data are well correlated with the likelihood of human recreational use (Horton et al. 2013). Antagonist experiments were conducted to confirm that the behavioral effects of these compounds are mediated by CB1 receptors.
Methods
Subjects
Male Sprague-Dawley rats were obtained from Envigo. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day, which included the food received during sessions. Water was continuously available in the home cage.
Discrimination procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via Med Associates interfaces (East Fairfield, VT). The computers were programmed in Med-PC for Windows, version IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Rats were first trained to discriminate Δ9-THC (3 mg/kg) from vehicle (ethanol/Cremophor EL/0.9% saline in a ratio of 1:1:18) using a two-lever choice methodology. Thirty minutes prior to the training sessions, rats received an injection of either saline or Δ9-THC and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses (FR10) on a designated injection-appropriate lever. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets. Rats were used in tests of substitution of the experimental compounds once they had achieved 9 of 10 sessions at 85% or greater injection-appropriate responding for both the first reinforcer and total session, which occurred after approximately 60 training sessions. The training sessions occurred on separate days in a double alternating fashion (drug-drug-vehicle-vehicle-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one vehicle and one drug session occurred between each test (drug-vehicle-test-vehicle-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
Fifty-four rats drawn from a larger pool of Δ9-THC trained rats were used for testing the compounds in the present study, and may have previously been used to test other compounds. During test sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. For dose-effect experiments, sessions lasted until 20 reinforcers were obtained or for a maximum of 20 min. Each compound was tested in a group of at least six rats using a repeated-measures design such that each rat was tested at all doses of a given drug. Vehicle (1 ml/kg) and Δ9-THC (3 mg/kg) controls were tested before the start of each compound evaluation. Doses of Δ9-THC (0.01 – 0.1 mg/kg), BB-22 (0.01 – 0.25 mg/kg), FUB-PB-22 (0.025 – 1 mg/kg), 5F-AMB (0.025 – 1 mg/kg), NM2201 (0.01 to 0.1 mg/kg), MAB-CHMINACA (0.025 – 0.25 mg/kg), and cocaine (10 and 25 mg/kg) were tested. A dose range was tested from no effect (<20% Δ9-THC-appropriate responding) to full effect (≥80% Δ9-THC-appropriate responding or suppression of responding to less than 20% of vehicle control). Doses were tested in no particular order. Pretreatment times were based on the time of peak depression for each compound in previous locomotor activity testing (data not shown), and a time-course study was conducted using the dose producing peak effect to confirm an appropriate pretreatment period. All compounds were tested using a 30-min pretreatment. 5F-AMB was also tested using a 60-min pretreatment.
For the time course experiments, a repeated-measures design was used, such that each rat was tested at several time points following a single administration of the test compound, except MAB-CHMINACA, which was tested in separate groups of rats for each time point. The lowest dose that fully substituted without significant rate effects in the dose-effect studies was selected. The rats were injected with the test compound and placed in the test chambers 5 min after administration. Sessions lasted until 5 reinforcers were obtained, or for a maximum of 5 min, and the rats were immediately removed from the chambers. Testing was repeated at 15, 30, 60, and 120 min after administration. If necessary, testing was continued at 4, 8, 24, and 48 h after administration until Δ9-THC-appropriate responding had decreased to below 30–40%.
Antagonism studies were conducted in a between-subjects design such that separate groups of 6 rats received a dose of agonist (selected from peak effects in the dose-effect studies) and either vehicle or a dose of rimonabant. There was also a vehicle-alone control group. Each of the synthetic cannabinoids was tested against three doses of rimonabant (0.025, 0.25 and 2.5 mg/kg) except FUB-PB-22 (0.25, 2.5 and 5 mg/kg) and Δ9-THC (1.2, 1.8 and 2.4 mg/kg). Rimonabant was administered 10 min before administration of agonist.
Drugs
Δ9-Tetrahydrocannabinol, BB-22 (1-(cyclohexylmethyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester), FUB-PB-22 (quinolin-8-yl 1-[(4-fluorophenyl)methyl]-1H-indole-3-carboxylate), 5F-AMB (methyl (2S)-2-{[1-(5-fluoropentyl)-1H-indazol-3-yl]formamido}-3-methylbutanoate), NM2201 (naphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate), MAB-CHMINACA (N-[(2S)-1-amino-3,3-dimethyl-1-oxobutan-2-yl]-1-(cyclohexylmethyl) indazole-3-carboxamide) and cocaine hydrochloride were provided by the National Institute on Drug Abuse Drug Supply Program. Rimonabant was obtained from Cayman Chemical (Ann Arbor, MI). All drugs were dissolved in ethanol/Cremophor EL/0.9% saline (in a ratio of 1:1:18) and were administered i.p. in a volume of 1 ml/kg. Cremophor EL was obtained from Sigma Aldrich (St. Louis, MO).
Data Analysis
Drug discrimination data were expressed as the mean percentage (± standard error) of drug-appropriate responses occurring in each test period. Rate of responding was calculated by dividing the total number of responses for each rat tested by the session time. Response rate data are expressed as the mean (± standard error) of all rats tested. Because response suppression may compromise stimulus control, rats failing to complete at least 10 responses during the test session were excluded from the analysis of the discriminative stimulus effects of that dose of test compound. If three or more of the rats did not complete the first reinforcer at a given dose, the discrimination data for that dose is not shown. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio, whereas all rats are shown for the response rate data.
Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. The potencies of BB-22, FUB-PB-22, 5F-AMB, NM2201, and MAB-CHMINACA were calculated by fitting straight lines to the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). Straight lines were fitted to the linear portion of dose-effect curves, including not more than one dose producing <20% of the maximal effect and not more than one dose producing >80% of the maximal effect. Other doses were excluded from the analyses. Response-rate data were analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The criterion for significance was set a priori at p<0.05.
Results
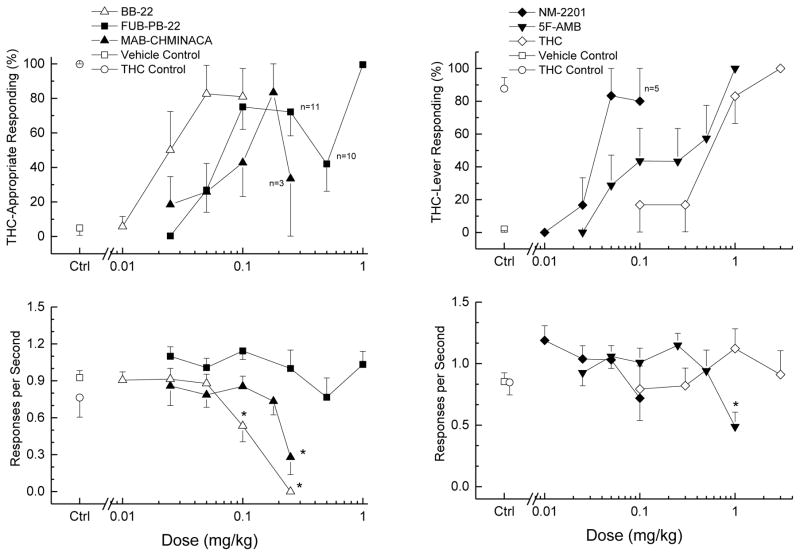
BB-22, FUB-PB-22, 5F-AMB, NM2201, and MAB-CHMINACA produced full substitution for the discriminative stimulus effects of Δ9-THC, although MAB-CHMINACA produced an inverted U-shaped dose-effect function. Dose-effect curves are illustrated in Figure 2, ED50 values are shown in Table 1, and time course functions are illustrated in Figure 3. The slopes of the dose-effect curves were not parallel F(5,41)=2.84; p=0.027) so the ED50 values may not be comparable. The slopes for FUB-PB-22 and 5F-AMB were significantly lower than those for BB-22 and NM-2201 (p<0.05). Cocaine failed to substitute for THC (data not shown), producing a maximum of <1% drug-appropriate responding at 10 mg/kg, and almost completely suppressing responding at 25 mg/kg F(2,10)=55.83; p<.001.
Fig. 2.
Substitution for the discriminative stimulus effects of Δ9-THC: Dose-Effect. Upper panels show mean percentage of total responses (± standard error of the mean) made on the drug-appropriate lever as a function of dose, for doses with three or more rats completing the first fixed ratio. Bottom panels show rate of responding (± standard error of the mean) in responses per second (r/s). All of the cannabinoids fully substituted for the discriminative stimulus effects of Δ9-THC (>80% drug-appropriate responding). Sample size for BB-22, MAB-CHMINACA, NM2201, and 5F-AMB was n=6 except where shown; for FUB-PB-22, sample size was n=12. Ctrl indicates vehicle and drug control. * indicates response rate different from vehicle control (p < 0.05).
Table 1.
ED50 values (mg/kg) for the locomotor depressant effects in mice and the discriminative stimulus effects of cannabinoids in Δ9-THC-trained rats. Data are depicted as mean with 95% confidence interval.
| Drug | Locomotor Activity | Drug Discrimination |
|---|---|---|
| Δ9-THC | 12.46 (1.09 to 142.75) | 0.61 (0.08 to 4.72) |
| BB-22 | 0.06 (0.01 to 0.39) | 0.03 (0.004 to 0.15) |
| FUB-PB-22 | 0.07 (0.01 to 0.33) | 0.13 (0.001 to 7.45) |
| 5F-AMB | 0.61 (0.15 to 2.43) | 0.19 (0.001 to 9.39) |
| NM-2201 | 0.29 (0.12 to 0.67) | 0.03 (0.01 to 0.16) |
| MAB-CHMINACA | 0.08 (0.01 to 0.65) | 0.07 (0.01 to 0.91) |
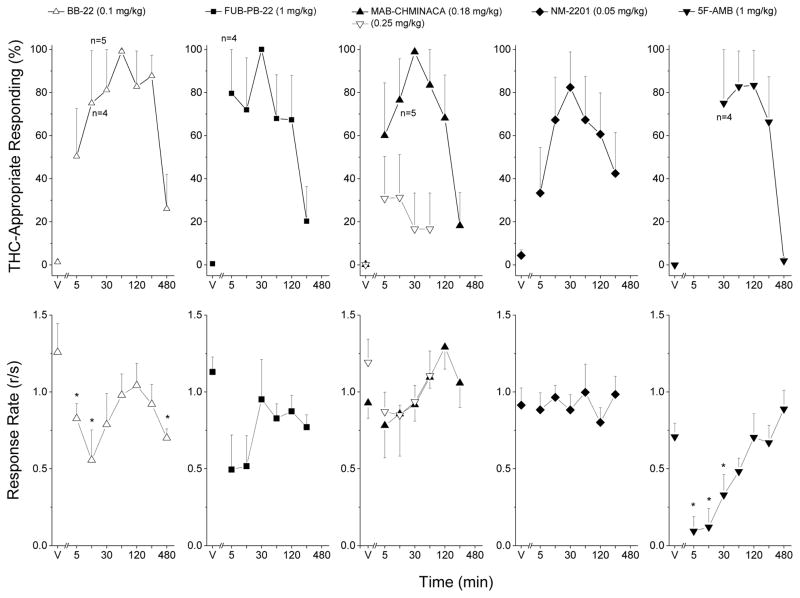
Fig. 3.
Substitution for the discriminative stimulus effects of Δ9-THC: Time-course. Upper panels show mean percentage of total responses (± standard error of the mean) made on the drug-appropriate lever as a function of time, for doses with three or more rats completing the first fixed ratio. Bottom panels show rate of responding (± standard error of the mean) in responses per second (r/s). Cannabinoids, n=6 except where shown. V indicates the vehicle control values. * indicates response rate different from vehicle control (p < 0.05).
BB-22 (30-min pretreatment) fully substituted for the discriminative stimulus effects of Δ9-THC at 0.05 and 0.1 mg/kg. BB-22 decreased response rate following 0.1 and 0.25 mg/kg doses F(5,25)=29.98, p<.001, and completely suppressed responding in all 6 rats following 0.25 mg/kg. Similarly, 0.1 mg/kg BB-2 fully substituted from 30 to 120 min during the time course study. Peak effect was observed at 60 min. Effects had diminished by 4 h after administration. Response rate was decreased such that 2 of 6 rats failed to complete the first reinforcer 15 min after administration, and 1 of 6 rats failed to complete the first reinforcer 30 min after administration of BB-22 F(7,35)=4.123, p=0.002.
FUB-PB-22 (30-min pretreatment, n=12) fully substituted for the discriminative stimulus effects of Δ9-THC at 1 mg/kg without affecting rate of responding. In the time course study, 1 mg/kg FUB-PB-22 fully substituted for Δ9-THC at 30 min, and effects had diminished by 4 h after administration. Responding was decreased at 5 and 15 min after administration F(6,30)=2.534, p=0.042.
MAB-CHMINACA (30-min pretreatment) produced an inverted-U shaped dose effect such that full substitution for the discriminative stimulus effects of Δ9-THC was observed at 0.18 mg/kg, whereas a higher dose (0.25 mg/kg) produced only 34% drug-appropriate responding. MAB-CHMINACA decreased response rate at 0.25 mg/kg such that 3 of 6 rats did not complete the first reinforcer F(5,25)=4.06, p=.008. In the time course study, 0.18 mg/kg fully substituted at 30 and 60 min after administration, with peak effect following 30 min. Response rate was not affected at any time point following 0.18 mg/kg MAB-CHMINACA. A time course of 0.25 mg/kg was also conducted to confirm the low level of drug-appropriate responding. Peak effect was 31% drug-appropriate responding, which is similar to the peak effect observed in the dose-effect experiment (Fig. 4).
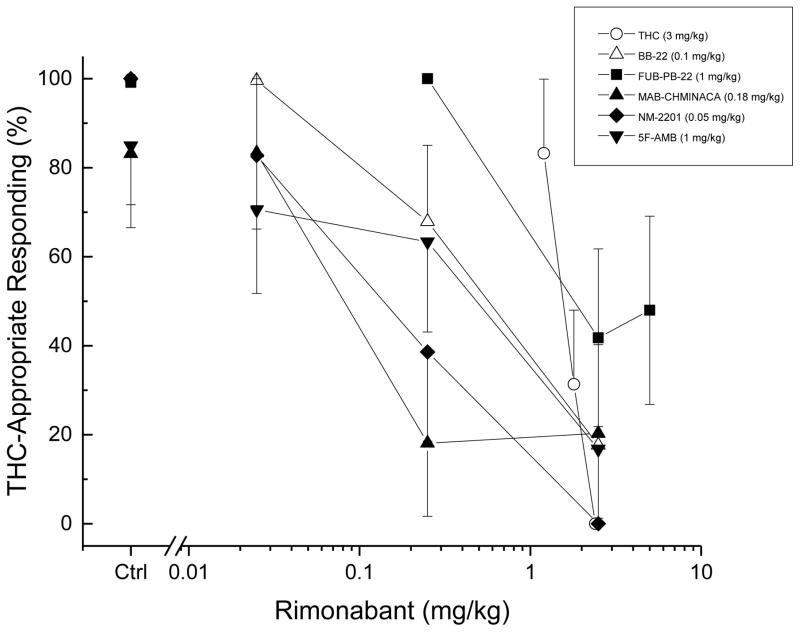
Fig. 4.
Antagonism of the discriminative stimulus effects of BB-22, FUB-PB-22, MAB-CHMINACA, NM2201, and 5F-AMB by the CB1 receptor inverse agonist rimonabant. The figure shows show mean percentage of total responses (± standard error of the mean) made on the drug-appropriate lever as a function of dose of rimonabant, for doses with three or more rats completing the first fixed ratio. Figure legend shows the dose of cannabinoid tested. Cannabinoids, n=6 except where shown. Ctrl indicates the effects of cannabinoid alone.
NM-2201 (30-min pretreatment) fully substituted for the discriminative stimulus effects of Δ9-THC at 0.05 and 0.1 mg/kg without affecting rate of responding. In the time course study, effects of 0.05 mg/kg peaked at 30 min and diminished by 2 h after administration. Rate of responding was not affected at any time point following 0.05 mg/kg NM-2201.
5F-AMB (60-min pretreatment, n=7) fully substituted for Δ9-THC at 1 mg/kg. Response rate was decreased following 1 mg/kg 5F-AMB F(6,36)=5.352, p<.001. In the time course study, 1 mg/kg 5F-AMB fully substituted at 60 to 120 min following administration. Responding was suppressed in 5 of 6 rats at 5 and 15 min following administration, and in 2 of 6 rats at 30 min F(6,30)=7.653, p<0.001. In a preliminary experiment, 3 of 5 rats failed to complete the first reinforcer following 2.5 mg/kg (30-min pretreatment), and convulsions were observed in 2 of 5 rats.
Rimonabant (1.2 to 2.4 mg/kg) dose-dependently blocked the discriminative stimulus effects of the training dose of Δ9-THC (Figure 6). Rimonabant (0.025 to 2.5 mg/kg) dose-dependently antagonized the ability of the peak doses of BB-22 (0.1 mg/kg), 5F-AMB (1 mg/kg), NM2201 (0.05 mg/kg), and MAB-CHMINACA (0.18 mg/kg) to substitute for the discriminative stimulus effects of Δ9-THC. In contrast, rimonabant 2.5 and 5 mg/kg only reduced drug-appropriate responding produced by FUB-PB-22 (1 mg/kg) to 42–48%. The slopes for the synthetic cannabinoids were parallel to each other; however, the slope for Δ9-THC was 2-fold steeper.
Discussion
All five of the test compounds fully substituted for the discriminative stimulus effects of Δ9-THC. Evidence for the full substitution of the test compounds for Δ9-THC was robust, as full substitution was observed in the time course and antagonism studies using separate groups of rats at the same doses and time points as observed in the dose-effect studies. Adverse effects were not observed up to doses that fully substituted; however, a higher dose of 5F-AMB (2.5 mg/kg) produced convulsions. These findings agree with earlier findings that synthetic cannabinoids fully substitute for the discriminative stimulus effects of Δ9-THC in rats (Gatch and Forster 2014, 2015, 2016, Järbe et al. 2011; Wiley et al. 2014), mice (Wiley et al. 2013, 2015) and monkeys (Ginsburg et al. 2012). In general, the cannabinoids tested have not produced adverse effects at doses which fully substitute, although one other compound, JWH-210, produced tremors (Gatch et al., 2016). These compounds come from several different structural classes with a wide range of structural substitutions, yet all act at CB1 receptors and produce full substitution for Δ9-THC. Taken together, these findings suggest that constructing novel cannabinoid molecules to evade detection and control by law enforcement will continue to be easy and profitable.
However, it should be noted that MAB-CHMINACA and FUB-PB-22 produced nonlinear dose-effect curves. MAB-CHMINACA produced an inverted U-shaped dose effect in the dose-effect study, such that the 0.25 mg/kg dose produced lower Δ9-THC-appropriate responding than 0.18 mg/kg. As previously noted, MAB-CHMINACA 0.18 mg/kg fully substituted in the dose-effect, time-course and antagonism studies in separate groups of rats. The decrease in Δ9-THC-appropriate responding following 0.25 mg/kg was replicated in a time course study using a different group of rats.
FUB-PB-22 produced a peak that nearly reached full substitution, followed by a sharp decrease in drug-appropriate responding, and then nearly 100% drug-appropriate responding at the top dose. In a previous study, RCS-4 produced a similar effect, although the initial peak was much lower (Gatch et al. 2016). Both FUB-PB-22 and RCS-4 produced a wide range of variability in the middle doses, ranging from 40% to 83% drug-appropriate responding. For this reason, a sample size of 12 was reported for the FUB-PB-22 data in the present study.
It is not clear why FUB-PB-22, MAB-CHMINACA and RCS-4 produced non-linear dose-effect curves. It would be convenient if they possessed a common structural moiety that conferred the effects; however, the three compounds come from different structural classes of cannabinoids and are more structurally similar to compounds that produced linear, full substitution than they are to each other. It is also possible that the descending limb of the MAB-CHMINACA was due to the rate-decreasing effects at that dose. However, the rate-decreasing effects were not observed during the time-course experiment, but the drop in Δ9-THC-appropriate responding was still present.
The Δ9-THC-like discriminative stimulus effects produced by the five test compounds in the present study were attenuated by rimonabant (2.5 mg/kg). This is consistent with earlier findings that the discriminative stimulus effects of synthetic cannabinoids are mediated by CB1 receptors (Ginsberg et al. 2014; Hruba and McMahon 2017; Rodriguez and McMahon 2014). However, the effects of FUB-PB-22 were only partially attenuated. The dose-effect functions of rimonabant in blocking the discriminative stimulus effects of the test compounds had similar slopes; however, the slope of rimonabant antagonism of Δ9-THC was significantly steeper, which may limit the validity of direct comparison of potencies.
In our previous reports, adverse effects were not observed following most of the synthetic cannabinoids at the doses tested, although JWH-210 produced tremors in mice (Gatch et al. 2014, 2015, 2016). In the present study, 5F-AMB produced sustained vocalization and convulsions when tested in rats at 2.5 mg/kg. As previously mentioned, 5F-AMB and MAB-CHMINACA produce serious adverse effects in humans, including lethality (Hasegawa et al. 2015a, 2015b, Katz 2016; Shanks and Behonick 2016). Adverse effects besides suppression of responding were not observed in the present study during testing of the other synthetic cannabinoid compounds, but there is a possibility that adverse effects would be observed if higher doses were tested. Because the endpoint of interest was substitution for the discriminative stimulus effects of Δ9-THC, doses were not tested once full substitution was observed.
In summary, these compounds have substantial abuse liability, since their chemical structures (fig. 1) are similar to those of abused cannabinoids, they bind to CB1 receptors and are high-efficacy agonists (Banister et al. 2016; De Luca et al. 2016; Hess et al. 2016), and data from the present study indicate that they produce discriminative stimulus effects very similar to those of Δ9-THC and other synthetic cannabinoid compounds known to be abused. Whether the synthetic cannabinoids produce effects at other receptors that may contribute to their abuse liability or adverse effect profile has not been determined. The inverted U-shaped dose-effect curve produced by MDA-CHMINACA may indicate that it has a very narrow window of Δ9-THC-like effects and may be of less interest to recreational users. In general, the compounds tested in the present study produced full Δ9-THC-like effects at doses that did not produce adverse effects. However, given that 5F-AMB produced toxicities at a higher dose in the present study and has produced substantial toxicity in recreational users, inadvertent overdosing of these compounds may be more dangerous than using marijuana.
Acknowledgments
Funding was provided by the Addiction Treatment Discovery Program of the National Institute on Drug Abuse for the behavioral data (NIH N01DA-13-8908). Program staff was involved in selection of compounds and test parameters. The ATDP had no further role in study design; the collection, analysis, and interpretation of data; or the writing of the report. They have granted permission for the submission of this data for publication.
Footnotes
Compliance with ethical standards All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
References
- Adamowicz P, Gieroń J. Acute intoxication of four individuals following use of the synthetic cannabinoid MAB-CHMINACA. Clin Toxicol (Phila) 2016;54:650–4. doi: 10.1080/15563650.2016.1190016. [DOI] [PubMed] [Google Scholar]
- Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, Mack JB, Glass M, McGregor IS, Connor M, Kassiou M. Pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem Neurosci. 2016;7:1241–54. doi: 10.1021/acschemneuro.6b00137. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther. 2012;340:787–800. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Castelli MP, Loi B, Porcu A, Martorelli M, Miliano C, Kellett K, Davidson C, Stair JL, Schifano F, Di Chiara G. Native CB1 receptor affinity, intrinsic activity and accumbens shell dopamine stimulant properties of third generation SPICE/K2 cannabinoids: BB-22, 5F-PB-22, 5F-AKB-48 and STS-135. Neuropharmacology. 2016;105:630–8. doi: 10.1016/j.neuropharm.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: temporary placement of the synthetic cannabinoid MAB-CHMINACA into Schedule I. Final order. Fed Regist. 2016;81:6171–5. [PubMed] [Google Scholar]
- Erdozain AM, Diez-Alarcia R, Meana JJ, Calladoa LF. The inverse agonist effect of rimonabant on G protein activation is not mediated by the cannabinoid CB1 receptor: Evidence from postmortem human brain. Biochem Pharmacol. 2012;83:260–268. doi: 10.1016/j.bcp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav Pharmacol. 2014;25:750–757. doi: 10.1097/FBP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids found on the gray market. Behav Pharmacol. 2015;26:460–8. doi: 10.1097/FBP.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology. 2016;233:1901–1910. doi: 10.1007/s00213-016-4237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24(5–6):437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O. Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol. 2015a;33:45–53. [Google Scholar]
- Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O. Postmortem distribution of MAB-CHMINACA in body fluids and solid tissues of a human cadaver. Forensic Toxicol. 2015b;33:380–387. doi: 10.1007/s11419-015-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE. Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice. Forensic Toxicol. 2016;34:329–343. doi: 10.1007/s11419-016-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:410–436. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Hruba L, McMahon LR. Apparent affinity estimates and reversal of the effects of synthetic cannabinoids AM-2201, CP-47,497, JWH-122, and JWH-250 by rimonabant in Rhesus monkeys. J Pharmacol Exp Ther. 2017;362:278–286. doi: 10.1124/jpet.117.240572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Δ(9)-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol. 2011;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz KD, Leonetti AL, Bailey BC, Surmaitis RM, Eustice ER, Kacinko S, Wheatley SM. Case series of synthetic cannabinoid intoxication from one toxicology center. West J Emerg Med. 2016;17:290–4. doi: 10.5811/westjem.2016.2.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol. 2013;16:2273–84. doi: 10.1017/S1461145713000709. [DOI] [PubMed] [Google Scholar]
- Kondrasenko AA, Goncharov EV, Dugaev KP, Rubaylo AI. CBL-2201. Report on a new designer drug: Napht-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate. Forensic Sci Int. 2015 Dec;257:209–13. doi: 10.1016/j.forsciint.2015.08.023. Erratum in Forensic Sci Int. 2016 Apr;261:70. [DOI] [PubMed] [Google Scholar]
- Lobo Vicente J, Chassaigne H, Holland MV, Reniero F, Kolaář K, Tirendi S, Vandecasteele I, Vinckier I, Guillou C. Systematic analytical characterization of new psychoactive substances: A case study. Forensic Sci Int. 2016;265:107–15. doi: 10.1016/j.forsciint.2016.01.024. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Nelson ME, Bryant SM, Aks SE. Emerging drugs of abuse. Emerg Med Clin North Am. 2014;32:1–28. doi: 10.1016/j.emc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Odoardi S, Romolo FS, Strano-Rossi S. A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci Int. 2016;265:116–20. doi: 10.1016/j.forsciint.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, McMahon LR. JWH-018 in rhesus monkeys: Differential antagonism of discriminative stimulus, rate-decreasing, and hypothermic effects. Eur J Pharmacol. 2014;740:151–9. doi: 10.1016/j.ejphar.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks KG, Behonick GS. Death after use of the synthetic cannabinoid 5F-AMB. Forensic Sci Int. 2016;262:e21–4. doi: 10.1016/j.forsciint.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Baranovsky A, Shafran Y. Synthetic cannabinoids as designer drugs: new representatives of indol-3-carboxylates series and indazole-3-carboxylates as novel group of cannabinoids. Identification and analytical data. Forensic Sci Int. 2014;244:263–75. doi: 10.1016/j.forsciint.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Tanda G. Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology. 2016;233:1845–1866. doi: 10.1007/s00213-016-4244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid–related illnesses and deaths. New Eng J Med. 2015;373:103–107. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- Tynon M, Homan J, Kacinko S, Ervin A, McMullin M, Logan BK. Rapid and sensitive screening and confirmation of thirty-four aminocarbonyl/carboxamide (NACA) and arylindole synthetic cannabinoid drugs in human whole blood. Drug Test Anal. 2016 doi: 10.1002/dta.2096. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 2013;31:223–240. [Google Scholar]
- UNODC. Global synthetic drugs assessment: Amphetamine-type stimulants and new psychoactive substances. United Nations; New York, New York: 2014. [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA. Cross-substitution of Δ(9)-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav. 2014;124:123–128. doi: 10.1016/j.pbb.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, Patel PR, Grabenauer M, Moore KN, Thomas BF. AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and potency of novel synthetic cannabinoids in producing Δ9-tetrahydrocannabinol-like effects in mice. J Pharmacol Exp Ther. 2015;354:328–39. doi: 10.1124/jpet.115.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]